Abstract
Although atmospheric particles are often non-spherical, Mie theory is commonly used to acquire aerosol optical depth, composition, and transport information from satellite retrievals. In the infrared (IR) region, the radiative effects of aerosols, usually modeled with Mie theory, are subtracted from satellite spectral data to determine key atmospheric and oceanic properties. To gain a better understanding of the infrared radiative effects of aerosols and the methods used to model them, an instrument has been designed to simultaneously measure infrared extinction spectra and particle size distributions obtained from a scanning mobility particle sizer (SMPS) and an aerodynamic particle sizer (APS). Infrared extinction spectra are simulated with Mie theory using the measured particle size distributions and available literature optical constants. As a result, the errors associated with using Mie theory to model the infrared extinction of mineral dust aerosol can be quantitatively examined. Initial results for this instrument are presented here. For ammonium sulfate, the Mie theory simulation is in good agreement with our measured extinction spectrum. This is in accordance with the nearly spherical shape of ammonium sulfate particles. However, for illite, an abundant clay mineral, there is poor agreement between the experimental spectrum and the Mie simulation. This result is attributed to particle shape effects.
INTRODUCTION
Mineral dust aerosol has a significant effect on the physical and chemical equilibrium of the atmosphere. Like all particles, mineral dust in the atmosphere will influence the global radiation balance through the direct scattering and absorption of light across the spectrum. Indeed, the direct radiative forcing of atmospheric aerosols represents one of the largest uncertainties in the current understanding of climate change (CitationPenner et al. 2001). Mineral dust particles in the atmosphere also serve as cloud condensation nuclei and as reaction sites for heterogeneous chemistry thereby affecting the climate forcing through indirect means (CitationGibson et al. 2006a; CitationKaufman et al. 2005; CitationRosenfeld et al. 2001; CitationUsher et al. 2003; CitationYin et al. 2002).
Modeling the impact of mineral dust aerosol on atmospheric processes requires accurate knowledge of the dust loading, as well as information about the dust composition, shape, and size distribution. Aerosol optical depths may be determined from remote sensing studies including high resolution or narrow band infrared (IR) spectral data from satellites (CitationAckerman 1997; CitationPierangelo et al. 2004). The infrared region is of particular importance as satellite measurements determine key atmospheric and oceanic properties such as the atmospheric temperature profile, water vapor and trace gas concentrations, and sea surface temperature through IR spectral measurements (CitationSokolik 2002). Under high dust loading conditions, the effect of atmospheric dust must be subtracted from the spectral measurements to accurately determine these atmospheric properties (CitationDeSouza-Machado et al. 2006). When the effects of dust are not properly included in satellite retrievals, the error can influence the interpretation of climate trends (CitationAckerman 1997). For example, a recent study found a change of 3 K in surface temperature determined from Advanced Very High Resolution Radiometer (AVHRR) data, when aerosols were included in the model (CitationHighwood et al. 2003).
Mie theory is commonly used for modeling the optical properties of aerosols in both radiative forcing and satellite retrieval algorithms (CitationConant et al. 2003; CitationMoffet and Prather 2005; CitationWang et al. 2002; CitationDeSouza-Machado et al. 2006). Mie theory is easy to apply for a given particle size distribution and set of optical constants. However, Mie theory is derived assuming uniform spherical particles (CitationBohren and Huffman 1983). Atmospheric mineral dust aerosol particles, on the other hand, are often inhomogeneous, complex mixtures of particles and particle aggregates of varying composition (CitationClaquin et al. 1999) and, moreover, are typically non-spherical (CitationDick et al. 1998), which can have a significant effect on the optical properties (CitationKalashnikova and Sokolik 2002). This is especially true in the neighborhood of IR absorption resonances, where particle shape effects can cause significant variations in spectral line positions and band profiles even for small particles (CitationBohren and Huffman 1983). These effects can be important since field studies have shown that as much as 30% of the total submicron aerosols can be mineral dust during a dust event (CitationArimoto et al. 2006). This could result in errors in the dust retrievals depending on the overlap between the actual and calculated line profiles, and specific narrow band IR sensor channels that are used to determine dust composition and aerosol optical depth (CitationAckerman 1997; CitationPierangelo et al. 2004).
To quantitatively investigate the radiative effects of mineral dust aerosol and the methods used to model them, an instrument capable of simultaneously measuring size distributions and infrared extinction spectra of aerosols has been designed and implemented. A scanning mobility particle sizer (SMPS) and an aerodynamic particle sizer (APS) are used to measure particle size distributions. Mie theory is then used to generate an ab initio simulated spectrum from the measured size distribution and available literature optical constants. This allows for an absolute comparison between the simulated and measured IR extinction spectra, with no adjustable parameters, thereby providing a quantitative evaluation of the errors associated with using Mie theory to model the optical properties of mineral dust aerosol.
This paper describes the experimental methodology, and the design, calibration, testing, and initial results from a newly designed and constructed instrument, the Multi-Analysis Aerosol Reactor System (MAARS). The application of these methods to study IR spectra for a series of important components of mineral dust aerosol will be described in a forthcoming publication (CitationHudson et al. 2007). The apparatus, including the aerosol generation, flow, and particle characterization methods are described first. Then a method to combine the measured SMPS and APS size distributions onto a common, volume equivalent diameter scale is shown.
The instrument and experimental approach are tested through measurements on both monodisperse polystyrene latex spheres (PSLs), and on ammonium sulfate aerosol. Ammonium sulfate is chosen because the particle shape and size characteristics and IR optical properties have been thoroughly characterized through earlier work (CitationKhlystov et al. 2004; CitationZelenyuk et al. 2006; CitationToon et al. 1976; CitationWeis and Ewing 1996). Our results show excellent quantitative agreement between Mie theory simulations and experimental extinction spectra for ammonium sulfate. As an application of this method to examine non-spherical systems, preliminary results for illite, an abundant clay component of mineral dust aerosol, are also presented.
THEORETICAL BACKGROUND
Combining SMPS and APS Data to Obtain a Full Size Distribution
Due to the wide variability in the size of ambient aerosol particles, it is often necessary to combine measurements from multiple instruments to yield a full size distribution. This is difficult when the measurement principles, such as electrical mobility and settling velocity, differ between the instruments. Previous studies have specifically outlined methods used to determine relationships between mobility and aerodynamic diameter measurements for the purpose of combining size distributions measured by the two methods. CitationKhlystov et al. (2004) created an algorithm to combine the mobility and aerodynamic size distributions using equivalencies between measurement principles outlined by CitationHinds (1999). CitationHand and Kreidenweis (2002) additionally used data from an optical particle counter (OPC) to retrieve the aerosols real refractive index and effective density (ρe) (defined as the ratio of the particle density (ρp) to the dynamic shape factor (χ)).
Here we expand on the definitions relating aerodynamic (Da) and mobility diameter (Dm) from CitationHinds (1999) and CitationKhlystov et al. (2004). Because atmospheric aerosol particles are often non-spherical, there is no unique definition for particle “size.” This issue has been discussed recently by CitationKalashnikova and Sokolik (2004). Our interest is in the optical properties of aerosol particles, in which case it is common to use either the volume equivalent diameter for “small” particles or the projected surface area equivalent diameter for “large” particles, depending on whether absorption or scattering is likely to dominate the optical properties (CitationBohren and Huffman 1983). The work we describe here focuses on studies of the IR resonance absorption for relatively small particles (D ≪ λ). Since absorption for small particles scales like the particle volume it is most appropriate to use the volume equivalent diameter (Dve) to describe the particle size distribution.
It is often assumed that particles in aerosol measurements are spherical such that the volume equivalent diameter is equal to the measured mobility diameter. Our interest, however, lies in examining the properties of non-spherical particles. Therefore, particle shape effects must be carefully considered in the conversion of measured mobility and aerodynamic diameters to volume equivalent diameter. According to CitationHinds (1999), the volume equivalent diameter is related to the aerodynamic diameter by:
It is important to note that our goal is not to determine precise aerodynamic shape factors, but rather to constrain the particle size distribution for use in Mie theory simulations of extinction spectra. Small errors in the shape factor or particle density have relatively little impact on the Mie simulations and will not significantly affect our results (vide infra). It is also worth pointing out that there is no direct correlation between aerodynamic shape and the optical properties of nonspherical particles.
Mie Theory
Mie theory can be used to predict the optical properties of uniform spherical particles. Of particular importance are the angle-integrated extinction (Cext), absorption (Cabs), and scatter cross-sections (Csca), given by:
The Mie simulation code used here (adapted from CitationHung and Martin 2002) calculates an extinction spectrum for a given size distribution and set of optical constants. The code essentially computes the extinction spectrum for a given size bin, and then sums the spectra over the size bins weighted by the particle number density in each bin to obtain the final spectrum. Mie simulations and optical constants available in the literature are used here for comparison to the experimentally measured spectra as is typical for modeling the radiative effects of atmospheric dust in many applications (CitationSokolik and Toon 1999).
EXPERIMENTAL METHODS
The Multi-Analysis Aerosol Reactor System (MAARS) apparatus has been described in detail elsewhere for other types of aerosol measurements (CitationGibson et al. 2006b). The experimental configuration used in the present study is shown in . The experiment consists of an atomizer (TSI, Inc. Model 3076) for aerosol generation, a Fourier-transform infrared (FTIR) spectrometer (Thermo Nicolet Nexus Model 670) with a liquid nitrogen cooled external MCT-A detector, and two particle sizing instruments, a scanning mobility particle sizer (SMPS) (TSI, Inc. Model 3936), consisting of a differential mobility analyzer (DMA) (TSI, Inc. Model 3080) and condensation particle counter (CPC) (TSI, Inc. Model 3025A), and an aerodynamic particle sizer (APS) (TSI, Inc. Model 3321). Briefly, the particles follow a flow stream from the aerosol generator through the path of the IR beam to the particle sizing instrumentation by a combination of conductive tubing and glass flow tubes. Size distributions and infrared extinction spectra are then simultaneously measured for each atomized aerosol. A suspension of 701 ± 6 nm polystyrene latex spheres (PSLs), a 1 wt% by volume ammonium sulfate solution, or a suspension of illite are used in this study.
FIG. 1 The main components of the Multi-Analysis Aerosol Reactor System (MAARS) designed to simultaneously measure aerosols IR extinction spectra and size distributions as a function of mobility and aerodynamic diameter with an SMPS and APS, respectively, are shown. A detailed view of the IR observation tube is shown with physical lengths for the glass tube, distance between window purges and aerosol stream in and out ports. See text for further detail.
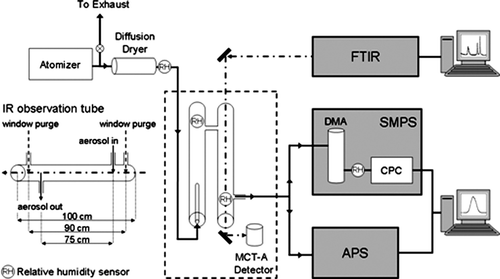
The particle flow stream from the atomizer passes through a diffusion dryer (TSI, Inc. Model 3062) typically resulting in an aerosol flow with relative humidity (RH) less than 15%. The glass flow tube system consists of an initial conditioning tube and an observation cell that is collinear with the IR beam. In these experiments, dry air is added to the particle stream in the conditioning tube to allow for sufficient flow to the particle sizing instrumentation. The observation tube length from window-to-window is 100 cm. The cell also has window purge ports, 90 cm apart, used for blowing dry air over the windows which additionally mixes with the particle stream. The distance between the particle stream entrance and exit ports in the observation tube is 75 cm. The observation tube is sealed at both ends with barium fluoride (BaF2) windows with a transmission range from 800–4000 cm−1 in the infrared spectral region. All IR spectra were measured by co-adding 256 scans at an instrument resolution of 8 cm−1.
After the observation tube, the particle stream is divided by a 3/8″ stainless steel cross directing the particle stream to the SMPS, APS, and an exhaust. The flow conditions are such that there is enough flow exiting the observation tube to supply the flow needs of both the SMPS (sheath flow = 2.0 lpm, aerosol flow = 0.2 lpm) and the APS (5 lpm). For high particle concentrations, one or two diluters (TSI, Inc. Model 3302A) are used with the APS to decrease the concentration to measurable levels. The SMPS measures the mobility diameter of the particle size distribution from 20–900 nm and the APS measures the aerodynamic diameter of the particle size distribution from 550 nm–20 μ m. The scan times for the IR, SMPS, and APS (210 sec total) are synchronized for simultaneous measurement of the size distribution with the corresponding infrared spectrum. The SMPS data is reported in either 32 or 64 bins per diameter decade and has been charge corrected for multiply charged particles. The APS data is reported in 32 bins per diameter decade.
For additional particle shape information, particle samples were collected on a piece of mica affixed to a scanning electron microscope (SEM) stub after exiting the diffusion dryer. The SEM stub was exposed to the particle stream for 15 to 30 minutes depending on the concentration of the sample. The SEM images were acquired with a Hitachi S-4000.
RESULTS AND DISCUSSION
701 nm PSL Spheres
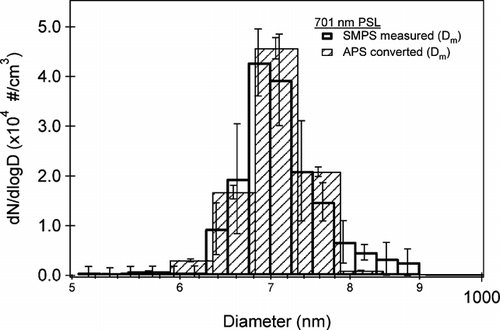
Monodisperse PSL spheres are used as an initial test of the particle sizing instruments and for testing the methodology to overlap the SMPS and APS data. The PSLs (Duke Scientific, Cat. # 3700A, Lot # 28824) have a known size (701 ± 6 nm), shape (χ = 1) and density (ρ = 1.05 g cm−3). shows an average of five measured size distributions for 701 nm PSL spheres from the SMPS and APS. The error bars represent the standard deviation of the measurements. The APS data has been adjusted from aerodynamic to mobility diameter according to Equation (Equation1). In this case, volume equivalent diameter and mobility diameter are equivalent because the PSLs are spherical. The SMPS data is shown with 64 bins per diameter decade and the APS is shown with 32 bins per diameter decade. As can be seen from the PSL data shown in , the agreement between the SMPS and the APS at the maximum peak position is quite good. Some disagreement is noted at mobility diameters greater than 750 nm, toward the upper limit of SMPS range, but the APS data is more reliable at these larger diameters and is used preferentially.
FIG. 2 The average size distribution, with respect to mobility diameter, of 701 ± 6 nm polystyrene latex spheres (PSLs) measured by the SMPS (open bars) and APS (hatched bars). The APS data has been adjusted to mobility diameter according to Equation (Equation1) using ρp = 1.05 g cm−3 and χ = 1. The error bars represent the standard deviation on the average value of five measurements.
Although the APS measures aerodynamic diameters as small as 550 nm, counting efficiency falls off rapidly for small particles (e.g., see first three APS size bins for the ammonium sulfate example in ). The manufacturers reported minimum counting efficiencies as a function of particle size are: 100% above 701 nm, 85% at 519 nm, 70% at 404 nm, and 35% at 360 nm; but every individual instrument varies somewhat (CitationKramlinger 2005). Note that the sampling efficiencies reported by the manufacturer are determined using PSL spheres. Therefore, the reported efficiency is quoted with respect to aerodynamic diameter. For spherical particles this is essentially equivalent to the mobility and volume equivalent diameters. However, for non-spherical particles, the sampling efficiency reported as a function of volume equivalent diameter can be quite different and will depend on the particle density and morphology.
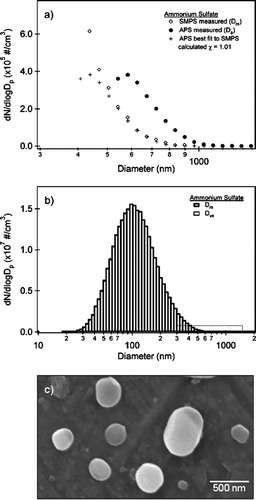
FIG. 3 (a) A partial size distribution of ammonium sulfate showing the overlap region between the SMPS (open diamonds) and APS. The APS data are shown as a function of aerodynamic diameter (filled circles) and adjusted to mobility diameter (plusses) according to Equation (Equation3). Using a density ρp = 1.769 g cm−3, a dynamic shape factor is calculated, χ = 1.01. The process of overlapping the SMPS and APS is discussed in detail in the text. (b) The full size distribution of ammonium sulfate on a log scale from 10 to 2000 nm. The size distributions are shown as a function of mobility diameter (Dm) and volume equivalent diameter (Dve). The dotted box highlights the overlap region between the SMPS and APS shown in . (c) SEM image of ammonium sulfate collected in the particle stream. The small particles, < 200 nm, are spherical where as larger particles can deviate in shape from that of a sphere.
Ammonium Sulfate: A Nearly Spherical Test Aerosol
Merging SMPS and APS Size Distributions
shows partial (a) and full (b) size distributions for ammonium sulfate aerosol obtained by atomizing a 1 wt% by volume ammonium sulfate solution and drying the particle stream as described in the experimental section. SEM images of a sample of ammonium sulfate particles collected from the particle stream directly onto the SEM stub are shown in . The partial size distribution in shows the overlap region between the SMPS and the APS. The APS data is shown as a function of both aerodynamic and mobility diameter. It should be noted that although the data are shown as individual points, each point represents the number concentration, dN/dlogDp, where the point is the median bin diameter. The data shown here are for an individual measurement. The overlap region between the SMPS and APS is selected with mobility diameters greater than 600 nm mobility diameter (∼800 nm aerodynamic diameter).
The following describes the process by which the APS data are shifted from aerodynamic to mobility diameter to match the SMPS data. Similar to the method described by CitationKhlystov et al. (2004), the SMPS and APS systems overlap in a size range for which the SMPS and APS both have good sampling efficiencies, and where the counting results for the two instruments are in good agreement. As noted above, the effective range of the APS, measured as a function of volume equivalent diameter, is dependent on particle density and shape, and the effective instrumental overlap region (in terms of mobility diameter) changes as a function of particle characteristics. The effective overlap region between the SMPS and APS for ammonium sulfate aerosol lies between 600 and 750 nm mobility diameter. To create the full size distribution as a function of mobility diameter, the SMPS data is used for diameters less than 600 nm and the APS data, adjusted to mobility diameter, is used for diameters greater than 600 nm. This method capitalizes on the performance strengths of each instrument within its optimal operating diameter range. Furthermore, it is assumed that over the narrow region of overlap (600–750 nm) the shape factor is a constant. CitationKhlystov et al. (2004) similarly assumed a constant size-correction factor within the overlapping size range. This assumption results in a negligible error in the final Mie simulated spectrum.
Using the relationship between the measured APS aerodynamic diameter, the derived APS mobility diameter, and the density of ammonium sulfate, ρ = 1.769 g cm−3, the dynamic shape factor can be calculated according to Equation (Equation3). The calculated dynamic shape factor for the size distributions shown in is χ = 1.01. The average result from more than 50 measurements of ammonium sulfate collected over a period of several weeks is χ = 1.01 ± 0.02. This is consistent with the common assumption that small ammonium sulfate particles are nearly spherical, and agrees with results from studies by CitationZelenyuk et al. (2006) on small ammonium sulfate particles (D < 200 nm), who report χ = 1.03 ± 0.01.
In order to compare the experimental IR spectrum to the Mie simulation, the full size distribution as a function of mobility diameter is converted to a volume equivalent diameter. shows the full size distribution of ammonium sulfate as a function of both mobility diameter and volume equivalent diameter calculated using Equation (Equation2). Because the dynamic shape factor is close to one, the two distributions are nearly indistinguishable. The volume equivalent size distribution can then be converted to a number concentration (dN), and used in the Mie simulation, together with optical constants from CitationToon et al. (1976).
Comparison of Experimental IR Data and Mie Simulation
In order to calculate a Mie simulation to compare to the ammonium sulfate experimental spectrum, the size distribution, optical constants, and IR cell path length are required. Because the infrared cell has been designed to eliminate deposition on the IR windows, the path length is not simply equal to the IR window-to-window cell dimensions of 100 cm. In fact, the path length should fall somewhere between 75 cm (aerosol inlet to outlet distance) and 90 cm (window to window air purge distance). The effective path length has been experimentally determined based on comparisons of our experimental IR spectrum of ammonium sulfate to previous results reported by CitationWeis and Ewing (1996).
The infrared spectrum of ammonium sulfate is shown in with the corresponding Mie simulation using the volume equivalent diameter size distribution. The ammonium sulfate optical constants used are from CitationToon et al. (1976). This spectral region shows three of the four IR-active vibrational modes of the ammonium sulfate salt: ν3(NH4 +) (3230 cm−1), ν4(NH4 +) (1425 cm−1), and ν3(SO4 2−) (1117 cm−1). The ν4(SO4 2−) is at 620 cm−1, below the measurement range of the instrument due to the BaF2 windows. The difference between the Mie simulation and the measured IR spectrum is shown in . Infrared absorptions attributed to gas-phase water present in the spectrum from slight changes in the observation tube have been subtracted out. Gas-phase carbon dioxide (CO2) is also kept to a minimum using a commercial dry air generator (Parker Balston, Model 75–62) but with small changes in the conditioning of the IR observation tube the CO2 concentration can change and is observed in the spectrum as a doublet centered at 2348 cm−1.
FIG. 4 (a) The experimental IR spectrum (black line) acquired simultaneously with the size distribution shown in and a Mie simulation (dotted line) using the CitationToon et al. (1976) ammonium sulfate optical constants and the measured size distribution. The area of the ammonium region (integrated from 2500 to 3700 cm−1) from the Mie simulation has been matched to that of the experimental spectrum to determine the path length of the IR cell. The path length for this spectrum was found to be 78 cm in good agreement with the experimental setup. b) The difference between the two spectra (Mie simulation—experimental). The ν3(NH4 +) peak in the Mie simulation is red shifted 44 cm−1 relative to the experimental spectrum. The largest differences are observed in the peak height of the ν3(SO4 2−) and ν4(NH4 +) peaks. The ratio of the peak area of these peaks between the Mie simulation and experimental spectrum are within 10%. These are also slightly shifted (10 cm−1) in the Mie simulation relative to the experimental spectrum.
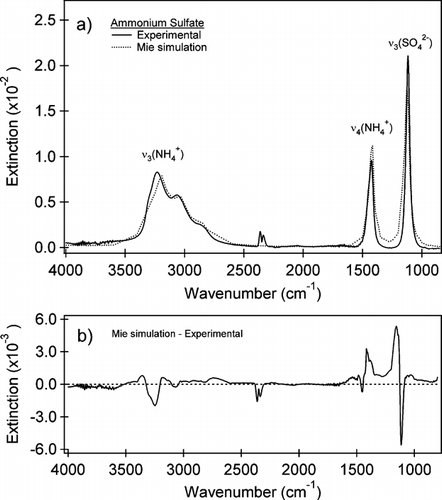
The integrated area of the simulated ν3(NH4 +) absorption is used to determine our effective optical path length as the experimental integrated area of this band, ν3(NH4 +), was shown to be in good agreement with Mie theory (CitationWeis and Ewing 1996). Matching the integrated area of the experimental spectrum to that of the Mie simulation, within the ammonium ν3 region (2500 to 3700 cm−1), yields an effective path length of 78 cm. This is clearly within the range of possible path lengths and is in good agreement with the experimental configuration as shown in . The average of 50 similar measurements gives a mean value for the path length of 78 ± 7 cm.
Further comparison of our data to that presented by CitationWeis and Ewing (1996) show a slight difference in the ratio of the peak heights of ν3(SO4 2−) to ν4(NH4 +). However, the ratio of the peak areas is in excellent agreement. Although there are some slight differences, overall the agreement between our experimental spectra and that of CitationWeis and Ewing (1996) is excellent.
In general, good agreement is found between the measured IR extinction spectrum and that generated by the Mie calculation. The ammonium sulfate test system helps to validate the instrument calibration and our experimental method. Furthermore, the results provide a confirmation of the quality of the published optical constants for ammonium sulfate.
Illite: A Clay Component of Mineral Dust Aerosol
Merging SMPS and APS Size Distributions
As a further application of the instrument, preliminary data for illite is presented. Illite is a potassium aluminum silicate hydroxide hydrate mineral in the clay group found to be an important component of atmospheric mineral dust. A suspension of illite (Source Clays Repository, IMt-1, Lot 1) and Optima water are atomized as described in the experimental section. shows the resulting partial (a) and full (b) size distributions for illite. SEM images of several illite particles collected from the flow stream are shown in . The SMPS and APS overlap region is shown in . The overlap procedure between the SMPS and APS is similar to that described previously. As was noted in for ammonium sulfate, the agreement between the SMPS and APS is quite good with the exception of the first few APS bins. The dynamic shape factor for illite can be calculated from Equation (Equation3) using the density of illite, ρ = 2.8 g cm−3, resulting in χ = 1.30 ± 0.02. Note the σ is determined in part by experimental uncertainty and in part by uncertainty in the bulk density value. The density of illite used here is an average of previously reported densities. This χ is appreciably larger than that for ammonium sulfate, as might be expected since most clays are plate-like, which would yield a larger value for χ (CitationHinds 1999).
FIG. 5 (a) A partial size distribution of illite showing the overlap region between the SMPS (open diamonds) and APS. The APS data are shown as a function of aerodynamic diameter (filled circles) and adjusted to mobility diameter (plusses) according to Equation (Equation3). Using a density ρp = 2.8 g cm−3, a dynamic shape factor is calculated, χ = 1.30. The process of overlapping the SMPS and APS is discussed in detail in the text. (b) The full size distribution of illite on a log scale from 10 to 2000 nm. The size distributions are shown as a function of mobility diameter (Dm) and volume equivalent diameter (Dve). The dotted box highlights the overlap region between the SMPS and APS shown in . (c) SEM image of illite collected in the particle stream. The illite particles appear plate-like supporting the large value of the experimentally determined χ.
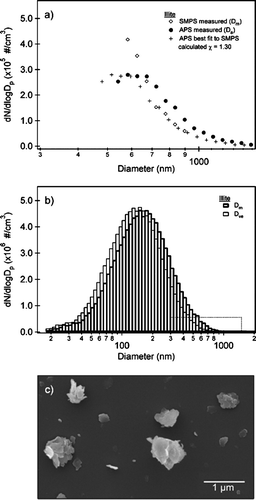
The full size distribution for illite is shown in as a function of both mobility diameter and volume equivalent diameter. Due to the larger χ, a notable difference is observed between the mobility diameter and the volume equivalent diameter. Thus, the common practice of equating the mobility and volume equivalent diameters would lead to a significant error in this case.
Comparison of Experimental IR Data and Mie Simulation
shows the experimental extinction spectrum for illite (acquired simultaneously with the size distribution shown in ), and the Mie theory based simulated spectrum. The Mie simulation uses the effective path length of 78 cm, determined from the ammonium sulfate experiments, optical constants from CitationQuerry (1987) and the volume equivalent size distribution shown in . The spectral region from 850–1350 cm−1 is expanded for clarity in . The peaks in the experimental spectrum are identified as the Al—Al—OH deformation at 916 cm−1, the prominent Si—O stretch at 1036 cm−1, and the inner O—H stretch at 3616 cm−1. A shoulder is also present in the experimental spectrum at ca. 1086 cm−1. shows the difference spectrum between the Mie simulation and the experimental spectrum. Note that in the resonance region, the differences between the simulated and measured spectra are much larger than for ammonium sulfate ().
FIG. 6 (a) Experimental IR spectrum (black line) acquired simultaneously with the size distribution shown in and a Mie simulation (dotted line) using the CitationQuerry (1987) optical constants for illite and the volume equivalent diameter size distribution (Dve). The peaks identified are for the Al—Al—OH deformation at 916 cm−1, the ν (Si—O) at 1036 cm−1 and the inner OH stretch at 3616 cm−1. Some gas-phase CO2 and H2O absorptions are observed in the experimental spectrum. Key differences between the simulations and the experimental spectrum are seen in the slope of the baseline from 2500–3500 cm−1 and a blue shift of the ν (Si—O) at 1036 cm−1 to 1080 cm−1 in the Mie simulations relative to the experimental spectrum. (b) An expansion of the IR spectrum for the two spectra in from 850 to 1350 cm−1. The experimental peaks and shoulder are underlined for clarity. A small blue shift in the position of the Al—Al—OH deformation of the Mie simulation can also be seen. (c) The difference between the Mie simulation and the experimental spectrum for the spectral range from 850–4000 cm−1. The largest difference is observed between the peak positions of the ν (Si—O) in the resonance region.
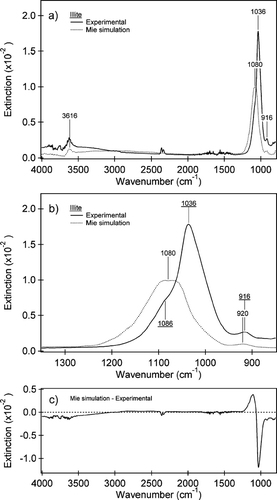
A major discrepancy is apparent in the strong Si—O stretch resonance region, where there are significant differences in band position, band shape, and peak intensity between the experimental and simulated spectra. The experimental Si—O resonance peak is red shifted by ∼ 44 cm− 1 from the Mie simulation and the experimental peak amplitude is roughly a factor of two larger. This spectral range is particularly important because the silicate stretch region is often used to characterize mineral dust loading from narrow band and high-resolution satellite spectral data (CitationSokolik 2002; CitationDeSouza-Machado et al. 2006). In contrast, the peak positions for the Al—Al—OH deformation and inner O—H stretch are in fairly good agreement between the experimental spectrum and Mie simulation. In addition to the discrepancy in the Si—O resonance region, the baseline slope in the 2500–4000 cm−1 range also differs somewhat.
It is important to consider possible reasons for the observed differences between the Mie simulation and experimental spectrum. For example, the differences in the slope of the scattering region above 3000 cm−1 could result from the presence of residual adsorbed water in the clay particles, which commonly gives a broad absorption feature in the 3000–3500 cm− 1 range, associated with the O—H stretching mode of adsorbed water. Consistent with this hypothesis, we also note a weak absorption band near 1640 cm− 1 that can be assigned to the H2O bending mode of adsorbed water. Alternatively, a sloping baseline in the range above 2000 cm− 1 could result from scattering by a small number of large particles that are not properly detected and counted in the APS. In particular, there may be some transmission losses between the extinction cell and the SMPS/APS instruments, specifically for large particles. In addition, uncertainties in the bulk density and aerodynamic shape factor can lead to some error in the recovered size distribution. Finally, there can be slight variations in resonance line profiles associated with differences in mineralogical samples.
To investigate these effects we have carried out a series of modeling studies and sensitivity tests, varying the parameters over the range of uncertainties and observing the effect on the Mie simulation. For example, the reported density of illite ranges from 2.65 to 2.9 g cm−3 corresponding to a possible range in derived dynamic shape factor of 1.28 to 1.31. The variation in size distribution that results from uncertainty in the density and shape factors can affect the Mie simulation. There is also a 9% uncertainty in the optical pathlength. In addition, we consider the possibility that large particles may be undercounted due to transmission losses. Based on these modeling studies and sensitivity analyses we estimate the largest uncertainty to be ∼ 12% in the amplitude of the calculated resonance peak. In contrast, these possible errors in the size distribution have a negligible effect on the resonance peak position or band shape. The shift in the size distribution due to changing the density and shape factor changes the resonance peak position by < 2 cm−1. In conclusion, possible errors in the size distribution that could arise from assumptions such as a size independent shape factor or density averaging have a small effect on the magnitude of the simulated spectrum and a negligible effect on the position and line shape of that spectrum.
A previous study has shown that the presence of adsorbed water does not lead to a significant change in the silicate Si—O resonance peak structure (CitationFrinak et al. 2005); studies of Na-Montmorillonite clay (a swellable clay) have shown a slight red shift in the Si—O stretch with water uptake, but even in this case the shift is very small relative to the 44 cm−1 shift between the experimental and Mie simulated spectrum observed here.
Studies in our lab involving attenuated total reflectance measurements on bulk clay powders have also shown that there can be some slight differences in line position and band shape among kaolinite and montmorillonite clay samples from different sources, though these variations are smaller than the 44 cm− 1 line shift observed here (CitationSchuttlefield et al. 2007). In this context it is also interesting to note that the original CitationQuerry (1987) reflectance measurements (from which the optical constants were derived), and an absorption spectrum measured by CitationHunt et al. (1950), are in very good agreement with our aerosol-based measurement in peak position and overall band shape. There are slight spectral differences, as might be expected, since the earlier reflectance and absorption measurements were carried out on compressed bulk powder samples of illite dust from different sources. The similarities are interesting because they clearly show that the results are largely independent of clay source and sample preparation and history.
While it is possible that the CitationQuerry (1987) optical constants might be in error, we think that unlikely. While we have not found another source for illite refractive index data, we have compared the CitationQuerry (1987) optical constant data sets for montmorillonite and kaolinite with results from other independent measurements (CitationRoush et al. 1991), and they show excellent agreement with the CitationQuerry (1987) data in the silicate stretch region. (In addition, the errors in the Mie theory simulation shown here are not unique to illite; we will present similar results in forthcoming publications for several other common constituents of mineral dust aerosol including kaolinite, montmorillonite, quartz, calcite, and others (CitationHudson et al. 2007).) Rather, the most likely reason for the discrepancy between the Mie simulation and the experimental spectrum is due to the breakdown of Mie theory as a result of particle shape effects. It is well known that Mie theory does a poor job in predicting the resonance line positions and band shapes for non-spherical particles, even for small particles that fall in the Rayleigh regime, D ≪ λ (CitationBohren and Huffman 1983). This effect is commonly recognized in the astronomy literature (CitationFabian et al. 2001) where spectral simulations often use analytic model results derived in the Rayleigh limit for different characteristic particle shapes such as distributions of disks, needles, or ellipsoids (CitationBohren and Huffman 1983). In a forthcoming paper we will explore these analytic model results for “shaped particles” and carry out a quantitative comparison with the Mie theory analysis and our experimental spectra (CitationHudson et al. 2007).
The difference in the peak position of the illite resonance feature between the experimental spectrum and the Mie simulation of the infrared spectrum could have an important effect on aerosol retrieval algorithms for the determination of dust loading and composition. CitationSokolik (2002) has proposed that high-resolution remote sensing could use the spectral region from 1099–1220 cm−1 to characterize wind-blown atmospheric dust as this region is highly sensitive to dust composition. However, Mie theory may incorrectly predict the position of key features in this spectral region due to particle shape effects, and as a result, determinations of mineralogical composition could be compromised. Further, inaccurate simulations of these absorption resonances can have implications for IR satellite retrievals of important atmospheric and oceanic properties such as sea surface temperature or ozone concentration (CitationDeSouza-Machado et al. 2006). These issues will be addressed in more detail in a future publication (CitationHudson et al. 2007).
CONCLUSIONS AND FUTURE WORK
An instrument has been designed to simultaneously measure particle size distributions, from 20 nm to 20 μ m, and IR extinction spectra. The complete size distribution is measured using mobility and aerodynamic diameters with an SMPS and APS, respectively. Using relationships between the Dm and Da, mobility and aerodynamic size distributions have been successfully combined as verified by measurements on 701 nm PSL and ammonium sulfate. Through the overlap of the mobility and aerodynamic size distributions the dynamic shape factor, χ, is determined yielding information about the aerodynamic shape of the particle. The size distributions can be converted to volume equivalent diameters that are then used as input into Mie simulations. Using the ammonium sulfate optical constants from CitationToon et al. (1976) combined with the measured size distribution, a Mie-based spectrum is found to be in excellent agreement with the experimental spectrum. This experiment verifies the optical constant data set of CitationToon et al. (1976), and allows a validation of the experimental method.
This instrument allows us to quantitatively evaluate errors that arise when applying Mie theory to non-spherical aerosol particles. Current investigations on a wide range of mineral dust aerosols including carbonates, sulfates, oxides, and clays are underway. Furthermore we plan to extend this work to include mixtures and coatings, as most atmospheric particles are rarely found as individual components but in fact are complex mixtures of aggregates and coated particles.
This material is based upon work supported by the National Science Foundation under Grant No. ATM-0425989. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors would like to thank Professor Scot Martin for providing us with the Mie simulation code and Professor Kuo-Ho Yang for his continued work on this code. PKH would like to thank Dr. Charles Brock for helpful thoughts and discussions on size distributions and aerosol measurements.
REFERENCES
- Ackerman , S. A. 1997 . Remote Sensing Aerosols Using Satellite Infrared Observations . J. Geophys. Res. , 102 : 17069 – 17079 . doi:10.1029/97JD01552
- Arimoto , R. , Kim , Y. J. , Kim , Y. P. , Quinn , P. K. , Bates , T. S. , Anderson , T. L. , Gong , S. , Uno , I. , Chin , M. , Huebert , B. J. , Clarke , A. D. , Shinozuka , Y. , Weber , R. J. , Anderson , J. R. , Guazzotti , S. A. , Sullivan , R. C. , Sodeman , D. A. , Prather , K. A. and Sokolik , I. N. 2006 . Characterization of Asian Dust during ACE-Asia . Global Planet. Change. , 52 : 23 – 56 .
- Bohren , C. F. and Huffman , D. 1983 . Absorption and Scattering of Light by Small Particles , New York : Wiley .
- Claquin , T. , Schulz , M. and Balkanski , Y. J. 1999 . Modeling the Mineralogy of Atmospheric Dust Sources . J. Geophys. Res. , 104 : 22243 – 22256 . doi:10.1029/1999JD900416
- Conant , W. C. , Seinfeld , J. H. , Wang , J. , Carmichael , G. R. , Tang , Y. H. , Uno , I. , Flatau , P. J. , Markowicz , K. M. and Quinn , P. K. 2003 . A Model for the Radiative Forcing During ACE-Asia Derived from CIRPAS Twin Otter and R/V Ronald H. Brown Data and Comparison with Observations . J. Geophys. Res. , 108 : 8661 doi:10.1029/2002JD003260
- DeCarlo , P. , Slowik , J. , Worsnop , D. , Davidovits , P. and Jimenez , J. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory . Aerosol Sci. Technol. , 38 : 1185 – 1205 .
- DeSouza-Machado , S. G. , Strow , L. L. , Hannon , S. E. and Motteler , H. E. 2006 . Infrared Dust Spectral Signatures from AIRS . Geophys. Res. Lett. , 33 : L03801 doi:10.1029/2005GL024364
- Dick , W. D. , Ziemann , P. J. , Huang , P. F. and McMurry , P. H. 1998 . Optical Shape Fraction Measurements of Submicrometre Laboratory and Atmospheric Aerosols . Meas. Sci. Technol. , 9 : 183 – 196 .
- Fabian , D. , Henning , T. , Jager , C. , Mutschke , H. , Dorschner , J. and Wehrhan , O. 2001 . Steps Toward Interstellar Silicate Mineralogy—VI. Dependence of Crystalline Olivine IR Spectra on Iron Content and Particle Shape . Astron. Astrophys. , 378 : 228 – 238 .
- Frinak , E. K. , Mashburn , C. D. , Tolbert , M. A. and Toon , O. B. 2005 . Infrared Characterization of Water Uptake by Low-Temperature Na-Montmorillonite: Implications for Earth and Mars . J. Geophys. Res. , 110 : D09308 doi:10.1029/2004JD005647
- Gibson , E. R. , Hudson , P. K. and Grassian , V. H. 2006a . Aerosol Chemistry and Climate: Laboratory Studies of the Carbonate Component of Mineral Dust and Its Reaction Products . Geophys. Res. Lett. , 33 : L13811 doi:10.1029/2006GL026386
- Gibson , E. R. , Hudson , P. K. and Grassian , V. H. 2006b . Physicochemical Properties of Nitrate Aerosol: Implications for the Atmosphere . J. Phys. Chem. , 110 : 11785 – 11799 . doi:10.1021/jp063821k
- Hand , J. L. and Kreidenweis , S. M. 2002 . A New Method for Retrieving Particle Refractive Index and Effective Density from Aerosol Size Distribution Data . Aerosol Sci. Technol. , 36 : 1012 – 1026 .
- Highwood , E. J. , Haywood , J. M. , Silverstone , M. D. , Newman , S. M. and Taylor , J. P. 2003 . Radiative Properties and Direct Effect of Saharan Dust Measured by the C-130 Aircraft During Saharan Dust Experiment (SHADE): 2. Terrestrial spectrum . J. Geophys. Res. , 108 : 8578 doi:10.1029/2002JD002552
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : Wiley .
- Hudson , P. K. , Gibson , E. R. , Young , M. A. , Kleiber , P. D. and Grassian , V. H. 2007 . Coupled Infrared Extinction and Size Distribution Measurements for Several Clay Components of Mineral Dust Aerosol . J. Geophys. Res. , Submitted
- Hung , H. M. and Martin , S. T. 2002 . Infrared Spectroscopic Evidence for the Ice Formation Mechanisms Active in Aerosol Flow Tubes . Appl. Spectrosc. , 56 : 1067 – 1081 .
- Hunt , J. M. , Wisherd , M. P. and Bonham , L. C. 1950 . Infrared Absorption Spectra of Minerals and Other Inorganic Compounds . Anal. Chem. , 22 : 1478 – 1497 .
- Kalashnikova , O. V. and Sokolik , I. N. 2002 . Importance of Shapes and Compositions of Wind-Blown Dust Particles for Remote Sensing at Solar Wavelengths . Geophys. Res. Lett. , 29 : 1398 doi:10.1029/2002GL014947
- Kalashnikova , O. V. and Sokolik , I. N. 2004 . Modeling the Radiative Properties of Nonspherical Soil-Derived Mineral Aerosols . J. Quant. Spectrosc. Radiat. Transfer. , 87 : 137 – 166 .
- Kaufman , Y. J. , Koren , I. , Remer , L. A. , Rosenfeld , D. and Rudich , Y. 2005 . The Effect of Smoke, Dust, and Pollution Aerosol on Shallow Cloud Development Over the Atlantic Ocean . Proc. Natl. Acad. Sci. USA , 102 : 11207 – 11212 .
- Khlystov , A. , Stanier , C. and Pandis , S. 2004 . An Algorithm for Combining Electrical Mobility and Aerodynamic Size Distributions Data when Measuring Ambient Aerosol . Aerosol Sci. Technol. , 38 : 229 – 238 .
- Kramlinger , C. 2005 . TSI Inc. personal communication
- Moffet , R. C. and Prather , K. A. 2005 . Extending ATOFMS Measurements to Include Refractive Index and Density . Anal. Chem. , 77 : 6535 – 6541 .
- Penner , J. E. , Andreae , M. , Annegarn , H. , Barrie , L. , Feichter , J. , Hegg , D. A. , Jayaraman , A. , Leaitch , R. , Murphy , D. , Nganga , J. and Pitari , G. 2001 . Aerosols, Their Direct and Indirect Effects , 881 New York : Cambridge University Press .
- Pierangelo , C. , Chedin , A. , Heilliette , S. , Jacquinet-Husson , N. and Armante , R. 2004 . Dust Altitude and Infrared Optical Depth from AIRS . Atmos. Chem. Phys. , 4 : 1813 – 1822 .
- Querry , M. R. 1987 . Optical Constants of Minerals and Other Materials from the Millimeter to the Ultraviolet , Aberdeen, MD : U.S. Army .
- Rosenfeld , D. , Rudich , Y. and Lahav , R. 2001 . Desert Dust Suppressing Precipitation: A Possible Desertification Feedback Loop . Proc. Natl. Acad. Sci. USA , 98 : 5975 – 5980 .
- Roush , T. , Pollack , J. and Orenberg , J. 1991 . Derivation of Midinfrared (5–25 μ m) Optical Constants of Some Silicates and Palagonite . Icarus. , 94 : 191 – 208 .
- Schuttlefield , J. D. , Cox , D. and Grassian , V. H. 2007 . An Investigation of Water Uptake on Clays Minerals using ATR-FTIR Spectroscopy Coupled with Quartz Crystal Microbalance Measurements . J. Geophys. Res. , in preparation
- Sokolik , I. N. 2002 . The Spectral Radiative Signature of Wind-Blown Mineral Dust: Implications for Remote Sensing in the Thermal IR Region . Geophys. Res. Lett. , 29 : 2154 doi:10.1029/2002GL015910
- Sokolik , I. N. and Toon , O. B. 1999 . Incorporation of Mineralogical Composition into Models of the Radiative Properties of Mineral Aerosol from UV to IR Wavelengths . J. Geophys. Res. , 104 : 9423 – 9444 . doi:10.1029/1998JD200048
- Toon , O. B. , Pollack , J. B. and Khare , B. N. 1976 . The Optical Constants of Several Atmospheric Aerosol Species: Ammonium Sulfate, Aluminum Oxide, and Sodium Chloride . J. Geophys. Res. , 81 : 5733 – 5748 .
- Usher , C. R. , Michel , A. E. and Grassian , V. H. 2003 . Reactions on Mineral Dust . Chem. Rev. , 103 : 4883 – 4939 .
- Wang , J. , Flagan , R. C. , Seinfeld , J. H. , Jonsson , H. H. , Collins , D. R. , Russell , P. B. , Schmid , B. , Redemann , J. , Livingston , J. M. , Gao , S. , Hegg , D. A. , Welton , E. J. and Bates , D. 2002 . Clear-Column Radiative Closure during ACE-Asia: Comparison of Multiwavelength Extinction Derived from Particle Size and Composition with Results from Sun Photometry . J. Geophys. Res. , 107 : 4688 doi:10.1029/2002JD002465
- Weis , D. D. and Ewing , G. E. 1996 . Infrared Spectroscopic Signatures of (NH4)2SO4 Aerosols . J. Geophys. Res. , 101 : 18709 – 18720 .
- Yin , Y. , Wurzler , S. , Levin , Z. and Reisin , T. G. 2002 . Interactions of Mineral Dust Particles and Clouds: Effects on Precipitation and Cloud Optical Properties . J. Geophys. Res. , 107 : 4724 doi:10.1029/2001JD001544
- Zelenyuk , A. , Cai , Y. and Imre , D. 2006 . From Agglomerates of Spheres to Irregularly Shaped Particles: Determination of Dynamic Shape Factors from Measurements of Mobility and Vacuum Aerodynamic Diameters . Aerosol Sci. Technol. , 40 : 197 – 217 .