Abstract
Because the health effects of manganese are dependent its oxidation-state, we have improved upon oxidation-state resolved methods to quantify soluble manganese in atmospheric aerosols. Two spectrophotometric methods were adapted for measurements in atmospheric aerosols in order to measure total soluble manganese (Mn sol ) and soluble oxidized manganese [Mn(III) and Mn(IV), Mn ox ]. Using the formaldoxime method, we noted a detection limit two orders of magnitude better than past studies using trace-metal clean techniques and a 1 meter path-length spectrophotometric cell. Extractions of co-located aerosol samples were performed in four environmentally or biologically relevant extract solutions and processed for soluble manganese analysis. The quantity of manganese extracted was a strong function of the fluid, and the greatest amount of manganese was extracted in the rain-water surrogate (acetate buffered solution). Mn sol in East St. Louis, IL, USA (6–20% of the total manganese) was less than the Mn sol in aerosols collected in Toronto, ON, Canada (40% of the total). Mn ox was not detected in the PM10 samples collected in East St. Louis, however Mn ox accounted for around 30% of the PM2.5 soluble manganese in Toronto. Mn ox was not detected in the coarse fraction in Toronto, which may imply that soils are not a source of Mn ox at this site. Oxidized manganese was not recoverable from extracts of samples from East St. Louis spiked with 1 μg Mn ox L−1. This implies that a soluble component of the aerosol is responsible for reduction of oxidized manganese and that the chemical form of manganese in aerosols can quickly change when it comes into contact with a fluid.
INTRODUCTION
Studies of atmospheric ambient aerosols in the United States, United Kingdom, and Canada have reported total manganese air concentrations in the range of < 1 to 50 ng m− 3(CitationAllen et al. 2001; CitationCrump 2000; CitationKidwell and Ondov 2004). Ambient atmospheric manganese can be attributed both natural (e.g., resuspended soil) and anthropogenic (e.g., welding, fungicides, batteries, and steel production) sources (CitationMergler and Baldwin 1997). Atmospheric manganese has also been associated with automobile wear and, in regions where the anti-knock fuel additive methylcyclopentadienyl manganese tricarbonyl (MMT) is used in automobile engines, manganese is a component of automobile exhaust (CitationRessler et al. 2000).
Manganese is an essential element, required for proper functioning of several enzymes in mammals (CitationBrown and Taylor 1999; CitationHeilbronn and Eriksson 1997). When ingested or inhaled in larger doses, however, the toxic effects of manganese can be quite severe, with neurological disorders such as manganism (a disorder similar to Parkinson's Disease) manifesting (CitationCalne et al. 1994; CitationWitholt et al. 2000). In smaller doses, studies have suggested that, due to their ability to transfer electrons and create reactive oxygen species, transition metals, like manganese, may play a large part in the toxicity of atmospheric particulate matter (CitationGwiazda et al. 2002; CitationValavanidis et al. 2005). Research efforts to elucidate the exact mechanisms of manganese toxicity are ongoing, and, while there are still many unanswered questions, it is generally agreed upon that manganese toxicity is likely a function of oxidation state. Studies suggest that Mn(III) may be significantly more toxic in-vivo than Mn(II) compounds as Mn(III) is able to form much stronger complexes with biological ligands, much like Fe(III) (CitationReaney et al. 2002). Oxidized forms of manganese (as Mn3 +) have been shown to be involved in the formation of reactive oxygen species (ROS), and thus compromising biological function, while no ROS were formed in vivo with Mn2 + (CitationAli et al. 1995).
Manganese can exist in several different oxidation states [Mn(0), Mn(II), Mn(III), Mn(IV), Mn(VI), and Mn(VII)] depending on the surrounding red-ox environment. However, in most environmental and biological fluids, the rapid oxidation of Mn(0) and the instability of Mn(VI) and Mn(VII) in dilute solutions results in most manganese to be in the forms of Mn(II), Mn(III), and Mn(IV), with inter-conversion between the forms possible. For example, both photo-oxidation and photoreduction of manganese species have been observed in seawater (CitationNico et al. 2002; CitationSunda and Huntsman 1994) while catalytic reactions have been shown to oxidize manganese on the surface in streams (CitationScott et al. 2002). In the presence of dissolved organic matter, the reductive dissolution of manganese oxides can be significant in a matter of hours. Upon illumination, significant dissolution of manganese oxides occur within minutes (CitationScott et al. 2002). Oxidation of Mn(II) compounds have also been observed in the presence of humic substances upon illumination, in a time-scale of one hour. This is attributed to the reactive oxygen species which are created by the humic substances (CitationNico et al. 2002). Because atmospheric particulate matter contains a large fraction of organic matter, as well as humic-like substances (CitationGraber and Rudich 2006), it is possible that similar reactions could occur in atmospheric aerosols. Studies by Stone and Morgan (1984) have shown that dissolution and reduction of Mn(III) and Mn(IV) oxides can occur in certain conditions (CitationStone and Morgan 1984a; CitationStone and Morgan 1984b). CitationMorgan (2005) has also shown that Mn(II) may slowly oxidize (half-life = 200 to 300 days) in aqueous solutions, but this process is accelerated by bacteria and surface catalysts (CitationDiem and Stumm 1984; CitationGounot 1994; CitationMorgan 2005).
Because of the difference in solubility, Mn(II) and Mn(ox) may exert different effects on the toxicity (CitationAdamson et al. 1999). While the solubility of Mn(ox) species depends on the chemical environment, Mn(II) species are, in general, much more soluble than Mn(ox) species. However, oxidized manganese is not completely insoluble and its solubility has been found to depend on the fluid in question. One study showed that 1–1.5% of manganese (IV) oxides will solubilize under physiological conditions (pH = 7.4 saline solution) over the course of 24 hours with this rate increasing at around pH = 4.0 (CitationElder et al. 2006; CitationLundborg et al. 1984). Also, it was found that, if ligands of appropriate type and concentration are present, then insoluble Mn(III) can be brought into solution (CitationKlewicki and Morgan 1999).
Extraction techniques, whether single reagent of sequential in design, are designed to recover metals with “functionally” different chemical forms or associations. However, in practice these fractionations are more operational in nature, because of the complexity of environmental materials and poor selectivity of extractants. The list of fractions described in literature is quite extensive, including, but not limited to: ion-exchangable; easily or moderately reducible; carbonate associated; organic-carbon associated; residual or alumino-silicate associated. Labile and/or bioavailable fractions are also commonly described, and CitationUre (1990) details a sequential extraction procedure which is designed to address different labile pools of trace metals (CitationUre 1990) (the BCR method). Some of the extract steps include harsh, strong acid reagents that clearly overestimate the environmentally labile metal pools. The US EPA standard method also includes a harsh acid extraction in order to address labile manganese (1995). Direct methods (i.e., not requiring wet-chemical extractions) are also available to address metal oxidation state in solids. For an example of how such techniques can be applied to iron speciation in atmospheric aerosols, refer to CitationMajestic et al. (2007). In the current study, the extractions were designed to mimic simple environmental or physiological relevant fluids that an aerosol may contact over its lifetime.
Due to the low concentration of manganese in atmospheric particles, its quantification can present many challenges. For example, in ambient aerosols, manganese levels are routinely in the range of 1 ng m− 3 air, which corresponds to total manganese levels around 1 μ g Mn L− 1 (ppb) solution (assuming 13 m3 air and a 10 mL extraction). Several methods have been proposed to speciate manganese (CitationChiswell and O'Halloran 1991; CitationKargosha and Noroozifar 2003; CitationNonova and Evtimova 1973) and to determine the oxidizing equivalents of manganese (CitationKessick et al. 1972) in lakewaters and streams. In many cases, it is difficult to apply these techniques to atmospheric particulate matter as the detection limits are not suitable or because of gross interferences which are inherent in the method. Only the leucocrystal violet technique has been used for the quantification of oxidized manganese in atmospheric aerosols and this often resulted in measurements which were below the detection limit or Mn(IV) species measured being greater than the overall manganese (CitationSiefert et al. 1998). One technique which shows great promise for the measurement of total soluble manganese is the formaldoxime (FAD) method from CitationMorgan and Stumm (1965). This method has the advantages in that the starting materials are very inexpensive, the Mn-FAD complex has a very high molar absorptivity, the required apparatus is easy to use, and it is relatively free from interferences (except iron). We report here on the modified version of this approach for application to atmospheric aerosols. To compliment the FAD method, we also demonstrate in this paper that the oxidation of o-tolidine is suitable to estimate oxidized species of manganese at levels commonly encountered in atmospheric particulate matter.
MATERIALS AND METHODS
Particulate Matter Sampling
Atmospheric particulate matter (PM) was collected from the U.S. Environmental Protection Agency (US EPA)–funded St. Louis Midwest Supersite in East St. Louis, IL. The site is in an urban residential/light commercial area and is about 3 km east of St. Louis, MO. With samplers built specifically for this study, two PM10 inlets (URG Corp.) were used to collect atmospheric PM at a total flow of 64 liters per minute (Lpm). Downstream of each inlet, flow was separated into five air streams and passed through five aluminum filter holders (URG Corp.), thus allowing for 10 co-located filters each sampling day. The 10 co-located PM samples were collected at a flow rate of 6.5 Lpm for 24 hours on 47 mm acid-cleaned Teflon filters (Teflo, 2.0 μ m pore size, 99.99% collection efficiency at 0.3 μ m, Pall Life Sciences Inc.). The use of aluminum filter holders resulted in elevated aluminum levels in the field blanks, but did not have any significant effect on soluble manganese. Analysis of field blanks for total soluble manganese and oxidized soluble manganese content averaged at or below the instrumental detection limit. Sampling was carried out every other day for 7 days in East St. Louis from March 13 to March 31, 2005. Air flows were calibrated in the laboratory and checked in the field before and after sampling each day.
The sampling site near Toronto was located on a third floor balcony in a residential neighborhood just west of Toronto, ON, Canada. This site was chosen because the manganese-based fuel additive, methylcyclopentadienyl manganese tricarbonyl (MMT), is used in automobiles here, but not in East St. Louis. The site is in-between two subway stations and one block north of the main thoroughfare through the neighborhood. One Sioutas personal cascade impactor sampler (PCIS) (CitationMisra et al. 2002; CitationSingh et al. 2003) was employed operating at 9 Lpm for 24 hours. This sampler separates the PM into five size-fractions (> 2.5 μ m, 2.5–1 μ m, 1–0.5 μ m, 0.5–0.25 μ m, and < 0.25 μ m) allowing for size-fractionated chemical-speciation data. The sampler was equipped with Teflon (25 mm Zefluor, 3.0 μ m) impactor substrates and a Teflon (37 mm Teflo, 2.0 μ m) after-filter. The specific size-cut-points and collection efficiency of each stage of the PCIS can be found elsewhere (CitationMisra et al. 2002). Twenty-four-hour samples were collected over the period of December 30, 2005 to January 22, 2006 (12 days) and the particulate matter was then analyzed for total soluble manganese and soluble oxidized manganese.
The pre-cleaned filters were weighed on a microbalance (Mettler) in a constant humidity and temperature environment pre- and post-sampling. For all sampling and analysis, trace-metal clean techniques were used throughout the preparation, sampling, and analysis processes. All pre- and post-sampling handling of the substrates were performed in a laminar flow HEPA hood or in a dedicated trace-metal clean room. Before use, all collection substrates were subjected to a rigorous acid-washing procedure which has been shown to minimize trace-metal contamination. For the Teflon filters, this procedure includes a 5 min soak in 30 mL 2 N high-purity HCl. The acid is then pumped through the filter at a rate of 3 mL/min using an oil-less vacuum pump. This step is then repeated with 30 mL 2 N HNO3. Finally, 60 mL of > 18.0 MΩ water is pumped through the filter at 3 mL / min. The filter is then placed in an acid-cleaned polystyrene dish and dried under a laminar flow clean bench.
Total Manganese Determination
Total manganese was determined by High Resolution (magnetic-sector) Inductively Coupled Plasma—Mass Spectrometry (HR-ICPMS) at the Wisconsin State Laboratory of Hygiene. Total dissolution of aerosols collected on the pre-cleaned Teflon membranes was effected by microwave-assisted acid digestion in miniature pre-cleaned Teflon bombs. An automated, temperature-regulated, trace analysis microwave system (Milestone Ethos+) was utilized [microwave program: 15 minute temperature ramp to 200°C followed by a 30 minute hold at 200°C]. The acid chemistry employed a small volume mix of ultra-high purity acids (1.5 mL 16N HNO3, 0.50 mL 12N HCl, 0.2 mL 28N HF) to realize very low blanks. A low final dilution volume (15 mL) enhanced signal to noise for subsequent HR-ICPMS analysis. A typical digestion batch consisted of 22 unknowns, 6 standard reference materials (SRMs), 4 matrix blanks, 2 method blanks, and 2 matrix spikes. The SRMs used to monitor digestion performance were selected to represent actual aerosol phases or significant aerosol components. These included the NIST SRMs: Recycled Auto Catalyst (#2556), Urban Dust (#1649a), and San Joaquin Soil (#2709). Recovery of Mn from SRMs #1649a and #2709 averaged 94.7 ± 6.8%, and spike recoveries were quantitative (98.2 ± 4.3%). Manganese method blanks were typically a small fraction of sample signal.
Soluble Manganese Determination
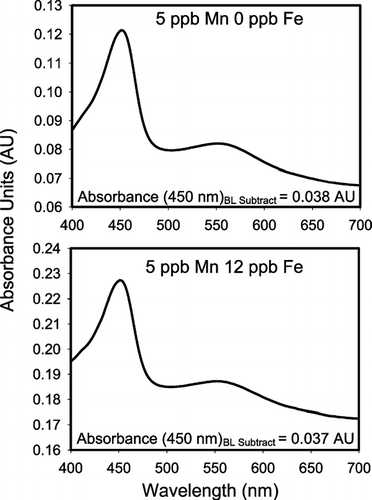
Total soluble manganese was quantified using the adapted formaldoxime (FAD) method (CitationMorgan and Stumm 1965) where a detection limit of 10 μ g soluble Mn L− 1 (ppb) has been previously reported (CitationBrewer and Spencer 1971). FAD strongly complexes both manganese and iron in solution with the complexes exhibiting peak absorption at 450 and 525 nm, respectively. The FAD-iron complex also shows a smaller absorption band at around 450 nm, which gives rise to a potential interference with the total manganese measurement. This interference was quantified here as adding 25% false manganese signal at a given iron and manganese concentration [(μ g Fe)/(μ g Mn) = 1], which is similar to other iron interference studies (CitationGoto et al. 1962). Given that soluble Fe ≫ Mn in most atmospheric particulate matter, it is important to find ways to remove this interference. Therefore, the method of CitationGoto et al. (1962) was employed by adding EDTA and lowering the pH. In this study, the pH of the extract was reduced to approximately 7.5 using hydroxylamine hydrochloride (HA). In these conditions, the iron-FAD complex breaks down while the manganese-FAD complex is stable for the analysis time (about 5 minutes). As the experiments of CitationGoto et al. (1962) were performed at manganese concentrations around 1000 times of those found in atmospheric aerosols, this method was adapted for the lower iron concentrations encountered in atmospheric particulate matter and the spectra are presented in . presents spectra of 5 ppb Mn with 12 ppb Fe and 5 ppb Mn with no Fe addition. The peak absorbance at 450 nm in (as in all of the sample spectra) was determined using a baseline subtraction. A linear baseline was calculated from 400–500 nm and the sample absorption at 450 nm was found by subtracting the calculated baseline value at 450 nm from the actual absorption at 450 nm. These absorption spectra show that the addition of EDTA and HA completely remove the iron interference up to at least 12 ppb Fe, which is the maximum expected soluble iron in our particulate matter samples (CitationMajestic et al. 2006), while the manganese signal is not compromised.
FIG. 1 Example spectra of the formaldoxime (FAD) method. Almost identical spectra are obtained for two solutions of equal manganese concentration, but varying iron concentration, showing that the iron interference can be eliminated at ppb levels by complexation with EDTA at slightly basic conditions (pH = 7.5). Baseline (BL) subtracted Absorbance values are shown at 450 nm.
For analysis, stock FAD solution (20 mM) was prepared in the laboratory by combining equimolar amounts of formaldehyde and HA. A stock 100 mg L− 1 Mn(II) solution was prepared from Mn(II)Cl2salt in 1% HCl (Fisher, trace-metal grade). Dilutions of this stock solution were prepared for calibration standards. To 1.950 mL of standard or sample, the following reagents were added in the indicated order:
1) 10 μ L 2.5% NaOH
2) 20 μ L 20 mM FAD
3) 10 μ L 0.08 N EDTA
4) 8.5 μ L 600 mM HA (approximate amount to lower pH of solution to 7.5)
We found that the measurement was more stable by adding the reagents separately, rather than pre-mixed, which is similar to CitationKessick and Morgan (1975). After approximately 5 minutes from the addition of HA, about 1.5 mL of sample was drawn into a 1 m pathlength liquid waveguide capillary cell (LWCC) (600 μ L) using a peristaltic pump and the absorbance spectrum obtained from 400 to 700 nm using a D2H light source and the Tidas I detector (World Precision Instruments, Inc., Sarasota, FL). The LWCC is a capillary cell with an internal volume of 600 μ L (for the 1 m path-length cell). The outside of the capillary is composed of a low-refractive index polymer, which results in complete internal light reflection when an aqueous solution is passed through the cell. As in past studies, the cell was interfaced to the light source and detector via fiber optic cables (CitationMajestic et al. 2006). The spectra were baseline subtracted and the absorbance was recorded at 450 nm. The Mn-FAD calibration curve from 0.1–5 ppb (1–50 ng filter− 1assuming 10 mL extract) can be found in the Supplementary Material. The measured slope = 0.0142 absorbance units (AU) ppb− 1 and the r2 value = 1.00. Based on the calibration curves, we could reliably detect approximately 0.09 ppb Mn, or 0.11 ng Mn m− 3 for 24-hour sampling at 6 liters per minute assuming a 10 mL extraction volume.
The reduction in pH necessary to remove the iron interference is associated with a decrease in maximum absorption. To test the sensitivity of the color-development to pH, Mn-FAD calibration curves were prepared at pH = 6.0, 6.9, 7.2, 7.5, and > 9.0. For these pH values, the observed slopes (in AU ppb− 1) were 9.55 × 10 − 3, 0.013, 0.013, 0.014, and 0.019. A 26.3% decrease in sensitivity is observed when decreasing the pH from > 9.0 to 7.5, however, this value remains relatively stable between pH of 6.9 and 7.5. Further, as shown by duplicate samples analyzed from the East St. Louis Midwest Supersite, we are able to achieve reasonable precision (9.1% RSD, n = 7) and detection limit (0.09 ppb) after decreasing the pH.
Soluble Oxidized Manganese Determination
Soluble oxidized manganese was measured using an adapted o-tolidine method (CitationMorgan and Stumm 1965). In the presence of higher oxidation states of Mn [Mn(III), Mn(IV)], o-tolidine is oxidized and exhibits an absorption maximum at 440 nm. Mn(III) will oxidize one molecule of o-tolidine while Mn(IV) [and Mn(VII)] compounds will oxidize two molecules of o-tolidine. As a result, this technique measures oxidizing equivalents of manganese, rather than absolute oxidized manganese concentration. Therefore, calibration curves were prepared from two oxidized manganese sources; Mn(III) and Mn(VII). It should also be noted that the HA which is used in the soluble manganese assay will reduce any oxidized manganese, which would result in low-biased oxidized manganese values. Therefore, the soluble oxidized manganese measurement was performed independent of the total soluble manganese measurement (i.e., different vials were used).
A stock solution of 100 ppm Mn(III) pyrophosphate was prepared as described in Klewicki (CitationKlewicki and Morgan 1998) at pH = 7.3 and a 50 times molar excess of pyrophosphate ligand relative to total manganese. Dilutions of the stock solution were initially prepared in > 18.0 MΩ water, but were found to degrade within minutes. Dilutions were then made in pyrophosphate solution in the same concentration as the stock solution and stability of the Mn(III) compound was verified spectrophotometrically and found to be stable for at least 30 minutes. In practice, experiments were always completed within 5 minutes of Mn(III) addition. Stock Mn(VII) was purchased from LabChem Inc (Pittsburgh, PA) as a 5% potassium permanganate solution and diluted as needed.
For analysis, 1.750 mL of standard (or filtered sample) was pipetted into a 2 mL acid-cleaned vial. 10 μ L of 0.1% o-tolidine was then added, followed by 142 μ L (750 mM) of concentrated perchloric acid (HClO4). Preliminary experiments showed that o-tolidine will oxidize in this environment in a predictable manner even when no manganese is present, likely due to ambient light or dissolved oxygen. To control for this, the blanks and samples were placed in the dark for 30 minutes and allowed to come to equilibrium before analysis and measured in triplicate. This was proven to remove any extra oxidation effect in the standards and subsequent blanks. Therefore, immediately following the addition of HClO4, the samples were placed in the dark for 30 minutes and then analyzed within 2 minutes. Calibration curves from 0.10 ppb to 5 ppb [Mn(III)] and 0.30 to 1.0 ppb [Mn(VII)] were prepared using this method and are can be seen in the Supplementary Material. It should be noted that the slope in the Mn(VII) plot (0.14 AU/ppb) is almost exactly twice that of Mn(III) (0.063 AU/ppb), indicating twice the oxidizing equivalents.
Precision of the Total Soluble and Oxidized Soluble Manganese Methods
Co-located PM10 samples collected at the East St. Louis Midwest Supersite were used to test the precision of the soluble manganese measurements. The samples were extracted for 2 hours under laboratory conditions (22 to 25°C, diffuse light) while gently agitated. The extract solutions were filtered through a 0.2 μm polypropylene filter (Whatman). Both total soluble manganese and oxidizing manganese equivalents were measured within 2 hours of filtration. Seven co-located PM10 samples from March 19, 2005 were extracted in MQ water and an air concentration of 0.83 ± 0.08 ng soluble Mn m− 3 air was obtained. This corresponds to a relative standard deviation of 9.1% and represents the overall precision for the FAD technique, which includes sampling, extraction, and analytical uncertainties. No oxidized manganese was detected in any of these extracts.
Because oxidized manganese levels were below detection in the precision test from the samples collected at the East St. Louis Midwest Supersite, eight test solutions each of equal concentrations of Mn(VII) were prepared at 0.46 and 0.92 ppb. The variations (as % RSD) for the 0.46 and 0.92 ppb Mn(VII) were found to be 13.8 and 8.5%, respectively, with the absorbance values ranging from 0.046 to 0.069 AU and 0.110 to 0.134 AU. While great care was taken to prepare samples in a standard fashion as well as ensuring that the standards were used within minutes of dilution, it is still likely that the scatter, especially at the lower concentration, is due to one or both of the following factors:
1) Oxidation of o-tolidine due to ambient light or light from the spectrophotometer during analysis
2) Breakdown of the very dilute (< 1.0 ppb) Mn(VII) standard to lower oxidation states of manganese, which will not oxidize o-tolidine, thus causing a lower absorbance signal.
RESULTS AND DISCUSSION
East St. Louis Samples
The co-located PM10 samples previously described were used to evaluate the manganese extraction efficiency of the following four extraction solutions, with contrasting but relevant properties, from real-world samples:
1) pH = 7.4 sodium bicarbonate (140 μ M)
2) Sodium chloride solution (140 μ M)
3) pH = 4.3 acetate buffer (500 μ M acetate) (rain-water surrogate)
4) Milli Q water (> 18.0 MΩ water)
The bicarbonate extractant was chosen because carbonate systems at this pH are physiologically relevant (CitationSun et al. 2001). The purpose of the sodium chloride solution was to match the ionic strength of the sodium bicarbonate buffer, but with a minimal buffering capacity. The acetate buffer was selected as it is a lower pH buffer with environmental applications in that it is a good approximation of fog and rain water and similar extractants have been used to define labile metal pools in other studies (CitationSiefert et al. 1998). Finally, the Milli Q water was utilized as a clean, simple, standard solution to be compared to all other leaches. We observe in that the most efficient reagent tested here for the release of manganese was the pH = 4.3 acetate buffered solution (a proxy for rain-water). CitationNico and Zasoski (2001) have shown that Mn(III) has a moderate affinity (log K = 4.4) for the acetate ion (CitationNico and Zasoski 2001). Therefore, the excess soluble manganese (i.e., the incremental amount extracted beyond the other three extractants) may be explained, in part, by the interaction of Mn(III) in the PM with the acetate ion and the subsequent dissolution of Mn(III). Studies have shown that, at pH < 5, soluble Mn(II) is the most stable Mn species (CitationMorgan 2000; CitationStumm and Morgan 1981). At this point, however, it is unclear which of these two mechanisms is primarily responsible for the release of manganese from atmospheric aerosols (lowering the pH or the presence of the acetate ion). The other three solutions all showed similar ability to extract manganese and all released approximately 1/2 the manganese extracted by the acetate buffer. The fraction of total manganese extracted (i.e., soluble %) (total manganese determined by ICPMS) of manganese is shown in . For the test dates, 6–20% of the manganese was soluble depending on the extractant. It should also be noted that no oxidized manganese was detected in these PM10 samples collected on March 17, March 19, and March 21, 2005 at the Midwest Supersite in East St. Louis, IL.
TABLE 1 Comparison of leachable manganese with total manganese values
FIG. 2 Plots of how the extract solution affects the soluble manganese present in PM10 samples collected in East St. Louis, IL. Each date consists of four co-located samples submitted to a different extract solution. In acetate solution, the fraction of the total manganese extracted is 20% and 13% for March 17 and 21, respectively.
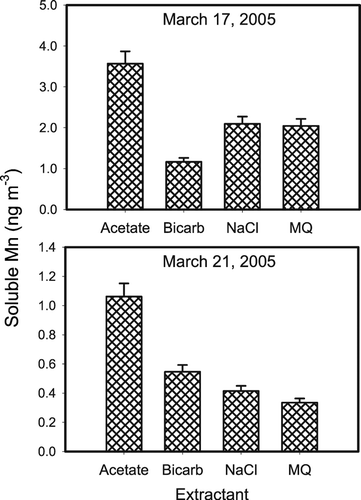
To examine the stability of manganese and to address the long-term precision of the measurement techniques, duplicate measurements for total soluble and soluble oxidized manganese were obtained in each leachate 24 hours after extraction for the March 17, 2005 sampling date. The total soluble manganese concentrations measured ranged from 3% (acetate buffer) to 31% (bicarbonate) higher than the original measurements shown in , while still no oxidized manganese was detected. These results imply that the manganese measurement is moderately reproducible even after samples are exposed to laboratory conditions for 24 hours. The ability to accurately recover low concentrations (0.5 ppb) of manganese is essential to successfully measure soluble manganese in personal exposure samples. To examine manganese recovery from various reagents, each extract from March 17, 2005 was spiked with 0.5 ppb excess Mn(II) (as MnCl2). The spike recoveries of the total soluble manganese ranged from 94% (MQ) to 126% (Bicarbonate). This suggests that the soluble manganese detection method described here can successfully determine manganese in the extract solutions at the low levels typical of personal exposure sample extracts.
Using all four of the extract solutions listed previously, seven aerosol extracts, from samples collected in East St. Louis, were spiked with 2.0 μ equivalents oxidized manganese L− 1 [2 ppb Mn(III) or 1 ppb Mn(VII)] to evaluate oxidized manganese recovery. In all of these samples, oxidized manganese was not observed either before or after the addition of the oxidized manganese. The fact that oxidized manganese was not detected in the actual samples after the spike (with oxidized manganese quantitatively recovered in the standard matrix) implies that a soluble component of the aerosol is responsible for the reduction of oxidized manganese in solution. Past studies have shown that Fe(II) can quickly (within minutes) reduce about 70% of the oxidized manganese present in a sample (CitationVillinski et al. 2001; CitationVillinski et al. 2003). In an effort to confirm the Fe(II) reductant hypothesis, aerosol extracts from East St. Louis samples were spiked with the Ferrozine (FZ) ligand, which forms a strong complex with Fe(II) (CitationStookey 1970), thus making Fe(II) unavailable to reduce the oxidized manganese. Upon spiking the FZ-ammended East St. Louis extracts with oxidized manganese, peaks at 440 nm were observed, indicating that oxidized manganese was now present in the spiked solutions. Oxidized manganese quantification was not possible, however, as the very strong Fe(II)-FZ3 peak (maximum = 562 nm) strongly interfered with the relatively small oxidized manganese peak (i.e., 10 μ g Fe(II) L− 1 vs. 0.10 μ g Mn(ox) L− 1 in the acetate buffer). These studies indicate that Fe(II) is, at least partially, responsible for the rapid reduction of oxidized manganese in our samples. It should be noted that soluble oxidized manganese was only observed in the East St. Louis samples when they were spiked with excess oxidized manganese in the presence of FZ. Even in the presence of FZ, however, no oxidized manganese was observed in the unspiked samples, indicating either very rapid reduction of Mn(ox) by Fe(II), or that Mn(ox) levels were below detection limits.
Toronto Samples
presents a plot showing size-resolved total soluble manganese for two days in Toronto using the rain-water surrogate (acetate buffered solution). The greatest air concentration of soluble manganese was found in the coarse fraction, with the air concentration decreasing with particle size. For particle sizes < 0.5 μ m, there was no detectable soluble manganese. This result is consistent with that reported by Allen et al., where total manganese levels measured by ICPMS were not detected in PM below 0.2 μ m in size (CitationAllen et al. 2001). Total soluble manganese data from Toronto are compared with data from the East St. Louis site in . It was observed that, on these sampling days, the air concentration of manganese was generally greater at the East St. Louis sampling site, and the particle concentration is roughly similar at both of the sites. shows the relative contribution of leachable manganese to the total manganese content for the sampling days in Toronto. The soluble manganese per cent of total manganese was found to differ greatly between the two sites (compare with ). Almost 40% of the manganese in the Toronto composite sample was soluble while a maximum of 20% of the manganese was soluble in the samples collected at East St. Louis (see ). Researchers analyzing particulate matter in occupational settings (manganese alloy plants) have found that about 40–60% of the total manganese is addressable by a dilute acetic acid solution (the pH and acetate concentration were not provided) (CitationThomassen et al. 2001). This range is comparable with the values determined in this study for Toronto, but far greater than the labile fraction found in East St. Louis. Another study used a surrogate lung fluid to extract labile metals from PM10 samples from various sampling sites (CitationSchauer et al. 2006). In this study, the labile manganese ranged from 20–100% of the total manganese depending on the sampling site and time of year.
TABLE 2 Comparison of soluble (pH = 4.3 acetate buffer) manganese air and particle concentrations for PM10 samples collected in East St. Louis and Toronto. PM10 mass values for Toronto are summed for each stage of the PCIS
TABLE 3 Comparison of soluble manganese versus soluble oxidized manganese concentrations for PM10 and PM2.5 samples collected in Toronto. PM10 and PM2.5 mass values are summed for each stage of the PCIS
FIG. 3 Size-fractionated soluble manganese () and soluble oxidized manganese () air concentrations measured in Toronto by the formaldoxime method and the o-tolidine method, respectively. Note that soluble manganese was detected in particles < 0.5 μ m. Also, note that no oxidized manganese was detected in the coarse fraction, which was the most dominant overall soluble manganese fraction.
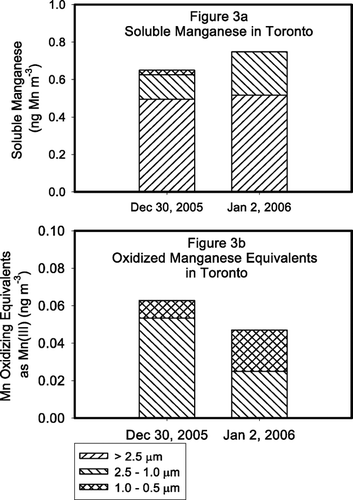
also details the relative abundance of oxidized manganese in the total soluble fraction in Toronto. Unlike in the East St. Louis samples, oxidized manganese is a significant fraction of the total soluble manganese, with the oxidized manganese ranging from 20–40% of the total soluble manganese [as Mn(III)] in the PM2.5 fraction. The limited sampling days presented here, however, should not be used to represent the sites at all times during the year. Size-fractionated total soluble oxidized manganese equivalents are shown in for the December 30, 2005 and January 2, 2006 samples at the Toronto site. Oxidized manganese was not detected in either day in the coarse mode, the size fraction with the greatest overall soluble manganese. In both the December 30, 2005 and January 2, 2006 samples, soluble oxidized manganese was found only in the intermediate size fractions ranging from 2.5 to 0.5 μ m. As was expected (since no soluble manganese was detected), no soluble oxidized manganese was present in the fractions < 0.5 μ m. The January 13 and 19 composite sample was very similar to these two days, except a very small (< 2.5% of total soluble) amount of oxidized manganese was detected in the coarse fraction (> 2.5 μ m).
IMPLICATIONS
The results indicate that the residence time of oxidized manganese in aerosol water layers and extracts may be dependant on the other components of the aerosol. We found that oxidized manganese was not detected by the o-tolidine method when excess oxidized manganese was spiked into aerosol sample extract solutions. However, in calibration standards prepared in the same extractant, oxidized manganese was reliably detected. This finding implies that the soluble components [in part, Fe(II)] of the aerosol may quickly reduce any soluble oxidized manganese present on the filter or initially in the extract. Thus, as particulate matter encounters various fluids or humidity regimes, the quantity and red-ox speciation of the soluble fraction will change. These complex interactions will not only depend on the composition of the fluid, but also on the chemical composition of the aerosol.
These new manganese speciation tools have allowed us to determine differences in the forms of manganese at two urban sites. The manganese speciation results presented here suggest that the two sites are quite different with respect to manganese sources. Similar total soluble manganese concentrations were measured in the particulate matter at each site. However, the aerosols collected in Toronto showed a significant amount of oxidized manganese in the PM2.5 fraction while the oxidized manganese in the PM10 samples from East St. Louis samples were below detection (even in samples containing Ferrozine). We also found that soluble oxidized manganese was below detection in the coarse PM fraction in Toronto, the source of which is primarily resuspended soils. The fact that oxidized manganese was detected only in the PM2.5 fraction implies that the source of the soluble oxidized manganese is combustion-related. According to the National Pollutant Release Inventory (NPRI) compiled by Environment Canada, there are no significant manganese point sources in the greater Toronto area (2006). Based on the NPRI data and the high solubility of the MMT combustion products, the differences in manganese speciation between East St. Louis and Toronto may be the result of products from the combustion of the manganese-based fuel additive, methylcyclopentadienyl manganese tricarbonyl (MMT).
Acknowledgments
This study was supported by the Health Effects Institute (HEI) grant #02-11. We thank Jay Turner, Jay Hill, and Eric Ryszkiewicz at the Midwest Supersite in East St. Louis, IL for providing us access to the site and assistance with obtaining samples. We also thank Helen Manolopolous for collecting PM samples in Toronto. We thank Jeff DeMinter, John Strauss, Chris Worley, Dustan Helmer, Joel Overdier, and Noel Stanton at the Wisconsin State Laboratory of Hygiene for their roles in the ICPMS analysis and filter preparation.
REFERENCES
- Adamson , I. Y. R. , Prieditis , H. and Vincent , R. 1999 . Pulmonary Toxicity of an Atmospheric Particulate Sample is due to the Soluble Fraction . Toxicol. Appl. Pharmacol. , 157 ( 1 ) : 43 – 50 .
- Ali , S. F. , Duhart , H. M. , Newport , G. D. , Lipe , G. W. and Slikker , W. 1995 . Manganese-Induced Reactive Oxygen Species—Comparison Between Mn+2 And Mn+3 . Neurodegeneration , 4(3) : 329 – 334 .
- Allen , A. G. , Nemitz , E. , Shi , J. P. , Harrison , J. P. and Greenwood , J. C. 2001 . Size Distributions of Trace Metals in Atmospheric Aerosols in the United Kingdom . Atmos. Environ. , 35 ( 27 ) : 4581 – 4591 .
- Brewer , P. G. and Spencer , W. 1971 . Colorimetric Determination of Manganese in Anoxic Waters . Limnol. Oceanogr. , 16 ( 1 ) : 107 – 110 .
- Brown , S. and Taylor , N. L. 1999 . Could Mitochondrial Dysfunction Play a Role in Manganese Toxicity? . Environ. Toxicol. Pharmacol. , 7 ( 1 ) : 49 – 57 .
- Calne , D. B. , Chu , N. S. , Huang , C. C. , Lu , C. S. and Olanow , W. 1994 . Manganism and Idiopathic Parkinsonism—Similarities and Differences . Neurology , 44(9) : 1583 – 1586 .
- Chiswell , B. and O'Halloran , K. R. 1991 . Comparison of 3 Colorimetric Methods for the Determination of Manganese in Fresh-Waters . Talanta , 38 ( 6 ) : 641 – 647 .
- Crump , K. S. 2000 . Manganese Exposures in Toronto During Use of the Gasoline Additive, Methylcyclopentadienyl Manganese tricarbonyl . J. Expo. Anal. Environ. Epidemiol. , 10 ( 3 ) : 227 – 239 .
- Diem , D. and Stumm , W. 1984 . Is Dissolved Mn-2+ Being Oxidized By O-2 in absence of Mn–Bacteria or Surface Catalysts . Geochim Cosmochim Acta , 48 ( 7 ) : 1571 – 1573 .
- Elder , A. , Gelein , R. , Silva , V. , Feikert , T. , Opanashuk , L. , Carter , J. , Potter , R. , Maynard , A. , Finkelstein , J. and Oberdorster , G. 2006 . Translocation of Inhaled Ultrafine Manganese Oxide Particles to the Central Nervous System . Environ. Health Perspect , 114 ( 8 ) : 1172 – 1178 .
- Environment Canada's National Pollutant Release Inventory (NPRI) . 2006 . http://www.ec.gc.ca/pdb/npri/npri_online_data_e.cfm. Accessed December 11, 2006
- Goto , K. , Tsuyoshi , K. and Furukawa , T. 1962 . Rapid Colorimetric Determination of Manganese in Waters Containing Iron—A Modification of the Formaldoxime Method . Anal. Chim. Acta , 27 : 331 – 334 .
- Gounot , A. M. 1994 . Microbial Oxidation and Reduction of Manganese—Consequences in Groundwater and Applications . FEMS Microbiol. Rev , 14 ( 4 ) : 339 – 349 .
- Graber , E. R. and Rudich , Y. 2006 . Atmospheric HULIS: How Humic-Like are They? A Comprehensive and Critical Review . Atmos. Chem. Phys , 6 : 729 – 753 .
- Gwiazda , R. H. , Lee , D. , Sheridan , J. and Smith , D. R. 2002 . Low Cumulative Manganese Exposure Affects Striatal GABA but not Dopamine . Neurotoxicol , 23 ( 1 ) : 69 – 76 .
- Heilbronn , E. and Eriksson , H. 1997 . “ Implications of Manganese in Disease, Especially Central Nervous System Disorders ” . In Mineral and Metal Neurotoxicology , Boston : CRC Press .
- Kargosha , K. and Noroozifar , M. 2003 . Flow Injection Speciation Analysis of Manganese in Real Samples by Diphenylcarbazide-Spectrophotometric Determination . Turkish J. Chem. , 27 ( 2 ) : 227 – 233 .
- Kessick , M. A. and Morgan , J. J. 1975 . Mechanism of Autoxidation of Manganese in Aqueous-Solution . Environ. Sci. Technol , 9 ( 2 ) : 157 – 159 .
- Kessick , M. A. , Vuceta , J. and Morgan , J. J. 1972 . Spectrophotometric Determination of Oxidized Manganese with Leuco Crystal Violet . Environ. Sci. Technol. , 6 ( 7 ) : 642 – 644 .
- Kidwell , C. B. and Ondov , J. M. 2004 . Elemental Analysis of Sub-Hourly Ambient Aerosol Collections . Aerosol Sci. Technol , 38 ( 3 ) : 205 – 218 .
- Klewicki , J. K. and Morgan , J. J. 1998 . Kinetic Behavior of Mn(III) Complexes of Pyrophosphate, EDTA, and Citrate . Environ. Sci. Technol , 32 : 2916 – 2922 .
- Klewicki , J. K. and Morgan , J. J. 1999 . Dissolution of Beta-MnOOH Particles by Ligands: Pyrophosphate, Ethylenediaminetetraacetate, and Citrate . Geochim. Cosmochim. Acta , 63 ( 19–20 ) : 3017 – 3024 .
- Lundborg , M. , Lind , B. and Camner , P. 1984 . Ability of Rabbit Alveolar Macrophages To Dissolve Metals . Exp. Lung Res. , 7 ( 1 ) : 11 – 22 .
- Majestic , B. J. , Schauer , J. J. and Shafer , M. M. 2007 . Application of Synchrotron Radiation for Measurement of Iron Red-Ox Speciation in Atmospherically Processed Aerosols . Atmos. Chem. Phys , 7 : 2475 – 2487 .
- Majestic , B. J. , Schauer , J. J. , Shafer , M. M. , Turner , J. R. , Fine , P. M. , Singh , M. and Sioutas , C. 2006 . Development of a Wet-Chemical Method for the Speciation of Iron in Atmospheric Aerosols . Environ. Sci. Technol. , 40 ( 7 ) : 2346 – 2351 .
- Mergler , D. and Baldwin , M. 1997 . Early Manifestations of Manganese Neurotoxicity in Humans: An Update . Environ. Res , 73 ( 1–2 ) : 92 – 100 .
- Misra , C. , Singh , M. , Shen , S. , Sioutas , C. and Hall , P. A. 2002 . Development and Evaluation of a Personal Cascade Impactor Sampler (PCIS) . J. Aerosol Sci , 33 ( 7 ) : 1027 – 1047 .
- Morgan , J. J. 2000 . Manganese in Natural Waters and Earth's Crust: Its Availability to Organisms . Metal Ions in Biological Systems , 37 : 1 – 34 .
- Morgan , J. J. 2005 . Kinetics of Reaction between O–2 and Mn(II) Species in Aqueous Solutions . Geochim. Cosmochim. Acta. , 69 ( 1 ) : 35 – 48 .
- Morgan , J. J. and Stumm , W. 1965 . “ Analytical Chemistry of Aqueous Manganese ” . In J. Amer. Water Works Assoc 107 – 119 .
- Nico , P. S. , Anastasio , C. and Zasoski , R. J. 2002 . Rapid Photo-Oxidation of Mn(II) Mediated by Humic Substances . Geochim. Cosmochim. Acta , 66 ( 23 ) : 4047 – 4056 .
- Nico , P. S. and Zasoski , R. J. 2001 . Mn (III) Center Availability as a Rate Controlling Factor in the Oxidation of Phenol and Sulfide on Delta–MnO2 . Environ. Sci. Technol , 35 ( 16 ) : 3338 – 3343 .
- Nonova , D. and Evtimova , B. 1973 . Spectrophotometric Study and Analytical Application of Reaction Between Manganese(II) and 4-(2-Pyridylazo)Resorcinol . Talanta , 20 ( 12 ) : 1347 – 1351 .
- Reaney , S. H. , Kwik-Uribe , C. L. and Smith , D. R. 2002 . Manganese Oxidation State and Its Implications for Toxicity . Chem. Res. Toxicol , 15 ( 9 ) : 1119 – 1126 .
- Ressler , T. , Wong , J. , Roos , J. and Smith , I. L. 2000 . Quantitative Speciation of Mn–Bearing Particulates Emitted from Autos Burning (Methylcyclopentadienyl)Manganese Tricarbonyl–Added Gasolines Using XANES Spectroscopy . Environ. Sci. Technol , 34 ( 6 ) : 950 – 958 .
- Schauer , J. J. , Lough , G. C. , Shafer , M. M. , Christensen , W. F. , Arndt , M. F. , DeMinter , J. T. and Park , J. 2006 . Characterization of Metals Emitted from Motor Vehicles . Health Effects Institute , 133
- Scott , D. T. , McKnight , D. M. , Voelker , B. M. and Hrncir , D. C. 2002 . Redox Processes Controlling Manganese Fate and Transport in a Mountain Stream . Environ. Sci. Technol , 36 ( 3 ) : 453 – 459 .
- Siefert , R. L. , Johansen , A. M. , Hoffmann , M. R. and Pehkonen , S. O. 1998 . Measurements of Trace Metal (Fe, Cu, Mn, Cr) Oxidation States in Fog and Stratus Clouds . J. Air Waste Manage. Assoc , 48 ( 2 ) : 128 – 143 .
- Singh , M. , Misra , C. and Sioutas , C. 2003 . Field Evaluation of a Personal Cascade Impactor Sampler (PCIS) . Atmos. Environ. , 37 ( 34 ) : 4781 – 4793 .
- Stone , A. T. and Morgan , J. J. 1984a . Reduction and Dissolution of Manganese(III) and Manganese(IV) Oxides by Organics. 1. Reaction with Hydroquinone . Environ. Sci. Technol , 18 ( 6 ) : 450 – 456 .
- Stone , A. T. and Morgan , J. J. 1984b . Reduction and Dissolution of Manganese(III) and Manganese(IV) Oxides by Organics. 2. Survey of the Reactivity of Organics . Environ. Sci. Technol , 18 ( 8 ) : 617 – 624 .
- Stookey , L. L. 1970 . Ferrozine—A New Spectrophotometric Reagent for Iron . Anal. Chem , 42 ( 7 ) : 779 – 781 .
- Stumm , W. and Morgan , J. J. 1981 . Aquatic Chemistry , New York : John Wiley & Sons .
- Sun , G. B. , Crissman , K. , Norwood , J. , Richards , J. , Slade , R. and Hatch , G. E. 2001 . Oxidative interactions of synthetic lung epithelial lining fluid with metal-containing particulate matter . Am. J. Physiol. Lung Cell. Mol. Physiol , 281 ( 4 ) : L807 – L815 .
- Sunda , W. G. and Huntsman , S. A. 1994 . Photoreduction of Manganese Oxides in Seawater . Mar. Chem. , 46 ( 1–2 ) : 133 – 152 .
- Thomassen , Y. , Ellingsen , D. G. , Hetland , S. and Sand , G. 2001 . Chemical speciation and sequential extraction of Mn in workroom aerosols: Analytical methodology and results from a field study in Mn alloy plants . J. Environ. Monit , 3 ( 6 ) : 555 – 559 .
- United States Environmental Protection Agency (USEPA) . 1995 . Test Methods for Evaluating Solid Waste Physical/Chemical Methods Vol. 1A , 846 SW .
- Ure , A. M. 1990 . “ Methods of Analysis for Heavy Metals in Soils ” . In Heavy Metals in Soils , Edited by: B. , J. Alloway . 40 – 73 . Glasgow : Blackie and Son .
- Valavanidis , A. , Fiotakis , K. , Bakeas , E. and Vlahogianni , T. 2005 . Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter . Redox Report , 10 ( 1 ) : 37 – 51 .
- Villinski , J. E. , O'Day , P. A. , Corley , T. L. and Conklin , M. H. 2001 . In situ spectroscopic and solution analyses of the reductive dissolution of MnO2 by Fe(II) . Environ. Sci. Technol. , 35 ( 6 ) : 1157 – 1163 .
- Villinski , J. E. , Saiers , J. E. and Conklin , M. H. 2003 . The effects of reaction-product formation on the reductive dissolution of MnO2 by Fe(II) . Environ. Sci. Technol , 37 ( 24 ) : 5589 – 5596 .
- Witholt , R. , Gwiazda , R. H. and Smith , D. R. 2000 . The neurobehavioral effects of subchronic manganese exposure in the presence and absence of pre-parkinsonism . Neurotoxicol. Teratol , 22 ( 6 ) : 851 – 861 .