Abstract
Monitoring of ambient bioaerosol concentrations through the characterization of outdoor particulate matter (PM) has only been performed on a limited basis in North Carolina (NC) and was the goal of this research. Ambient samples of PM 2.5 (fine) and PM 10−2.5 (coarse) were collected for a six-month period and analyzed for mold, endotoxins and protein. PM 2.5 and PM 10−2.5 concentrations of these bioaerosols were reported as a function of PM mass, as well as volume of air sampled. The mass of PM 2.5 was almost twice that of the PM 10−2.5 ; however, the protein and endotoxin masses were greater in the coarse than the fine PM indicating an enrichment in the coarse PM. The protein and mold results demonstrated a seasonal pattern, both being higher in the summer than in the winter. Except for an occasional excursion, the endotoxin data remained fairly constant throughout the six months of the study.
INTRODUCTION
Exposures to bioaerosols are a major component in evaluating total long-term exposures to both PM10−2.5 (< 10 μ m in aerodynamic diameter > 2.5 μ m) and PM2.5 fraction (< 2.5 μ m in aerodynamic diameter) particulate matter (PM). Ambient PM exposure may occur in outdoor or indoor air since PM originating from ambient sources and penetrating into the indoor environment can result in individuals being exposed while indoors (CitationSamet and Spengler 1991).
It is widely accepted in the indoor air quality (IAQ) research community that bioaerosols can induce allergic, toxic, and infectious responses in exposed individuals. Symptoms of exposed individuals include coughing, wheezing, runny nose, irritated eyes or throat, skin rash, diarrhea, aggravation of asthma, headache, and fatigue. Immunological reactions can include asthma, allergic rhinitis, and hypersensitivity pneumonitis. In addition, the exposure of children to Stachybotrys chartarum is under investigation for a possible association with pediatric pulmonary hemorrhage (CitationLevetin 1995; CitationHusman 1996).
Bioaerosols or particulate matter, which is biological in origin (BioPM), are complex mixtures that may include plant pollen and microorganisms (mold and bacteria), as well as their parts and components (CitationSalvaggio and Aukrust 1981; CitationMenetrez, Foarde, and Ensor 2000). Although pollen is widely studied as an aeroallergen, historically, somewhat less has been known about ambient concentrations of fungal spores. CitationSalvaggio and Aukrust (1981) found Cladosporium spores in ambient air at a concentration 1000 times the concentration of pollen grains. Aerometric sampling devices have collected spores from 20,000 to 40,000 species of fungi. Of these, four major groups have been identified as being potential allergens: Phycomycetes, Ascomycetes, Basidiomycetes, and Deuteromycetes (CitationSalvaggio and Aukrust 1981).
Bacteria and fungi are important components of outdoor or atmospheric aerosols in addition to being important components of indoor aerosols. The size range of individual airborne bacteria is from 0.5 to 2.0 μ m (Bacillus spp., Pseudomonas spp., Xanthomonas spp., and Arthrobacter spp.), while many mold spores are significantly larger; 2.5 to 3.0 μ m for Aspergillus fumigatus, 3.5 to 5.0 μ m for Aspergillus niger, 3.0 to 4.5 for Penicillium brevicompactum, 7 to 17 μ m by 5 to 8 μ m for Cladosporium macrocarpum, 15.0 to 25 μ m for Epiccocum nigrum, and 2.8 to 3.2 for Trichoderma harizanum (CitationSamson and Hoekstra 1995). Desiccated non-viable fragments of microorganisms as well as clumps of microorganisms are also common in samples of ambient air. These fragments have been identified in the sub-micron size range. Studies indicated that a sizable fraction of both coarse and fine PM in both indoor and outdoor samples was of biological origin (CitationMenetrez, Foarde, and Ensor 2001). This biological component can be identified specifically by species or collectively accounted for in the measurement of protein concentrations that reflect the levels of all PM that is biological in origin (CitationMenetrez, Foarde, and Ensor 2001). This includes plant debris and related organic material [Humic-Like Substances (HULIS)], whole and fragmented mold spores, bacteria, and pollen. These non-viable fragments may remain toxigenic or allergenic depending upon the specific organism or organism component.
One of the most important constituents of BioPM potentially responsible for a variety of adverse health effects are endotoxins. Endotoxins or lipopolysaccharides (LPS) are derived from the cell walls of gram-negative bacteria. The endotoxin component of PM when inhaled stimulates alveolar macrophages and respiratory epithelial tissue to release cytokines or chemottractants that initiate an inflammatory cascade (CitationThorne 2000). A significant association has been found between endotoxin levels in home environments and the clinical severity of asthma (CitationMichel et al. 1996). In fact, studies have singled out endotoxins as the most significant component associated with the development and progression of airway disease. Endotoxins are recognized as an occupational hazard in agricultural and manufacturing industries (CitationSchwartz et al. 1995; CitationDouwes et al. 2002, Citation2003; CitationGordon et al. 1992). Environmental monitoring of endotoxins has been reported repeatedly in literature, mainly in occupational settings and indoor dust samples collected from homes (CitationHeederik et al. 2003; CitationMenetrez et al. 2001).
Exposure to ambient PM has been associated with harmful effects. The exact constituents of PM air pollution that induce disease and the mechanisms involved are unknown. Studies to determine the components of PM that contribute to airway inflammation and irritation have been attempted (CitationBonner et al. 1998; CitationDonaldson and MacNee 2001; CitationSoukup and Becker 2001; CitationBoehlecke et al. 2003). Aerodynamic size fractions of PM have been studied including coarse (PM10−2.5) and fine (PM2.5), and recently fine and submicrometer fractions (Pope 2000). Yet the coarse PM fraction remains recognized as being associated with significant adverse effects on the bronchiolar region of conducting airways that remains the primary site of asthma and associated airway inflammation (CitationMonn and Becker 1999; CitationSoukup and Becker 2001). Although bioaerosols have been identified and linked to adverse health effects, they have not been extensively studied for their prevalence in ambient PM. A better understanding of the risks presented by bioaerosols will inform the processes of exposure management and potentially provide information that could lead to improvements in medical treatment and management of disease.
The goal of the present study was to monitor ambient samples of PM2.5 and PM10−2.5 for mass, culturable mold, endotoxins, and total protein. Furthermore, a key objective was to utilize the same filter for each of the analyses. While mold and endotoxins were selected because of their associated health implications for exposure, protein was selected as a non-specific measure of the amount of PM that was biological in origin. The data was reported as a function of PM mass and volume of air sampled. A limited time frame of six months was the period of sample collection. We present and discuss the analysis and results of these bioaerosol investigations.
MATERIALS AND METHODS
Filter samples were collected from August 22, 2003 through January 28, 2004 for a total of 12 sampling periods. Each sampling period was composed of continuous daily (24 hour) sample collection of 5 to 15 days duration resulting in as few as 5 to as many as 15 sets of coarse and fine filter samples. Control samples were also collected during each time period. All PM10−2.5 and PM2.5 filter samples were analyzed for culturable mold spores (cfu), endotoxins (EU), and protein (ng).
A total of 342 filter samples of ambient air were analyzed successfully for endotoxins and protein: 151 coarse fraction, 151 fine fraction, and 40 control samples. A total of 304 filters were successfully analyzed for mold: 113 coarse fraction, 151 fine fraction control, and 40 control samples. The analysis of the PM2.5 fraction filters for culturable mold resulted in no growth as expected (due to the particle size being smaller than even the smallest intact mold spore) and was performed as part of the control. Of the filters analyzed for mold, 38 could not be completed.
SAMPLE COLLECTION
Fine and coarse ambient particle samples were collected using a Dichotomous Partisol Plus 2025 sequential air sampler, manufactured by Rupprecht and Patashnick Company Inc. (R&P). The air samples were collected on 47 mm PolyTetraFluoroEthylene (PTFE) filters (Gilman Teflo 47 mm, Cat. No. R2P-J047). The particle size fractions ranged from 0.1 to 2.5 μ m for the fine fraction and 2.5 to 10.0 μ m for the coarse fraction. The dichotomous sampler was located on a roof-top platform (the intake was 10.0 meters above the surrounding ground level) on the EPA building on the campus of the University of North Carolina in Chapel Hill. The flow rate of the dichotomous sampler was constant at 16.7 L/min. The flow is split such that the fine fraction rate is 15 L/min and the coarse fraction is 1.7 L/min. The total volume of air sampled varied with the length of the collection period and ranged from 21.13 to 24.90 m3, except for week 9 when only 12 m3 was sampled. The number of samples collected during the 2003–2004 time periods of collection are listed in (Sample periods for collection).
TABLE 1 Sample periods for collection
Filter cassettes coming into contact with the PTFE filters were wrapped in precleaned aluminum foil (rinsed with methylene chloride and allowed to air dry). The instruments were baked in a laboratory oven at 120°C for a period of not less than 12 hours. The instruments were then allowed to cool to room temperature within the lab oven, removed and stored within a sterile environmental chamber (sprayed and wiped with 95% ethanol and allow to air dry). Sterilization of the 47 mm PTFE filters was performed by placing them (polyethylene ring side up) in plastic petri dishes (with caps removed and placed beside each dish) in a biological hood (Class II Type A/B3) and exposing them to UV light for 8–12 hours. After exposure, the lids were placed on the petri dishes containing the exposed filters and wrapped in groups of 8 to 15 filters with aluminum foil.
Prior to sampling, each filter was each removed from its cassette using sterilized stainless steel tweezers and placed into an individually numbered and labeled sterilized plastic petri plate. The Petri plates were placed into a controlled environmental chamber for a minimum of 24 hours undisturbed to allow the filters to equilibrate prior to gravimetric weighing and sample recording. The chamber was maintained at a temperature of 20–23°C +/– 2°C, and relative humidity of 30–40% +/– 5%.
A minimum of 15% of the total anticipated number of filters were prepared as blanks. Of these blanks, during each sample period one blank was processed through each R&P sampler sleeve with zero exposure time (field blanks). One blank was carried to the field and returned with the technician (transport blank) and one blank remained in the laboratory hood (laboratory blank). The remaining blanks remained in the environmental chamber and were not removed. In order to control for contaminants, the blank filters were analyzed in the same manner as the air sample filters.
Gravimetric Analysis
After samples were collected, filters were placed in a petri dish, the dish was labeled, and the date and volume of air sampled was recorded. Filters were then weighed, again following a 24-hour equilibration period, and their total mass load determined. Filter gravimetric change was measured with a Mettler-Toledo MX-5 microbalance using the instruments internal calibration procedure. Documentation of the filter weight and volume of air drawn through each filter was archived. After weighing, each filter was placed back into the petri dish, and put back into the environmental chamber until biological analysis was performed.
BioPM Analysis
For the biological analysis, contents on the filters were extracted simultaneously for enumeration of fungal colony forming units, as well as lipopolysaccharide/endotoxin and protein determinations. Each filter was placed into a separate sterile container with 5 ml of sterile, pyrogen-free 0.01% triethylamine and shaken vigorously for 30 minutes to elute the collected particles.
One of the goals of the study was to utilize a single filter to quantify mass and for biological analyses. This was also the primary limitation of the study, as the use of PTFE filters increased the difficulty of the biological component extractions. While PTFE filters were chosen for this study because they are optimal for mass determinations, they are known to be less than optimal for biological measurements. While additional work is needed to maximize the use of PTFE filters for biological analyses, the first step was the selection of an extraction buffer. A number of studies have been performed to determine the optimal filter material, extraction protocol, and reagents (CitationDouwes et al. 1995; CitationThorne et al. 1997; CitationOlenchock et al. 1989). None of the studies have been successful in defining a “best” protocol that results in high extraction efficiency for multiple components. It appears that the “best” may be dependent upon the environment in which the sample is going to be collected (CitationReynolds and Milton 1993). Therefore, for this study we extracted the filters in sterile, pyrogen-free 0.01% triethylamine with vigorous shaking for 30 minutes since similar conditions previously resulted in satisfactory endotoxin extraction (CitationReynolds and Milton 1993).
A series of experiments were undertaken to evaluate the extraction efficiency for endotoxins from a variety of filters to compare extraction from PTFE filter to other commonly used filters. A series of outdoor air samples were collected simultaneously for each of the filter types. The extraction procedure was as described above. These experiments were limited to PM2.5 samples. It is unknown if the size fraction would influence the extraction efficiency. However, previous experiments (CitationMenetrez et al. 2001) with pure endotoxin pipetted and then dried on the filter surface yield similar endotoxin recoveries, suggesting that the size fraction may be less important than other filter characteristics in influencing extraction efficiency. Our feasibility experiments showed that the highest endotoxin extraction efficiency was achieved with the mixed cellulose ester filters. From polycarbonate filters, we were able to extract approximately 68% of the endotoxin compared to the MCE. While from the PTFE filter, approximately 47% of the endotoxin was extracted as compared to the MCE filter. Therefore, while the absolute levels in sampled air are undoubtedly underestimated, the relative levels can be compared. The preliminary extraction efficiencies were consistent within filter types in that relative standard deviations for MCE, polycarbonate and PTFE were 41%, 50%, and 42%, respectively. Similar extraction efficiencies would be expected for the protein and the culturable fungi.
Fungal Analysis
The eluted sample/buffer suspension was diluted as necessary and 0.1 mL plated in duplicate on 2% malt extract agar with chloramphenicol. The plates were incubated at room temperature, and counted when moderate growth became visible for culturable colony forming units (CFUs). The CFUs were then related to the unit of mass collected on the filter and the unit of air volume passed through the filter. These filter samples provided the core data used to indicate the ambient concentration of viable mold. An additional limitation for the use of PTFE filters is the possibility that some of the fungal spores may have desiccated with the extended sampling times employed in this study.
Endotoxin Analysis
For the endotoxin analysis, 50 μ L of the eluted sample/buffer suspension was placed in duplicate wells. Endotoxin activity was measured using the Limulus Amebocyte Lysate Assay (Cambrex BioScience, Walkersville, MD) following the manufacturer's instructions. This assay is sensitive to endotoxin activity as low as .01 EU/ml. The level of endotoxin activity in a sample was determined by the reaction of endotoxins in the specimen with the lysate and a substrate, producing a color change over time, and comparing to similar reactions of a known standard endotoxin reference. The level of color change was quantified using a Bio-Tek Microplate reader measuring the optical density at 405 nm. When the optimal optical density was exceeded, the sample/buffer suspension was diluted and reanalyzed.
Protein Analysis
For the protein analysis, 10 μ L aliquots from each eluted sample/buffer suspension were analyzed using the Nano-Orange Protein Quantification Kit (Molecular Probes, Eugene, OR) following the manufacturer's instructions. Quantification of the level of protein in a sample was obtained by the reaction of protein in the specimen with a diluted Nano-Orange agent, heated to 95.0°C for 10 minutes and then allowed to cool to room temperature. A fluorescent reaction was produced which was compared with bovine serum albumin standards. The level of fluorescence was measured using a Turner Digital Fluorometer Model 450. Protein level was used as a general indicator of biologically based PM.
RESULTS AND DISCUSSION
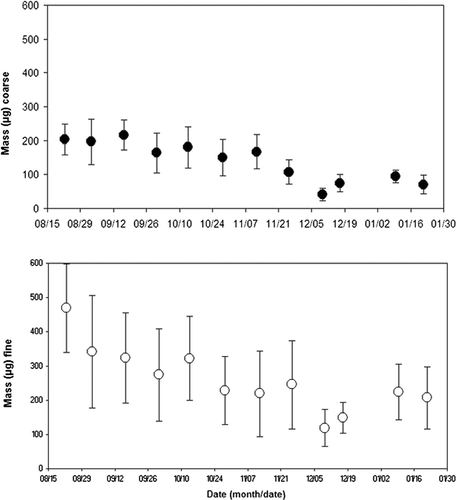
The individual PM10 − 2.5 and PM2.5 mass data were averaged for each of the 12 monitoring periods and the standard deviations calculated and shown as error bars on . As can be seen in , the masses measured for PM2.5 was higher than those for PM10−2.5 were. This is similar to the findings of other studies conducted in the eastern United States that compared fine and coarse PM abundance (EPA 2004). In addition, the ratio of fine to coarse PM was most likely affected by location of the samplers on the roof of the EPA building, 10 m above ground level. At that height, the amount of coarse aerosol is expected to be less than at ground level (Chen et al. 2006).
All of the individual BioPM filter data was averaged for each of the 12 monitoring periods. The averaged result ± the standard deviation is shown in , , , , for mold, endotoxin, and protein for each of the 12 monitoring periods
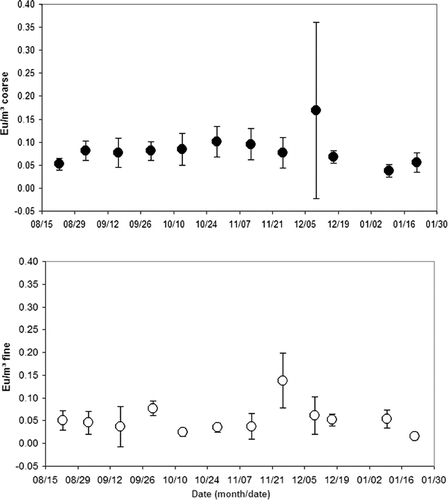
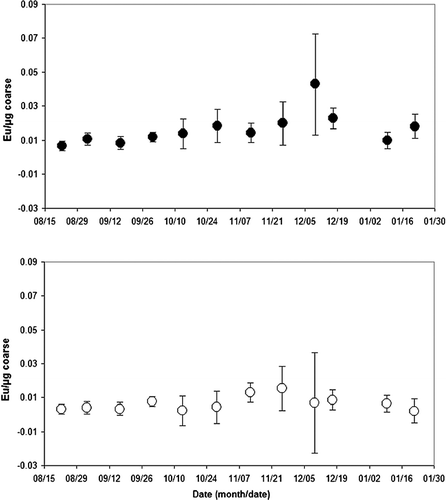
FIG. 3 Endotoxin (Eu/μ g) of coarse PM10−2.5 and fine PM2.5 recovered in extracts from PTFE filters.
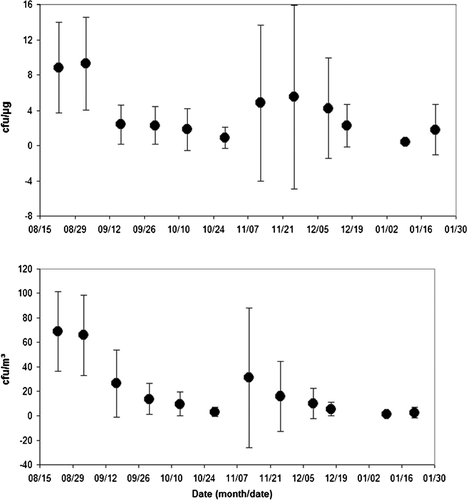
presents the coarse sample concentration of fungal mold spores (CFUs) by sample weight (μ g) and by air volume (m3) respectively. The average concentration of mold spores by sample weight (CFUs/μ g) peaked during the period from 8/27/2003 to 9/9/2003 (13 samples) at 9.285 ∀ 5.251 CFU/μ g, and an average sample weight of 184.0 ∀ 48.0 μ g. The lowest average concentration of viable mold spores by sample weight occurred from 1/6/2004 to 1/13/2004 (5 samples) at 0.380 ∀ 0.155 CFU/μ g, and an average sample weight of 96.0 ∀ 24.0 μ g (see ).
The average concentration of viable mold spores by air volume (CFUs/m3) peaked during the period from 8/19/2003 to 8/26/2003 (13 samples) at 68.8 ∀ 32.8 CFU/m3. The lowest average concentration of mold spores by sample air volume occurred from 1/6/2004 to 1/13/2004 (5 samples) at 1.5 ∀ 0.55 CFU/m3. As expected, the results of the fine filter samples indicated no growth, or below the detectable limit of the analysis, for all samples analyzed for fungal mold spores. As described above, the size range of commonly found intact mold spore species is greater than the upper size limit of the PM2.5 sample. The seasonality of culturable fungal spore levels has been studied (CitationHalwagy 1989). This data agrees with the general trends reported by CitationHalwagy (1989) with higher numbers in the summer and lower numbers in the winter (Burge 1995).
and show the coarse and fine sample concentration of endotoxins (EUs) by sample weight (μ g), and by air volume (m3), respectively. Overall, the levels remained fairly constant for the six months of the study, however, there were some weeks where the mean levels peaked. For the coarse fraction, the average concentration of endotoxins by sample weight (EU/μ g) peaked during the period from 12/6/2003 to 12/13/2003 (14 samples) at 0.045 ∀ 0.030 EU/μ g, and an average sample weight of 41 μ g (see ). The lowest coarse fraction average concentration of endotoxins by sample weight occurred from 8/19/2003 to 8/26/2003 (13 samples) at 0.008 ∀ 0.004 EU/mg, and an average sample weight of 204 μ g (see ).
The average concentration of endotoxins by sample weight (EU/mg) for the fine fraction peaked during the period from 11/19/2003 to 12/2/2003 (12 samples) at 0.016 ∀ 0.006 EU/μ g, and an average sample weight of 245 μ g (see ). The lowest fine fraction average concentration of endotoxins by sample weight occurred from 1/15/2004 to 1/28/2004 (14 samples) at 0.002 ∀ 0.001 EU/μ g, and an average sample weight of 207 μ g (see ). Additional periods of low fine fraction average concentrations of endotoxins were recorded from 10/7/2003 to 10/20/2003 (15 samples) at 0.002 ∀ 0.002 EU/μ g, and an average sample weight of 322 μ g (see ), and from 8/19/2003 to 8/26/2003 (13 samples) at 0.003 ∀ 0.003 EU/μ g, and an average sample weight of 469 μ g (see ).
In all 12 sample periods, the endotoxins were in greater concentrations in the coarse fraction when compared based on mass concentration. In two sample periods the coarse fraction was notably greater in endotoxin concentration, specifically from 12/6/2003 to 12/13/2003 [an average difference (coarse - fine) of 0.038 EU/μ g in the 28 samples analyzed] and from 1/15/2004 to 1/28/2004 [an average difference (coarse - fine) of 0.021 EU/μ g in the 28 samples analyzed].
In all but 2 of the 12 sample periods, endotoxin concentrations were at least as high in the coarse fraction when compared based on air volume concentration. The average concentration of coarse fraction endotoxins by air volume (EU/m3), shown in , peaked during the period from 12/6/2003 to 12/13/2003 (14 samples) at 0.16 ∀ 0.2 EU/m3. The lowest average concentration of endotoxins by sample air volume occurred from 1/6/2004 to 1/13/2004 (9 samples) at 0.03 ∀ 0.01 EU/m3.
The average concentration of endotoxins by sample air volume (EU/m3) for the fine fraction peaked during the period from 11/19/2003 to 12/2/2003 (12 samples) at 0.15 ∀ 0.07 EU/m3. The lowest fine fraction average concentration of endotoxins by air volume occurred from 1/15/2004 to 1/28/2004 (14 samples) at 0.02 ∀ 0.01 EU/m3.
In 7 of the 12 sample periods listed in , the comparison of coarse to fine fraction showed that endotoxins were in greater concentrations in the coarse fraction based on volume concentration. In one period, the greater concentration of endotoxins was found in the fine fraction and in four periods, they were nearly equal. In one sample period, the coarse fraction was significantly greater in endotoxin concentration, specifically from 12/6/2003 to 12/13/2003 (an average difference of 0.09 EU/m3 in the 28 samples analyzed).
While there were weeks when the mean levels of endotoxins peaked, overall the mean levels were constant with the coarse fraction exceeding the fine fraction. Those weeks with peaks generally had large standard deviations, suggesting that a specific event may have resulted in an unusually high level for one day. While the original source of endotoxins is the cell wall of Gram-negative bacteria, endotoxins are ubiquitous in nature and are found in soil and water but at varying concentrations. It is likely that during one of the spikes there was an event such a dust cloud created by a construction or building project disturbing a high organic reservoir resulting in an exceptionally high endotoxins level that day with no accompanying increase in dust mass.
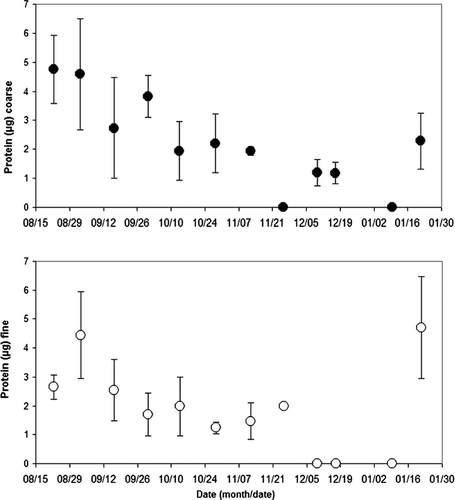
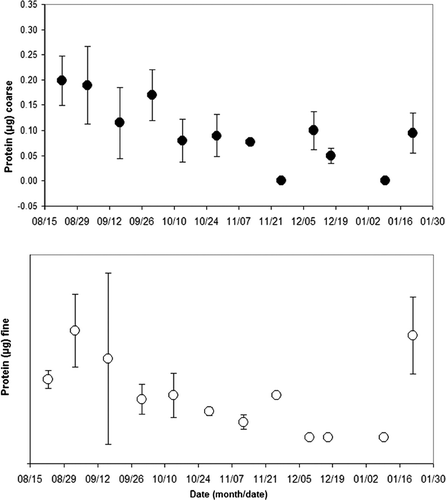
and show the coarse and fine sample concentration of protein (ng) by sample weight (μ g), and by air volume (m3), respectively. Samples analyzed for protein with results below the detectable limit (BDL) of 0.1 μ g/μ L (42 of 136 samples) were omitted from the calculation of the average concentration, but were listed as zero when all of the samples in that period were BDL. For the coarse fraction, the average concentration of protein (μ g), shown in , peaked during the period from 8/19/2003 to 8/26/2003 (12 samples) at 4.8 ∀ 1.0 μ g, and an average sample weight of 204 μ g (see ). The lowest coarse fraction average concentration of protein by sample weight occurred from 11/19/2003 to 12/2/2003 (12 samples) and 1/6/2004 to 1/13/2004 (9 samples) when all of the samples were BDL, and an average sample weight of 108 μ g (see ).
For the fine fraction, the average concentration of protein (μ g) peaked during the period from 8/27/2003 to 9/9/2003 (14 samples) at 4.5 ∀ 1.5 μ g, and an average sample weight of 342 μ g (see ). The lowest fine fraction average concentration of protein by sample weight occurred from 12/6/2003 to 12/13/2003 (14 samples), 12/15/2003 to 12/19/2003 (5 samples), and 1/6/2004 to 1/13/2004 (9 samples) when all of the combined 28 samples were BDL, and an average sample weight of 119.0 ∀ 53.0 μ g, 149.0 ∀ 45.0 μ g, and 224.0 ∀ 82.0 μ g (see ), respectively.
In 11 of 12 sample periods shown in , the comparison of coarse to fine fraction revealed that protein was in greater concentrations in the coarse fraction. In one period 11/19/03 to 12/2/03 the fine fraction (4 samples averaging 2.0 ∀ 0.1 μ g of protein, 8 BDL samples) was greater than the coarse fraction (12 BDL samples). However, this occurrence may be due to the presence of numerous BDL sample results and the technique used to account for these results.
The average concentration of coarse fraction protein by air volume (ug/m3) () peaked during the period from 8/19/2003 to 8/26/2003 (13 samples) at 0.20 ∀ 0.05 μ g/m3. The lowest coarse fraction average concentration of protein by sample air volume occurred from 11/19/2003 to 12/2/2003 (12 samples) during which all of the samples were BDL.
For the fine fraction, the average concentration of protein by sample air volume (μ g/m3) peaked during the period from 8/27/2003 to 9/9/2003 (14 samples) at 0.21 ∀ 0.07 μ g/m3. The lowest fine fraction average concentration of protein by sample air volume occurred from 12/6/2003 to 12/13/2003 (14 samples), 12/15/2003 to 12/19/2003 (5 samples), and 1/6/2004 to 1/13/2004 (9 samples) when all of the combined 28 samples were BDL. A comparison of coarse to fine fraction protein concentrations indicated that in six periods the coarse fraction was in greater concentration, in five periods the fine fraction was in greater concentration, and in one period both were BDL.
As with the mold data, we observed the summer periods produced higher protein levels and the winter lower levels, except for the final week of the study 1/15/04–1/28/04. The protein measurements reflected some seasonal differences is most likely due to the various proteinaceous components in BioPM. For example, in times of high pollen, protein levels would be expected to be elevated. Bacteria and fungi are likely a small fraction of the total protein load.
It should be noted that for all of the biological components measured, that some weeks had notably larger standard deviations. An examination of the daily data that went into the weekly average showed that usually at least 2 days were markedly higher than the other days. This variability is typical of the weather related patterns generally observed for bioaerosol data. For example, during periods of rain, mold spore levels are significantly lower, whereas afterwards, depending upon the season, a spike would be anticipated.
One of the goals of the study was to utilize a single filter to quantify mass and for biological analyses. We met this goal by using PTFE filters, which are ideal for accurate mass determination. Since PTFE filters are, however, suboptimal for BioPM recovery, the absolute levels of BioPM presented here are undoubtedly under representations.
CONCLUSIONS
Ambient samples of PM2.5 and PM10−2.5 were collected and analyzed for mold, endotoxins, and protein, and reported as a function of PM mass and volume of air sampled. Throughout the 6-month study, the presence of endotoxins and protein within both size ranges, and culturable mold in the coarse fraction, was shown to be present. The coarse PM fraction contained a higher percent of biological mass as was demonstrated by the protein measurements. The results also indicated that higher concentrations of endotoxins were present in course PM than were present in fine PM. Both protein and mold levels were seasonal; however, endotoxin levels remained generally constant. The strong correlation between endotoxins and course PM is consistent with findings from a similar study of southern California urban air (CitationMueller-Anneling et al. 2004).
Ambient concentrations of fungal mold spores peaked during the August summer period and reached the lowest level during the January winter period. A comparison of coarse and fine mold samples was not possible due to the absence of viable CFUs in the fine fraction.
The concentrations of ambient bioaerosols documented in this study contribute to more fully understanding exposure to airborne biological allergens. The larger intact spores and clumps of organisms, and the smaller organism fragments, as well as nucleated combinations of these particles, occupy the entire spectrum of PM10−2.5 and PM2.5—the respirable size range (CitationVerhoeff et al. 1992; FDA 1994; CitationLiccorish et al. 1985; CitationTargonski, Persky, and Rameskrishnan 1995). This article describes the first major study of urban bioaerosol concentrations in North Carolina and addresses their prevalence in the fine and coarse fractions. Additional studies are needed to further characterize seasonal and yearly variations of bioaerosols, and endotoxins in particular, as well as the role they play in effecting respiratory conditions.
REFERENCES
- Boehlecke , B. , Hazucha , M. , Alexis , N. E. , Jacobs , R. , Reist , P. , Bromberg , P. A. and Peden , D. B. 2003 . Low-Dose Airborne Endotoxin Exposure Enhances Bronchial Responsiveness to Inhaled Allergen in Atopic Asthmatics . J Allergy Clin. Immunol. , 112 ( 6 ) : 1241 – 3 .
- Bonner , J. C. , Rice , A. B. , Lindroos , P. M. , O'Brian , P. O. , Dreher , K. L. and Rosas , I. 1998 . Introduction of the Lung Myofibroblast PDGF Receptor System by Urban Ambient Particles from Mexico City . Amer. J. Respir. Cell. Mol. Biol. , 19 ( 4 ) : 672 – 680 .
- Chen , F. , Williams , R. , Svendsen , E. , Yeatts , K. , Creason , J. , Scott , J. , Terrell , D. and Case , M. 2007 . Coarse Particulate Matter Concentrations from Residential Outdoor Sites Associated with the North Carolina Asthma and Children's Environmental Studies (NC-ACES) . Atmos. Environ. , 41 : 1200 – 1208 .
- Donaldson , K. and MacNee , W. 2001 . Potential Mechanism of Adverse Pulmonary and Cardiovascular Effects of Particulate Air Pollution (PM10) . Intl. J. Hygiene and Environ. Health , 203 ( 5-6 ) : 411 – 415 .
- Douwes , J. and Heederick , D. 2002 . Does Environmental Endotoxin Exposure Prevent Asthma? . Thorax , 57 ( 1 ) : 86 – 90 .
- Douwes , J. , Versioot , P. , Hollander , A. and Heederik , G. 1995 . Influence of Various Dust Sampling and Extraction Methods on the Measurement of Airborne Endotoxin . Appl. Environ. Microbiol. , 61 ( 5 ) : 1763 – 1769 .
- Douwes , J. , Thorne , P. , Pearce , N. and Heederick , D. 2003 . Bioaerosol Health Effects Assessment Progress and Prospects . Ann. Occupat. Hygiene , 47 ( 3 ) : 187 – 200 .
- Food and Drug Administration . 1994 . Center for Biological Evaluation and Research . Federal Register , 59 ( 225 ) : 60362 – 3 . Docket No. 94N-0012
- Gordon , T. , Galdanes , K. and Brosseau , L. 1992 . Comparison of Sampling Media for Endotoxin-Contaminated Aerosols . Appl. Occup. Environ. Hyg. , 7 ( 7 ) : 472 – 477 .
- Halwagy , M. 1989 . Seasonal Airspora at Three Sites in Kuwait, 1977–1982 . Mycol. Res. , 93 : 209
- Heederik , D. Biological Agents Monitoring and Evaluation of Bioaerosol Exposure . International Modern Industrial Hygiene, Vol. 2, Biological Aspects . Edited by: Perkins , J. L. pp. 293 – 327 . Cincinnati, OH : American Conference of Governmental Industrial Hygienists .
- Husman , T. 1996 . Health Effects of Indoor-Air Microorganisms . Scand. J. Work Environ. Health. , 22 : 5 – 13 .
- Levetin , E. 1995 . “ Fungi ” . In Bioaerosols , Edited by: Burge , H. Boca Raton, FL : CRC Press .
- Liccorish , K. , Novey , H. S. , Kozak , R. D. and Wilson , A. F. 1985 . Role of Alternaria and Penicillium Spores in the Pathogenesis of Asthma . J. Allerg. Clin. Immunol. , 76 : 819 – 825 .
- Menetrez , M. Y. , Foarde , K. K. and Ensor , D. S. 2000 . Fine Biological PM: Understanding Size Fraction Transport and Exposure Potential (Extended Abstract). The Air and Waste Management Association Specialty Conference, PM2000: Particulate Matter and Health—The Scientific Basis for Regulatory Decision-making. January 24–28
- Menetrez , M. Y. , Foarde , K. K. and Ensor , D. S. 2001 . An Analytical Method for the Measurement of Nonviable Bioaerosols . J. Air & Waste Manag. Assoc. , 51 : 1436 – 1442 .
- Michel , O. , Kips , J. , Duchateau , J. , Vertanger , F. , Robert , L. , Collet , H. , Pauwels , R. and Sergysels , R. 1996 . Severity of Asthma is Related to Endotoxin in House Dust . Amer. J. Respir. Critical Care Med. , 154 : 1641 – 1646 .
- Monn , C. and Becker , S. 1999 . Cytotoxicity and Induction of Proinflammatory Cytokins from Human Monocytes Exposed to Fine (PM2.5) and Coarse (PM10 − 2.5) in Outdoor and Indoor Air . Toxicol. Appl. Pharmacol. , 155 ( 3 ) : 245 – 252 .
- Mueller-Anneling , L. , Avol , E. , Peters , J. M. and Thorne , S. 2004 . Ambient Endotoxin Concentration in PM10 from Southern California . Environ. Health Perspect. , 112 : 583 – 588 .
- Olenchock , S. A. , Lewis , D. M. and Mull , J. C. 1989 . Effects of Different Extraction Protocols on Endotoxin Analysis of Airborne Grain Dusts . Scand. J. Work, Environ. Health , 15 : 430 – 435 .
- Pope , C. A. 1999 . Mortality and Air Pollution Associations Persist with Continued Advances in Research Methodology . Environ. Health Perspect. , 107 : 613 – 614 .
- Reynolds , S. J. and Milton , D. K. 1993 . Comparison of Methods for Analysis of Airborne Endotoxin . Appl. Occup. Environ. Hyg. , 8 ( 9 ) : 761 – 767 .
- Salvaggio , J. and Aukrust , L. 1981 . Allergy and Clinical Immunology . J. Allerg. Clin. Immunol. , 68 ( 5 )
- Samet , J. M. and Spengler , J. D. 1991 . Indoor Air Pollution—A Health Perspective , Baltimore : Johns Hopkins University Press .
- Samson , R. , Hoekstra , E. , Frisvad , J. C. and Fillenborg , O. 1995 . Introduction to Food Borne Fungi , The Netherlands : Centraalbureau voor Schimmelcultures .
- Schwartz , D. A. , Thorne , P. S. , Yagla , S. J. , Burnmeister , L. F. , Denchuck , S. A. , Watt , J. L. and Quinn , T. J. 1995 . The Role of Endotoxin in Grain Dust Induced Lung Disease . Amer. J. Respir. Crit. Care Med. , 152 ( 2 ) : 503 – 600 .
- Soukup , J. M. and Becker , S. 2001 . Human Alveolar Macrophage Responses to Air Pollution Particulates are Associated with Insoluble Components of Coarse Material, Including Particulate Endotoxin . Toxicol. Appl. Pharmacol. , 171 ( 1 ) : 20 – 26 .
- Targonski , P. , Persky , V. and Rameskrishnan , V. 1995 . Effect of Environmental Molds on Risk of Death from Asthma During the Pollen Season . J. Allerg. Clin. Immunol. , 95 : 955 – 961 .
- Thorne , P. S. , Reynods , S. J. , Milton , D. K. , Bloebaum , P. D. , Zhang , X. , Whitten , P. and Burmeister , L. F. 1997 . Field Evaluation of Endotoxin Air Sampling Methods . Amer. Indust. Hygiene Assoc. J. , 58 : 792 – 799 .
- Thorne , P. S. 2000 . Inhalation Toxicology Models of Endotoxin and Bioaerosol Induced Inflammation . Toxicol. , 152 ( 1–3 ) : 13 – 23 .
- US Environmental Protection Agency . 2004 . Air Quality Criteria for Particulate Matter. Vol. 1 EPA/600/P-99/002af
- US Environmental Protection Agency . 2005 . Sources of Indoor Air Pollution http://www.epa.gov/iaqAvailable online at
- Verhoeff , A. P. and Van Wijnen , J. H. 1992 . Presence of Viable Mold Propagules in Indoor Air in Relationship to House Damp and Outdoor Air . Allergy , 47 : 83 – 91 .