Abstract
In this article we present observations on the detection efficiency of a recently developed TSI 3785 Water Condensation Particle Counter (WCPC). The instrument relies on activation of sampled particles by water condensation. The supersaturation is generated by directing a saturated airflow into a “growth tube,” in which the mass transfer of water vapor is faster than heat transfer. This results in supersaturated conditions with respect to water vapor in the centerline of a “growth tube.” In this study, the cut-off diameter, that is, the size, where 50% of the sampled particles are successfully activated, varied from 4 to 14 nm for silver particles as a function of temperature difference between the saturator and the growth tube. The solubility of the sampled particles to water played an important role in the detection efficiency. Cut-off diameters for ammonium sulphate and sodium chloride particles were 5.1 and 3.6–3.8 nm, respectively at nominal operation conditions. Corresponding cut-off diameter for hydrophobic silver particles was 5.8 nm.
1. INTRODUCTION
The condensation particle (nucleus) counters (CPC, CNC) are widely used for measuring number concentrations of submicrometer aerosol particles. As most of the particles in atmosphere in terms of number are below 1 micrometer in diameter, the results of CPC measurements are very close to total number concentration above the smallest detectable size. In a CPC the aerosol particles are grown by condensing supersaturated vapour onto the surface of the particles. This enables particles to grow large enough to be optically detected. This is a very powerful method to detect particles that contain small amount of mass and are therefore difficult to detect by any other means (CitationMcMurry 2000).
The properties of the vapor (and particles) and the degree of the supersaturation determine the lowest size of a particle onto which the condensation can be activated (CitationMertes et al. 1995; CitationSem 2002). In addition, a number of other factors (e.g., geometry, inlet design) affect the true smallest detectable size (CitationStolzenburg and McMurry 1991). The most often used substances as the condensing vapor are water and alcohols. The supersaturation of the vapor is very high and therefore the response of the CPC is typically insensitive to the composition of the aerosol particles.
There are several methods used to produce the supersaturated vapor. Until recent years, three different principles are used, conductive cooling of the working fluid (CitationBricard 1976; CitationStolzenburg and McMurry 1991), (turbulent) mixing of cool and warm saturated air streams (CitationKousaka 1982; CitationMavliev 2002) and adiabatic expansion of the aerosol-vapor-mixture (CitationAitken 1897; CitationMetnieks and Pollak 1959; CitationKürten et al. 2005). The conductive cooling type is usually operated in continuous flow and therefore it can be used in connection with size classifiers working on constant flow principle (e.g., differential mobility analyser and diffusion battery). This type is presently widely used and several models are available commercially. All the models, however, have limitations concerning maximum concentration, lowest detectable particle size, and so on. Different types of the CPCs and their history have been recently reviewed by CitationMcMurry (2000).
The type of a CPC and its construction determine the performance characteristics of the device. The central characteristic of the condensation particle counters is the detection efficiency as a function of particle size. More practical parameter is a cut-off size. It is a diameter at which the detection efficiency drops to 50%. The cut-off size depends on the aerosol losses in the inlet of the CPC, the efficiency of the optical system, and the particle activation efficiency. However, CitationStolzenburg and McMurry (1991) has shown that the cut-size is mostly a function of the activation efficiency alone. Thus for conductive cooling CPC it can be determined by the difference of temperatures between saturator and condenser. The characteristics, including the cut-off diameter of the commercially available CPCs have been investigated by several researchers and are rather well known.
Recently, a new type of CPC was designed and produced commercially (TSI 3785). It has a unique design that allows this device to use water as its working liquid in a laminar flow construction. The general characteristics of this CPC have been presented earlier by CitationHering et al. (2005). They showed the smallest detectable size for various types of aerosols involving water soluble salts, organic compounds and ambient aerosol. In addition, the response of the instrument to high concentrations was investigated. The physical principles of the operation were investigated by CitationHering and Stolzenburg (2005). Summarizing the main findings of these papers one can conclude that the basic operation of the new instrument is well understood and experimental results show values close to those predicted by theoretical calculations. The water based CPC was compared against more widely used butanol based CPC (TSI model 3022A) by CitationBiswas et al. (2005). They found good correlation and agreement up to particle concentration of 40 000 cm− 3. They concluded that WCPC is a reliable counter up to this concentration. The abovementioned studies showed clearly the influence of the aerosol material on the cut-off diameter, indicating the importance of the heterogeneous nucleation probability.
In this study, the particle detection efficiency of the TSI 3785 was experimentally investigated in 1–20 nm particle diameter range. The calibrations were done using different aerosol materials in order to study the WCPC response to both hygroscopic and hydrophobic compounds which have different heterogeneous nucleation probabilities within the growth tube. The investigations were done using silver, ammonium sulphate, and sodium chloride particles. Silver is widely used in calibrations and these particles are spherical and water insoluble. The salt particles are water soluble and have a crystal structure. The true shape and structure in nanometer size range is, however, not well known. We investigated the sensitivity of the cut-off diameter as the temperatures of the saturator and the growth tube were changed. This is not a standard way of operating the CPC. However, the method of changing temperatures and therefore also supersaturation within the growth tube has been used for butanol based CPCs by several researchers (e.g., CitationMertes et al. 1995). This method allows investigations of the size distribution in the nanometer size range. The cut-off diameter was derived from the experimental data by fitting a mathematical function. This provides a practical way to describe the CPC characteristics when doing, for example inversion of size distribution measurements.
2. EXPERIMENTAL SETUP
The operation of a condensation particle counter (CPC) relies on the activation of sampled aerosol particles to optically detectable sizes. The smallest observable particle size, that is cut-off size of the CPC is determined as a size, where 50% of incoming particles are successfully accounted for. This is the most important parameter, which determines the suitability of a given condensation particle counter to a particular purpose. In this study, detection efficiency D50 is determined experimentally for a TSI model 3785, which utilizes water as a size amplification agent. The detection efficiency of the CPC was determined using a measurement method presented in CitationScheibel and Porstendörfer (1983). A schematic figure of the calibration setup is presented in .
FIG. 1 Experimental setup, which was used to determine the detection efficiency of a TSI 3785 water condensation particle counter (WCPC).
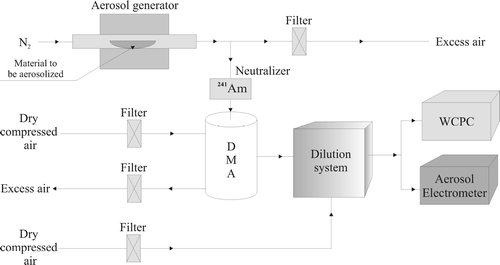
A tube furnace (Carbolite Furnaces MTF 12/388) was used to produce a polydisperse aerosol sample. Particles were generated evaporating silver (Ag), ammonium sulphate ((NH4)2SO4), or sodium chloride (NaCl) to a passing nitrogen flow at a variable temperature (280–1100°C, depending on the material. As the flow cooled down outside of the heated section of a tube furnace, the vaporised material nucleated and formed polydisperse aerosol particles. Actual flow rate through the furnace as well as the oven temperature varied between the experiments, since the produced aerosol size distribution depends on both the residence time within the heated section and the temperature. As the aim was to determine detection efficiency of a particle counter using an electrometer as a reference instrument, high enough concentrations of particles in an ultra-fine size range had to be generated. This translates into a fine-tuning of flow rates through the generator and the temperature within so that the monodisperse sample was taken from close to a modal mean diameter of the produced aerosol particles.
A sample flow of 2.0 l min− 1 was extracted from the tube furnace generated polydisperse aerosol population at ambient pressure. They were charged with an alpha-active 241Am bipolar source. Then, a monodisperse fraction was selected with a differential mobility analyser (DMA VIE-08, Hauke, length 0.109 m). The DMA was operated with 2.0 l min− 1 aerosol and 20.0 l min− 1 sheath and excess air flows. The flows were measured with a Gillian bubble flow meter and controlled with needle valves.
The sample flow containing the monodisperse aerosol particles was directed to a condensation particle counter (CPC) and to a reference instrument (TSI 3068 electrometer). The sample flow for the electrometer was 3.7 l min−1 and the TSI 3785 water CPC had a nominal flow rate of 1.035 l min−1. Monodisperse sample was diluted to a concentration below 104 cm− 3. This ensured that the condensation particle counter was able to detect single particles within the optics of the instrument making the coincidence negligible. At the same time, the concentration of the charged fraction had to be high enough to be reliably detected with the reference electrometer. The particles of below 20 nm in size have only one charge after the radioactive neutralizer (CitationWiedensohler 1988). This justified the use of an electrometer as a reference for the total concentration of monodisperse particles.
2.1. Data Analysis
Concentrations of size-segregated aerosol samples measured with the condensation particle counters (CPC) were normalized to the readings of the reference electrometer. Detection efficiency (E (Dp)) curve of the CPC is determined as a ratio between the concentrations measured with the CPC and the electrometer. An exponential function was fitted in a least squares sense to the measurement data. The fitted function was
The temperature of the saturator and the growth tube of the TSI 3785 CPC can be changed by the user. This alters the supersaturation that the sampled particles experience inside the growth tube of the instrument. At higher temperature differences, smaller and smaller particles are activated and accounted for by the counting optics. The D50 values obtained from equation Equation1 were plotted against the corresponding temperature differences Δ T. Their relation was in accordance with a function
3. RESULTS AND DISCUSSION
3.1. Detection Efficiency
Detection of sub-micron aerosol particles relies on the activation of these particles to optically active sizes of several micrometers in diameter. In a TSI model 3785 condensation particle counter (CPC) the size of the sampled aerosol particles is amplified by water condensation (CitationHering and Stolzenburg 2005). Particle laden sample air is first conditioned to almost 100% relative humidity with a wetted wick on the outer wall of the tube. The temperature of this saturator wall is held constant (20°C) in normal operating conditions. The actual size enhancement of the sampled aerosol particles occurs in the growth tube, in which the wick is kept wetted but the temperature is increased to 60°C (CitationHering et al. 2005). Close to the wick, the partial pressure of water vapor is close to equilibrium vapor pressure at the wick temperature. As the passing flow in the growth tube has a lower partial pressure of water vapor, diffusion of water vapor takes place towrd the centerline of the sample flow. Since mass diffusivity of water vapor is higher than thermal diffusivity of the air, a region of supersaturation with respect to water vapor is generated in the centre of the growth tube (CitationHering and Stolzenburg 2005). In fact, in the water-based condensation particle counter, supersaturated region can be generated either with a positive or negative temperature difference between the saturator and the growth tube. However, the profile changes: absolute supersaturation is higher and the maximum value occurs at the centerline of the tube, when the conditioner has a lower temperature as the growth tube. This will lead to better size amplification and lower cut-off sizes (CitationHering and Stolzenburg 2005).
The key parameter determining the amount of supersaturation obtained within the growth tube is the temperature difference between the saturator and the growth tube. The detection efficiency study of the TSI 3785 was performed with 1 to 20 nm silver particles. The measured detection efficiency curves are presented in . We examined the influence of changes in temperature difference to the detection efficiency (D50) values. The experiments were conducted by changing both the saturator and the growth tube temperatures.
FIG. 2 The effect of temperature difference in the detection efficiency of the CPC TSI model 3785 WCPC for silver particles (a) using a constant growth tube temperature (50°C) and varying the saturator temperature (10, 15, 20, 25, and 30°C, squares, diamonds, asterisks, circles, and pluses, respectively), (b) using a constant saturator temperature of 15°C and varying the growth tube temperature (65, 60, 55, 50, 45, and 40°C, crosses, squares, diamonds, asterisks, circles, and pluses, respectively). Continuous lines present a fit through experimental points. As the temperature difference increases, the cut-off diameter decreases.
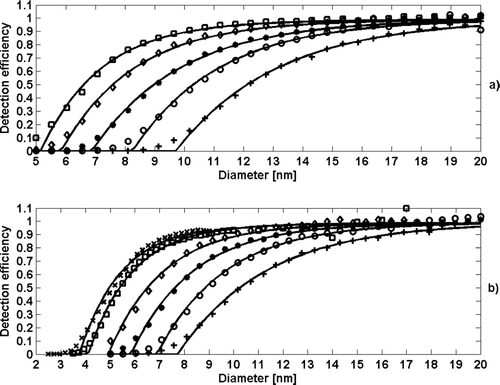
As the saturator temperature was kept in a fixed 15°C temperature and the growth tube temperature was scanned from 40 to 60°C, the cut-off diameter changed from 7 nm to 12 nm (). Additionally, detection efficiency measurements were also conducted when the growth tube temperature was kept constant (50°C) and the saturator temperature was varied (). The D50 values varied from 5 to 10 nm.
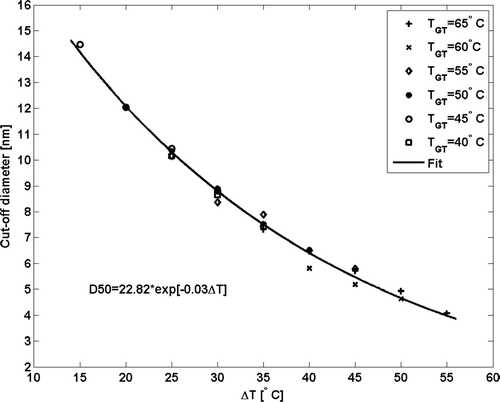
Essentially, the detection efficiency depended only on the temperature difference. Based on the Clausius-Clapeyron equation, the cut-off diameter has a exponent dependency on the saturation ratio, that is temperature difference between the saturator and the growth tube. The D50 data from is presented as a function of temperature difference (Δ T) in . As the Δ T increased, the cut-off size decreased. This is a result of elevated supersaturation inside the growth tube and subsequent activation of smaller particles. This enables tuning of the cut-size to a desired value in the range between 4 nm and 14 nm. However, a care should be taken when tuning the temperature settings of the instrument. By setting the optics temperature equal to the growth tube temperature, water condensation into the optical head of the instrument is suppressed. In addition, the alignment of the CPC optics can be altered due to the changing temperature settings. This can deteriorate the counting efficiency of the instrument at high particle concentrations.
FIG. 3 The detection efficiency of a TSI model 3785 CPC as a function of temperature difference between the saturator and the growth tube for silver particles. Higher temperature difference leads to higher supersaturation inside the growth tube. As a result, heterogeneous nucleation onto initially smaller particles is initiated and they are successfully counted with the optics of the instrument. Continuous line represents a least squares fit through the experimental points.
3.2. The Effect of Chemical Composition to the Detection Efficiency
As noted by CitationHering et al. (2005) detection efficiency of a water-based condensation particle counter depends on the chemical composition of sampled particles. They compared a TSI model 3785 WCPC with a TSI model 3025, which utilizes butanol as the growing agent. Particles composed of water soluble inorganic salts (ammonium sulphate, ammonium nitrate, sodium chloride) were detected more efficiently and had a cut-off size of 4.5, 4.7, and 3.6 for the aforementioned compounds. For a pure dioctyl sebacate (DOS) the cut-off size was as large as 30 nm, but mixed with just 4 ppt of sodium chloride decreased the D50 value down to 13 nm in diameter.
We tested the effect of chemical composition on WCPC detection efficiency by generating inorganic salts (ammonium sulphate and sodium chloride) and inert silver particles and determined the cut-off diameters by fitting an exponential curve (Equation Equation1) to the experimental data. The results are presented in and in . As expected, the solubility of the particle material had an effect on the cut-off diameter (D50). For nominal operating conditions (20°C for saturator and 60°C for the growth tube), the cut-off diameter for silver particles was at 5.8 nm. For ammonium sulphate, the corresponding value was 5.1 nm. CitationHering et al. (2005) reported a slightly lower cut-off diameter (4.5 nm) for ammonium sulphate.
FIG. 4 The detection efficiencies for particles of different chemical composition for TSI model 3785 CPC. For a normal operation conditions (dT = 20–60°C), the effect of chemical composition to cut-off size is 1 nm, but for a smaller temperature difference, the cut-off depends more crucially on the chemical composition and in particular, water solubility, of the sampled aerosol particles.
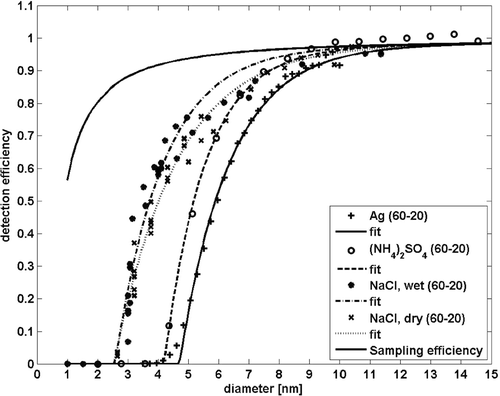
TABLE 1 Cut-off diameter (D50) and lowest observable particles (D0) for silver, ammonium sulphate, and sodium chloride particles as well as a theoretical detection limit from CitationHering et al. (2005) for normal TSI model 3785 temperature settings (saturator at 20°C and growth tube at 60°C). Estimated errors for the D50 values are 0.3 nm, which correspond to the half-width of the transfer function through the particle sizing setup.
The D50 decreased even further as sodium chloride particles were used, as already reported by CitationHering et al. (2005). According to their experiments, the cut-off diameter for sodium chloride particles was at 3.6 nm. Our results are equal, in a case, when we humidified the oven-generated particles to 44 ± 1% relative humidity prior size segregation with a differential mobility analyzer (presented as “wet” in ).
A plausible explanation to the cut-off diameter difference between ammonium sulphate and sodium chloride particles could be explained by the differences in their hygroscopic properties. Prior to exposing sampled aerosol particles to supersaturation with respect to water vapor, the particles are placed to an environment, where the relative humidity is close to 100%. In this stage, hygroscopic sodium chloride and ammonium sulphate particles already grow in size by condensation of water vapor considerably (CitationHämeri et al. 2000, Citation2001). Activation inside the growth tube occurs easier, since the particles are already bigger and the phase transition from solid crystal to a liquid droplet has already commenced. In a case of hydrophobic components, the phase transition and condensation presumably takes place only within the growth tube. The size amplification in the sub-saturated conditions will also lead to smaller diffusion losses within the saturator of the CPC as the hygroscopic particles are enlarged in the sub-saturated conditions, as argued also by CitationHering et al. (2005).
Although both ammonium sulphate and sodium chloride are hygroscopic, there is a difference in their water vapor affinities. Hämeri et al. (Citation2000, Citation2001) experimentally measured hygroscopic growth factors for ultra-fine particles in sub-saturation for ammonium sulphate and sodium chloride, respectively. At relative humidity of 90% and room temperature of 22°C initially 8 nm ammonium sulphate particles grew to 10.8 nm indicating a growth factor of 1.35 (CitationHämeri et al. 2000). At the same RH, on the other hand, sodium chloride crystals exhibited growth by a factor of 1.8, that is, the eventual size of 14.5 nm. The ratio of the growth factors (1.35) remains approximately the same for larger particles. Assuming that this water vapor affinity difference can be extrapolated to smaller sizes and higher humidities, this will lead to 33% lower cut-off size for sodium chloride than for ammonium sulphate particles. Based on our results, the D50 for wetted sodium chloride particles was 30% larger than that of ammonium sulphate particles, showing a good agreement with the scaled hygroscopic growth factor differences.
Additional difference between the two salts is the shape of solid crystals. Large sodium chloride crystals have a cubic shape whereas the solid ammonium sulphate is (more) spherical. Due to cubic shape, solid sodium chloride experience higher drag in a differential mobility analyser (DMA). This will lead to an overestimation of their physical size based on their electrical mobility. In the conversion from electrical mobility to a physical size, a shape factor has to be taken into account. For a cubical shape this factor is 1.08 (CitationHinds 1999). However, it is still an open question, whether the shape of the nm sized sodium chloride particles have a cubical shape or not (CitationKrämer et al. 2000).
Detection efficiency of the WCPC for sodium chloride was determined for both dry and humidified situation. In dry experiment, the sodium chloride was generated with a heated oven and size segregated with a DMA. In this case, the microstructure and shape of NaCl is not well defined and there might be voids within the particles (CitationKrämer et al. 2000). The cut-off diameter was in this case 3.8 nm. When an external humidifier was used to increase the relative humidity (RH) just after the oven to 44 ± 1.4 %, the D50 of 3.6 was observed (). The D50 values differ only slightly, so the actual differences in the microstructure remain unknown.
4. SUMMARY AND CONCLUSIONS
In this article, we presented evaluation of the water based CPC concerning the cut-off diameter and detection efficiency for nanometer size range particles. The measurements were done using silver, ammonium sulphate and sodium chloride particles. The calibrations done using the standard operation conditions of the WCPC agree sufficiently well with earlier studies as well as the theoretical calculations. Small differences found are within uncertainties of both the physical conditions within the CPC and the true size and shape of the particles. The diameter of the particles was determined using mobility diameter based classification (DMA) and is not always the same as the geometrical diameter.
The investigations were performed for a range of temperatures and supersaturations in order to study the response of the instrument and physical characteristics at non-standard conditions. These studies indicate that for non-soluble particles (silver), the cut-off diameter can be varied between about 4 and 14 nm by changing the temperature difference between saturator and the growth tube from 55°C to 15°C (standard value 40°C). This allows one to investigate the fraction of particles between these diameters and can be a valuable tool for investigating for example the atmospheric nucleation (CitationKulmala et al. 2005). Similar differences of the cut-off diameter for soluble particles were also observed, but with different absolute values.
The solubility of sampled particles had an effect on the detection efficiency of the WCPC. At nominal operation conditions, WCPC cut-off for hydrophobic silver particles was 5.8. For ammonium sulphate, the cut-off decreased to 5.1 nm. For even more hydrophilic compound, sodium chloride, the cut-off decreased further to 3.8 nm. In addition, a slight change in the cut-off was observed as the oven-generated sodium chloride particles were exposed to a small amount of water vapor (relative humidity 44 ± 1%) prior to size selection with a differential mobility analyzer. This could be a result of a reorganization of the salt microstructure and resulting change in electrical equivalent diameter.
The variable detection efficiency is reflected also to ambient measurements. Atmospheric particles are always a mix of different compounds leading to variable solubility to water vapor and thus different total concentrations measured with the WCPC (CitationBiswas et al. 2005). The ratio has both temporal and spatial variability (CitationBiswas et al. 2005). This is a drawback considering for example ambient aerosol particle number concentration monitoring, since the composition of ultra-fine particles is not known with a high time resolution. However, the changing cut-off diameter as a function of sampled particle composition could be utilized, when a water-based Condensation Particle Counter is operated in parallel with a butanol based counter with a same nominal cut-off size. Then the ratio between the two instrument could reveal information about the chemical characteristics the ultra-fine particles.
Acknowledgments
Discussions with Susanne Hering and Mark Stolzenburg concerning the operation and design of the WCPC are acknowledged. Financial support from Academy of Finland is gratefully acknowledged.
REFERENCES
- Aitken , J. A. 1897 . On Some Nuclei of Cloudy Condensation, Part I , Transactions of the Royal Society of Edinburgh . XXXIX.
- Baron , P. A. and Willeke , K. 2001 . Aerosol Measurement—Principles, Techniques, and Applications , (2nd edition) , pp. 1131 New York : John Wiley and Sons .
- Biswas , S. , Fine , P. M. , Geller , M. D. , Hering , S. V. and Sioutas , C. 2005 . Performance Evaluation of a Recently Developed Water-Based Condensation Particle Counter . Aerosol Sci. Technol. , 39 : 419 – 427 . [CSA]
- Bricard , J. A. , Delattre , P. , Madeleine , G. and Pourprix , M. 1976 . Detection of Ultra-fine Particles by Means of a Continuous Flux Condensation Nucleus Counter , Edited by: Liu , B. Y.H. pp. 565 – 580 . New York : Fine Particles: Aerosol Generation, Measurement, Sampling, and Analysis. Academic Press .
- Hämeri , K. , Väkevä , M. , Hansson , H. -C. and Laaksonen , A. 2000 . Hygroscopic Growth of Ultrafine Ammonium Sulphate Aerosol Measured Using an Ultrafine Tandem Differential Mobility Analyser . J. Geophys. Res. , 105 : 22231 – 22242 . [CSA] [CROSSREF]
- Hämeri , K. , Laaksonen , A. , Väkevä , M. and Suni , T. 2001 . Hygroscopic Growth of Ultrafine Sodium Chloride Particles . J. Geophys. Res. , 106 : 20749 – 20757 . [CSA] [CROSSREF]
- Hering , S. V. and Stolzenburg , M. R. 2005 . A Method for Particle Size Amplification by Water Condensation in a Laminar, Thermally Diffusive Flow . Aerosol Sci. Technol. , 39 : 428 – 436 . [CSA] [CROSSREF]
- Hering , S. V. , Stolzenburg , M. R. , Quant , F. R. , Oberreit , D. R. and Keady , P. B. 2005 . A Laminar-Flow, Water-Based Condensation Particle Counter (WCPC) . Aerosol Sci. Technol. , 39 : 659 – 672 . [CSA] [CROSSREF]
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behaviour, and Measurement of Airborne Particles , p. 504 New York : Wiley-Interscience .
- Kousaka , Y. , Niida , T. , Okuyama , K. and Tanaka , H. 1982 . Development of a Mixing Type Condensation Nucleus Counter . J. Aerosol Sci. , 15 : 231 – 240 . [CSA] [CROSSREF]
- Kulmala , M. , Lehtinen , K. E. J. , Laakso , L. , Mordas , G. and Hämeri , K. 2005 . On the Existence of Neutral Atmospheric Clusters . Boreal Env. Res. , 10 : 79 – 87 . [CSA]
- Kürten , A. , Curtius , J. , Nillius , B. and Borrmann , S. 2005 . Characterization of an Automated, Water-Based Expansion Condensation Nucleus Counter for Ultrafine Particles . Aerosol Sci. Technol. , 39 : 1174 – 1183 . [CSA] [CROSSREF]
- Krämer , L. , Pöschl , U. and Niessner , R. 2000 . Microstructural Rearrangement of Sodium Chloride Condensation Aerosol Particles on Interaction with Water Vapor . J. Aerosol Sci. , 31 : 673 – 685 . [CSA] [CROSSREF]
- Mavliev , R. 2002 . Turbulent Mixing Condensation Nucleus Counter . Atmos. Res. , 62 : 303 – 314 . [CSA] [CROSSREF]
- Mertes , S. , Schröder , F. and Wiedensohler , A. 1995 . The Particle Detection Efficiency Curve of the TSI-3010 CPC as a Function of Temperature Difference Between Saturator and Condenser . Aerosol Sci. Technol. , 23 : 257 – 261 . [CSA]
- McMurry , P. H. 2000 . The History of CPCs . Aerosol Sci. Technol. , 33 : 297 – 322 . [CSA] [CROSSREF]
- Metnieks , L. and Pollak , L. W. 1959 . Introduction for Use of Photo-Electric Condensation Nucleus Counters , School of Cosmic Physics, Dublin Institute of Advanced Studies .
- Sem , G. J. 2002 . Design and Performance Characteristics of Three Continuous-Flow Condensation Particle Counters: A Summary . Atmos. Res. , 62 : 267 – 294 . [CSA] [CROSSREF]
- Scheibel , H. G. and Porstendörfer , J. 1983 . Generation of Monodisperse Ag- and NaCl-Aerosols with Particle Diameters Between 2 and 300 nm . J. Aerosol Sci. , 14 : 113 – 126 . [CSA] [CROSSREF]
- Stolzenburg , M. R. and McMurry , P. H. 1991 . An Ultrafine Aerosol Condensation Nucleus Counter . Aerosol Sci. Technol. , 14 : 48 – 65 . [CSA]
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Range . J. Aerosol Sci. , 19 : 387 – 389 . [CSA] [CROSSREF]