The study describes a 25-mm filter cassette constructed with a plastic masking disk covering the bottom of the cassette cowl, reducing the area of exposed filter by 95%. Two rectangular sample traces, each with a size of 1.7 mm × 5.5 mm (9.35 mm2) were produced. The traces were sufficiently small to be analyzed by direct microscopic examination without requiring a sample transfer step. Direct microscopy of fungal spores using the new Bi-Air (BA) filter cassette was compared to the Air-O-Cell (AOC) slit impaction cassette using Penicillium chrysogenum and Stachybotrys chartarum spores. The comparisons involved the collection of side-by-side samples in a quiescent settling chamber.
The distribution of P. chrysogenum and S. chartarum spores between the duplicate BA sample traces was uniform. The deposition of spores across the sample traces was also similar for the BA and AOC, with a coefficient of variation (CV) between traverses of 27% for the BA and 29% for the AOC. The average retention of Penicillium spores was equivalent for mixed cellulose ester (MCE) filters with pore sizes of 1.2 um and 3 um. However, a 5 um pore size resulted in about a 20% reduction in the average retention for 3 um Penicillium spores. The average ratios of BA to AOC concentrations for P. chrysogenum spores were 2.0, 1.8, and 1.7 at airflow rates of 1 lpm, 2 lpm, and 3 lpm, respectively. The average ratio of BA to AOC concentrations for S. chartarum at the three airflow rates was 1.1.
The edges of the BA sample trace were well defined and bounded, and the sample area did not vary between samples. The constant size of the BA sample trace allowed less than 100% of the sample to be analyzed without affecting the precision of the analysis.
INTRODUCTION
Slit impaction samplers such as the Air-O-Cell (AOC) cassette and Allergenco Sampler (Zefon International, St. Petersburg, FL) are commonly used to collect airborne fungal spores for analysis by light microscopy. They are simple to use, relatively inexpensive, easily analyzed by a large number of laboratories, and widely accepted within the indoor air quality community. A significant advantage of slit impaction samplers is the relatively small area of the sample trace, which allows the sample to be analyzed by direct microscopic examination without requiring a sample transfer step. However, the impaction of fungal spores onto adhesive media also has several limitations as a collection method.
First, impaction samplers are characterized by a cutoff size. Spores with an aerodynamic diameter below the cutoff size are collected with less than 50% efficiency, resulting in the underreporting of those spores. This limitation especially affects Aspergillus/Penicillium (Asp/Pen) like spores, which are frequently encountered as contaminant spores in the indoor environment (CitationMcGrath et al. 1999; CitationEngelhart and Exner 2002; CitationBaxter et al. 2005). Second, impaction samplers are limited to relatively short sampling durations, and may be problematic for the collection of time-weighted average (TWA) or personal samples. The short-term samples collected using impaction samplers are typically more variable then TWA samples, requiring larger sample sizes to discriminate between concentration distributions. In addition to being more variable, short-term samples are generally less representative of the average exposure when the sample size is small (CitationMcDevitt et al. 2005).
A third limitation is that the airflow over the collection medium is generally parallel to the adhesive strip. Spores that rebound from the adhesive surface are captured by an air stream that is moving parallel to the collection medium, reducing the probability they will be retained by the sampler. Therefore, spore morphology as well as spore size may affect collection efficiency. Finally, the relatively high airflow rate of 10–15 lpm typically used with impaction samplers reduces the theoretical aspiration efficiency for larger spores, which may be especially important for the efficient capture of spore clusters (CitationWilleke and Baron 1993).
Filter samplers avoid many of these limitations. They have high collection efficiencies, the collection efficiency is less dependent on spore size and morphology, they can be operated at low airflow rates, the airflow rate can typically be varied to suit the sampling environment, samples may be collected for long periods of time, and they are a common method for collecting personal samples. These characteristics make them a potentially useful collection medium for fungal spores. In fact, filter samplers have been used for the collection of airborne fungal spores for many years (CitationPalmgren et al. 1986; CitationEduard et al. 1990; CitationMcGrath et al. 1999; CitationAizenberg, Grinshpun et al. 2000; CitationEngelhart and Exner 2002; CitationMcDevitt et al. 2005).
The large surface area of filters may offer some advantage when sampling high particulate concentrations, or when sampling dusty environments. But, in general, the large size of the sample trace has been a limitation for the quantitative analysis of spores by direct microscopy. For example, a typical 25-mm filter cassette has an exposed filter area of 380 mm2, whereas a slit impaction sampler produces a sample trace with an area of about 30 mm2 (CitationNIOSH 1994; CitationAizenberg, Reponen, et al. 2000). The large exposed surface area of filters has typically required the spore concentration to be determined using an intermediate sample transfer step, which is prone to sample loss (CitationPalmgren et al. 1986; CitationEduard et al. 1990; CitationKate et al. 2002). A second option was to estimate the concentration by analyzing a small percentage of the total sample area. (CitationAizenberg, Grinshpun et al. 2000; CitationAdhikari et al. 2003; CitationMcDevitt et al. 2005).
For example, the analytical protocol described by Aizenberg, CitationGrinshpun et al. (2000) involved the analysis of 40 microscopic fields of view (FOV) for each filter sample, with each FOV having an area of 0.0025 mm2. This protocol utilized 0.1 mm2 of filter area, equivalent to analyzing 0.026% of the total sample. CitationAdhikari et al. (2003) analyzed 5.8 mm2 of a 25 mm filter, or 1.5% of the total sample area. CitationMcDevitt et al. (2005) analyzed a maximum area of 6.25 mm2, which was equivalent to analyzing a maximum of 1.6% of the collected sample. Each of these protocols involved the analysis of less than 2% of the total filter area, with over 98% of the sample discarded. These examples simply demonstrate the difficulty of using typical filter samplers for the direct microscopy of fungal spores.
Purpose
The large exposed surface area of the filter limits the utility of many filter samplers for the direct analysis of fungal spores by microscopy. The purpose of this study is to describe a new 25 mm filter cassette that does not have this limitation. A plastic masking disk with two rectangular slits covers the bottom of the cassette cowl, limiting sample deposition to the area of the two slits. The slotted disk reduces the area of exposed filter by 95%; and, produces duplicate rectangular sample traces with well defined boundaries. The sample traces produced by the Bi-Air (BA) filter cassette (Bi-Air Corp., Placentia, CA) are sufficiently small to be analyzed by direct microscopic examination without requiring a sample transfer step, thus avoiding one of the primary limitations of many of the available filter samplers.
The utility of the BA filter cassette for the direct microscopic examination of fungal spores has been illustrated by comparing the relative performances of the BA and the AOC slit impaction cassette, which is commonly used for the collection of airborne fungal spores. The relative comparison of the two samplers was based on the collection of the smaller Penicillium chrysogenum spore and the larger Stachybotrys chartarum spore. The comparison involved the collection of side-by-side samples in a quiescent settling chamber. The quiescent settling chamber that was used to conduct the comparisons is briefly described. The chamber was used to evaluate the effect of pore size on the collection efficiency of mixed cellulose ester (MCE) filters. MCE filters with pore sizes of 1.2 μ m, 3 μ m, and 5 μ m were evaluated. Second, the chamber was used to assess the aspiration efficiency of the BA at airflow rates of 1, 2, and 3 lpm. Third, the variation in the distribution of P. chrysogenum spores across the BA and AOC sample traces was also compared.
METHODS
Test Chamber
Samples were collected in a quiescent settling chamber that was similar in concept to the quiescent settling chamber described by CitationFeather and Chen (2003). The chamber was hexagonal, with a height of 122 cm and an interior volume of approximately 2.3 m3. A sample injection port was centered in the top of the chamber, and could be capped after introducing the sample. Two mixing fans with 11 cm diameters were installed near the top of the chamber. The test section was the lower 30 cm of the chamber, which included six sampling ports and a pressure relief port (37 mm open-face cassette) in the floor of the chamber.
Air velocities in the test section of the chamber were measured using a Mannix Air Master air velocity meter. The measurements were made at a total sampling rate of 90 lpm, with all six air sampling pumps operating at 15 lpm. This was the highest sampling rate used in the chamber. The air velocity in the test section was less than 30.5 cm/sec (61 fpm), which was the minimum sensitivity of the air velocity meter.
An Alnor Model 530 Micromanometer (±1 Pa) was used to measure differential pressures within the chamber. An open-face 37 mm filter cassette was positioned in the center of the chamber floor to minimize pressure differentials during sampling. The maximum differential pressure within the chamber at a sampling rate of 90 lpm was −3 Pa with the top port open and −4/5 Pa with the top port sealed.
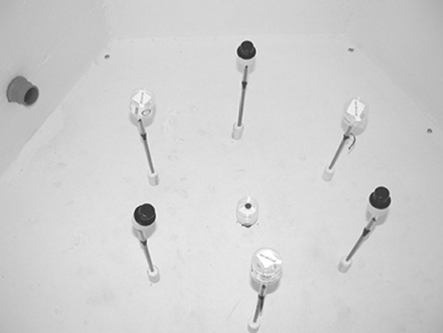
The test section was located at the bottom of the chamber. The positioning of the six sampling ports is illustrated in . The ports were equally spaced, and located a distance of 30 cm from the side panels and 23 cm above the floor of the chamber. Tests were conducted by attaching the test cassettes to the six sample ports and the door of the chamber was closed. The two 12-volt mixing fans (11 cm diameter) and the six air sampling pumps were turned on at t = 0 minutes. The sample inlet in the top of the chamber was opened, and the spores were introduced into the chamber by a rapid series of manual injections using a squeeze-bulb atomizer beginning at t = 0.5 minutes and ending at t = 1 minute. The inlet was typically closed at t = 1.5 minutes, and the two fans were turned off at t = 2 minutes. Sampling times were measured from t = 1 minute. Following completion of the test, the chamber was evacuated by attaching a HEPA-filtered vacuum to a port in the side of the chamber.
FIG. 1 Arrangement of the six sample ports in the test section of the settling chamber, with alternating AOC and BA cassettes attached.
The actual spore concentrations in the test chamber were not measured, although an attempt was made to measure the average number of spores contained in each injection of the alcohol suspension. The test solution was sprayed into the cowl of an open-face 25-mm cassette sampling at 20 lpm. The filter was cut into quarters, and one quarter of the filter was then analyzed at a magnification of 600 × starting at the point of the cut filter and moving towards the perimeter (CitationNIOSH 7400, 1994). However, the spore counts from multiple traverses on the same filter were too variable to provide a reliable average, and this approach was not pursued.
Sample Collection and Analysis
The P. chrysogenum and S. chartarum spores were identified by an outside laboratory and provided as culture plates. The spores were collected from the culture plates with sterile swabs and transferred into 30 ml plastic bottles containing a final volume of 10 ml of 70% isopropanol (USP). Glass beads (4 mm) were added to the test solution in order to mix any settled spores, with the solution being shaken by hand immediately prior to each use. The alcohol preserved the spores without subjecting them to excessive dehydration, and evaporated more rapidly than an aqueous suspension. Dehydration of collected P. chrysogenum spores was not observed until after about four weeks, at which time the spores were no longer used.
The isopropanol test suspension was delivered into the settling chamber using manual injections with a simple squeeze-bulb atomizer purchased at a local pharmacy. The intent had been to use a syringe pump combined with a low-flow nebulizer to deliver the spores. However, during preliminary tests of the chamber design the precision achieved using the simpler and less costly atomizer was sufficient to achieve the objectives of the study. Therefore, this delivery method was used throughout the study. The collected fungal spores were counted at a magnification of 600×.
The samples were analyzed by bright field microscopy using a Nikon Alphaphot microscope (Nikon America, New York) equipped with a 10× eyepiece and a planachromat 60× objective.
The AOC samples were collected at an airflow rate of 15 lpm with the cassette oriented in a vertical position. The sampling pumps were turned on prior to introduction of the spores into the settling chamber, which prevented the passive settling of spores into the vertical inlet of the AOC. The maximum sample collection time for each AOC cassette was limited to 10 minutes. Following sample collection, the cassette was opened and the adhesive sample strip was removed. A drop of Cytoseal 60 liquid adhesive (Richard-Allan Scientific, Kalamazoo, MI) was placed on a glass slide, and the bottom of the sample slide was placed on the adhesive. The sample was stained with lactophenol cotton blue and a cover slip was placed on the sample. The AOC samples were analyzed by beginning the analysis near one end of the sample trace, but within the sample area. Every other traverse was analyzed until a maximum of 11 traverses, or about 22% of the sample trace, had been analyzed. In comparison, CitationBaxter et al. (2005) analyzed 19% (10 traverses) of the AOC sample trace.
The BA samples were collected at an airflow rate of 1, 2, or 3 lpm with the BA oriented in a vertical position. Following sample collection, the cassette was opened and the MCE filter (Millipore, Bedford, MA) was placed on the support pad. The two sample traces were separated by cutting the filter in half using an Xacto Knife with a ½ inch blade. One sample trace was placed on a glass slide, and the slide inserted into a Quick Fix® acetone vaporizer (EMS, Charleston, SC) to collapse and clear the MCE filter. The slide was removed, and the sample was stained with lactophenol cotton blue and covered with a cover slip. The BA samples were analyzed by starting at one edge of the sample trace and counting every traverse until a maximum of ten traverses had been analyzed. The first traverse, adjacent to the edge of the trace, was not analyzed because of possible edge effects. A total of 10 traverses were analyzed, or about 52% of the BA sample trace.
Filter Cassette
The BA filter cassette was a 25 mm filter cassette constructed of electrically dissipative polystyrene containing 4% carbon. is a side view of the BA, showing one of the four 3-mm square sample inlets. The cap was simply rotated to open and seal the cassette; with the slotted cowl and cap forming an enclosed capture chamber during sample collection. All tests were conducted with the four inlet slots in the fully open position.
is a photo of the interior of the cassette cowl showing the positions of the duplicate sample traces. The 25 mm filter was covered by a plastic masking disc containing two rectangular slits with dimensions of 1.7 mm × 5.5 mm. Each of the two sample slits had an area of 9.35 mm2, with a combined area of 18.7 mm2.
Filter Media
The effect of filter pore size on spore retention and recovery was evaluated by attaching BA cassettes to the six sampling ports in the chamber in an alternating pattern. Two cassettes each contained MCE filters with pore sizes of 1.2 μ m, 3 μm, and 5 μ m. The airflow rate for each BA was adjusted to 2 lpm, and 30-minute samples were collected. The effect of filter pore size was evaluated by comparing the relative concentrations of spores retained by the three MCE filters.
Airflow Rate
The cutoff size of the AOC has been characterized as 2.7 μ m at an airflow rate of 15 lpm, which was similar to the size of the P. chrysogenum spores (CitationAizenberg, Reponen et al. 2000). Therefore, the effect of airflow rate on aspiration efficiency could be evaluated by comparing the BA concentrations at the three airflow rates to the average AOC concentration. A ratio of approximately 2 for BA versus AOC concentrations was interpreted as being equivalent to approximately a 100% collection efficiency for the BA.
The effect of airflow rate on aspiration efficiency of the BA was evaluated by attaching BA cassettes containing 3.0 μm MCE filters to sampling ports 2, 4, and 6 in the settling chamber. The airflow rates of the three BA cassettes were adjusted to 1, 2, and 3 lpm, respectively. Three AOC cassettes were attached to sampling ports 1, 3, and 5, and their airflow rates were adjusted to 15 lpm. The three BA samples were collected for 30 minutes, while the three AOC samples were collected in series, with each AOC sampling for consecutive 10-minute periods.
RESULTS
Test Chamber
contains the average concentrations of P. chrysogenum and S. chartarum spores contained in each injection of test solution into the chamber. The data in were calculated based on three series of six injections (4,000 spores/m3), three series of 12 injections (8,000 spores/m3), ten series of 15 injections (10,000 spores/m3), and six series of 20 injections (13,340 spores/m3). One injection of the test solution was equivalent to an average concentration of 667 spores/m3 of P. chrysogenum spores and 38 spores/m3 of S. chartarum spores. The total coefficient of variation (CV) between injections was 20% for the higher concentrations of P. chrysogenum spores and 26% for the lower concentrations of S. chartarum spores. All the S. chartarum spores were detected as single spores, and most (99%) of the P. chrysogenum spores were also observed as single spores. Only a few groups consisting of two to three P. chrysogenum spores were detected, with a single aggregate of eight P. chrysogenum spores detected.
TABLE 1 The average spore concentrations (spores/m3) and variability per injection of the alcohol spore suspension into the chamber. The data are for multiple series of 6, 12, 15, and 20 injections
The equivalence of the six sampling ports was evaluated by collecting six side-by-side AOC samples for a period of ten minutes, using Penicillium spores to make the comparison. The sample results for each of the six ports and the CV measuring the variation in concentration between the ports are contained in . The Penicillium concentrations at the six sampling ports ranged from 9,947spores/m3 to 12,410 spores/m3, with an average concentration of 10,783 spores/m3. The CV between the six ports was a relatively low 9%.
TABLE 2 Variability of P. chrysogenum spore concentrations between the six sampling ports in the settling chamber for a single 10-minute test
The total CV (collection and analysis) between the measured concentrations at the six sampling ports decreased with concentration. The CV was 32% at an average S. chartarum concentration of 500 spores/m3, 9% at an average P. chrysogenum concentration of 11,000 spores/m3, and 6% at an average P. chrysogenum concentration of 17,000 spores/m3. The between-port variability was within the range of 3% to 15% at the two higher Penicillium concentrations, which met the EPA indoor equivalency requirements for sampler performance in microenvironments (Wiener and Rodes 1993). Therefore, the lower variability associated with the higher P. chrysogenum concentrations provided a better basis for the comparisons.
Sample Trace
The corner of a typical BA sample trace viewed at 100X magnification is illustrated in . A similar view of the corner of a typical AOC sample trace is illustrated in . These particular samples were collected outdoors, not in the settling chamber. They were used as illustrations because they are sufficiently dense to be photographed at the lower magnification. The sample in illustrates the well defined boundary and reproducible area of the BA sample trace. The masking disk was constructed with a small ridge encircling the bottom edge of each slit, which compressed the filter. The compression ridge confined the spores within the sample trace, and allowed the sample traces to be clearly observed when the filter was removed from the cassette.
FIG. 4 The corner of a typical BA sample trace at 100× magnification. The sample trace was well defined and the 1.7 mm × 5.5 mm area did not vary between samples.
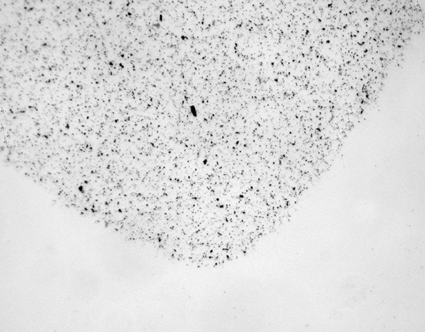
FIG. 5 The corner of a typical AOC sample trace at 100× magnification. The edges of the sample trace were diffuse and the 2 mm × 14.5 mm area often varied between samples.

The distribution of P. chrysogenum spores between the two BA sample traces was compared based on the sum of the spores detected in a series of tests. The total P. chrysogenum concentrations detected for 49 samples was 18,508 spores/m3 in trace A and 18,376 spores/m3 in trace B; a ratio of 0.993. A similar comparison of 42 S. chartarum samples yielded an average of 683 spores/m3 in trace A and 692 spores/m3 in trace B; a ratio of 0.987. When the ratios for individual samples were averaged, the average ratios were 0.995 for P. chrysogenum and 0.89 for S. chartarum. Tape lift samples were collected from the plastic strip separating the two sample slits in four cassettes. Although the sample size was small, no spores were detected. The uniformity of spore deposition across the sample trace was evaluated for both the BA and AOC. contains the average number of spores within a single traverse for six representative AOC and BA sample traces that were randomly selected. The average CV for the variation of spore counts between the traverses within each sample is also reported. The data in are arranged by the average number of spores per FOV. The spore density ranged from 14 to 38 for the smaller BA samples, and from 18 to 48 for the larger AOC samples. The average spore density for the BA was 21 spores per FOV with a CV of 27%. The average spore density for the AOC was 35 spores per FOV with a CV of 29%.
TABLE 3 The CV between microscopic traverses for six representative sample traces with various spore densities. An average of 11 traverses for AOC samples and 10 traverses for BA samples
Filter Media
The percentages of the spores recovered in are relative to those obtained with a filter having a 1.2 μ m pore size in order to provide a simple comparison. The average retention of P. chrysogenum spores was equivalent for filters with pore sizes of 1.2 μ m and 3 μ m. However, the use of filters with a 5 μ m pore size resulted in about a 20% reduction in the average retention for the smaller P. chrysogenum spores. The retention rates for the larger S. chartarum spores did not vary with pore size in the range tested. The CV values in were calculated based on the four sample traces obtained in two tests for each pore size, a relatively small sample size.
TABLE 4 Effect of filter pore size on collection efficiency for Penicillium and Stachybotrys Spores
Airflow Rate
The average spore concentrations obtained at airflow rates of 1, 2, and 3 lpm are contained in . The ratios of the BA concentrations to the AOC concentration indicate the performance of the BA relative to that of the AOC at the three airflow rates. The average ratios of BA to AOC concentrations for P. chrysogenum spores were 2.0, 1.8, and 1.7 at airflow rates of 1 lpm, 2 lpm, and 3 lpm, respectively, for three to seven replicate tests. The ratios of BA to AOC concentrations for S. chartarum varied between 1.4 and 1.0 for the three airflow rates, with an average of 1.1.
TABLE 5 Effect of airflow rate on spore retention for the Bi-Air cassette. Average Penicillium concentrations (spores/m3) for 30-minute tests at airflow rates of 1, 2 and 3 lpm
DISCUSSION
Sample Trace
A primary advantage of the BA compared to other filter samplers is that the sample was restricted to less than 5% of the total area of a 25 mm filter, as illustrated in . The area of each BA sample trace was about a third of that typically produced by the AOC, which is commonly used for the direct microscopic examination of fungal spores. Therefore, the small size of the BA sample trace allowed the sample to be easily analyzed by microscopy. The BA provided an effective method for utilizing the potential benefits offered by filter samplers for the collection of airborne fungal spores.
However, the MCE filters were often brittle, and the compression ridges frequently caused the filters to tear. The tear in the filter, as could be observed using either a stereoscope or at a magnification of 100×, occurred outside the compression ridge and did not affect the sample collection area nor the integrity of the sample. In practice, the excess filter was typically cut away prior to analysis to reduce the volume of acetone required to collapse and clear the filter.
A second advantage of the BA compared to other spore samplers is that the deposition of the spores was restricted to the areas of the two rectangular slits by the compression ridges. The corner of a typical BA sample trace is illustrated in at 100× magnification. The edges of the sample trace were well defined and bounded. This resulted in a sample area that did not vary between samples. The constant size of the BA sample trace allowed less than 100% of the sample to be analyzed without affecting the precision of the analysis. In comparison, the sample trace produced by a typical slit impaction sampler, because of the gap between the inlet and the collection medium, is less defined and can vary in area between samples. The effect of the gap between the inlet and the collection is illustrated for an AOC sample in , which is also the corner of a sample trace at 100× magnification.
A third potential benefit of the BA design is the production of duplicate sample traces. One sample trace was available for microscopic examination, while the second trace was available for culturing or other methods of analysis. In addition, culturing may be delayed until after the microscopic examination of the sample. This allows culturing to be restricted to those samples in which contaminant spores were detected. Since the spores in the test chamber were essentially delivered as individual spores, the distribution of spores between the two traces was uniform. However, when collecting actual samples, the presence of spore clusters may alter this ratio. The cluster will only be collected in one of the two sample traces, potentially altering the uniformity of the spore concentrations in each trace. Anecdotal evidence based on the collection of duplicate outdoor samples for six hours at an airflow rate of 1 lpm suggested the effect of spore clusters was minimized for collection times exceeding three hours; or sample volumes exceeding about 180 liters.
The variation in the distribution of spores across the BA and AOC sample traces was also compared, as indicated in . The variation of spore counts between traverses was essentially equivalent for the two samplers. In addition, a pattern in the spore concentration along the longitudinal axis of the sample trace was generally not detected for either sampler. Therefore, it was concluded that the spores were deposited with an equivalent uniformity by the two samplers.
Filter Cassette
The four inlets on the BA provided a total inlet area of 0.36 cm2 when in the fully open position, resulting in an inlet velocity of 46 cm/sec at an airflow rate of 1.0 lpm. Therefore, inlet velocities for airflow rates of 2 lpm or less were similar to the range of air velocities detected in indoor spaces (CitationWilleke and Macher 1999; CitationASHRAE 2004). The design of the BA, as illustrated in , is typical of a blunt sampler rather than a thin-wall sampler. This design, which is also typical of slit impaction samplers, was expected to be more efficient for the low air velocities encountered indoors, but less efficient for sampling in the presence of substantial wind velocities outdoors (Hering 1989).
At an airflow rate of 1 lpm, the face velocity at the filter surface in the BA would be expected to be about 90 cm/sec, with a range of 45 cm/sec to 270 cm/sec at airflow rates of 0.5 lpm to 3 lpm. Penetration of spores into the filter matrix would be expected to decrease at the high face velocities in the BA (CitationBrock 1983; Hering 1989). This was expected to help retain the spores near the surface of the filter; an advantage for analysis by microscopy. Probably fewer than 5% of the smaller P. chrysogenum spores appeared as “ghosts.” These were spores that were embedded within the upper surface of the filter, but were still visible and could be identified.
Particle bounce may be an issue for both silt impaction samplers and filter samplers, and might be expected to increase at high filter face velocities (CitationWilleke and Baron 1993; CitationHinds 1999; CitationVincent 1999). However, this did not appear to be a significant issue. When bounce occurs in an impaction sampler, the particle rebounds into an air flow that is essentially moving parallel to the collection medium, decreasing the probability that the particle will be retained.
In a filter sampler, the air flow is perpendicular to the collection medium, and rebounding particles are moving countercurrent to the air flow. When bounce occurs, some of the kinetic energy of the particle is lost, and the air stream again directs the now lower-energy particle into repeated contact with the collection medium. In addition, MCE filters have a rough surface, which tends to increase spore retention. Therefore, sample loss in the BA due to particle bounce, even at high filter face velocities, was expected to be of less importance relative to the loss incurred in slit impaction samplers.
Filter Media
The maximum airflow rate through an MCE filter with a 3 μ m pore size is nominally 30 lpm/cm2 of exposure surface area (Millipore, Bedford, MA). Since the exposed filter area in the BA was 0.187 cm2, the maximum theoretical airflow rates obtained with a high-volume air sampling pump were about 3.7 lpm for a 1.2 μ m filter and 5.6 lpm for a 3 μ m filter. Sustainable airflow rates for 3 μ m MCE filters were about 1 lpm when using a low-volume personal sampling pump rated at 5 lpm, and 2.5 lpm for a low-volume pump rated at 12 lpm. In addition, the maximum airflow rate that could be obtained through an MCE filter varied between filter lots, and individual filters within a lot, due to variations in the thickness of the filters. Therefore, the airflow rate was calibrated for each cassette.
The rated pore size of a filter is the maximum pore size, essentially the 99th percentile pore size. Therefore, the collection efficiency for filters was expected to be very high, even for particles as small as half the diameter of the stated filter pore size (CitationBrock 1983; CitationHinds 1999).
A small number of preliminary tests had demonstrated that MCE filters with 0.8 μ m and 1.2 μ m pore sizes retained an equivalent number of the small spores. The relative concentrations of P. chrysogenum spores in indicated that the retention of these smaller spores was also equivalent for filters with pore sizes of 1.2 and 3 μ m, but a 20% reduction in the reported concentration of P. chrysogenum spores occurred for a filter pore size of 5 μ m. Therefore, an MCE filter with a 3 μ m pore size was the largest pore size that could be used without resulting in the loss of some of the small P. chrysogenum spores. However, even using a 5 μ m pore size at airflow rates of 2 lpm or less would be expected to result in a reported P. chrysogenum concentration 60% higher than that reported using an AOC.
The CV values in were proportional to the pore size. This may have been due to a greater difficulty in detecting the spores as the pore size increased. The larger pores allowed a deeper penetration of the spores into the upper layer of the filter media, resulting in a larger percentage of “ghosts.” These spores, although both visible and identifiable, required more care to detect and count.
Airflow Rate
contains the concentrations of P. chrysogenum spores collected in side-by-side samples at airflow rates of 1 lpm, 2 lpm, and 3 lpm. The concentration ratios decreased from 2.1 at an airflow rate of 1 lpm to 1.6 at 3 lpm. These results suggested the BA was an efficient sampler for small spores at airflow rates of 1 lpm, but that the retention of small spores relative to AOC may have decreased with an increase in airflow rate.
This result suggested that turbulence may have developed at inlet velocities exceeding approximately 92 cm/sec, which was equivalent to an airflow rate of 2 lpm. Based on the observed ratios, it was estimated that the concentrations of smaller spores may be under reported by approximately 30% at an airflow rate of 3 lpm. However, even at an airflow rate of 3 lpm, the concentration of P. chrysogenum spores expected to be reported with the BA would be 70% higher than with the AOC. The average BA concentration was 1.1 times the average AOC concentration for the larger S. chartarum spores. Therefore, the aspiration efficiency of the BA did not decrease substantially for airflow rates of 1 to 3 lpm when collecting the larger spores.
Sampling Time
The ability of a sampler to collect samples over extended periods of time without overloading is required for the reliable collection of TWA and personal samples. Overloading by debris with the BA is less of an issue as compared to adhesive-strip samplers when sampling ambient air. The lower airflow rates used with the BA minimizes the collection of larger particulate, which generally have a higher density than spores. Many of the particulate that are entrained are similar in size to small spores, reducing obscuration of the sample.
The BA has been routinely used to collect 60-and 90-minute samples during field investigations; it has been used to collect outdoor samples for periods of up to six hours; and it has been used to collect some 2,000 area and personal samples during mold remediation projects. However, one of the more critical applications has been its use during post-remediation verification sampling in an intensive care unit (ICU) in a hospital.
The Healthcare Infection Control Practices Advisory Committee (HICPAC) suggests a minimum Aspergillus clearance concentration of 0.99 cfu/m3 (CitationSehulster et al. (2004). The BA allowed the ICU to be cleared to a concentration of 0.8 spores/m3 forAsp/Pen like spores by collecting 1,280 liter samples over a seven hour period. As a precaution, all collected Asp/Pen like spores were assumed to be Aspergillus fumigatus, a human pathogen. For those samples in which Asp/Pen like spores were detected by microscopy, the second sample trace was submitted to a health agency for additional analysis. In addition, only one sample was collected at each of the 13 sampling locations within the ICU. Achieving a similar level of clearance using 10-minute samples would have required nine samples to be collected at each of the 13 sampling locations.
LIMITATIONS
The experimental design did not include a method for determining actual spore concentrations. One objective of the study was to demonstrate the utility of the BA filter cassette for the direct microscopic examination of spores. This was a qualitative objective, and could be demonstrated without determining actual concentrations. The second objective was to demonstrate that the performance of the BA was at least comparable to that of the AOC, a commonly used sampler for the collection of airborne spores. The lack of information on actual spore concentrations in the settling chamber was addressed by only collecting side-by-side samples using both samplers. Therefore, this was considered to be an acceptable limitation in the experimental design.
The comparison of the two samplers was based on a relatively small number of samples. However, the CVs were kept small by basing the primary comparisons on high spore concentrations in the settling chamber. For example, at a P. chrysogenum concentration of 10,000 spores/m3, the CV between the six sampling ports in the settling chamber was a relatively low 9%.
Only a limited range of spore concentrations were evaluated. The maximum concentration of P. chrysogenum spores was 30,000 spores/m3; and the maximum S. chartarum concentration was 1,200 spores/m3. The concentration ranges included in the study were typical of those often encountered in field studies. However, the results of the study may vary at concentrations higher than those included in the study. In addition, results may vary for environments in which large clusters of spores are present.
Finally, the comparison was limited to only two spore types, the smaller 3 μm P. chrysogenum spore and the larger S. chartarum spore. However, the comparisons based on a representative small and large spore provided sufficient data for a meaningful comparison of the two samplers. In addition, Aspergillus/Penicillium like spores are often the dominant spore type detected in contaminated indoor environments, and Stachybotrys spores are of interest in many mold investigations.
SUMMARY
A filter sampler has been described that allows fungal spores to be analyzed by direct microscopic examination without an intermediate sample transfer step. The BA filter cassette produced duplicate sample traces, with each trace having an area of 9.35 mm2. The total sample area for the duplicate samples was 18.7 mm2, or less than 5% of the 380 mm2 of exposed filter area in a typical 25 mm filter cassette. In comparison, the area of a slit impaction sampler is typically 30 to 40 mm2.
The BA cassette combined the inherent advantages of a filter sampler with the small sample area typical of slit impaction samplers. The MCE filter could be cleared using acetone vapor, and the spores could be analyzed by direct microscopic examination without an intermediate sample transfer step. The cleared filter provided a smooth, transparent surface for analysis. The duplicate sample traces produced by the BA were rectangular with well defined boundaries. Even the smooth P. chrysogenum spores adhered to the rough surface of the MCE filter, and remained within the area of the sample trace when a staining solution was applied. In addition, trapped air bubbles could be dislodged without disturbing the sample by gently tapping on the cover slip. This is sometimes difficult to accomplish with a water-filled gel collection medium.
The gap separating the slit from the collection medium in an impaction sampler results in an unbounded sample trace with often diffuse edges and variable in size. This characteristic makes it difficult to analyze anything less than 100% of the sample trace with precision. The duplicate sample traces produced by the BA were bounded and the spores confined to a well defined rectangular area on the filter. The bounded sample area of the BA allowed less than 100% of the sample to be analyzed quantitatively.
The reported cutoff size for the AOC is 2.7 μ m, resulting in an expected collection efficiency of about 50% for a 3 μ m P. chrysogenum spore. The ratio of the BA concentration to the AOC concentration for 3 μ m P. chrysogenum spores was 2.0 at an airflow rate of 1 lpm. This was the expected ratio if the collection and recovery of spores with the BA was about 100%. However, the ratio was 1.8 at an airflow rate of 2 lpm and 1.5 at an airflow rate of 3 lpm. Therefore, the apparent aspiration efficiency of the BA for P. chrysogenum spores was 90% at an airflow rate of 2 lpm and 75% at 3 lpm. The retention of the larger S. chartarum spores by the BA was about equal to that of the AOC at all three airflow rates.
The use of an MCE filter with a larger pore size resulted in higher maximum airflow rates. The maximum theoretical airflow rate for a BA loaded with an MCE filter with a 3 μ m pore was 5.6 lpm. MCE filters with pore sizes of 1.2 μ m and 3 μ m retained 3 μ m P. chrysogenum spores with the same efficiency, as predicted by filtration theory. However, a filter pore size of 5 μ m resulted in a 20% reduction in the measured concentration of P. chrysogenum spores. The larger S. chartarum spores were retained with the same efficiency at all three pore sizes.
REFERENCES
- Adhikari , A. , Martuzevicius , D. , Reponen , T. , Grinshpun , S. , Cho , S. , Sivasubramani , S. , Zhong , W. , Levin , L. , Kelley , A. , St. Clair , H. and LeMasters , G. 2003 . Performance of the Button Personal Inhalable Sampler for the Measurement of Outdoor Aeroallergens . Atmos. Environ. , 37 : 4723 – 33 . [CSA]
- Aizenberg , V. , Grinshpun , S. A. , Willeke , K. , Smith , J. and Baron , P. A. 2000 . Performance Characteristics of the Button Personal Inhalable Aerosol Sampler . AIHAJ , 61 : 398 – 404 . [INFOTRIEVE] [CROSSREF] [CSA]
- Aizenberg , V. , Reponen , T. , Grinshpun , S. A. and Willeke , K. 2000 . Performance of Air-O-Cell, Burkard and Button Samplers for Total Enumeration of Airborne Spores . AIHAJ , 61 : 855 – 864 . [INFOTRIEVE] [CROSSREF] [CSA]
- ASHRAE . 2004 . Thermal Environmental Conditions for Human Occupancy , Atlanta, GA. : American Society of Heating, Refrigerating and Air-Conditioning Engineers . (ASHRAE Standard 55–2004)
- Baxter , D. M. , Perkins , J. L. , McGhee , C. R. and Seltzer , J. M. 2005 . A Regional Comparison of Mold Spore Concentrations Outdoors and Inside “Clean” and “Mold Contaminated” Southern California Buildings . JOEH , 2 : 8 – 18 . [INFOTRIEVE] [CROSSREF] [CSA]
- Brock , T. D. 1983 . Membrane Filtration: A User's Guide and Reference Manual , Madison, WI : Science Tech, Inc. .
- Eduard , W. , Lacey , J. , Karlsson , K. , Palmgren , U. , Strom , G. and Blomquist , G. 1990 . Evaluation of Methods for Enumerating Microorganisms in Filter Samples from Highly Contaminated Occupational Environments . AIHAJ , 51 : 427 – 436 . [CROSSREF] [CSA]
- Engelhart , S. and Exner , M. 2002 . Short-Term Versus Long-Term Filter Cassette Sampling for Viable Fungi in Indoor Air: Comparative Performance of the Sartorius MD 80 and GSP sampler . Int. J. Hyg. Environ. Health , 205 ( 6 ) : 443 – 51 . [INFOTRIEVE] [CROSSREF] [CSA]
- Feather , G. A. and Chen , B. T. 2003 . Design and Use of a Settling Chamber for Sampler Evaluation under Calm-Air Conditions . Aerosol Sci. Technol. , 37 : 261 – 270 . [CROSSREF] [CSA]
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, , 2nd Ed. , New York : John Wiley & Sons .
- Kate , T. , Durand , H. , Mullenberg , M. L. , Burge , H. A. and Seixas , N. S. 2002 . Effect of Sampling Time on the Culturability of Airborne Fungi and Bacteria Sampled by Filtration . Ann. Occup. Hyg. , 46 ( 1 ) : 113 – 118 . [CROSSREF] [CSA]
- McDevitt , J. J. , Lees , P. E. J. , Merz , W. G. and Schwab , K. J. 2005 . Use of Green Fluorescent Protein-expressing Aspergillus fumigatus conidia to Validate Quantitative PCR Analysis of Air Samples Collected in Filters . JOEH , 2 ( 12 ) : 633 – 640 . [INFOTRIEVE] [CROSSREF] [CSA]
- McGrath , J. J. , Wong , W. C. , Cooley , J. D. and Straus , D. C. 1999 . Continually Measured Fungal Profiles in Sick Building Syndrome . Curr. Microbiol. , 38 ( 1 ) : 33 – 6 . [INFOTRIEVE] [CROSSREF] [CSA]
- NIOSH . 1994 . Method 7400: Asbestos and Other Fibers by PCM, , Fourth Edition , Washington, D.C. : NIOSH Manual of Analytical Methods . 8/15/94, DHHS/CDC/NIOSH
- Palmgren , U. , Strom , G. , Blomquist , G. and Malmberg , P. 1986 . Collection of Airborne Microorganisms on Nucleopore Filters, Estimation and Analysis—CAMNEA method . J. Appl. Bacteriol. , 61 : 401 – 406 . [INFOTRIEVE] [CSA]
- Sehulster , L. M. 2004 . “Guidelines for Environmental Infection Control in Health-Care Facilities: Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee” . Chicago, IL : (HICPAC), ASHE/AHA .
- Vincent , J. H. , ed. 1999 . Particle Size-Selective Sampling for Particulate Air Contaminants , Cincinnati, OH : ACGIH .
- Willeke , K. and Baron , P. A. , eds. 1993 . Aerosol Measurement: Principles Techniques and Applications , New York : Van Nostrand Reinhold .
- Willeke , K. and Macher , J. M. , eds. 1999 . Bioaerosols: Assessment and Control , Cincinnati, OH : ACGIH .

