Light absorption by aerosols is one of the most uncertain parameters associated with the direct and indirect aerosol effects on climate and is one of the most difficult quantities to measure. This article describes the development of a sensitive method of measuring aerosol absorption at 532 nm with excellent time response (detection limit: 0.08 Mm−1, 60 second average) using photoacoustic absorption spectroscopy. An accurate calibration method (accuracy of 1–2%) at atmospherically relevant absorption levels and independent validation of the photoacoustic technique is presented. An upper limit to the instrument precision for aerosol absorption measurement is ∼6% (2σ, 30 sec) while instrument accuracy is calculated to be ∼5%. A standard for aerosol absorption measurement techniques using well characterized absorbing aerosol is also proposed.
1. INTRODUCTION
1.1. Aerosol Absorption and Climate
Uncertainties in current estimates of climate forcing are at the core of the debate of climate science today. One of the largest uncertainties in global radiative forcing is the contribution from aerosols. Current estimates suggest (CitationIPCC 2001; CitationPenner et al. 2001) that the net cooling caused by the radiative forcing due to direct and indirect effects of aerosol is potentially half to two-thirds the warming caused by greenhouse gases. However the uncertainties quoted for the aerosol contribution are as large as or larger than the estimates themselves (CitationIPCC 2001; CitationSchwartz 2004). Aerosols that scatter and absorb radiation can have a cooling or warming effect depending on the magnitude of the absorption term and the albedo of the underlying surface. Whether cooling or warming results, the magnitude of the effect is directly related to the quantity of light an aerosol absorbs. Aerosol absorption is an important contributor to: (1) the aerosol single scatter albedo (ωo) or the ratio of aerosol scattering (σsp) to total extinction (σep), where σep is the sum of scattering and absorption (σap) and (2) Aerosol Optical Depth (AOD), the vertical integration of extinction. Given its contribution to these parameters, it is critical that the absorption term be measured accurately.
1.2. Measuring Aerosol Absorption
The two most common techniques for measuring aerosol absorption are the so-called difference method, where absorption is derived from the difference between extinction and scattering, and from filter based methods that measure the attenuation of a filter accumulating aerosol. An additional technique, although not nearly as wide spread in its application, is photoacoustic absorption spectroscopy. Here, these techniques are assessed briefly.
The combination of instrumentation that provides the best measure of absorption by difference is through cavity ring down spectroscopy to measure aerosol extinction (with accuracies of 2% or better, CitationSmith and Atkinson 2001; CitationStrawa et al. 2003; CitationPettersson et al. 2004) and the integrating nephelometer to measure scattering, which has a reported accuracy of ∼7% (CitationAnderson and Ogren 1998; CitationBond et al. 1999). The major disadvantage of this method is the large uncertainties derived from the propagation of errors through the difference of the two quantities, particularly at low absorption levels in the atmosphere (CitationBond et al. 1999). Filter based methods, including the aethelometer and Particle Soot Absorption Photometer (PSAP) have found widespread application for field measurements of absorption and have been evaluated using the σep–σsp calculated absorption (CitationBond et al. 1999; CitationWeingartner et al. 2003; CitationSheridan et al. 2005). The PSAP and aethalometer measurements, having precision somewhere in the range of 20–25% (CitationBond et al. 1999; CitationMagi et al. 2003) and 30–35% (CitationWeingartner et al. 2003; CitationSchmid et al. 2005) respectively, form the basis for most field absorption and albedo data. These filter based techniques require many correction factors that limit the quality of the derived data. The works by CitationBond et al. (1999), CitationAnderson et al. (2003), CitationWeingartner et al. (2003), CitationArnott et al. (2005a), CitationSchmid et al. (2005), and CitationVirkkula et al. (2005) are important references in understanding the strengths and weaknesses of these filter based techniques. The corrections needed for filter based methods have been found to be source specific (CitationWeingartner et al. 2003; CitationSchmid et al. 2005) and determination of these corrections are not easily performed in the field and so general corrections calculated during inter-comparisons are usually applied to all PSAP or aethalometer units.
The photoacoustic technique, which is a direct method that can be easily calibrated, avoids the fundamental difficulties associated with filter based measurements or in situ measurements using the difference of extinction and scattering. The photoacoustic technique has recently been developed into a stable method of measuring aerosol absorption and is fast becoming an accepted standard for comparisons to the filter based methods and the difference method (CitationMoosmuller et al. 1998; CitationArnott et al. 1999, Citation2003, Citation2005a, Citation2005b; CitationSchmid et al. 2005; CitationSchnaiter et al. 2005; CitationSheridan et al. 2005). These photoacoustic systems have reported accuracies of 5–10% depending on laboratory or field locations and a number of methods of calibration. Excluding the calibration methods used for black carbon mass monitors (CitationPetzold and Niessner 1995; CitationKramer et al. 2001) two types of calibrations have been used for photoacoustic instruments measuring aerosol absorption. CitationArnott et al. (2000) measured the acoustic response to the light absorption of NO2 and compared this to the absorption measured using a transmittance measurement. The photoacoustic signal can also be calculated using a theoretical calibration which requires calibrated laser power, measured resonator quality factor and calibrated microphones as described in CitationRosenwaig (1980). Probably the largest issue relating to the application of the photoacoustic technique to aerosol absorption is the potential to evaporate volatile species from the aerosol. Under high RH conditions evaporation may result at the expense of an acoustic response. CitationArnott et al. (2003) and CitationRaspet et al. (2003) investigated this phenomenon for ambient and theoretical aerosols, respectively, and suggest that aerosol be dried below 65–70% RH to avoid evaporation biases.
We draw the following conclusions regarding the current state of aerosol absorption measurements:
| 1. | Uncertainties in the regular field measurement of aerosol absorption need to be reduced so that the quality of related quantities is improved. Continued development of fast, accurate, precise, and direct measurement techniques of aerosol absorption is desired to achieve this end, and to further understand the measurements produced from the current methods; | ||||
| 2. | Field instruments should be calibrated regularly in the field, ideally with an absorbing aerosol standard. This is an important step for filter based methods, so that the uncertainties in these measurements can be further reduced. | ||||
In this manuscript, we describe further development and validation of a photoacoustic spectrometer (PAS) with optimized sensitivity and precision for atmospheric measurements that can significantly reduce the uncertainties in measurements of ambient aerosol absorption. We present a calibration at atmospherically relevant absorption levels (< ∼25 Mm−1) using ozone and a potential aerosol absorption standard consisting of dyed mono-disperse polystyrene spheres. Combining the fast and sensitive photoacoustic technique with fast and sensitive cavity ring down systems will provide a sensitive, well calibrated, low uncertainty instrument configuration to measure aerosol optical properties with particular capabilities of measuring the single scatter albedo with reduced uncertainty.
2. EXPERIMENTAL DETAILS
2.1. Instrument Description
The photoacoustic principle is well described (e.g., CitationMiklos et al. 2001) and significant work has been performed in the application of the technique to gas phase absorption and also describing its theoretical properties (CitationBinjnen 1995; CitationRaspet et al. 2003; CitationRey et al. 2005). Here, the method is summarized briefly. When power modulated light is absorbed by a particle, the energy is released as heat creating pressure waves that propagate away from the particle. In a cavity, these waves resonate at the characteristic radial and longitudinal frequencies of the cavity. When the light source is modulated at a resonant frequency of the cavity, the propagated sound wave is amplified and can be detected by sensitive microphones.
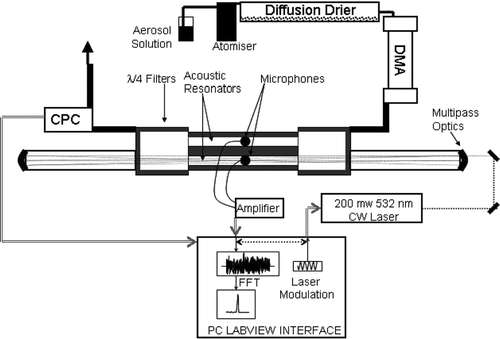
shows a schematic of the photoacoustic system described in this work. It takes aspects of the designs of CitationMiklos et al. (2001), CitationKramer et al. (2001) and CitationNagele and Sigrist (2000). In this instrument 532 nm continuous wave (CW) laser light (200 mw) is introduced into a cavity consisting of two 150 mm long (1/2 λ) 25 mm diameter tubes (resonators) machined into a 100 mm diameter aluminium rod. The sample flow passes through both resonators while the light passes through one. Sensitive microphones (Knowles AcousticsFootnote 1 EK3132) are placed at the center of each tube (anti-node of the sound wave), one detecting background noise, the other detecting background noise and photoacoustic signal. The microphones are attached to a short length of threaded rod which is screwed into the resonator block. The microphones are orientated in the same position relative to one another while the back of the microphone plate is in direct contact with the sample volume via a small channel to allow for pressure equalization. The signals are subtracted and amplified using a differential preamplifier–amplifier combination optimized for the 1–2 KHz region. The main cavity block is capped on both ends with 1/4 λ expanded volumes (75 mm in length and diameter) which act as the boundary for the resonators and as acoustic filters (total sample volume of the instrument is ∼750 cm3). The laser path length is increased through the use of a Herriot cell multi-pass arrangement (CitationHerriot and Schulte 1965) where spherical mirrors (reflectivity >99.5% at 532 nm, 1 m radius of curvature) are placed ∼85 cm apart in line with the signal resonator. The multi-passed light (40-fold increase in path) passes through this resonator. The Herriott cell optics and the photoacoustic cell are separated by anti-reflective coated quartz windows at the expanded volumes. The laser light passes through these windows with a small loss of overall power (∼5%) and negligible contribution to noise within the cell. The amplitude of the laser is modulated electronically with a square wave function at the resonant frequency of the resonator. Laser modulation and reading of the microphones are synchronized so that user-defined sampling periods are triggered from a zero crossing of the modulation signal. A Fast Fourier Transform is applied to the signal and the resulting resonance peak is integrated in the frequency domain.
2.2. Particle Generation
Two types of absorbing aerosol were used in this study. Nigrosin dye particles were generated in a collision type atomizer (CitationLiu and Lee 1975) using a solution of Nigrosin dye (C48N9H51) in deionized water. The particles were dried over 13X molecular sieve. Downstream of the drying stage relative humidity (RH) was monitored and for all experiments was ≤5%; well below the RH at which any effects of evaporation on the acoustic signal are expected (CitationArnott et al. 2003). The size distribution was varied by changing the concentration of the dye introducing an absorbing component to the spheres. The particles were then passed through a custom built Differential Mobility Analyzer, DMA (CitationKnutson and Whitby 1975) producing a size-selected near-monodisperse size distribution. DMA flows were calibrated initially and checked on a regular basis while the performance of the DMA size selection capability was monitored regularly by analysing a range of diameters of non-absorbing polystyrene spheres. Particle concentrations for all experiments were measured using a Condensation Particle Counter. The CPC flow was initially calibrated and then checked on a regular basis using a wet test meter. Number concentrations were stable (variations ≤ ± 5%) over the sampling periods (often ten minutes or more). An initial sample of dried particles was deposited on a conductive filter and analysed using Transmission Electron Microscopy (TEM). shows an example of the micrographs of the atomized and dried Nigrosin solution. All micrographs appeared to show spherical particles. Absorbing Polystyrene Spheres (APSS) were obtained from Duke Scientific.Footnote 2 These aqueous solutions of monodisperse polystyrene spheres are treated with an absorbing dye. Characterization of the spheres is not provided. The aerosol is generated by atomization of the solution. A range of sizes (nominally 150 nm, 300 nm, 450 nm, 600 nm, 800 nm, and 1020 nm) were obtained for this analysis. Again, particles were dried over molecular sieve and the humidity monitored (always ≤5% RH) prior to size selection and interrogation by the instrumentation.
2.3. Uncertainties
Uncertainties in the voltage applied to the DMA, DMA pressure, sheath flows, and sample flows were included in particle size calculations. Cumulatively, there is estimated to be about ±3% uncertainty in the calculated diameter. Uncertainties in extinction measurements were included and PAS calibration uncertainties were also considered. Uncertainties in the particle number concentrations generated in the atomizer (measured by the CPC) and CPC flow rate calibration (± 5%) were considered, as were uncertainties in temperature (±0.25°C) and pressure (±1 Torr). The contributions of multiply charged particles that exit the DMA with the singly charged particles of interest was quantified using a method similar to that described in CitationHoppel (1978) and their optical effect was accounted for in the analysis. Although laser power within the PAS system was not monitored, the repeated stability of gas phase calibrations and monodisperse aerosol over many months of operation indicated a very stable light source.
3. RESULTS AND DISCUSSION
3.1. PAS Calibration
We have used the Cavity Ring-Down Aerosol Extinction Spectrometer (CRD-AES) system developed by CitationPettersson et al. (2004) to measure the gas phase absorption of ozone and calibrate the photoacoustic response. The CRD-AES system uses a pulsed 532 nm laser whereas the PAS system uses a CW 532 nm laser at slightly different wavelength (Δλ = 0.1 nm). Ozone was chosen as the calibration gas because its broadband absorption structure around 532 nm minimizes any potential absorption differences derived from the two lasers (calculated to be ∼0.1% difference using cross section data from CitationBurkholder and Talukdar 1994). Also, the ozone calibration technique is suitable for field application because ozone is easy to generate (a commercially available mercury lamp generator was used in this study) and quantify.
3.1.1. Resonant Frequency and Quality Factor
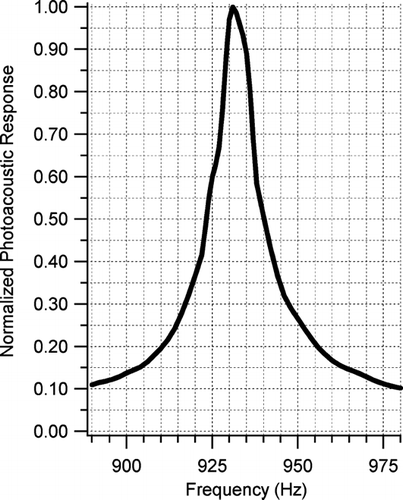
The resonant frequency of the photoacoustic cavity changes as temperature and gas density change. In this laboratory system, oxygen and ozone are introduced into the cell and the amplitude of the photoacoustic response due to ozone absorption is measured across a frequency range surrounding the expected fundamental resonant frequency (FR). shows these measurements and pinpoints the resonant frequency during an ozone calibration as 932 Hz. The resonant cavity quality factor (Q = FR ÷ FWHM) is determined to be 52. This Q is low for a resonant cavity and hence the acoustic signal sensitivity to changes in temperature and composition of the sample is small. Using the relation outlined in CitationMiklos et al. (2001) a 1°C change in temperature will cause just less than a 1% change in measured signal for this resonator. It was also calculated that RH changes of 10% will cause a 1% change to the measured signal (due to gas density changes). For this study, the resonant frequency was stable; temperatures did not vary more than ±0.25°C and the RH of the system varied less than ±2%.
3.1.2. PAS Response Calibration
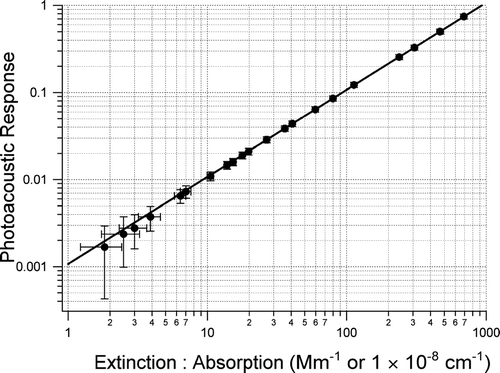
The photoacoustic system was calibrated with ozone at known optical absorption levels measured with the cavity ring down technique. Ozone loss through the CRD-AES and PAS systems was negligible (<1% loss through instrument surfaces and plumbing). The CRD-AES system measured extinction to an accuracy and precision of better than 1%; this sensitivity enabled accurate calibration of the PAS system across 3 orders of magnitude down to levels approaching the instrument sensitivity. depicts the calibration using the CRD-AES system and shows a linear response of the PAS system across this large absorption range. The precision in this calibration (2σ in the calculated slope) is 0.09% across the full range and 0.39% across the atmospherically relevant range (<∼25 Mm−1). Combining this precision for the gas phase calibration and the accuracy in the CRD-AES measurement gives a total accuracy for the PAS instrument, for the gas phase calibration of 1 to 2%.
CitationArnott et al. (2000) calibrated their aerosol photoacoustic instrument by measuring the light transmission through the photoacoustic cell and the photoacoustic response in the presence of NO2. They used NO2 concentrations that gave gas phase absorption levels of ∼ 1.5 × 105 Mm−1. This method assumes linearity in the calibration down to the detection limit of the instrument. They have also compared their calibration method to the difference between aerosol extinction and scattering (CitationSheridan et al. 2005) and the theoretical calibrations outlined by CitationRosenwaig (1980). The agreement between these three methods provides confidence in the gas phase absorption calibration and that saturation at high levels used for calibration and other potential sources of non-linear dependencies are minimal. The calibration presented here covers a range extending from near the instrument detection limit up through atmospherically relevant values, and confirms the linear dependence at levels nearing the detection limits of the currently available instrumentation. The precision of the calibration is also a significant advance in minimizing uncertainties in absorption measurements. This calibration method can also utilize other methods of measuring ozone if a CRD-AES system is not available. Limited calibration trials using a commercial ozone monitor were performed and compared to the CRD-AES calibration. These calibrations were similar suggesting that commercial ozone monitors for calibration could be used for calibrations (ozone absorbs at 0.007 Mm−1/ppb at STP using cross section data of CitationBurkholder and Talukdar (1994)).
3.2. Sensitivity
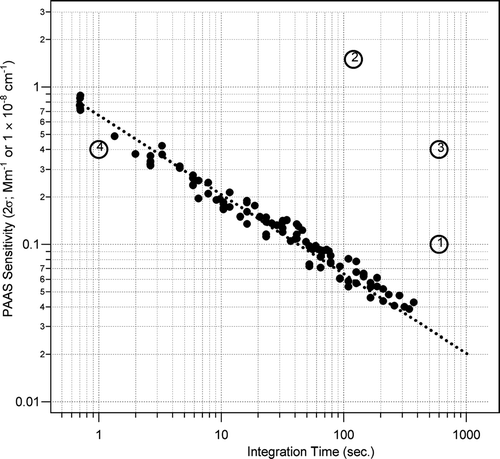
Using the calibration from the PAS laboratory sensitivity was determined (defined as 2σ in background signal). The instrument sensitivity was measured for a range of integration times and flow rates between 1 to 5 LPM. shows a modified Allan Variance plot, where variance has been converted to absorption using the calibration from . The small variability around the fitted slope indicates long term (3 months) stability in microphone response, small differences in laboratory ambient noise and PAS insensitivity to sample flows up to 5 LPM. indicates a PAS sensitivity of ∼ 0.8 Mm−1 for a 1 second integration time and ∼0.08 Mm−1 for a 60 second integration time. also shows some reported sensitivities for other instrumentation measuring aerosol absorption. A significant contribution to the improvement in sensitivity for this instrument, compared to literature values 1, 2, and 3 from , is the effective laser power. This system uses 40 passes of a 200 mW laser, resulting in 8 W of effective power. The large mass surrounding the resonators, expanded volumes and differential measurement also combine to improve sensitivity by significantly reducing ambient noise. Under conditions where ambient noise was minimized as much as possible, flow noise from flows up to 5 LPM contributed about 15% of the noise while the background electronic noise of the microphones (∼ 80%) appeared to limit the instrument sensitivity. Amplifier electronic noise contributed the remaining 5%. (CitationArnott et al. 2006, point 4 in ) reported uncertainties of 0.4 Mm−1 for a 1 second integration time for their most recent photoacoustic system. This improvement relative to their previous system was due to increases in laser power.
FIG. 5 Modified Allan Variance plot showing PAS sensitivity relative to other techniques. Dashed line shows theoretical profile of noise scaled to 1/√ N. Numbered points indicate literature sensitivities values for various instruments measuring aerosol absorption. 1: PSAP (CitationBond et al. 1999), 2: multi-angle absorption photometer (MAAP) (CitationPetzold et al. 2005), 3: Photoacoustic (CitationArnott et al. 1999), 4: Photoacoustic (CitationArnott et al. 2006).
3.3. Validation Using Nigrosin Dye
Nigrosin is a water soluble black dye (C48N9H51) that has been used previously to generate absorbing aerosol for instrument comparisons (CitationBond et al. 1999) and has a reported refractive index of 1.67 + 0.26i (CitationGarvey and Pinnick 1983). The PAS response was validated using atomized and dried water soluble Nigrosin dye particles and a laboratory technique similar to that employed by CitationPettersson et al. (2004). First, the performance of the experimental configuration, source generation and size selection was confirmed using CRD-AES and non-absorbing polystyrene spheres. The tests performed showed results consistent with the published refractive index for polystyrene (Real RI of 1.59, CitationBoundy et al. 1952) and Mie theory. The size dependence of extinction and absorption for Nigrosin particles was measured by size selecting Nigrosin aerosol and passing this flow through the CRD-AES, PAS, and CPC system.
shows the linear correlation between aerosol extinction () (absorption ()) and number concentration (αep = σepNep; (αap = σapNp)) for three selected Nigrosin particles sizes. Individual extinction and absorption values are the average of 30 points of 1 second data. shows the linear regression analysis of each size particle interrogated. The slope of these weighted linear regressions represent the averaged extinction (σep) or absorption (σap) optical cross section for that size particle. The standard deviation (σ) of these cross sections is also shown in brackets next to the cross section value. The precision within each of these cross sections (2σ) is also shown in and gives a representation of PAS instrument precision. This precision value incorporates the precision from the gas phase calibration and aerosol number concentration measurements. For large particles, generating stable and sufficient number concentrations is difficult, thus creating larger measurement uncertainties. For particles up to 950 nm diameter the precision of PAS was 6% or better. This reported precision (2σ, 30 sec) is considered an upper limit for measuring aerosol absorption using PAS as it is dominated by the uncertainty from aerosol number concentrations (ozone calibration contributes a small uncertainty). Aerosol extinction and aerosol absorption optical cross sections obtained from the slopes of the linear fits (e.g., ) were converted to Q values; the efficiency of a particle to absorb, scatter or extinguish light relative to its geometrical cross sectional area. Optical cross sections were divided by the geometrical cross section of the particles to calculate QEXT and QABS, after the effect of multiply charged particles was removed. shows the experimental QEXT and QABS values measured for dry Nigrosin dye particles. The TEM microscopy of the Nigrosin particles appeared to show particles that were spherical (). Using this as the basis for assuming spherical particles, Mie theory was used to find the best fit values of the real (RIREAL) and imaginary (RIIMAG) refractive index that fit the QEXT data. Chi square minimization with RIREAL and RIIMAG as the only free parameters returned a best fit refractive index of 1.70 (±0.04) + 0.31i (±0.05i). Quoted uncertainties are one standard deviation (1σ) calculated from the chi square minimization as detailed in (CitationPress et al. 1986, p. 533). A non-spherical particle is known to “wash out” the resonance structure in extinction and scattering and the deviations between experimental and theoretical QEXT at larger diameters is consistent with a small non-spherical component to the aerosol at these sizes. The best fit refractive index compares favorably to the previously reported RI of 1.67 + 0.26i reported by CitationGarvey and Pinnick (1983), although no uncertainties in these values were reported. The similarity in the refractive indices provides further confidence in the CRD-AES system and allows us to base further analysis on the CRD-AES. Using the best fit RI values, Mie theory was used to calculate the expected QABS. This is overlaid against the experimental QABS values in and shows excellent agreement and is an independent validation of the photoacoustic technique as applied to aerosol absorption. The average deviation of the experimental QABS from the modeled QABS gives an indication of PAS accuracy related to measurement of aerosol absorption. This provides an accuracy value of 4.5% although this value is dominated (50%) by one large deviation at the smallest diameter. The source of this deviation is unknown, although the corrections for multiply charged particles are largest at this diameter.
FIG. 6 Nigrosin Aerosol Extinction (a) and Absorption (b). Response to Aerosol Number Concentrations. Particle sizes presented here are 850 nm (•), 650 nm (♦), 450 nm (⊗). Aerosol number concentration ranges were dictated by atomization of the dye solution.
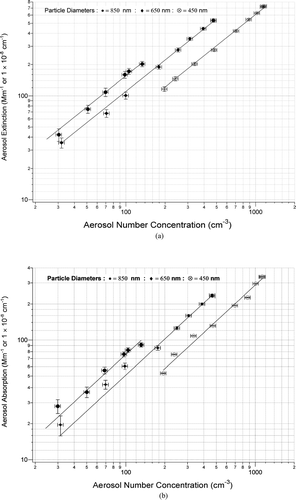
FIG. 7 Experimental QEXT (▪) and QABS (▴) measured using CRD-AES and PAS, respectively. Solid line through QEXT data indicates the best fit to the real and imaginary refractive index using Mie Theory (1.70 + 0.31i). Solid line through QABS data is the independent Mie Theory calculated QABS from the QEXT best fit. Dashed lines indicate QEXT and QABS calculated using the literature refractive indices for Nigrosin from CitationGarvey and Pinnick (1983).
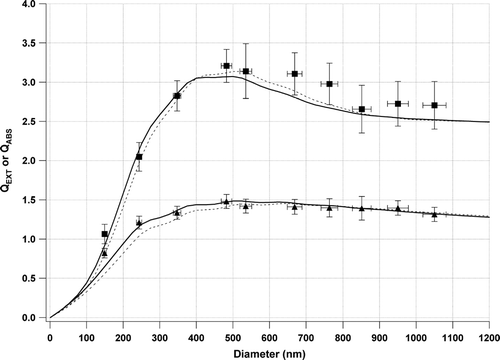
TABLE 1 Precision in extinction and absorption data for nigrosin aerosol using 30 seconds of data for both extinction and absorption
3.4. Standardized Absorbing Aerosol
An ideal calibration for any method of measuring aerosol absorption would be well characterized and easily generated absorbing aerosol. Most instrumentation for measuring aerosol absorption use calibration methods that do not initially involve aerosol and are then compared to other methods using a common aerosol, usually laboratory generated black carbon. This method has provided the basis for many inter-comparisons. However, the fractal nature of the particles and lack of characterizability of the particles prior to use limits it ability to reduce uncertainties in absorption measurements. In an effort to develop a direct absorbing aerosol standard and simplify the calibration of absorbing aerosol instrumentation, and perhaps improve the accuracy of calibrations, samples of dyed mono-disperse polystyrene spheres were obtained (APSS). Solutions were atomized and passed through the DMA producing a nearly monodisperse absorbing aerosol. The CRD-AES, PAS, and CPC system was used to measure total aerosol extinction, absorption and number concentrations. Again, comparisons between aerosol number concentration and PAS absorption () show a linear correlation with a maximum precision (2σ, 30 sec) of 6.7%, within the linear fit across the diameter range of 150–800 nm. This includes uncertainties due to aerosol number concentrations and sampling fluctuations. shows the optical cross sections derived from the weighted linear regressions and the measured precision in the measurements. QEXT values were again calculated and used in a refractive index fitting analysis similar to that used for Nigrosin dye particles. In this analysis, a single refractive index that provided a good fit the Q data (real and imaginary) could not be determined. This suggested that the dye did not penetrate completely through the larger particles, indicating a refractive index that varied with particle size. Despite this, the absorption optical cross sections for each particle size can be repeatabley measured to high precision which could then be used individually to calibrate the PAS or any other absorption measurement instrument. Also, the range of single scatter albedo values across the particle size range (presented in ) will be of value in investigating possible albedo dependence of some absorption measurement methods. This calibration tool is tied to the original CRD-AES/PAS ozone calibration, and will be dependant on the particular APSS batch from the manufacturer. This method will allow for assessment of instrument response to aerosol rather than a gas or mass calibration. Considering the high precision in the ozone calibration, it is anticipated that any calibration using DMA size-selected APSS will be limited by uncertainties in the particle number concentrations. Non-size-selected APSS could very easily be used as a comparative tool between other instruments. The advantage of these procedures would be to allow laboratory standardized APSS that can be simply generated, to be used for regular field calibrations of instrumentation measuring absorption. The APSS would also have the following advantages: (1) allows calibration using particles. The APSS aerosol eliminates any uncertainties that may be introduced due to volatile species on soot particles, the fractal nature of soot or the subtle differences in soot optical properties produced from different fuels and fuel ratios. (2) Allows interrogation of the absorption, scattering and extinction response of instrumentation using Mie theory, given the characterized spherical particles. This may aid in investigations into scattering corrections (including particle stacking) for filter based methods.
FIG. 8 Absorption Response of Absorbing Polystyrene Spheres to Aerosol Number Concentrations. Particle sizes presented here are 800 nm (•), 600 nm (♦), 300 nm (⊗).
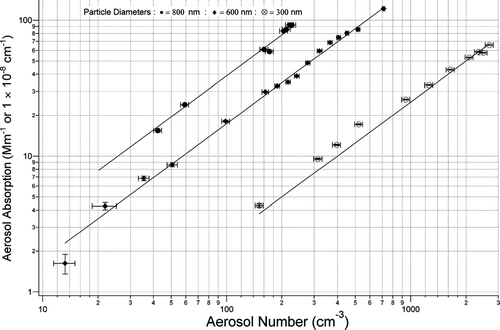
TABLE 2 Measured extinction and absorption for absorbing polystyrene spheres
4. SUMMARY
A fast, sensitive and direct method of measuring aerosol absorption has been developed using the photoacoustic technique. The instrument has been calibrated using ozone and a cavity ring down aerosol extinction spectrometer (CRD-AES). The calibration spans 3 orders of magnitude in signal and includes levels of aerosol absorption most commonly observed in the atmosphere (< ∼25 Mm−1). This calibration explicitly shows the linear dependence between absorption and photoacoustic response at these absorption levels, and provides a gas phase calibration accuracy of 1–2%. The sensitivity of the system has been calculated to be 0.08 Mm−1 for 60 seconds of integration. Here, the photoacoustic technique for measuring aerosol absorption has been independently validated using dry size selected absorbing Nigrosin dye aerosol. The PAS instrument responded to this aerosol as Mie theory predicted. This validation also provides confidence that the mechanism of generating acoustic signal from gas phase species is comparable to that producing acoustic signal from aerosol. The validation step also provides an estimation of the upper limit of the precision of aerosol absorption measurements, and is in the order of 6 % (2σ, 30 sec). This is believed to be dominated (2–3%) by uncertainties in aerosol number concentrations. This validation step also provided an estimate of the overall instrument accuracy, with respect to aerosol absorption, of about 5%. This compares to filter methods having precision of 20–35% (not inclusive of filter scattering response). The validated photoacoustic system was used to calibrate mono-disperse absorbing polystyrene spheres. These measurements confirmed the precision range of the instrument derived from the Nigrosin trials. These calibrated monodisperse dyed polystyrene spheres, can be used as a calibration standard for laboratory and field studies, independent of the refractive index. The precision and sensitivity improvements of this system compared to current methods of measuring aerosol absorption will contribute significantly to reducing the uncertainty in measured aerosol absorption. This system incorporates a number of features that enhances its applicability to aerosol systems. The large diameter resonators reduce the resonator quality (Q) which reduces signal sensitivity to changes in temperature and gas composition. This reduces signal sensitivity, although this sensitivity is returned and further enhanced using a Herriot multi-pass arrangement. This multi-pass configuration provides enhanced sensitivity (8 Watts of effective laser power) while providing only 200 mW of laser power onto a single particle. This minimizes the possibility of evaporation of adsorbed species. Also, the volume sampled by the multi-passed laser increases sampling volume, which contributes to reducing issues with aerosol sampling uncertainties. This instrument has been modified for field measurements and will be used in tandem with a field version of the cavity ring down aerosol extinction spectrometer to significantly improve the quality of absorption and single scatter albedo measurements.
Acknowledgments
This work was funded by NOAA's Climate program. The authors acknowledge Steven Ciciora, Bill Dubé, Richard McLaughlin, and Erik Richards for help in design, development, and construction. Thanks to Thomas Giddings at the University of Colorado for the TEM images.
Notes
1Certain commercial equipment, instruments, or materials are identified in this article in order to adequately specify the experimental procedure. Such identification does not imply recognition or endorsement by the National Oceanic and Atmospheric Administration, nor does it imply that the material or equipment identified are necessarily the best available for the purpose.
2See footnote 1.
REFERENCES
- Anderson , T. L. , Masonis , S. J. , Covert , D. S. , Ahlquist , N. C. , Howell , S. G. , Clarke , A. D. and McNaughton , C. S. 2003 . Variability of Aerosol Optical Properties Derived from In Situ Aircraft Measurements During ACE-Asia . J. Geophys. Res.-Atmos. , 108 ( D23 ) ACE 15-1
- Anderson , T. L. and Ogren , J. A. 1998 . Determining Aerosol Radiative Properties using the TSI 3563 Integrating Nephelometer . Aerosol Sci. Technol. , 29 ( 1 ) : 57 [CSA] [CROSSREF]
- Arnott , W. P. , Hamasha , K. , Moosmuller , H. , Sheridan , P. J. and Ogren , J. A. 2005a . Towards Aerosol Light-Absorption Measurements with a 7-Wavelength Aethalometer: Evaluation with a Photoacoustic Instrument and 3-Wavelength Nephelometer . Aerosol Sci. Technol. , 39 ( 1 ) : 17 [CSA] [CROSSREF]
- Arnott , W. P. , Moosmuller , H. , Rogers , C. F. , Jin , T. F. and Bruch , R. 1999 . Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description . Atmos. Environ. , 33 ( 17 ) : 2845 [CSA] [CROSSREF]
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. , Slaton , W. , Hand , J. L. , Kreidenweis , S. M. and Collet , J. L. Jr. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res. , 106 ( D1 ) AAC 15-1[CSA]
- Arnott , W. P. , Moosmuller , H. and Walker , J. W. 2000 . Nitrogen Dioxide and Kerosene-Flame Soot Calibration of Photoacoustic Instruments for Measurement of Light Absorption by Aerosols . Rev. Scientific Instruments , 71 ( 12 ) : 4545 [CSA] [CROSSREF]
- Arnott , W. P. , Walker , J. W. , Moosmuller , H. , Elleman , R. A. , Jonsson , H. H. , Buzorious , G. , Conant , W. C. , Flagan , R. C. and Seinfeld , J. H. 2006 . Photacoustic Insight for Aerosol Light Absorption Aloft from Meterological Aircraft and Comparison with Particle Soot Absorption Photometer Measurements: DOE Southern Great Plains Climate Research Facility and the Coastal Stratocumulus Imposed Perturbation Experiments . J. Geophys. Res. , 111 D05S02[CSA] [CROSSREF]
- Arnott , W. P. , Zielinska , B. , Rogers , C. F. , Sagebiel , J. , Park , K. , Chow , J. , Moosmuller , H. , Watson , J. , Kelly , K. , Wagner , D. , Sarofim , A. , Lighty , J. and Palmer , G. 2005b . Evaluation of 1047-nm Photoacoustic Instruments in Source-Sampling of Black Carbon Aerosol and Particle-Bound PAHs from Gasoline and Diesel Powered Vehicles . Environ. Sci. Technol. , 39 ( 14 ) : 5398 – 5406 . [INFOTRIEVE] [CSA] [CROSSREF]
- Binjnen , F. 1995 . Refined CO-Laser Photoacoustic Trace Gas Detection; Observation of Anaerobic Processes in Insects, Soil and Fruit , Ph.D. thesis 179 Department of Natural Sciences. Nijmegen, Catholic University of Nijmegen .
- Bond , T. C. , Anderson , T. L. and Campbell , D. 1999 . Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols . Aerosol Sci. Technol. , 30 ( 6 ) : 582 [CSA] [CROSSREF]
- Boundy , R. H. and Boyer , R. F. , eds. 1952 . Styrene. Its Polymers, Copolymers and Derivatives , New York : Reinhold Publishing .
- Burkholder , J. B. and Talukdar , R. K. 1994 . Temperature Dependence of the Ozone Absorption Spectrum over the Wavelength Range 410 to 760 nm . Geophys. Res. Lett. , 21 ( 7 ) : 581 – 584 . [CSA] [CROSSREF]
- Garvey , D. M. and Pinnick , R. G. 1983 . Response Characteristics of the Particle Measuring Systems Active Scattering Aerosol Spectrometer Probe (ASASP-X) . Aerosol Sci. Technol. , 2 : 477 [CSA]
- Herriot , D. R. and Schulte , H. 1965 . Folded Optical Delay Lines . Appl. Optics , 4 ( 8 ) : 883 [CSA]
- Hoppel , W. A. 1978 . Determination of the Aerosol Size Distribution from the Mobility Distribution of the Charged Fraction of Aerosols . J. Aerosol Sci. , 9 : 41 – 54 . [CSA] [CROSSREF]
- IPCC . 2001 . Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change , Cambridge, New York, , UK : Cambridge University Press . IPCC, 2001: Climate Change 2001: The Scientific Basis
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications . J. Aerosol Sci. , 6 ( 6 ) : 443 [CSA] [CROSSREF]
- Kramer , L. , Bozoki , Z. and Niessner , R. 2001 . Characterisation of a Mobile Photoacoustic Sensor for Atmospheric Black Carbon Monitoring . Anal. Sci. , 17 ( S ) : s563 [CSA]
- Liu , B. Y. H. and Lee , K. W. 1975 . An Aerosol Generator of High Stability . Amer. Indust. Hygiene Assoc. J. , 36 : 861 [CSA]
- Magi , B. I. , Hobbs , P. V. , Schmid , B. and Redemann , J. 2003 . Vertical Profiles of Light Scattering, Light Absorption, and Single Scattering Albedo During the Dry Biomass Burning Season in Southern Africa and Comparisons of In Situ Remote Sensing Measurements of Aerosol Optical Depths . J. Geophys. Res. , 108 ( D13 ) SAF 40-1[CSA]
- Miklos , A. , Hess , P. and Bozoki , Z. 2001 . Application of Acoustic Resonators in Photoacoustic Trace Gas Analysis . Rev. Sci. Instru. , 72 ( 4 ) : 1937 [CSA] [CROSSREF]
- Moosmuller , H. , Arnott , W. P. , Rogers , C. F. , Chow , J. , Frazier , C. A. , Sherman , L. E. and Dietrich , D. L. 1998 . Photoacoustic and Filter Measurements Related to Aerosol Light Absorption During the Northern Front Range Air Quality Study (Colorado 1996/1997) . J. Geophys. Res. , 103 ( D21 ) : 28149 – 28157 . [CSA] [CROSSREF]
- Nagele , M. and Sigrist , M. W. 2000 . Mobile Laser Spectrometer with Novel Resonant Multipass Photoacoustic Cell for Trace-Gas Sensing . Appl. Phys. B , 70 : 895 – 901 . [CSA]
- Penner , J. E. , Hegg , D. and Leaitch , R. 2001 . Unraveling the Role of Aerosols in Climate Change . Environ. Sci. Technol. , 35 ( 15 ) : 332A – 340A . [INFOTRIEVE] [CSA]
- Pettersson , A. , Lovejoy , E. R. , Brock , C. A. , Brown , S. S. and Ravishankara , A. R. 2004 . Measurement of Aerosol Optical Extinction at 532 nm with Pulsed Cavity Ring Down Spectroscopy . J. Aerosol Sci. , 35 ( 8 ) : 995 [CSA] [CROSSREF]
- Petzold , A. and Niessner , R. 1995 . Novel Design of a Resonant Photoacoustic Spectrophone for Elemental Carbon Mass Monitoring . Appl. Phys. Lett. , 66 ( 10 ) : 1285 [CSA] [CROSSREF]
- Petzold , A. , Schloesser , H. , Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. and Virkkula , A. 2005 . Evaluation of Multiangle Absorption Photometry for Measuring Aerosol Light Absorption . Aerosol Science and Technology , 39 ( 1 ) : 40 – 51 . [CSA] [CROSSREF]
- Press , W. H. , Flannery , B. P. , Teukolsky , S. A. and Vetterling , W. T. 1986 . Numerical Recipes. The Art of Scientific Computing , New York : Cambridge University Press .
- Raspet , R. , Slaton , W. , Arnott , W. P. and Moosmuller , H. 2003 . Evaporation-Condensation Effects on Resonant Photoacoustics of Volatile Aerosols . J. Atmos. Oceanic Technol. , 20 : 685 [CSA] [CROSSREF]
- Rey , J. M. , Marinov , D. , Vogler , D. E. and Sigrist , M. W. 2005 . Investigation and Optimisation of a Multipass Resonant Photoacoustic Cell at High Absorption Levels . Appl. Phys. B , 80 : 261 [CSA] [CROSSREF]
- Rosenwaig , A. 1980 . Photoacoustics and Photoacoustic Spectroscopy , New York : Wiley .
- Schmid , O. , Artaxo , P. , Arnott , W. P. , Chand , D. , Gatti , D. , Frank , G. P. , Hoffer , A. , Schnaiter , M. and Andreae , M. O. 2005 . Spectral Light Absorption by Ambient Aerosols Influenced by Biomass Burning in the Amazon Basin-1. Comparison and Field Calibration of Absorption Measurement Techniques . Atmos. Chem. Physics Discuss. , 5 : 9355 – 9404 . [CSA]
- Schnaiter , M. , Schmid , O. , Petzold , A. , Fritzsche , L. , Klein , K. F. , Andreae , M. O. , Helas , G. , Thielman , A. , Gimmler , M. , Mohler , O. , Linke , C. and Schurath , U. 2005 . Measurement of Wavelength-Resolved Light Absorption by Aerosols Utilizing a UB-VIS Extinction Cell . Aerosol Sci. Technol. , 39 : 249 – 260 . [CSA]
- Schwartz , S. E. 2004 . Uncertainty Requirements in Radiative Forcing of Climate Change . J. Air and Waste Manage. Associ. , 54 ( 11 ) : 1351 [CSA]
- Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. , Andrews , E. , Atkinson , D. B. , Strawa , A. W. , Varma , R. and Virkkula , A. 2005 . The Reno Aerosol Optics Study: An Evaluation of Aerosol Absorption Measurement Methods . Aerosol Sci. Technol. , 39 ( 1 ) : 1 [CSA] [CROSSREF]
- Smith , J. D. and Atkinson , D. B. 2001 . A Portable Pulsed Cavity Ring-Down Transmissometer for Measurement of the Optical Extinction of the Atmospheric Aerosol . Analyst , 126 : 1216 – 1220 . [INFOTRIEVE] [CSA] [CROSSREF]
- Strawa , A. W. , Castaneda , R. , Owano , T. , Baer , D. S. and Paldus , B. A. 2003 . The Measurement of Aerosol Optical Properties using Continuous Wave Cavity Ring-Down Techniques . J. Atmos. Oceanic Technol. , 20 ( 4 ) : 454 – 465 . [CSA] [CROSSREF]
- Virkkula , A. , Ahlquist , N. C. , Covert , D. S. , Arnott , W. P. , Sheridan , P. J. , Quinn , P. K. and Coffman , D. J. 2005 . Modification, Calibration and a Field Test of an Instrument for Measuring Light Absorption by Particles . Aerosol Sci. Technol. , 39 ( 1 ) : 68 [CSA] [CROSSREF]
- Weingartner , E. , Saathoff , H. , Schnaiter , M. , Streit , N. , Bitnar , B. and Baltensperger , U. 2003 . Absorption of Light by Soot Particles: Determination of the Absorption Coefficient by means of Aethalometers . Aerosol Sci. , 34 : 1455 – 1463 . [CSA] [CROSSREF]