Abstract
The contact angle, θ, and volume equivalent diameter of an (NH4)2SO4 aqueous droplet was measured using an environmental scanning electric microscope (ESEM), showing the hygroscopic growth of the solution droplet as the relative humidity (RH) increased from 80% to 98%. (NH4)2SO4 particles with diameters in the range 1–2 μ m were produced by an atomization technique, and collected onto a copper substrate that had been treated with polytetrafluoroethylene. To observe the hygroscope growth, the sample chamber of the ESEM was filled with water vapor at a pressure of 600 Pa, and the sample temperature was adjusted using a cooling stage to control the relative humidity inside the chamber. Before the observation of the hygroscopic growth, we determined the value of θ from overhead views of droplets on the stage at a tilted angle of 45°. The average value of θ was 96 ± 10°, and this value was used to estimate the droplet diameter. We measured the diameter of the (NH4)2SO4 droplets at different RH, and observed that the growth factor, G, increased with increasing RH. The experimental value of G was consistent with the theoretically estimated value. This shows that our method for determining the value of θ was valid, and that the ESEM technique can be used to measure the diameters of droplets of aqueous solutions.
INTRODUCTION
Atmospheric aerosols vary in terms of their composition, size, and phase. Their state (i.e., liquid or solid phase) varies with respect to their chemical composition, amount of water vapor present, and ambient temperature (e.g., Seinfeld and Pandis 1988). Information on the state of atmospheric aerosols is required to predict the rate of heterogeneous reactions on the surface of such particles, and the degree of light scattering and absorption by these particles (e.g., Seinfeld and Pandis 1988; CitationTang and Munkelwitz 1994). In this work, we have studied (NH4)2SO4aerosol particles, which are widely distributed in the atmosphere. The (NH4)2SO4 particles exist as a crystalline phase at low relative humidity (RH), and this phase is transformed into an aqueous solution phase as the RH increases above a threshold value, which is known as the deliquescence relative humidity (DRH). In the aqueous solution stage, the droplet changes in size on absorbing or evaporating water vapor due to changes in RH (hygroscopic growth). A hysteresis occurs, in that the aqueous solution recrystallizes at an RH lower than the DRH, and the RH where the recrystallization occurs is known as the efflorescence relative humidity (ERH).
The environmental scanning electron microscope (ESEM) has been used to observe morphological changes in individual aerosol particles on changes in RH, and has been used to determine their DRH and ERH values (CitationEbert et al. 2002; CitationHoffman et al. 2004). When coupled with an energy dispersive X-ray (EDX) analysis, the ESEM has been applied to studies on the hygroscopic behavior of atmospheric aerosols having a mixed composition (CitationLaskin et al. 2005). This application showed the advantages in using an ESEM. A shortcoming in the technique is in measuring the size of solution droplets that are collected on a flat surface. These droplets contact the surface at an angle, θ, which is dependent on the composition of the solution and on the substrate, affecting the shape of a droplet. Thus, hygroscopic growth is rarely discussed using ESEM data.
In this study, the ESEM technique was used to observe the hygroscopic growth of (NH4)2SO4 particles. The initial effort focused on measuring the values of θ of droplets of an aqueous solution of (NH4)2SO4 on a substrate using ESEM images, and then, we measured the increase in diameter of the droplets with increasing RH. We will show the hygroscopic growth, and will provide a comparison of the experimental and theoretical hygroscopic growth.
EXPERIMENTAL
We used an Environmental Scanning Electron Microscope (ESEM, Quanta 200 FEG, FEI Company, USA) to observe the hygroscopic properties of (NH4)2SO4 droplets. The ESEM is a special type of Scanning Electron Microscope (SEM) that is able to observe samples in a low vacuum, and allows for the introduction of gas into its sample chamber. Correspondingly, we introduced water vapor into our chamber for our hygroscopic observations.
The samples prepared were (NH4)2SO4 aerosols that were artificially generated by atomizing a 0.2–0.5 wt% solution of aqueous (NH4)2SO4 in deionized water using a nebulizer. The aerosols generated by the nebulizer were dried to an RH below 10% by mixing with a dilution air. Aerosols with droplet diameters in the range 1–2 μ m were collected on copper substrates that had been coated with polytetrafluoroethylene (PTFE). The PTFE treatment made the substrate hydrophobic, and PTFE-treated slides have been used previously in the observation of droplets (CitationPant et al. 2006).
We mounted a Peltier cooling stage inside the ESEM chamber to control the temperature of the sample, which also allowed us to control the RH, which is defined by
The cooling stage had a resistive temperature device that measured the temperature to the accuracy of ± 0.5°C. This uncertainty invoked an error of around 3% in the RH values.
As an observation run, we initially kept the sample at a RH value below 10% inside the ESEM for a period of about 10 min, and then, we began to take images at RH = 70%, and then increased RH by cooling the sample using a cooling rate of 0.1°C per 2 min, which was slow enough for heat transfer to occur over the whole sample. We took an image of the droplets at each temperature step and observed their hygroscopic growth.
The skill in monitoring was how to acquire clear images. Well-focused images can be taken, but at the sacrifice of the condition of the sample. However, we also had to take good care not to impose any damage on the sample at the same time as taking our images. To obtain a well-focused image with a sample remaining in good condition, we had to optimize our observational conditions, which were: accelerating voltage = 20 keV, use a mid-range scanning speed (line time = 5.3 ms), and use a magnification = 1,600×. We checked for damage on a sample under the observational conditions of P w = 600 Pa and temperature = 0.2°C, and found that the sample had no observable damage before it was exposed in the electron beam for a period of 1 h.
We measured the size of the droplets obtained in the images using an image analysis software package on a PC (Genesis Particle Analysis, EDAX Inc., USA), and calculated the growth factor, which is usually used to indicate the degree of hygroscopic growth. This value is defined by the ratio of the diameter at a given RH to that of a dry crystal particle (usually taking the diameter at RH to be < 10%), and is given by
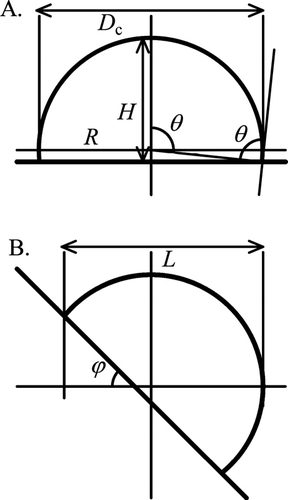
FIG. 1 Schematic diagrams of a spherical cap shape. (A) On a horizontal stage and (B) on a stage tilted at an angle ϕ, where θ is the contact angle, R and H are the radius and the width of the spherical cap, respectively, D c is the cross-sectional diameter, and L is the droplet length perpendicular to the tilt axis.
CONTACT ANGLE
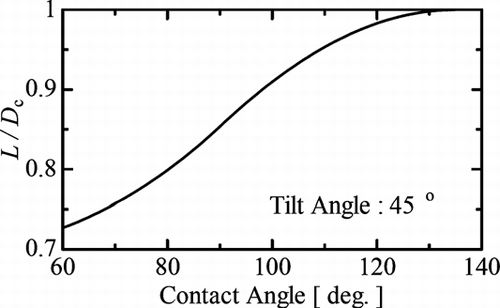
To determine the value of θ of the droplets on the substrate, we tilted the sample on a cooling stage at an angle of 45°, and then, we took an image, such as the image shown in . The overhead view of particle changed with respect to the tilt angle of the stage. If the particle has a spherical cap shape, then an overhead view of its outline can be depicted mathematically. For example, for a length, L, the length perpendicular to the tilt axis, as defined in and , is given by the formula
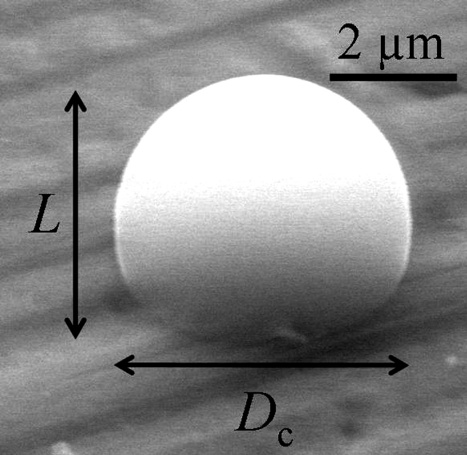
FIG. 2 An image of a spherical cap shape at a tilt angle of 45°. The particle in the image is an (NH4)2SO4 aqueous solution droplet. The tilt axis is in the horizontal direction.
Modifications to the shape of a droplet shape on a tilted surface have been discussed elsewhere (CitationFrenkel 1948; CitationFurmidge 1962). For micron-sized droplets, the original shape is maintained, even at a tilt angle of 90°.
To find the average value of θ, we measured the values of L and D c of 99 droplets of (NH4)2SO4 aqueous solution with a temperature variation of 0.8–1.2°C and over the RH range 90%–93%. Using a thermodynamic model, the fraction of salt in the solution was expected to be 24–29 wt% (CitationClegg et al. 1995). We found that the average value of θ was θ = 96 ± 10°. The reported value for pure water ranges from 100 to 117°, and it does not make a clear distinction with the value of θ = 96 ± 10° (CitationAdamson and Gast 1997; CitationPruppacher and Klett 1997). The substrate we used was not completely flat, and was rough on the nanometer scale. This roughness may have affected our measured error in the value of θ .
In this study, we used a value of θ of θ = 96 ± 10° to estimate the volume equivalent diameter of the solution droplets on the substrate. The volume, V, and the volume equivalent diameter, D v, of a cap-shaped droplet are given by
The value of θ depends on the surface tension of the aqueous solution, which varies with temperature and salt fraction. This can induce a significant error in the size estimation of a droplet. However, in our observation range, the fraction of the surface tension was expected to be 81–83 mJ/m2 using an empirical model (CitationChen 1994). If the interface energies between solid and gas and between solid and liquid were assumed to be constant, from the Young's relation (e.g., CitationAdamson and Gast 1997) the error in θ was estimated to be ± 1°, which was low when compared with the measurement error of ± 10°.
RESULTS AND DISCUSSION
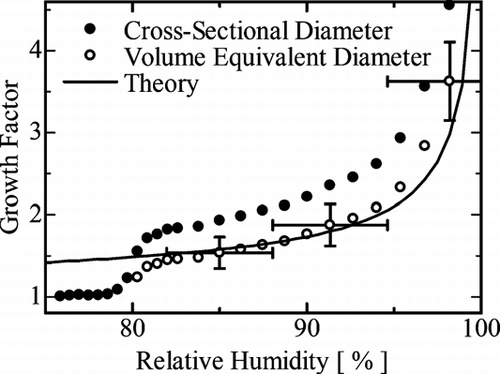
We observed the hygroscopic growth of (NH4)2SO4 droplets within the RH range 70–98%. This observation was made at constant vapor pressure of 600 Pa on decreasing the sample temperature from 4.7 to 0.0°C. We took microscope images at each RH value, and carried out image analysis. We assumed that the dry crystal particle was spherical and deduced the growth factor, G, using Equation (Equation3). shows the average value of G as a function of RH. The solid dots denote the value of G defined by the cross-sectional diameter, D c . This value increased markedly as the value of RH increased from 79% to 81%, indicating the deliquescence of the (NH4)2SO4 salt crystals. The deliquescence is gradual in this measurement whereas suspended particle techniques shows abrupt transition (e.g., CitationTang and Munkelwitz 1994; CitationHämeri et al. 2000; CitationGysel et al. 2002). This may be related to the pressure of operation of the ESEM. The particle size after the deliquescence point was defined by the volume equivalent diameter, D v, calculated using the contact angle θ = 96 ± 10°, and is denoted by the open circles in . The values of G obtained from D v were about 30% smaller than the values obtained from D c .
FIG. 4 The average growth factor as a function of RH. The RH was increasing during the data collection. The solid circles were obtained from the cross-sectional diameter. The open circles were obtained from the volume equivalent diameter after the deliquescence point. The solid curve is the theoretically calculated curve. The horizontal error bars arise from the uncertainty of the temperature of sample. The vertical error bars arise from the standard deviation plus the error in θ .
We evaluated the results of the growth factor measurements using the theoretically derived value of G expressed by
The experimental values of G from the volume equivalent diameter are in good agreement with the theoretical values within the RH range of 81% to 96%, and taking into account the error bars, the entire values after the deliquescence point are reasonable. Research into growth factors using a tandem differential mobility analyzer has also shown values that are consistent with theory (CitationHämeri et al. 2000; CitationGysel et al. 2002). Thus, we have confidence in our ability to measure the size of aqueous solution droplets using the ESEM technique.
CONCLUSIONS
In this study, we aimed to deduce the size of (NH4)2SO4 aqueous droplets from microscopic images, and establish their hygroscopic growth. To obtain the size of the droplets, we determined the contact angle, θ, from a top view of the droplet on a tilted stage. The average value of θ was 96 ± 10° for (NH4)2SO4 droplets on a PTFE-coated copper substrate. Using a value of θ = 96 ± 10°, we could measure a reasonable droplet particle size from the microscope images taken, which was measured with increasing values of RH to estimate the growth factor, G, of the (NH4)2SO4 droplets. The resulting value of G was consistent with the theoretically calculated values of G. This shows that our measurements of the size of aqueous solution droplets on a flat surface were correct.
Our method to determine the value of θ can be applied to droplets of other solutions and other substrates, such as a Cu-C supported grid, which is often used in ESEM hygroscopic observations, and can contribute to the study of the hygroscopic growth of droplets using an electron microscope. With the addition of an EDX analysis, we expect that the technique can be an applied to atmospheric aerosol hygroscopic growth. However we have to carry out an extra experiment with other salts or salt mixtures, and other particle size ranges to strengthen validity of our technique to determine the volume equivalent diameter of a liquid particle, and we will discuss these elsewhere.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science, Culture Grant-in-Aid for Exploratory Research (Grant No. 18654081).
REFERENCES
- Adamson , W. and Gast , A. P. 1997 . Physical Chemistry of Surfaces, , 6th ed. , New York : John Wiley and Sons .
- Chen , J.-P. 1994 . Theory of Deliquescence and Modified Köhler Curves . Atmos. Sci. , 51 : 3505 – 3516 .
- Clegg , S. L. , Ho , S. S. , Chan , C. K. and Brimblecombe , P. 1995 . Thermodynamic Properties of Aqueous (NH4)2SO4 to High Supersaturation as a Function of Temperature . J. Chem. Eng. Data , 40 : 1079 – 1090 .
- Ebert , M. , Inerle-Hof , M. and Weinbruch , S. 2002 . Environmental Scanning Electron Microscopy as a New Technique to Determine the Hygroscopic Behaviour of Individual Aerosol Particles . Atmos. Environ. , 36 : 5909 – 5916 .
- Gysel , M. , Weingartner , E. and Baltensperger , U. 2002 . Hygroscopicity of Aerosol Particles at Low Temperatures. 2. Theoretical and Experimental Hygroscopic Properties of Laboratory Generated Aerosols . Environ. Sci. Technol. , 36 : 63 – 68 .
- Frenkel , Y. I. 1948 . On the Behavior of Liquid Drops on a Solid Surface 1. The Sliding of Drops on an Inclined Surface . J. Exp. Theor. Phys. (USSR) , 18 : 659 – 667 . http://arxiv.org/abs/physics/0503051(In Russian). English translation available at
- Furmidge , C. G. L. 1962 . Studies at Phase Interfaces. I. The Sliding of Liquid Drops on Solid Surfaces and a Theory for Spray Retention . J. Colloid Science , 17 : 309 – 324 .
- Hämeri , K. , Väkevä , M. , Hanson , H.-C. and Laaksonen , A. 2000 . Hygroscopic Growth of Ultrafine Ammonium Sulphate Aerosol Measured Using an Ultrafine Tandem Differential Mobility Analyzer . J. Geophys. Res. , 105 : 22231 – 22242 .
- Hoffman , R. C. , Laskin , A. and Finlayson-Pitts , B. J. 2004 . Sodium Nitrate Particles: Physical and Chemical Properties During Hydration and Dehydration, and Implications for Aged Sea Salt Aerosols . J. Aerosol Sci. , 35 : 869 – 887 .
- Laskin , A. , Iedema , M. J. , Ichkovich , A. , Graber , E. R. , Taraniukb , I. and Rudich , Y. 2005 . Direct Observation of Completely Processed Calcium Carbonate Dust Particles . Faraday Discussions , 130 : 453 – 468 .
- Pant , A. , Parsons , M. T. and Bertram , A. K. 2006 . Crystallization of Aqueous Ammonium Sulfate Particles Internally Mixed with Soot and Kaolinite: Crystallization Relative Humidities and Nucleation Rates . J. Phys. Chem. A , 110 : 8701 – 8709 .
- Pruppacher , H. R. and Klett , J. D. 1997 . Microphysics of Clouds and Precipitation, , 2nd ed , Dordrecht, , Netherlands : Kluwer Academic Publishers .
- Seinfeld , J. H. and Pandis , S. N. 1998 . Atmospheric Chemistry and Physics: From Air Pollution to Climate Change , New York : John Wiley and Sons .
- Tang , I. N. and Munkelwitz , H. R. 1994 . Water Activities, Densities, and Refractive Indices of Aqueous Sulfates and Sodium Nitrate Droplets of Atmospheric Importance . J. Geophys. Res. , 99 : 18801 – 18808 .