Abstract
Understanding the characteristic behavior of ions produced from the interaction of a high energy laser pulse with nanoparticles is essential for quantitative determination of composition and size of nanoparticles from single particle mass spectrometry (SPMS). We employed a one-dimensional hydrodynamic model, where the laser field is coupled to the non-equilibrium time-dependent plasma hydrodynamics of the heated particles. We focus on regimes of laser width from 0.01 ns to 10 ns (532 nm wavelength, 100 mJ/pulse) and particle size (20–400 nm in diameter) most relevant to commonly used SPMS, and determine the properties of ions generated during the interaction with a strong laser pulse. We compare the simulation results with experiments conducted on aluminum nanoparticles.
The laser-particle interaction is separated into a “soft heating” regime followed by a hydrodynamic expansion. Simulation results showed that the ablation/ionization is effectively complete well before the laser ever reaches its peak intensity. As the pulse width decreased for a given pulse energy, the kinetic energy of ions increased, suggesting that too short a pulse laser (i.e., high laser intensity) would be undesirable because higher energetic ions lead to lower detection efficiency in the SPMS. Results also show that for particle sizes in the range of 100 nm ∼ 400 nm, as particle size increased, the kinetic energy of ions produced from the particle increased with a power law relationship, consistent with experiment. Lastly our simulations indicated that ions from the surface of the particle are of higher energy, and therefore have lower detection efficiency.
INTRODUCTION
Many advances in single-particle mass spectrometry for quantitative characterization of size and composition of particles have been made in the last decade (CitationSalt et al. 1996; CitationSuess and Prather 1999; CitationJayne et al. 2000; CitationMurray 2000; CitationNoble and Prather 2000; CitationReents and Ge 2000; CitationKane et al. 2001; CitationMahadevan et al. 2002). Typically for these experiments a pulsed laser is used as the ionization source, from which time-of-flight mass-spectrometry can be used to deduce composition. The common practice is to use some other method such as light scattering to deduce the size of the particle just prior to ionization, thereby obtaining a measure of both size and composition (CitationSalt et al. 1996). While the technique has proven itself to be highly reliable, it suffers from the limitations inherent to all light scattering, namely the high power dependence on particle size, which has generally limited the technique to particles greater than ∼200 nm. More generically a light scattering, or other particle selection approach however, (e.g., mobility selection, size selective aerodynamic focusing) does not take advantage of the inherent capability of the mass spectrometer, to not only provide composition information, but total mass as has been attempted by our group and the work of Reents (CitationReents and Ge 2000; CitationLee et al. 2005).
Recently, we and others, with the use of a highly focused pulse laser, determined both total particle mass (i.e., size) and composition quantitatively using only the ion signal from a laser ionization time-of-flight mass spectrometer (CitationReents and Ge 2000; CitationMahadevan et al. 2002; CitationLee et al. 2005; CitationPark et al. 2005). The quantification of composition and size of nanoparticles can be achieved with reasonable accuracy when two primary conditions are met. One is near complete atomization/ionization of the particle constituents (i.e., all neutral atoms are converted to ions with few molecular species), and two, ions produced from the particle should be detected independent of composition and size, or with a known relationship (CitationLee et al. 2005). Typically a strong laser peak fluence (∼ 1011 W/cm2) that is several orders of magnitude higher than the theoretical energy to atomize and singly ionize all atoms in the particle has been employed. On the other hand, the detection efficiency of ions in the single particle mass spectrometry is highly sensitive to their properties (e.g., kinetic energy) and the laser parameters (e.g., pulse width). The strong laser pulses might produce highly energetic ions so that their transmission efficiency to the detector in the aerosol mass spectrometry (usually through the time-of-flight tube) would be degraded (CitationLee et al. 2005). This suggests if size-dependent or composition-dependent energetic ions are formed, and their relationships are not understood, it will degrade our ability to quantify the composition and size of particles.
The objective of this article is to understand in greater detail the interaction of a laser pulse with a nanoparticle so as to determine the characteristic properties of ions produced from the particle. In the present study, we employed a modified 1-D hydrodynamic model based on prior work of CitationMilchberg et al (2001) to simulate the temporal evolution of ionization state, and energy as a function of particle size of aluminum that was heated and ionized by a nanosecond 532 nm Nd:YAG laser. The effects of the laser pulse width (i.e., laser intensity) on the ion properties produced after laser-particle interaction were also examined. The simulation results are compared to measurements obtained with a well characterized single particle mass spectrometer (SPMS) (CitationMahadevan et al. 2002; CitationLee et al. 2005).
MODEL DESCRIPTION
Several models to describe the interaction of clusters with a laser pulse have been developed (CitationDitmire et al. 1996; CitationLezius et al. 1998; CitationMilchberg et al. 2001). In the coulomb explosion model, laser-heated electrons can escape the cluster early in the laser-cluster interaction, leading to a charge buildup in the cluster. This charge buildup will lead electrostatic forces to be dominant in the cluster expansion, resulting in a so-called coulomb explosion. On the other hand, in the hydrodynamic model, the plasma dynamics are driven in response to hydrodynamic forces rather than electrostatic forces. Milchberg et al. showed that the hydrodynamic forces dominate electrostatic forces for clusters larger than 5 nm, and a laser peak intensity of > 1014 W/cm2. However, for typical single particle mass spectrometry operation the situation is typically more complicated. For a peak intensity ∼ 1011 W/cm2, and particles of 20–400 nm, a purely hydrodynamic model is only valid after sufficient laser heating has taken place to overcome the cohesive energy of the solid. Essentially we can think of the laser interaction process as taking place in two steps. First a rapid laser heating, with evaporation of neutrals from the particle surface followed by a second step of resonant absorption and hydrodynamic plasma expansion.
In a previous work (CitationRai 2006), we developed a phenomenological model to study the mass and energy transfer processes for the combustion of aluminum nanoparticles. In this study, a similar model was employed to account for the laser-particle heating, and particle evaporative cooling, and integrated into a one-dimensional hydrodynamic model to derive ion properties resulting from a laser-particle interaction (CitationMilchberg et al. 2001). While our calculations are focused on Aluminum, because of our experimental measurements and because modeling a mono-atomic particle are considerably easier, the results on the features of laser-particle interactions are expected to be qualitatively generic, and should lead to a greater mechanistic understanding, and as a guide for future experimental developments.
1. “Soft” Particle Heating
During the early stages of the laser pulse, the laser intensity is relatively low, such that the amount of energy absorbed is below the cohesive energy, so that the particle experiences a “soft” heating and results in neutrals being evaporated. The particle temperature and radius are determined by a coupling of laser heating and evaporative cooling. The effect of vaporization was examined by solving the energy balance in the free molecule regime (CitationMilchberg et al. 2001; CitationRai 2006). The energy absorbed from the laser, q abs , is balanced with the energy used to evaporate aluminum from the particle, q evap and the energy used to heat the particle, as follows.
2. Hydrodynamic Model
The hydrodynamic simulation is based on a one-fluid two temperature (i.e., electron and ion temperature) model, which includes thermal conduction, and a collision-radiative model for the ionization dynamics, the complete details for which are presented elsewhere (CitationMilchberg et al. 2001). Briefly, the laser's electric field (= E(r), 1-D solid angle averaged electric field) is used to heat, and ionize the particle, and to advance the non-equilibrium time-dependent plasma hydrodynamics of the heated particle. The electric near field is described by, ∇ · (ϵE) = 0, where ϵ (r) is the dielectric function, and is coupled to a 1-D radial Lagrangian hydrocode. A near field approximation is appropriate for the case when the product, ka ≪ 1, where k (= 2π /λ) is the laser wave number, and a is the particle radius. For example, for a 20 nm diameter particle, excited with a visible laser, ka∼ 0.1. The dielectric response (ϵ (r)) of the laser-heated plasma is taken to be a Drude form, which is appropriate for strongly heated near-solid-density plasmas with little electronic band structure (CitationParra et al. 2003). The 1-D radial Lagrangian hydrocode model is outlined below including the mass (9), momentum (10), and energy (11) equations.
SINGLE PARTICLE MASS SPECTROMETER
In order to experimentally determine the relationship between particle size and the amount of ions detected with the single particle mass spectrometer (SPMS), we first need to generate aerosols and transport them to the SPMS. For generation of aerosols, we dispersed commercial aluminum nanopowders (Aveka Inc.) in methanol, and silver nitrate and sodium chloride in deionized water, and suspended them in the air using a Collison atomizer. These particles were passed through several aerosol diffusion driers to remove the solvent. The aerosol was then passed through a differential mobility analyzer (DMA) (CitationKnutson and Whitby 1975) to select particles of known size for direct delivery to the inlet of the SPMS. Detailed descriptions on the SPMS can be found in our previous article (CitationMahadevan et al. 2002; CitationPark et al. 2005). Briefly, the SPMS consists of an aerodynamic lens inlet, three stage differential vacuum systems, a free firing pulsed laser for particle ionization, a linear time-of-flight mass spectrometer, and a 500 MHz digital oscilloscope and PC for data acquisition. The aerodynamic lens system produces a narrow collimated particle beam and transports particles of 30–300 nm into the high vacuum system with a high transmission efficiency. The free firing pulsed laser (a frequency-doubled Nd:YAG laser operated at 10 Hz, 532 nm wavelength), through a spherical plano-convex lens, is tightly focused at the extraction field of the mass spectrometer and intersects the particle beam with a laser beam diameter of ∼ 0.1 mm and a laser pulse duration of ∼ 5 ns. We have found that a pulse energy of ∼ 100 mJ/pulse, corresponding to a peak laser power density at the focal point of approximately ∼ 1011 W/cm2, provided sufficiently high level of ion currents to provide quantitative composition measurement. When the laser hits a particle successfully, positive ions formed from the particle are accelerated along ∼ 1 m time-of-flight tube and detected by microchannel plates (MCP).
RESULTS AND DISCUSSION
In our present (experimental) study, aluminum nanoparticles are irradiated with a 532 nm, 5 ns FWHM 100 mJ Gaussian pulse (Nd:Yag laser) with a peak laser intensity of ∼ 1.55 * 1011 W/cm2. As mentioned above, the particle first undergoes a soft heating process to overcome the cohesive energy of aluminum and then transitions to a hydrodynamic plasma expansion. The temporal and spatial profiles (1-D) for density, temperature, and velocity of electrons and ions from the laser-particle interaction can be obtained by a complete simulation of both models.
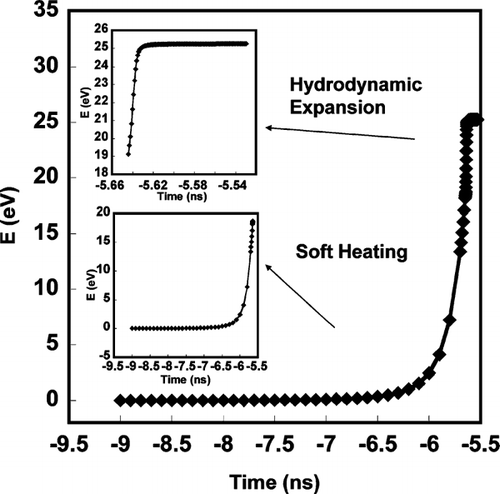
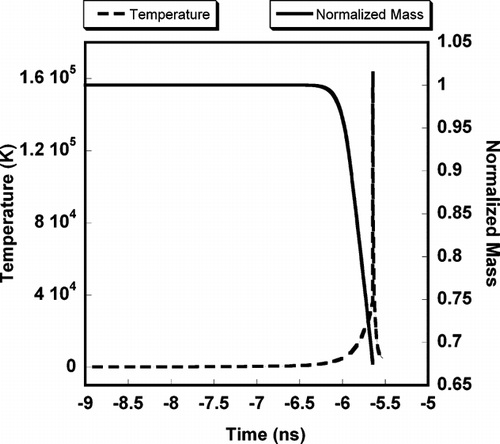
shows results for the temporal variation in temperature and particle mass, for the early stage of particle heating for aluminum of 100 nm diameter. The laser peak is at t = 0 and the interaction between laser and particle is observed from t = −9 ns. As discussed above, the cohesive energy of aluminum will delay the hydrodynamic expansion of the particle in the early stage of the laser-particle interaction, until the temperature reaches the cohesive energy threshold (∼3 eV). After the particle temperature reaches the cohesive energy threshold, electrons and ions energies are high enough such that the particle rapidly transitions into a dense plasma and expands in response to hydrodynamic forces, where the high-density collisional processes are dominant in particle ionization and heating. During hydrodynamic expansion, the particle is rapidly heated to a very high temperature (> 105 K) and then cools rapidly due to expansion cooling as shown in . It is also interesting to note that the temperature increases so rapidly that particle heating is much faster than the ability to evaporate mass, as evidenced by the very small evaporation loss from the particle during the soft heating.
FIG. 1 Simulation of soft laser heating of 100 nm Aluminum as a function to time. Time = 0 corresponds to the peak in the 532 nm, 5 ns FWHM 100 mJ Gaussian laser pulse.
The normalized electron density and electric field for a 100 nm diameter particle during the hydrodynamic expansion are presented in and . The initial electron density of aluminum is 6.026 * 1022/cm3. In the electron density (N e ) is normalized by the critical plasma density (N cr = m e ω2/4π e2 = 3.95 * 1021/cm3) where m e is the electron mass, ω is the laser frequency, and e is electron charge. Results are presented from the point in time when the temperature reaches the cohesive energy threshold, at −5.644 ns. The critical plasma density defines the point below which the plasma becomes transparent to the laser beam, and no further energy deposition takes place. indicates that the electron density profile is non-uniform during the expansion, and that there is a critical density surface (i.e., N e ∼ N cr ) where resonant laser light absorption occurs, and is maintained from −5.644 ns to −5.634 ns, about ∼10 ps. presents the vacuum level normalized electric field during the hydrodynamic expansion as a function of radial location and time. Near the region of the critical density surface (radial location) the corresponding electric field is significantly enhanced with respect to its vacuum value as shown in . This time (∼10 ps) can be defined as the critical density lifetime (τ cr ), which is the time for a laser heated cluster to expand to a local electron density, which is below the critical density (i.e., N e < N cr ). At times greater than the critical density lifetime (τ cr ), laser light absorption will decrease significantly.
FIG. 2 (a) Temporal and radial spatial variation of normalized electron density. (b) Temporal and radial spatial variation of the normalized electric field.
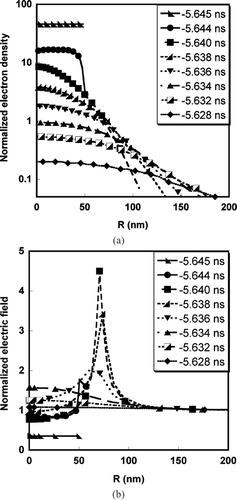
The results from and indicates that the laser-particle interaction occurs over a period of ∼3 ns (from −9 ns to −5.6 ns) for the Gaussian 5 ns FWHM laser pulse, and that most of the time is spend to overcome the cohesive energy threshold. On the other hand, if we look at the absolute energy deposited to the particle during the interaction (), it is clear that although the hydrodynamic plasma expansion occurs over a very short time (∼ 10 ps), the energy deposition rate is much faster (∼ 1012 ev/sec) than in the “soft heating” regime (18 eV in 3 ns). also shows that the energy deposition is completed after the critical density life time, which is consistent with the discussion above.
Thus, in our nanosecond laser pulse, the energy absorption of the particle only takes place during the leading edge of the pulse, and after τ cr , the coupling is no better than in a gas. In other words, the ablation/ionization is over well before a nanosecond laser ever reaches its peak intensity. This observation is qualitatively consistent with previous results for the interaction of the laser pulse (peak intensity > 1014 W/cm2) with argon clusters (CitationParra et al. 2003).
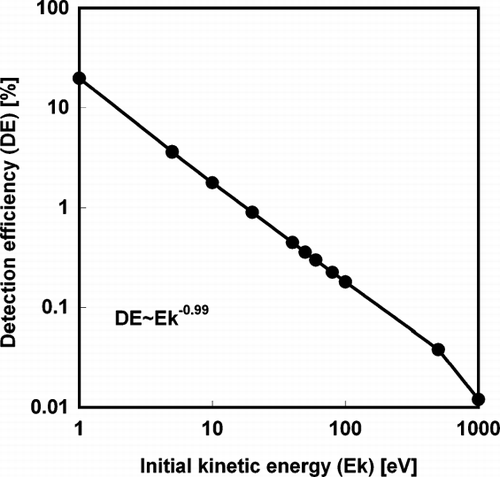
As mentioned early, the SPMS can be used to estimate particle size using the predetermined relationship between the ion signal (i.e., the amount of ions detected in the measured mass spectrum) and particle size. However, the formation of energetic ions will affect the transport efficiency through the SPMS time-of-flight tube and therefore sensitivity. In our previous study (CitationLee et al. 2005), we defined the detection efficiency (DE) as
Clearly, the initial kinetic energy of ions plays an important role in the application of SPMS: ions with high kinetic energy are harder to collimate with the extraction fields and as a consequence, both the detection sensitivity, and the ability of quantitatively characterize single nanoparticle are degraded. Thus we are especially interested in the kinetic energy of constituent ions produced from the laser pulse. The remainder of this article explores the influence of the laser pulse intensity on the kinetic energy of ions produced from the laser-particle interaction, as well as the particle size dependent energetic ion formation.
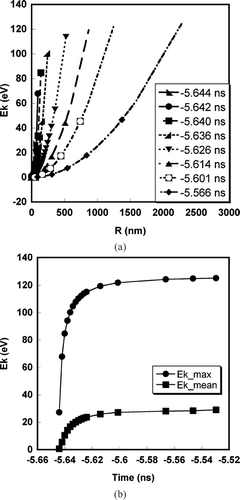
The kinetic energy of ions was computed using the velocity profiles obtained from the simulation results. shows the kinetic energy (= 1/2 mv2) profiles as a function of radial distance at various times, for an aluminum particle of initial diameter of 100 nm. Evidently the ions at the surface of the expanding plasma cloud have the highest kinetic energy. From the practical point of view a bias to higher kinetic energy for the surface atoms will lead to lower detection efficiency as shown in and therefore a bias against surface species. We have also plotted the number weighted average kinetic energy (i.e., E k _mean = Σ E k (r) * N i (r) / Σ N i where r is the radius and N is the ion number), and its maximum value against time in . Note that the kinetic energy increases rapidly initially, but reaches an asymptotic value after ∼−5.634 ns. This occurs because most of laser energy is absorbed onto the particle during the leading edge of the pulse till we achieve τ cr (∼ 10 ps).
FIG. 5 (a) Kinetic energy profile as a function of radial distance at various times for an aluminum particle of initial diameter = 100 nm. (b) Temporal variation of the average and maximum kinetic energy for an aluminum particle of initial diameter = 100 nm.
The hydrodynamic simulation results suggested that most of the laser energy is not deposited into the particle because of the poor coupling between laser pulse and the plasma. Obviously, for the single particle mass spectrometer, a hot plasma is preferable to ensure complete ionization, which can be achieved by employing a higher laser intensity or absorption efficiency. However, a hotter plasma should produce more energetic ions, which will result in greater ion loss during the transport in the TOF. In other words, too intense a photon flux or too much absorption may decrease the overall ability to quantify SPMS data, by possible species biases in the detection efficiency of ions, while too weak a laser cannot ensure complete conversion of constituent atoms to ions. Ideally one would like to understand where the optimum lies.
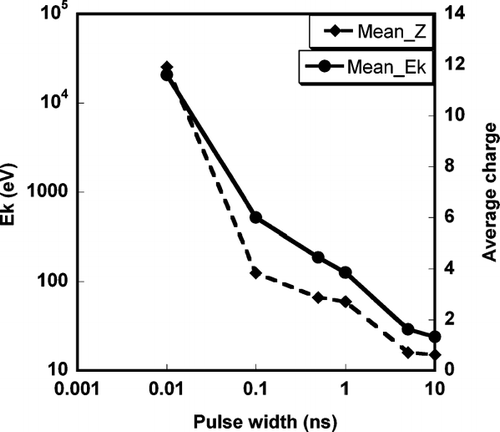
Based on the above discussion, we conducted simulations to investigate ion formation from a 100 nm aluminum nanoparticle after their interaction with laser light of varying pulse widths (10 ps ∼ 10 ns). The motivations are based on our previous discussion that indicated that the critical plasma density is achieved well before a nanosecond laser reaches its peak intensity. Furthermore all experimental work on particle mass spectrometry to date, at least to our knowledge has employed nanosecond lasers. To explore the role of pulse width we used the following conditions for our simulation; 10 ps (7.75 × 1013 W/cm2), 100 ps (7.75 × 1012 W/cm2), 0.5 ns (1.55 × 1012 W/cm2), 5 ns (1.55 × 1011 W/cm2), 10 ns (= 7.75 × 1010 W/cm2), plots the number weighted average kinetic energy and average ionization state of ions for different laser intensity. The results show a monotonic increase in both the mean kinetic energy and average charge of ions as the pulse width is decreased. The results indicate that going to a shorter laser pulse creates a much more aggressive laser interaction. If we consider the nano-second laser sources as our base case condition, it is quite clear that a longer pulse laser would be the wrong direction to go to ensure the complete ionization approach. The next question is if pico-second lasers which are readily available offer some advantages. The results suggested that for a pico-second laser, the mean kinetic energy and average ionization state of ions could be very high, such that complete ionization criterion can be guaranteed, however, the higher kinetic energy of ions will result in a low detection efficiency given standard ion optics employed in TOF systems. On the other hand the pico-second laser has the highest likelihood that poor absorbers of surface coated material that might preferentially be blown-off without being ionized may be detected with a shorter pulse laser providing a more aggressive ionization (CitationZhou et al. 2006).
FIG. 6 Effect of laser pulse width on average kinetic energy and average ionization state for an aluminum particle of initial diameter = 100 nm.
The above result invites the question, would a lower pulse energy for pico-second lasers have some advantages for SPMS application. Further simulations for 100 ps and 10 ps pulses were carried out with laser pulse energy of 50 mJ and 10 mJ. As one would expect, the simulations give lower mean ion kinetic energy and average charge of ions with the decrease of laser pulse energy, but in general is seen to be a an insensitive parameter, likely because the absorption time is shorted than the pulse duration in any case. Thus once a threshold energy is achieved the resulting ionization process becomes relatively insensitive to pulse energy. For example, as the laser pulse energy decreases from 100 mJ to 10 mJ, the resulting ions mean kinetic energy decreases by a factor of 1.3 (from 517 eV to 395 eV), and 1.04 (from 20762 eV to 19826 eV) for 100 ps and 10 ps lasers, respectively. This also implies that a pico-second laser with near threshold energy would provide enough ionization with low ion kinetic energy. Simulations on a 10 ps pulse with lower pulse energy show that the threshold energy is on the order of hundreds of microjoules. So a 1 mJ pulse would give a kinetic energy of 18186 eV while the resulting ions mean kinetic energy of a 0.5 mJ pulse is only 226 eV and the interaction takes place over the whole laser pulse. Further decrease of the pulse energy to 0.1 mJ, results in insufficient energy absorbed to overcome the cohesive energy threshold, and as a result no hydrodynamic plasma formation.
The present results suggest that a nano-second laser may in fact provide the optimal laser source, for the present design of time-of-flight optics. However, experiments on pico-second laser are warranted. In the latter case, other configurations, such as a high pressure ionization region where the kinetic energy of ions can be absorbed may offer interesting avenues for exploiting pico-second lasers (CitationWang et al. 2006).
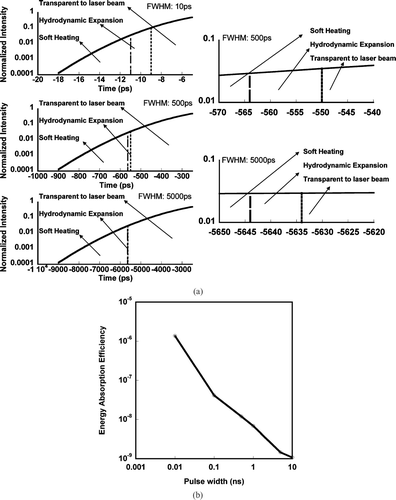
To further illustrate the effect of laser pulse width on the laser-particle interaction, the normalized Gaussian laser pulses are plotted in . For each laser pulse, we also define the temporal regions that correspond to the “soft heating” and hydrodynamic expansion, and the fraction of laser energy absorbed as a function of pulse width plotted in . Note that in , we should compare the relative fraction of “soft heating” and hydrodynamic expansion time in each pulse rather than the absolute interaction time. We can see that the hydrodynamic interaction regime become significantly longer as the laser pulse width is decreased. Considering that the energy absorption during this part of the interaction is much more aggressive, the result shown in , implies that there is more energy deposited onto the particle for a shorter laser pulse, which is manifested as an increase in ion energy and ionization state. This conclusion is confirmed by the calculation of energy absorption efficiency shown in , which indicates a monotonic increase in absorption efficiency as the pulse width is decreased.
FIG. 7 (a) Left: Gaussian laser pulses of various width overlayed with the “soft-heating” and hydrodynamic expansion regions for an aluminum particle of initial diameter = 100 nm. Right: Detailed view of “soft-heating” and hydrodynamic expansion regions for FWHM 5000 ps and FWHM 500 ps laser pulses. (b) Calculated fraction of laser energy absorbed in a 100 nm aluminum particle as a function of pulse width.
Next we turn our attention to the effect of particle size on the kinetic energy of ions produced. In our previous work, we hypothesized that the non-linear relationship between the ion signal and particle size observed in our experiment is due to the formation of size-dependent energetic ions (CitationLee et al. 2005). In this work, simulations of laser interactions with particles in size range of 20∼400 nm were carried out and the resulting ions energy were examined to understand their size dependence.
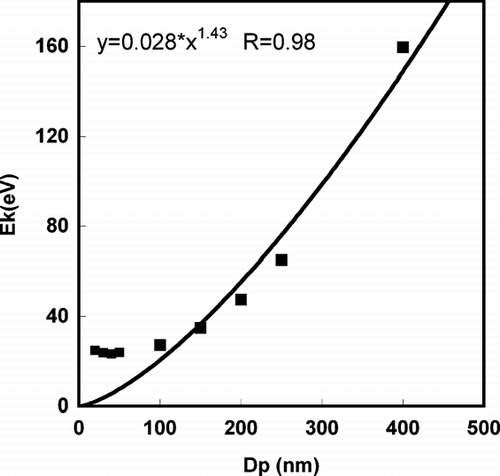
The number weighted average kinetic energy for particles in the 20–400 nm range are shown in . We found that as size increases, the average kinetic energy of ions increases, and for particle size ∼ 100 nm and larger, the kinetic energy follows a power law relationship in particle diameter (E k ∼ D p 1.43). One possible explanation for the size-dependent kinetic energy formation is that with increasing particle size, the critical density lifetime increases (i.e., it takes longer for the average density of the larger particle to drop below the critical density), and therefore the particle absorbs energy for a longer period of time. Increased absorption time results in greater heating, leading to a higher initial kinetic energy and charge state for larger particles.
We are now in a position to compare the simulation results with our experiment. In our previous study (CitationLee et al. 2005), we simulated the detection efficiency (DE) of ions traveling through the time-of-flight tube in the single particle mass spectrometry, and showed that the DE depends on their initial kinetic energy (E k ), providing the relationship DE (%) ∼ E k − 0.99. This indicates that DE decreases with higher kinetic energy ions. In that study, we hypothesized that the non-linear relationship between ion peak area, and particle size was caused by the more energetic ion formation from larger particles. Our current simulation results (E k ∼ D p 1.42) qualitatively showed that this should be true.
By employing the relationship (E k ∼ D p 1.43) from the current simulation results, and the DE (%) ∼ E k − 0.99 from the previous ion trajectory simulation, we obtain DE (%) ∼ D p − 1.42. Since the DE is related to the integrated peak area in a single particle mass spectrum by the equation of
FIG. 9 Comparison of the relationship between ion peak area and particle size from simulation results, and experimentally determined by the single particle mass spectrometer.
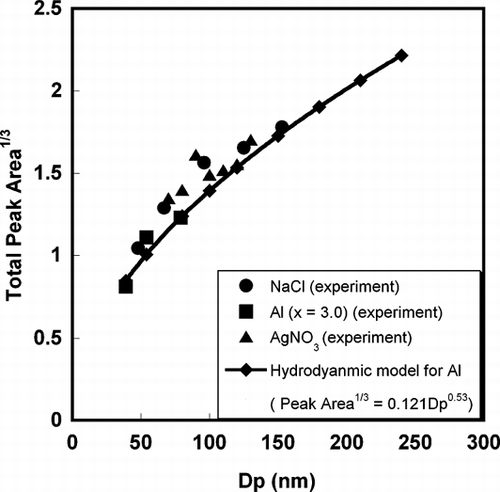
It is also interesting to note that for smaller particles, there is a discrepancy between the average kinetic energy and the power law relationship, E k ∼ D p 1.43, obtained above, the kinetic energy for particles in small size range have a relative higher value. The high kinetic energy suggests a relatively more aggressive energy absorption and particle ionization for a smaller particle. This result is consistent with a recent experimental observation by CitationWang et al. (2006) who developed a nanoaerosol mass spectrometer (NAMS) using the complete ionization technique. Quantitative chemical characterization for a particle with diameter ∼10 nm was achieved with a quadrupole ion guide and quadrupole ion trap system. In their work, multiple charged ions peaks (+4) were frequently observed in the spectra, which imply highly ionization states were achieved for particle sizes < ∼10 nm.
CONCLUSIONS
The objective of this article was to understand pulsed laser interactions with small particles as it applies to the implementation and quantification of single particle mass spectrometry. In this study we applied a one-dimensional hydrodynamic model to determine the characteristic behavior of ions produced from aluminum nanoparticles as a result of interaction with a strong laser pulse. During the early stages of interaction, the particle experiences a “soft heating” regime to overcome the cohesive energy of solid. Subsequently, the laser–particle interaction transitions to a resonant absorption and hydrodynamic plasma expansion. The effect of laser parameters such as pulse width, energy, and particle size were investigated in this work.
Our simulation results showed that the ablation/ionization process is finished well before the laser ever reaches its peak intensity. We found that shorter laser pulses lead to greater energy absorption and produce a more intense plasma, which will result in higher ionization state and higher ion kinetic energy. However the higher kinetic energy of the shorter pulse lasers will likely lead to a significantly degraded detection efficiency for ions in traditional TOF ion optics. The simulations suggested that nano-second lasers may, in fact, provide an optimized solution for SPMS application.
We also found particle size-dependent energetic ions are formed from the laser-particle interaction, and the kinetic energy of ions is proportional to the particle size with a power law relationship (E k ∼ D p 1.42). This result is show to be consistent with our experimental observation, and suggest that particle size-dependent energetic ions led to the power-law relationship between peak area and particle size observed in a single particle mass spectrometer.
Acknowledgments
Support for this work comes from the Army Research Office.
REFERENCES
- Ammosov , M. V. , Delone , N. B. and Kraînov , V. P. 1986 . Tunnel Ionization of Complex Atoms and Atomic Ions in a Varying Electromagnetic-Field . Zhurnal Eksperimentalnoi I Teoreticheskoi Fiziki , 91 ( 6 ) : 2008 – 2013 .
- Ditmire , T. , Donnelly , T. , Rubenchik , A. M. , Falcone , R. W. and Perry , M. D. 1996 . Interaction of Intense Laser Pulses with Atomic Clusters . Physical Review A: Atomic, Molecular, and Optical Physics , 53 ( 5 ) : 3379 – 3402 .
- Friedlander , S. F. 2000 . Smoke, Dust, and Haze , New York : Oxford University Press .
- Jayne , J. T. , Leard , D. C. , Shang , X. , Davidots , P. , Smith , K. A. , Kolb , C. E. and Worsnop , D. R. 2000 . Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles . Aerosol Sci. Technol. , 33 ( 1–2 ) : 49 – 70 .
- Kane , D. B. Oktem , B. 2001 . Nanoparticle Detection by Aerosol Mass Spectrometry . Aerosol Sci. Technol. , 34 ( 6 ) : 520 – 527 .
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications . J. Aerosol Science , 6 ( 6 ) : 443 – 451 .
- Lee , D. , Park , K. and Zachariah , M. K. 2005 . Determination of the Size Distribution of Polydisperse Nanoparticles with Single-Particle Mass Spectrometry: The Role of Ion Kinetic Energy . Aerosol Sci. Technol. , 39 ( 2 ) : 162 – 169 .
- Lezius , M. , Dobosz , S. , Normand , D. and Schmidt , M. 1998 . Explosion Dynamics of Rare Gas Clusters in Strong Laser Fields . Phys. Rev. Lett. , 80 ( 2 ) : 261
- Mahadevan , R. , Lee , D. , Sakurai , H. and Zachariah , M. R. 2002 . Measurement of Condensed-Phase Reaction Kinetics in the Aerosol Phase Using Single Particle Mass Spectrometry . J. Phys. Chem. A , 106 ( 46 ) : 11083 – 11092 .
- Milchberg , H. M. , McNaught , S. J. and Parra , E. 2001 . Plasma Hydrodynamics of the Intense Laser-Cluster Interaction . Phys. Rev. E: Statistical, Nonlinear, and Soft Matter Physics , 64 ( 5–2 ) 056402/1-056402/7
- Murray , V. J. 2000 . Sampling and Analysis of Individual Particles by Aerosol Mass Spectrometry . J. Mass Spectrom. , 35 ( 5 ) : 585 – 595 .
- Noble , C. A. and Prather , K. A. 2000 . Real-Time Single Particle Mass Spectrometry: A Historical Review of a Quarter Century of the Chemical Analysis of Aerosols . Mass Spectrom. Rev. , 19 ( 4 ) : 248 – 274 .
- Park , K. , Lee , D. , Rai , A. , Mukherjee , D. and Zachariah , M. R. 2005 . Size-Resolved Kinetic Measurements of Aluminum Nanoparticle Oxidation with Single Particle Mass Spectrometry . J. Phys. Chem. B , 109 ( 15 ) : 7290 – 7299 .
- Parra , E. , Alexeev , I. , Fan , J. , Kiong , Y. K. , McNaught , S. J. and Milchberg , H. M. 2003 . Hydrodynamic Time Scales for Intense Laser-Heated Clusters . J. Optical Soc. Amer. B: Optical Physics , 20 ( 1 ) : 118 – 124 .
- Rai , A. , Park , K. , Zhou , L. and Zachariah , M. R. 2006 . Understanding the Mechanism of Aluminum Nanoparticle Oxidation . Combustion Theory and Modeling , 10 ( 5 ) : 843 – 859 .
- Reents , W. D. and Ge , Z. 2000 . Simultaneous Elemental Composition and Size Distributions of Submicron Particles in Real Time Using Laser Atomization/Ionization Mass Spectrometry . Aerosol Sci. Technol. , 33 ( 1–2 ) : 122 – 134 .
- Salt , K. , Noble , C. A. and Prather , K. A. 1996 . Aerodynamic Particle Sizing versus Light Scattering Intensity Measurement as Methods for Real-Time Particle Sizing Coupled with Time-of-Flight Mass Spectrometry . Anal. Chem. , 68 ( 1 ) : 230 – 234 .
- Suess , D. T. and Prather , K. A. 1999 . Mass Spectrometry of Aerosols . Chem. Rev. , 99 ( 10 ) : 3007 – 3036 .
- Wang , S. , Zordan , C. A. and Johnston , M. V. 2006 . Chemical Characterization of Individual, Airborne Sub-10-nm Particles and Molecules . Anal. Chem. , 78 ( 6 ) : 1750 – 1754 .
- Zhou , L. , Rai , A. and Zachariah , M. R. 2006 . Component and Morphology Biases on Quantifying the Composition of Nanoparticles Using Single-Particle Mass Spectrometry . Intl J. Mass Spectrom , 258 ( 1–3 ) : 104 – 112 .