Abstract
A Particle-Into-Liquid Sampler (PILS) was modified and coupled with a Total Organic Carbon (TOC) Analyzer (Sievers 800T, GE Water Systems, Boulder, CO), in an attempt to measure particulate organic carbon (OC) online. The PILS droplet collection system was changed from an inertial impactor to a miniature cyclone to increase the efficiency of transferring insoluble carbonaceous aerosol to the liquid sample stream. The performance of the modified PILS was investigated with a variety of calibration aerosols through comparison with the Sunset Labs ECOC technique (NIOSH method 5040). Linear regression slopes of water-soluble organic compounds compared well with Sunset Labs measurement, agreeing to within 5%. However, a size dependence was observed when comparing insoluble carbonaceous aerosol (polystyrene latex spheres, PSL). The new method did not effectively measure insoluble particles with aerodynamic diameters greater than ∼ 110 nm due to inefficient analysis by the TOC. The OC measurement method was also compared with online Sunset Labs organic carbon (OC) measurements in two urban locations: Atlanta, GA, and Riverside, CA. Linear regression slopes between the PILS technique and Sunset Labs were near unity (101% to 93% ± 2 and 5%, respectively), and not statistically different from unity considering the measurement uncertainty of each method. However there was a significant (0.6 to 1.7 μ gC m − 3 ) non-zero intercept, with the Sunset Labs instrument measuring higher concentrations, possible due to the inability of the PILS to measure large, insoluble particles or positive artifacts with the non-blank corrected Sunset Labs filter-based collection method.
1. INTRODUCTION
Organic carbon (OC) represents one of the least understood fractions of atmospheric aerosols (CitationFuzzi et al. 2006; CitationKanakidou et al. 2005; CitationNovakov et al. 1997). However, since it typically comprises a large fraction of fine particle mass, it may play a role in climate (CitationIPCC 2007) by directly and indirectly affecting planetary radiation balance and may have effects on human and ecological health (CitationPope 2000; CitationThurston et al. 2005). OC aerosol is formed through both primary emissions and secondary processes from combustion, biogenic emissions, and other sources. Because it is chemically complex, quantitative measurements of OC are challenging.
Filter-based samples are a common method of OC measurement and typically require capturing aerosol on pre-baked quartz filters over substantial (∼ 1–24 hours) integration times (CitationSubramanian et al. 2004; CitationTurpin et al. 1994). The organic compounds may be extracted by solvents, such as purified water, hexane, benzene, etc, followed by various analytical methods, including UV/chemical oxidation, gas chromatography/mass spectroscopy, and ion chromatography (CitationSullivan and Weber 2006a, b; CitationSullivan et al. 2004; CitationZappoli et al. 1999; CitationZheng et al. 2002).
Analysis of carbonaceous material on filter media can also be determined by thermal-optical techniques following the protocols developed by CitationHuntzicker et al. (1982). This method has become one of the most commonly used approaches for measuring the mass of ambient aerosol carbon. It quantifies the total carbon (e.g., organic and elemental fractions) collected on a quartz filter by heating the filter continuously or in a stepped manner to ∼ 800°C in a non-oxidizing pure helium atmosphere. Organic material is volatilized and detected by Flame Ionization Detection (FID). The sample temperature is typically reduced to ∼ 600°C, and the sample atmosphere is replaced with 2% oxygen (with the balance helium). The temperature is then increased to ∼ 850°C and the remaining carbonaceous material is oxidized and subsequently detected by FID. Finally, the FID signal is internally calibrated with a known quantity of methane.
A limitation to this method is that during the first heating cycle, an unknown fraction of organic carbon may char on the filter and not be immediately detected. During the second heating cycle (in an oxidizing atmosphere), both the pyrolyzed organic material and elemental carbon fraction are evaporated from the filter. To delineate OC from EC, one of two optical techniques is typically used. Thermal-optical reflectance (TOR) determines the EC and OC split (CitationChow et al. 1993) by measuring laser reflectance from the filter surface. Reflectance is diminished by elemental carbon, as well as by any charred organic material that forms during the first heating cycle. As the sample analysis begins, the baseline reflectance of the filter (which is determined only from captured elemental carbon) is determined. The reflectance decreases as the organic material chars during the filter heating. During the second heating cycle (e.g., in an oxidizing atmosphere), this charred material oxidizes and results in increased filter reflectance. When filter reflectance returns to baseline, evolved carbon detected up to this point is considered the organic fraction. The remaining carbon that is detected by FID after this point is considered elemental carbon.
Thermal optical transmittance (TOT) is similar to TOR, except that in this case transmission of a laser beam through the filter is used to monitor concentrations of light absorbing material on the filter. The OC EC split is defined as the point when laser transmittance returns to baseline (CitationBirch 1998). This is used by the Sunset Labs TOT analyzer (Model 3, Sunset Labs, Forest Grove, OR), and is the basis for the National Institute of Occupational Safety and Health method 5040 (CitationNIOSH 1996).
The TOT and TOR methods produce organic and elemental carbon mass that is operationally defined by the method and are generally used for offline analysis of filter samples. Offline filter-based measurements of OC are susceptible to artifacts and limited by their lengthy sample integration time. In contrast, online systems can provide substantially more data that is less likely to be contaminated due to handling, and are often less expensive. Online measurements can be made with a Sunset Labs semi-continuous ECOC instrument (Model 3F, Sunset Labs, Forest Grove, OR), which is similar to the offline TOT analyzer, with a few exceptions. The instrument is configured with an internal filter that captures aerosol and then oxidizes and detects OC and EC with similar temperature and carrier gas profiles and transmittance-based pyrolysis correction. However, it employs a non-dispersed infrared detection (NDIR) system for quantification of evolved CO2 (carbon dioxide). This instrument is used extensively throughout this work and is taken as the standard for comparison with the PILS-based OC measuring technique investigated. The online OCEC detector is described in greater detail in Section 2.
2. INSTRUMENTATION
The Particle-Into-Liquid Sampler (PILS)
The Particle-Into-Liquid Sampler was originally developed for measurement of water-soluble inorganic aerosol components. It operates by increasing the size of particles through condensational growth in a super saturated water vapor environment to sizes easily captured by inertial methods. The droplets formed are focused by a nozzle and deposited at the center of a polycarbonate impaction plate. A film of water with dissolved aerosol components spreads from the center of the impaction plate to its edges, where a stainless steel mesh wicks the liquid from the polycarbonate impaction plate to a 0.508 mm (0.02 inch) ID polyetheretherketone (PEEK) collection tube. The mesh wick is continually washed with a stream of ultra pure deionized water injected at a known flow rate opposite to the sample collection point. Liquid sample collected from the impaction plate passes through a glass debubbler to remove entrained air bubbles, and is drawn by two glass syringes (Kloehn Ltd, Las Vegas, NV) that pump in an alternating tandem configuration to provide a smooth, continuous flow. The smallest possible syringe volumes were chosen (typically 500 ml or less) in order to minimize any potential mixing effects in the sample (CitationSorooshian et al. 2006). These syringes then force the liquid sample through a 0.22 μ m pore polypropylene filter and deliver it to a detector. A Sievers Total Organic Carbon (TOC) analyzer (Model 800T, Boulder, CO) has been used to measure the water-soluble organic components of the ambient aerosol. This instrument will be referred to here as a PILS-WSOC.
For carbon analysis, the Sievers TOC analyzer uses a combination of 25% ammonium persulfate solution (flow rate = 0.75 μ l min− 1) and ultraviolet light (λ = 254 nm and 184 nm) to oxidize the dissolved organics in the liquid sample to CO2. The sample is acidified by adding 6M phosphoric acid (pH typically < 2). The sample is split into two equal flows. One sample passes through a delay coil and into the CO2 detection sensor which measures the concentration of total inorganic carbon (TIC). The second sample passes through an ultraviolet light oxidation reactor, which photochemically oxidizes the organic compounds to form CO2. The sample is then pumped through a second CO2 sensor which detects total carbon in the sample (TC). The CO2 is isolated through a selective membrane into a high-purity water loop and is quantified by conductometric detection (CitationCarlson 1980). Thus, total organic carbon (TOC) is determined by difference between the two measurements (e.g., TC-TIC = TOC). The Sievers TOC analyzer can be operated in either a 3-s or 6-min sample integration mode.
During PILS-WSOC operation, ambient aerosol sample is periodically diverted through a Teflon filter by an inline computer-actuated valve. This removes entrained aerosol and allows for an assessment of any background interference from organic material. Possible sources of background interferences include captured semi volatile organic carbon (SVOC) that penetrated the denuder, or contamination of the ultra purified water used in the analysis. A background concentration is linearly interpolated between consecutive background measurements and is subtracted from the dataset. Further discussion of the specific operational techniques, including a discussion of possible background artifacts, can be found in CitationSullivan et al. (2004). From previous studies, the PILS-WSOC uncertainty is estimated at 8% and a limit of detection of 0.1 μ g Carbon m− 3 (μ gC m− 3).
A consequence of using an impaction plate to collect droplets is that while not likely having an effect on soluble compounds, insoluble compounds may adhere to the surface and not be efficiently transferred to the liquid stream. In ambient urban samples, the impactor visibly darkens over time due to the adhesion of elemental carbon. Thus, it is reasonable to expect some fraction of insoluble organic carbon to also adhere to the impactor. The inline 0.22 μ m liquid filter also inhibits measurements of insoluble organic particulates. Recent data from a ground site in Atlanta GA, and St. Louis, MO shows that typically the WSOC fraction of OC is 40–80% (CitationSullivan and Weber 2006a; CitationSullivan et al. 2004), with the remaining fraction likely being the insoluble fraction. During short events when local fresh emissions dominate, the WSOC/OC fraction can be considerably lower.
Attempts have already been made to measure total OC semi-continuously with a technique that in principle is similar to the PILS. Like the PILS, the steam-jet aerosol collector (SJAC) (CitationKhlystov et al. 1995) was originally designed for inorganic aerosol measurement. After mixing aerosol with a supersaturated water vapor, particles are grown in size and collected. Rather than using an impactor to collect particles, two cyclones are used in series to achieve a sampling collection efficiency of greater than 99%. Coupled to a Total Organic Carbon analyzer, CitationEven et al. (2000) tested the SJAC performance with various soluble and insoluble organic aerosols, however, the instrument was never field tested. Their results are further discussed in this article.
The Sunset Labs ECOC Analyzer
A Sunset Labs ECOC analyzer (Model 3F, Forest Grove, OR) was used for comparison in this study. Following NIOSH method 5040 (CitationBirch 1998; CitationNIOSH 1996), this instrument semi-continuously quantifies organic carbon that is deposited on an internal filter. The sample is denuded of volatile gases by an inline parallel plate carbon denuder (CitationEatough et al. 1993). The Sunset Labs ECOC analyzer is not equipped for automated blank measurement and correction. In recent work from St Louis, MO, CitationBae et al. (2004) estimated the Sunset Labs blank concentration of organic carbon at ∼ 0.94 ± 0.09 μ gC m− 3 by comparing two online systems with a 24-h integrated filter. To account for any possible positive sampling artifacts, CitationLim et al. (2003) used two Sunset Lab ECOC instruments in parallel configuration, simultaneously measuring ambient aerosol and a Teflon-filtered sample that collected SVOC only. The ambient carbonaceous particulate fraction is calculated from the difference. With this method, they report a limit of detection (LOD) of 0.3 μ gC m− 3 for a one-hour integrated measurement. CitationTakegawa et al. (2005) empirically determined an LOD of approximately 1.0 μ gC m− 3, for an instrument with no blank correction by comparison with an Aerosol Mass Spectrometer (Aerodyne, Billerica, MA) which had an approximate collection efficiency of 0.5. Using the commercially available instrument in this study, we estimate an instrument LOD of 0.5 μ gC m− 3, though this is somewhat uncertain due to the limited number of studies that reported limits of detection for the online Sunset Labs analyzer. The instrument was configured to collect sample for 45 minutes. Thus, higher time resolution organic carbon data from the PILS technique was averaged to this interval. Most research examining OC measurement technique uncertainty has focused on the offline method of TOT and TOR analysis (CitationBae et al. 2004; CitationHuebert et al. 2004; CitationLim et al. 2003; CitationSchauer et al. 2003; CitationTurpin et al. 1990; CitationTurpin et al. 1994; CitationTurpin et al. 2000). Offline TOT method analyzer uncertainty is typically in the range of 5–20% (depending on OC concentration, with higher uncertainty at lower concentrations). CitationHuebert et al. (2004) estimate combined uncertainty (due to flow rates, sample handling, and analyzer) at 26%. Uncertainty in the Sunset Labs online instrument has not been clearly defined by the literature. For this work, overall uncertainty in the online version of the Sunset Labs OC analyzer is estimated at ± 20%.
This article will discuss some of the key design changes made to the PILS-WSOC for OC measurement, assess performance when measuring calibration aerosol generated under controlled conditions, as well as discuss comparison with the Sunset OCEC measurement from two urban deployments.
3. INSTRUMENT DESIGN
Construction of a Mini-Cyclone
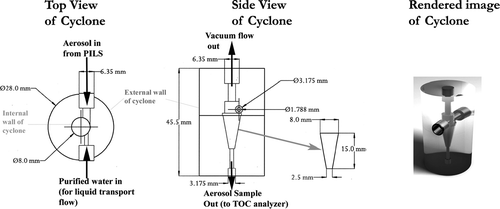
Following the approach of CitationEven et al. (2000) to improve transfer of insoluble OC to sample liquid, the PILS impactor was replaced with a mini cyclone. The cyclone was designed following the criteria of CitationGussman et al. (2002) and CitationKenny et al. (2000), and was constructed of polycarbonate with internal dimensions of approximately 23 mm total internal height, 8 mm maximum width that tapers to ∼ 2 mm diameter over 15 mm (see ). Vacuum flow is achieved by way of a 1/8″ OD stainless steel tube that is inserted through the top of the cyclone, connected to a critical orifice of known diameter, and then to a vacuum pump. To collect aerosol droplets, a 1/4′ scriptstyle 4” OD (1/8′ ID) stainless steel tube was press fit into the end of the standard PILS impactor nozzle, and inserted into the mini cyclone tangent to the internal wall, as shown in . The nozzle opening was positioned directly across from a small port that continually delivered 0.6 ml min− 1 of purified liquid water (typical resistivity: 18.2 Mohm-cm) into the cyclone. The liquid flow rate was the same as the transport flow of water introduced at the top of the impaction plate in the traditionally configured PILS (CitationSullivan et al. 2006). This liquid creates a small, continuous ribbon of purified water and entrained aerosol as it descends the inner wall of the cyclone until reaching the bottom, where water and particles were extracted and transported to the analyzer via syringe pumps. The inline liquid filter normally present on a PILS-WSOC instrument was removed and liquid sample lines shortened to minimize any OC loss to the tubing wall during transport to the detector. Loss of insoluble particles is quantified in the next section. The advantage of this method is that aerosol is not impacted directly onto a plate and is less likely to adhere to the surface. This modified instrument is referred to as a PILS-OC.
FIG 1 Schematic and rendered image of PILS-OC mini cyclone. On left, a top view of the minicyclone including basic dimensions and aerosol and liquid transport flow injection points. In center, a side profile view of mini cyclone, including main dimensions. Vacuum flow out and aerosol sample out are also depicted. The internal dimensions of the cyclone are present to the immediate right of middle figure. A computer rendered image of the mini cyclone is shown on the right and is oriented with the top of the cyclone at the top of the image.
4. LABORATORY CALIBRATIONS
Carbonaceous aerosol was generated using an atomizer and compressed nitrogen. Approximately 0.02 grams carbon of various organic compounds (see ) were added to 200 ml
TABLE 1 Summary of compounds tested for technique comparison. The table also includes molecular formula, vapor pressure of compound, and solubility in water
of purified (> 18 Mohm-cm) water, making a ∼ 0.1% carbon solution. Following the atomizer, water was removed from the aerosol via silica diffusion driers and aerosol neutralized by a polonium 210 ionization source. The dried and neutralized particles were then diluted in a 4 liter glass jar with room air, which had been filtered using a High-Efficiency Particulate Air filter and denuded by an activated carbon parallel-plate denuder (CitationEatough et al. 1993). The diluted aerosol was passed through a second parallel plate carbon denuder to remove any residual organic vapors. An inline, non-rotating MOUDI impactor (CitationMarple et al. 1991) restricted particle size to less than 1.0 μ m (except in the case of 1.96 μ m polystyrene latex aerosol, when no impactor was inline). Finally, the sample flow was split to the PILS and Sunset Labs instrument. After the split, flow tubing material, diameter, and length were nearly identical. This resulted in aerosol concentrations delivered to each instrument of approximately 10–20 μ g C m− 3.
Assessment of Mini-Cyclone Collection Efficiency
In order to assess cyclone collection efficiency, a comparative analysis between the cyclone (PILS-OC), impactor (PILS-WSOC), and Sunset Labs OC measurements were conducted. Laboratory-generated, polydispersed oxalate aerosol was simultaneous delivered to each instrument. Oxalate was chosen because it is highly soluble and has a relatively low vapor pressure (CitationCRC 2005). This minimizes the risk of loss by volatilization in either system, and ensures that the PILS systems (which use liquid water to transport dissolved aerosol) is able to quantify the sample. Both results were compared with the Sunset Labs OC measurement.
The ratio of OC determined by PILS-WSOC and Sunset Labs is investigated first. The experiment was repeated six times. By measuring polydispersed oxalate aerosol, the ratio of OC determined by PILS-WSOC compared to the Sunset Labs OC technique was 93.5% ± 1.2% (mean ± 1σ). PILS-WSOC has an instrument uncertainty of 8% (CitationSullivan et al. 2006), suggesting that this difference from unity is not significant.
The PILS impactor was replaced with a mini cyclone and the experiment was repeated. The ratio of oxalate aerosol concentration determined by PILS-OC (e.g., with mini cyclone) compared with the concentration determined by Sunset Labs OC was 69.3% ± 1.7% (1 σ) which is significantly lower than the PILS-WSOC/Sunset Labs ratio. The comparison was repeated 8 times. Since the instrument configuration for each experiment was identical (with the exception of using a mini cyclone instead of the impactor on the PILS), this difference is assumed to be due to a lower collection efficiency for the mini cyclone. Because the cyclone system has a collection efficiency of 69.3% ± 1.7% (1 σ) compared to the Sunset Labs method, this factor has been applied to the data throughout the remainder of this paper. Since the PILS-OC method is similar to the PILS-WSOC method (with the exception of using a mini cyclone for droplet collection), combined uncertainty (e.g., uncertainty associated with the PILS-WSOC plus the standard deviation of the collection efficiency) of the PILS-OC instrument is estimated to be similar to PILS-WSOC at ± 10%.
Tests with Calibration Aerosol
A variety of organic carbon aerosol was generated from single standard solutions and sampled using the PILS with mini cyclone and TOC detection. Several selected organic compounds that varied in molecular weight, vapor pressure, and solubility in water were tested. These compounds were chosen based partly on those described by CitationSaxena and Hildemann (1996) and are listed in .
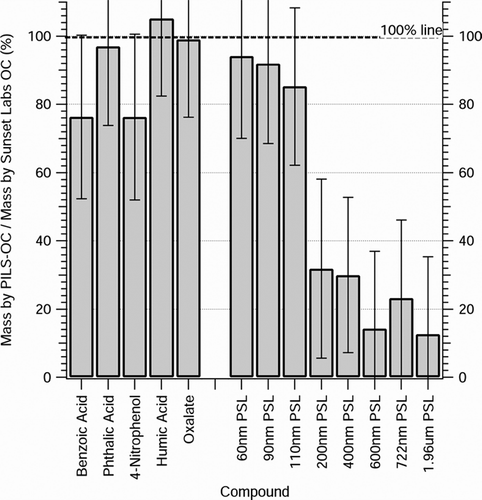
The fraction of aerosol measured by PILS-OC compared with Sunset Labs is shown in for the detected compounds. Oxalate, phthalic acid, and humic acid aerosol compared well, with agreement between the two instruments exceeding 95%. Benzoic acid and 4-nitrophenol aerosol was somewhat lower; PILS-OC typically measured ∼ 76% of the mass when compared to Sunset Labs, though the error bars () span 100%. Elemental carbon (EC) aerosol was generated in a similar manner as the dissolved organic compounds, with one exception: the atomizer was submerged in an ultrasonic bath in order to distribute the insoluble EC throughout the water matrix prior to atomization. Elemental carbon (EC) was not detected by the PILS-cyclone system with Sievers TOC analyzer. Elemental carbon calibration aerosol was, however, detected by the Sunset Labs analyzer in the form of EC implying that EC aerosol was successfully delivered to the instruments. Error bars are plotted for each compound and are calculated as the sum of squares of repeated measurement standard deviation (e.g., precision), and estimated instrument uncertainty in the PILS-OC and Sunset Labs analyzer.
FIG 2 Recovery fractions of various aerosols by PILS-OC when compared to Sunset Labs OC method. Water-soluble compounds are on the left side of figure, while insoluble compounds are on the right side of the figure. Error bars are quadratic sum of squares of propagated error including standard deviations of at least 8 repeated measurements and instrument uncertainty.
Similar findings were reported by CitationEven et al. (2000), who tested a variety of organic compound standards in solution (e.g., not generated as aerosol) by measuring with a Shimadzu TOC analyzer and Sievers TOC analyzer. The Shimadzu analyzer is a combustion-based catalytic oxidation method that efficiently quantifies all carbonaceous material in a liquid sample regardless of its solubility. Even et al. tested benzoic acid and humic acid standards which were equally measured by both the Sievers TOC analyzer and the Shimadzu analyzer (recovery = ∼ 100%). Black carbon was also tested, and was not efficiently recovered (∼ 0%) by the Sievers analyzer, but was detected by the Shimadzu.
To test completely insoluble particles, monodispersed polystyrene latex (PSL) spheres (for all sizes: 10% w/w, Duke Scientific, Palo Alto, CA) were generated in the lab using the technique previously described and to compare measurements of purely insoluble aerosol. For the tested PSL sizes, an apparent size dependence was observed—as particle size increased, the comparison between PILS-OC and Sunset Labs decreased, as shown in . When compared to Sunset Labs, PILS-OC effectively measured insoluble particles that were less than ∼ 110 nm in diameter. For 200 nm monodisperse PSL, PILS-OC measured approximately 31% of the mass that Sunset Labs analyzer detected. This is likely a result of the TOC analyzer's inability to adequately oxidize and detect large, insoluble organic particles. 90 nm and 200 nm PSL spheres in solution were also tested by CitationEven et al. (2000). As compared to the Shimadzu analyzer, the 90 nm spheres were well recovered (∼ 100%), and the 200 nm spheres where poorly recovered (∼ 25%) by the Sievers analyzer, similar to our results. Note that while trace concentrations of water-soluble organic surfactants were present in all PSL solutions, they are likely insignificant when compared to the carbon mass derived from PSL spheres. For example, no carbonaceous material (from PSL or from surfactants) was detected by PILS-OC when testing 1.96 μ m PSL ().
The consequence of the TOC analyzer's inability to quantify solid insoluble particles larger than ∼ 0.11 μ m will vary depending on ambient conditions. Purely insoluble organic aerosol typically originates from fresh combustion processes, and has been shown to be generally less than 150 nm in size (CitationKleeman et al. 2000) (e.g., most insoluble material is likely to be oxidized and detected). Particle growth processes, such as heterogeneous nucleation or coalescing, can produce bulk particles with sizes larger than those that can be detected by this instrument if they were completely insoluble. However, the PILS-OC collection method reduces the size of aerosol by dissolving most of the soluble components within an internally mixed aerosol leaving the remaining insoluble core. Aged aerosol that has undergone photochemical and oxidative chemistry have been shown to be mostly composed of oxygenated, and hence water-soluble, compounds (CitationZhang et al. 2007). Insoluble vegetative detritus, however, can be significantly larger than ∼ 0.11 μ m and so would not be measured by this technique.
In an attempt to measure larger insoluble particles, the Sievers TOC oxidizer flow rate was increased; however, it had little effect on the instrument's measurement efficiency for insoluble particles larger than 200 nm. Doubling the oxidizer flow rate to 1.5 μ l min− 1 had less than 5% improvement when measuring 200 nm PSL aerosol. Compared to Sunset Labs, this corresponds to PILS-OC measuring just ∼ 35% of the mass of 200 nm PSL. Oxidizer flow rates can not be further increased because gas bubbles form within the analyzer's liquid flow system causing erroneous carbon mass concentrations.
To quantify large insoluble OC, a combustion-based analytical technique, such as the Shimadzu TOC-Vcs (Shimadzu Scientific Instruments, Columbia, MD) could be coupled with the PILS instead of the liquid-based oxidation system of the Sievers TOC. Although the Shimadzu is significantly larger and more expensive, it is capable of online analysis with ∼4 minute sample integration times.
Loss to Sample Tubing
Replacing the impactor with a cyclone to capture the large droplets formed in the PILS appeared to improve collection of insoluble particles. The penetration of insoluble particles suspended in liquid while transported from the cyclone to detector was also tested. In order to assess particle loss within the liquid sample transport tubing, various lengths of polyetheretherketone (PEEK) tubing were used to connect the mini cyclone with the syringes used to pump the sample. A 110 nm PSL aerosol was generated and sampled by PILS-OC using various lengths of 0.508 mm (0.02 inch) internal diameter tubing. The longest length tested, 75 cm, resulted in an apparent loss of ∼ 6% of PSL aerosol (compared to Sunset Labs measurement). No detectable loss of large, insoluble particles was observed when tubing length was less than 30 cm. Under normal operating conditions, tubing length is generally less than 15 cm. During this study, the length of tubing from the syringes to the TOC analyzer sample inlet was approximately 13 cm.
Insoluble particles suspended in the sample stream may also be lost at the various plumbing connections, syringe assembly, or internal plumbing within the Sievers analyzer. Since good agreement was observed between the PILS-OC and Sunset Labs when measuring 110 nm PSL (suggesting minimal loss), we estimate these potential losses to be minor.
5. AMBIENT STUDIES
Ambient comparison studies were conducted during field studies in Atlanta GA and Riverside CA to investigate the capabilities of the PILS-OC for measuring urban emissions.
Atlanta, Georgia
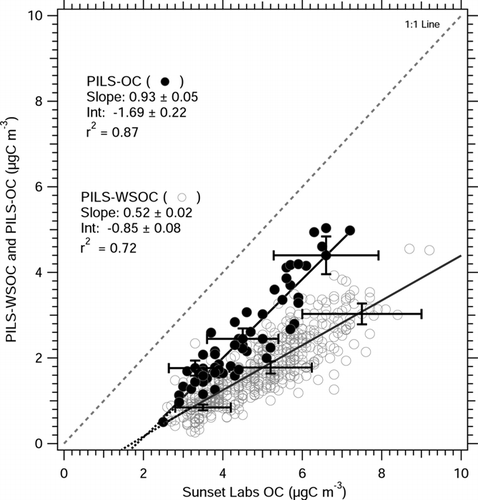
A PILS-WSOC (e.g., water soluble organic carbon system) and a Sunset Labs OC analyzer were co-located at a sampling site in Atlanta, Georgia from 17 April–13 September 2004. The sampling site is located within 400 m of a major highway and may be subjected to relatively fresh—and therefore less soluble—highway (mainly non-diesel) emissions. Heavy duty truck traffic (e.g., diesel powered) is not permitted on the highway so emissions are mainly from gasoline vehicles. Atlanta has been shown to have significant sources of both biogenic and anthropogenic aerosol, with a significant fraction of secondary organic aerosol (CitationLim and Turpin 2002; CitationSullivan and Weber 2006a; CitationWeber et al. 2007). Since secondary organic aerosols tend to be water soluble (CitationKondo et al. 2007), this fraction of organic aerosol can be detected by PILS-WSOC in Atlanta. Median carbon monoxide (CO) concentration in Atlanta during the comparison period was 0.5 ppmv. compares PILS-WSOC measurement of WSOC with Sunset Labs measurement of OC during this period. From the regression slope, WSOC measured by PILS-WSOC (e.g., use of impaction plate) measured approximately 72% ± 1% (1σ) of the organic carbon mass determined by the Sunset Labs instrument. Combined measurement uncertainty and precision for both instruments (10% PILS and 20% Sunset Labs), shown as error bars in , does not account for this difference from unity, thus, the remaining ∼ 28% is likely the insoluble fraction of OC that is not quantified by the PILS-WSOC.
FIG 3 PILS-WSOC (A) and PILS-OC (B) measurement of ambient organic carbon compared with Sunset Labs measured in Atlanta, Georgia. PILS-OC measurements are from 9 May–6 June, 2005. PILS-WSOC measurements are from 17 April–13 September 2004. PILS measurements are averaged to 45-minute integral of Sunset Labs OC measurement. Slope, intercept, and r2 are calculated from univariate linear regression. Sparse error bars are uncertainty associated with the instruments, estimated by sum of squares of instrument uncertainty. For clarity, only a few error bars are included.
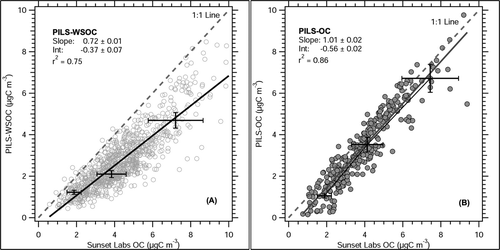
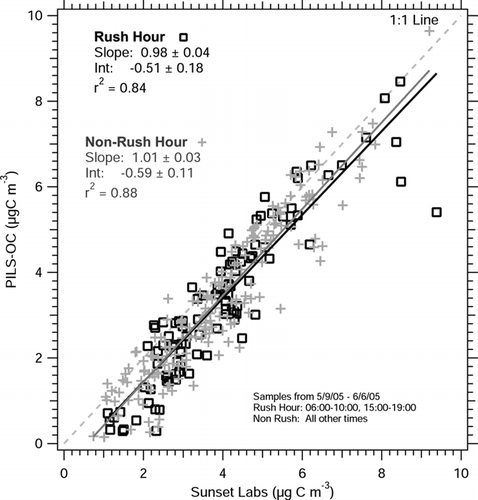
In contrast, a PILS-OC (e.g., with mini cyclone in place of the impactor) was deployed in the same location from 9May–6 June, 2005 using the same inlet and sampling configuration. By univariate linear regression, the PILS-OC measured ∼ 101% (± 2%, 1σ) of the organic carbon compared to Sunset Labs measurement of organic carbon (), a significant difference to PILS-WSOC. Considering the measurement uncertainty and precision of both instruments (included in ), the regression slope for PILS-OC is statistically indistinguishable from unity. PILS-OC also has a higher correlation coefficient (r2 = 0.87) with the Sunset Labs measurement, suggesting that the variability in organic carbon concentration is better captured by the PILS-OC instrument. For both PILS-WSOC and PILS-OC comparisons, a small but significant intercept was observed (). This intercept is discussed in detail in the Regression Intercept section.
In order to examine different aerosol sources, the comparison of PILS-OC and Sunset Labs organic carbon in Atlanta was categorized as rush-hour or non–rush-hour samples (). During this experiment, the median elemental carbon concentrations for both rush hour and non–rush hour periods were similar at 1.0 μ gC m− 3, however, rush hour periods were observed to have higher variability in EC concentration (1σ = 0.9 μ gC m− 3 for rush hour samples, and 1σ = 0.5 μ gC m− 3 for non–rush hour samples) caused by short spikes of EC with concentrations exceeding 2–3 μ gC m− 3. No significant difference is observed in regression slopes or intercepts between the data sets. The intercept in both rush hour and non-rush hour samples are statistically identical (), where the Sunset Labs measures ∼ 0.5 ± 0.1 μ gC m− 3 when the PILS-OC measures zero.
Riverside, California
An additional field deployment was conducted in Riverside, California as part of the Study of Organic Aerosols at Riverside (SOAR) field campaign. Conducted on the campus of the University of California/Riverside from 18 July–15 August, 2005, the sampling location was located approximately 600 meters from a major highway leading into Los Angeles, California. Organic aerosol in this region is thought to be largely primary emissions from condensed organic vapors from mobile sources (Hughes et al. Citation2000; CitationPang et al. 2002; CitationSardar et al. 2005a; CitationSardar et al. 2005b), including mobile sources from the nearby highway, as well as emissions transported from the Los Angeles basin. At times, secondary OC is also expected to be a significant fraction of ambient OC. Elemental carbon, a tracer for mobile source emissions, had distinct peaks during rush hours and often exceeded 3–4 μ gC m− 3, evidence that a significant fraction of sampling was from mobile source emissions. Median (± 1σ) morning rush hour elemental carbon concentrations were 1.5 ± 0.95 μ gC m− 3.
shows a comparison between PILS and Sunset Labs organic carbon measurements in Riverside. By univariate linear regression fit, PILS-WSOC (e.g., impactor) measured approximately 52% ± 2% (1σ) of carbonaceous material as compared to Sunset Labs method. This is lower than the percentage observed in Atlanta by PILS-WSOC and is consistent with a higher fraction of organic aerosol being from insoluble primary emissions. During the Riverside field campaign, median CO concentration was 0.65 ppmv which is ∼ 30% higher than Atlanta during PILS-OC test period in 2004. A larger fraction of insoluble organics were likely present in Riverside due to its proximity to the large highway and significant mobile source emissions from the Los Angeles, CA basin (i.e., higher fraction of primary emissions).
FIG 5 Comparisons between PILS-OC and Sunset Labs and PILS-WSOC and Sunset Labs in Riverside, California. PILS-OC measurements from 27–30 July 2005, and PILS-WSOC measurements are from 22–27 July and 31 July–9 August 2005. Lines through data are univariate regression least-squares fit, and have been extended to the intercept (dashed lines). Sparse error bars are uncertainty associated with the instruments, estimated by sum of squares of instrument uncertainty. For clarity, only a few error bars are included.
The PILS-WSOC impactor was replaced with a mini cyclone for approximately three days (27 July–30 July) near the middle of the measurement campaign, and a comparison with Sunset Labs is also provided in . By univariate linear regression slope, PILS-OC technique measured ∼ 93% ± 5% of the Sunset Labs OC mass, representing an enhancement over the PILS-WSOC method (though slightly less than the slope observed in Atlanta). The correlation coefficient was r2 = 0.87.
As in Atlanta, a significant intercept is observed () when PILS-OC is compared to Sunset Labs OC. By extrapolation, Sunset Labs tended to report approximately ∼ 1.7 μ gC m− 3 when PILS-OC detected ∼ 0 μ gC m− 3. Uncertainty plus precision associated with each measurement (error bars, ) does not explain this intercept. This is discussed further in the following section.
Regression Intercept
The significant intercept detected when comparing the PILS-OC technique with a measurement of OC by Sunset Labs could be caused by a combination of potential biases. Interestingly, the intercept is higher in the urban region with higher primary emissions (CO or EC) and hence higher fractions of insoluble particles. Sampling a significant fraction of large (e.g., > 110 nm) insoluble carbon particles, which are not detected by the PILS-OC method, would result in lower reported OC concentrations by PILS-OC, consistent with the observed intercept. It is also possible there was a positive artifact caused by penetration of SVOCs through the denuder in the Sunset Labs system that were absorbed by the filter and then detected by the analyzer as OC. For the Sunset Labs OC data presented here, instruments blanks were not performed. It is noteworthy that the intercept in the Atlanta study (0.56 μ gC/m3) is similar to the typical blank values reported in St Louis (0.90 ± 0.02 μ g m− 3 by CitationBae et al. (2004)). (Note that Atlanta and St. Louis emissions are more similar than Los Angeles versus St Louis.) To further investigate possible causes for the intercepts, the Sunset Labs measurements of OC were compared with an online measurement of aerosol mass.
In Atlanta, PM2.5 mass was measured by the State of Georgia Environmental Protection Division (GAEPD) with a Tapered Element Oscillating Microbalance (TEOM, model 1400ab, Thermo-Electron) located approximately 15 km to the southeast of our PILS-OC and Sunset Labs OC instruments. The GAEPD TEOM operates with a case temperature of 30°C, and utilizes a Nafion drier system to remove water vapor from the sample (resulting RH = ∼ 15%). It is noted that this configuration may result in loss of semi-volatile compounds.
For data collected throughout the summer of 2004, a regression intercept of 2.0 ± 0.04 μ gC m− 3 (r2 = 0.58) was observed when comparing a Sunset Labs OC measurement with the GAEPD TEOM (not plotted, i.e., ∼ 2 μ gC m− 3 of OC at zero mass). In May 2005 (the period of PILS-OC and Sunset OC comparison), the Sunset Labs instrument had an intercept of 1.4 ± 0.12 μ gC m− 3 (r2 = 0.66) compared to the TEOM. In contrast, the PILS-OC, when compared to the TEOM during this same period in May, had an intercept of 0.17 ± 0.11 μ gC m− 3. Furthermore, the PILS-WSOC (e.g., water-soluble fraction of OC) had an intercept of 0.66 ± 0.06 μ gC m− 3 when compared to the TEOM during summer 2004, which is a factor of three times lower than Sunset Labs during the same time period (when the OC intercept was 2.0 μ gC m− 3). Since the instruments were not collocated (and thus at times possibly not sampling identical sources), there is some scatter throughout the data. These results suggest that the Sunset Labs—TEOM intercept might be caused by two possibilities. First, there may be substantial volatilization of aerosol within the TEOM that results in decreased observed aerosol mass. However, this also requires a significant and nearly equal amount of undetected OC by the PILS-OC since this system was more consistent with TEOM (intercept closer to 0 μ gC m− 3). Or second, there could have been a positive artifact associated with the Sunset Labs OC measurement caused by SVOC penetration through the denuder, and unlike the PILS-OC had no instrument blank correction. A combination of these various factors is possible, however, both PILS-OC and TEOM comparisons to Sunset Labs OC are consistent with a non-zero Sunset OC blank.
Similar to the analysis in Atlanta, the Riverside Sunset Labs and PILS-OC measurements were compared with a PM2.5 mass measurement, in this case a beta attenuation monitor (BAM-1020, Met-One, Grants Pass, OR) operated by the California Air Resources Board during the co-sampling period (28–30 July 2005). The BAM site was located at Rubidoux-Riverside, CA, ∼ 8 km from the Riverside OC sampling site. When the BAM sample relative humidity exceeded 55%, the sample was heated to ∼ 3° above ambient temperature to avoid condensed water on the filter tape. With slight heating, it is possible for semi-volatile particle loss. By linear regression, the Riverside Sunset Labs OC measurement had an intercept of 2.49 ± 0.33 μ gC m− 3 compared to the BAM measurement, as shown in . In contrast, the intercept is ∼ 0.4 ± 0.3 μ gC m− 3 in the PILS-OC measurement. Like the Atlanta study, the instruments were not collocated and in this case there is significant scatter throughout the data (r2 = 0.42). However, the results are consistent with the Atlanta comparison; Sunset Labs intercept is significantly larger than that of the PILS-OC, despite similar regression slopes and r statistics. Similar trends have also been observed in other studies. Although not plotted, a linear regression between a Sunset Labs analyzer (with activated carbon denuder) and a co-located TEOM (inlet heated to 35°C and utilizing a Nafion drier) at a site ∼ 20 km to the north-northeast of Mexico City had an intercept of 3.62 ± 0.16 μ gC m− 3 (r2 = 0.40) (unpublished data).
FIG 6 PILS-OC and Sunset Labs OC measurements of organic carbon compared to a beta attenuation monitor in Riverside, California. Sample period was 28–30 July 2005.
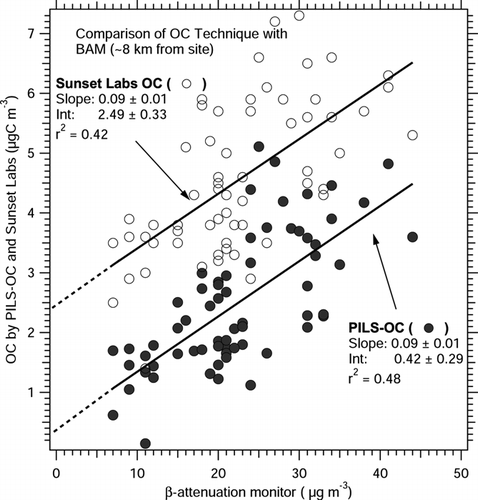
It is not possible with this data to conclusively specify what caused the intercepts between the various measurements. A systematic difference between Sunset Labs and bulk aerosol mass could be due to a positive Sunset OC artifact or negative artifact from in the PM measurement. Some studies suggest online mass measurements with the TEOM are subject to substantial loss of semi-volatile mass (CitationWilson et al. 2006), and other studies show positive artifacts associated with the online Sunset Labs OC measurement (CitationBae et al. 2004). Intercepts between Sunset OC and PILS-OC can also be due to one or more factors; positive artifacts associated with the Sunset Labs OC measurement, or under measurement of OC by the PILS-OC due to inability to measure insoluble particles larger than 0.1 μ m diameter. Overall, the most consistent explanation between all the measurements is a positive bias in OC measured by the Sunset Labs instrument.
6. CONCLUSIONS
Replacement of the PILS impactor with a mini cyclone appears to increase collection efficiency of small, insoluble particles. When corrected for a measured 69% droplet collection efficiency and coupled with a Sievers TOC analyzer for carbonaceous aerosol detection, linear regression slopes of 1.01 and 0.93 were observed when compared with a Sunset Labs OC measurement in Atlanta and Los Angeles, respectively. Considering the combined measurement error, the results are statistically not different from one. The PILS-OC measurements are significantly different then the measurement of only water-soluble organic carbon (WSOC) made with a standard PILS using inertial impaction for droplet collection. PILS-WSOC—Sunset Labs OC regression slopes were 0.72 and 0.52 in Atlanta and Los Angeles, consistent with an expected lower fraction of secondary organic aerosol (e.g., WSOC) in Los Angeles. However, there are limitations. A size dependence on insoluble particles was observed. Calibration with purely insoluble PSL spheres showed that particles greater than approximately 110 nm are not efficiently quantified by the Sievers TOC analyzer. This results in a poor or no detection of large, insoluble organic particles by this technique. Alternate methods of carbon detection, such as combustion techniques, could be used to solve this limitation, but such methods are generally not as fast as the Sievers analyzer and are significantly more expensive.
The PILS-OC technique was deployed in Atlanta, Georgia and Riverside, California to measure ambient aerosol carbon and was compared to OC determined by a Sunset Labs instrument. By linear regression slope (± 1σ), the PILS-OC measured ∼ 101% ± 2% of OC in Atlanta, and ∼ 93% ± 5% of OC in Riverside, not statistically different from one considering the combined uncertainties associated with each measurement (10% PILS-OC, 20% Sunset OC). The measurements were also highly correlated in each location (r2 = 0.86, r2 = 0.87, respectively). However, in both cases, a significant positive intercept was observed, where the Sunset OC measurement was significantly higher than PILS-OC. Both measurements were also compared to bulk aerosol mass measurements. At zero mass, the PILS-OC intercept was small whereas the Sunset Labs OC intercept was significantly larger than zero, with a larger intercept in regions of higher mobile source emissions. The observed intercepts could be associated with a number of causes, including the PILS-OC method's inability to adequately detect large insoluble OC, negative artifacts associated with online mass measurements, and positive artifacts with the online Sunset Labs OC measurement. However, for all comparisons a consistent but uncertain explanation for a significant portion of the intercept is a positive bias due to absorption and analysis of SVOCs by the Sunset Labs online instrument.
Acknowledgments
now at: Nelson Institute of Environmental Medicine, New York University School of Medicine New York
We gratefully acknowledge Jose-Luis Jimenez and Ken Docherty from the University of Colorado—Boulder, and Paul Ziemann from University of California—Riverside for assistance with site preparation and the invitation to participate in the Study for Organic Aerosol Research. We also acknowledge Jamie Schauer and David Snyder from the University of Wisconsin—Madison for providing online EC and OC data from the Riverside campaign. Funding sources for this study include California Air Resources Board Contract No. 98-316.
Notes
†Vapor pressure at 25°C;
‡ Humic acid is a complex carbonaceous material found in soils and natural water. It is considered to be water-soluble and have a low vapor pressure. A specific molecular formula is not available. For additional information, consult the International Humic Substances Society at http://www.ihss.gatech.edu.; a Elemental carbon as Carbon Black, Alfa Aesar, Stock #39724.
REFERENCES
- Bae , M. S. , Schauer , J. J. , DeMinter , J. T. , Turner , J. R. , Smith , D. and Cary , R. A. 2004 . Validation of a Semi-Continuous Instrument for Elemental Carbon and Organic Carbon Using a Thermal-Optical Method . Atmos. Environ. , 38 : 2885 – 2893 .
- Birch , M. E. 1998 . Analysis of Carbonaceous Aerosols: Interlaboratory Comparison . Analyst , 123 : 851 – 857 .
- Carlson , R. M. 1980 . Method and Apparatus for the Determination of Volatile Electrolytes #4,209,299. licensed by Sievers, USA
- Chow , J. C. , Watson , J. G. , Pritchett , L. C. , Pierson , W. R. , Frazier , C. A. and Purcell , R. G. 1993 . DRI Thermal/Optical Reflectance Carbon Analysis System: Description, Evaluation and Applications in U.S. Air Quality Studies . Atmos. Environ., Part A: General Topics , 27A : 1185 – 1201 .
- CRC . 2005 . Handbook of Chemistry and Physics , Cleveland, OH : Chemical Rubber Pub. Co. .
- Eatough , D. J. , Wadsworth , A. , Eatough , D. A. , Crawford , J. W. , Hansen , L. D. and Lewis , E. A. 1993 . Multiple-System, Multi-Channel Diffusion Denuder Sampler for the Determination of Fine-Particulate Organic Material in the Atmosphere . Atmos. Environ., Part A: General Topics , 27A : 1213 – 1219 .
- Even , A. , Kok , J. , Bakker , F. P. and Slanina , J. S. 2000 . “ Online Analysis of Organic Aerosol Samples—A Contribution to Subproject ‘AEROSOL’ ” . In EUROTRAC-2 Symposium , Edited by: Midgley , P. M. , Reuther , M. and Williams , M. Germany : Garmisch Partenkirchen .
- Fuzzi , S. , Andreae , M. O. , Huebert , B. J. , Kulmala , M. , Bond , T. C. , Boy , M. , Doherty , S. J. , Guenther , A. , Kanakidou , M. , Kawamura , K. , Kerminen , V. M. , Lohmann , U. , Russell , L. M. and Poschl , U. 2006 . Critical Assessment of the Current State of Scientific Knowledge, Terminology, and Research Needs Concerning the role of Organic Aerosols in the Atmosphere, Climate, and Global Change . Atmos. Chem. Phys. , 6 : 2017 – 2038 .
- Gussman , R. A. , Kenny , L. C. , Labickas , M. and Norton , P. 2002 . Design, Calibration, and Field Test of a Cyclone for PM1 Ambient Air Sampling . Aerosol Sci. Technol. , 36 : 361 – 365 .
- Huebert , B. , Bertram , T. , Kline , J. , Howell , S. , Eatough , D. and Blomquist , B. 2004 . Measurements of Organic and Elemental Carbon in Asian Outflow During ACE-Asia from the NSF/NCAR C-130 . J. Geophys. Res.—Atmospheres , : 109
- Hughes , L. S. , Allen , J. O. , Bhave , P. , Kleeman , M. J. , Cass , G. R. , Liu , D. Y. , Fergenson , D. P. , Morrical , B. D. and Prather , K. A. 2000 . Evolution of Atmospheric Particles Along Trajectories Crossing the Los Angeles Basin . Environ. Sci. Technol. , 34 : 3058 – 3068 .
- Huntzicker , J. J. , Johnson , R. L. , Shah , J. J. and Cary , R. A. 1982 . “ Analysis of Organic and Elemental Carbon in Ambient Aerosol by a Thermal-Optical Method ” . In Particulate Carbon: Atmospheric Life Cycle , Edited by: Wolff , G. T. and Klimisch , R. L. 79 – 88 . New York : Plenum Press .
- IPCC . 2007 . Climate Change 2007: The Physical Science Basis. Summary for Policymakers , Geneva, , Switzerland : IPCC Secretariat .
- Kanakidou , M. , Seinfeld , J. H. , Pandis , S. N. , Barnes , I. , Dentener , F. J. , Facchini , M. C. , Van Dingenen , R. , Ervens , B. , Nenes , A. , Nielsen , C. J. , Swietlicki , E. , Putaud , J. P. , Balkanski , Y. , Fuzzi , S. , Horth , J. , Moortgat , G. K. , Winterhalter , R. , Myhre , C. E. L. , Tsigaridis , K. , Vignati , E. , Stephanou , E. G. and Wilson , J. 2005 . Organic Aerosol and Global Climate Modelling: A Review . Atmos. Chem. Phys. , 5 : 1053 – 1123 .
- Kenny , L. C. and Gussman , R. A. 2000 . A Direct Approach to the Design of Cyclones for Aerosol-Monitoring Applications . J. Aerosol Sci. , 31 : 1407 – 1420 .
- Khlystov , A. , Wyers , G. P. and Slanina , J. 1995 . The Steam-Jet Aerosol Collector . Atmos. Environ. , 29 : 2229 – 2234 .
- Kleeman , M. J. , Schauer , J. J. and Cass , G. R. 2000 . Size and Composition Distribution of Fine Particulate Matter Emitted from Motor Vehicles . Environ. Sci. Technol. , 34 : 1132 – 1142 .
- Kondo , Y. , Miyazaki , Y. , Takegawa , N. , Miyakawa , T. , Weber , R. J. , Jimenez , J. L. , Zhang , Q. and Worsnop , D. R. 2007 . Oxygenated and Water-Soluble Organic Aerosols in Tokyo . J. Geophys. Res.—Atmospheres , : 112
- Lim , H.-J. and Turpin , B. J. 2002 . Origins of Primary and Secondary Organic Aerosol in Atlanta: Results of Time-Resolved Measurements During the Atlanta Supersite Experiment . Environ. Sci. Technol. , 36 : 4489 – 4496 .
- Lim , H. J. , Turpin , B. J. , Edgerton , E. , Hering , S. V. , Allen , G. , Maring , H. and Solomon , P. 2003 . Semicontinuous Aerosol Carbon Measurements: Comparison of Atlanta Supersite Measurements . J. Geophys. Res.—Atmospheres , : 108
- Marple , V. A. , Rubow , K. I. and Behm , S. M. 1991 . Microorifice Uniform Deposit Impactor (MOUDI): Description, Calibration, and Use . Aerosol Sci. Technol. , 14 : 434 – 446 .
- NIOSH . 1996 . “ Elemental Carbon (Diesel Particulate): Method 5040 ” . In NIOSH Manual of Analytical Methods , Edited by: Eller , P. M. and Cassinelli , M. E. Cincinnati : National Institute for Occupational Safety and Health .
- Novakov , T. , Hegg , D. A. and Hobbs , P. V. 1997 . Airborne Measurements of Carbonaceous Aerosols on the East Coast of the United States . J. Geophys. Res. , 102 : 30023 – 30030 .
- Pang , Y. , Eatough , N. L. and Eatough , D. J. 2002 . PM2.5 Semivolatile Organic Material at Riverside, California: Implications for the PM2.5 Federal Reference Method Sampler . Aerosol Sci. Technol. , 36 : 277 – 288 .
- Pope , C. A. 2000 . Review: Epidemiological Basis for Particulate Air Pollution Health Standards . Aerosol Sci. Technol. , 32 : 4 – 14 .
- Sardar , S. B. , Fine , P. M. , Mayo , P. R. and Sioutas , C. 2005a . Size-Fractionated Measurements of Ambient Ultrafine Particle Chemical Composition in Los Angeles using the NanoMOUDI . Environ. Sci. Technol. , 39 : 932 – 944 .
- Sardar , S. B. , Fine , P. M. and Sioutas , C. 2005b . Seasonal and Spatial Variability of the Size-Resolved Chemical Composition of Particulate Matter (PM10) in the Los Angeles Basin . J. Geophys. Res.—Atmospheres , : 110
- Saxena , P. and Hildemann , L. M. 1996 . Water-Soluble Organics in Atmospheric Particles: A Critical Review of the Literature and Application of Thermodynamics to Identify Candidate Compounds . J. Atmos. Chem. , 24 : 57
- Schauer , J. J. , Mader , B. T. , Deminter , J. T. , Heidemann , G. , Bae , M. S. , Seinfeld , J. H. , Flagan , R. C. , Cary , R. A. , Smith , D. , Huebert , B. J. , Bertram , T. , Howell , S. , Kline , J. T. , Quinn , P. , Bates , T. , Turpin , B. , Lim , H. J. , Yu , J. Z. , Yang , H. and Keywood , M. D. 2003 . ACE-Asia Intercomparison of a Thermal-Optical Method for the Determination of Particle-Phase Organic and Elemental Carbon . Environ. Sci. Technol. , 37 : 993 – 1001 .
- Sorooshian , A. , Brechtel , F. J. , Ma , Y. L. , Weber , R. J. , Corless , A. , Flagan , R. C. and Seinfeld , J. H. 2006 . Modeling and Characterization of a Particle-Into-Liquid Sampler (PILS) . Aerosol Sci. Technol. , 40 : 396 – 409 .
- Subramanian , R. , Khlystov , A. Y. , Cabada , J. C. and Robinson , A. L. 2004 . Positive and Negative Artifacts in Particulate Organic Carbon Measurements with Denuded and Undenuded Sampler Configurations . Aerosol Sci. Technol. , 38 : 27 – 48 .
- Sullivan , A. P. , Peltier , R. E. , Brock , C. A. , de Gouw , J. A. , Holloway , J. S. , Warneke , C. , Wollny , A. G. and Weber , R. J. 2006 . Airborne Measurements of Carbonaceous Aerosol Soluble in Water Over Northeastern United States: Method Development and an Investigation into Water-Soluble Organic Carbon Sources . J. Geophys. Res. , 111 : 1 – 14 .
- Sullivan , A. P. and Weber , R. J. 2006a . Chemical Characterization of the Ambient Organic Aerosol Soluble in Water. 1. Isolation of Hydrophobic and Hydrophilic Fractions with a XAD-8 Resin . J. Geophys. Res. , 111 : 9
- Sullivan , A. P. and Weber , R. J. 2006b . Chemical Characterization of the Ambient Organic Aerosol Soluble in Water: 2. Isolation of Acid, Neutral, and Basic Fractions by Modified Size-Exclusion Chromatography . J. Geophys. Res. , 111 : 19
- Sullivan , A. P. , Weber , R. J. , Clements , A. L. , Turner , J. R. , Bae , M. S. and Schauer , J. J. 2004 . A Method for On-Line Measurement of Water-Soluble Organic Carbon in Ambient Aerosol Particles: Results from an Urban Site . Geophys. Res. Lett. , 31 : 4
- Takegawa , N. , Miyazaki , Y. , Kondo , Y. , Komazaki , Y. , Miyakawa , T. , Jimenez , J. L. , Jayne , J. T. , Worsnop , D. R. , Allan , J. D. and Weber , R. J. 2005 . Characterization of an Aerodyne Aerosol Mass Spectrometer (AMS): Intercomparison with Other Aerosol Instruments . Aerosol Sci. Technol. , 39 : 760 – 770 .
- Thurston , G. D. , Ito , K. , Mar , T. , Christensen , W. F. , Eatough , D. J. , Henry , R. C. , Kim , E. , Laden , F. , Lall , R. , Larson , T. V. , Liu , H. , Neas , L. , Pinto , J. , Stolzel , M. , Suh , H. and Hopke , P. K. 2005 . Workgroup Report: Workshop on Source Apportionment of Particulate Matter Health Effects—Intercomparison of Results and Implications . Environ. Health Perspect. , 113 : 1768 – 1774 .
- Turpin , B. J. , Huntzicker , J. J. and Adams , K. M. 1990 . Intercomparison of Photoacoustic and Thermal Optical Methods for the Measurement of Atmospheric Elemental Carbon . Atmos. Environ. Part a—General Topics , 24 : 1831 – 1835 .
- Turpin , B. J. , Huntzicker , J. J. and Hering , S. V. 1994 . Investigation of Organic Aerosol Sampling Artifacts in the Los Angeles Basin . Atmos. Environ. , 28 : 3061 – 3071 .
- Turpin , B. J. , Saxena , P. and Andrews , E. 2000 . Measuring and Simulating Particulate Organics in the Atmosphere: Problems and prospects . Atmos. Environ. , 34 : 2983 – 3013 .
- Weber , R. J. , Sullivan , A. P. , Peltier , R. E. , Russell , A. G. , Yan , B. , Chen , Y. , Zheng , M. , De Gouw , J. A. , Warneke , C. , Brock , C. A. , Holloway , J. S. , Atlas , E. L. and Edgerton , E. 2007 . A Study of Secondary Organic Aerosol Formation in the Anthropogenic-Influenced Southeastern USA . J. Geophys. Res. , 112 : D13302 10.1029/2007J0008408
- Wilson , W. E. , Grover , B. D. , Long , R. W. , Eatough , N. L. and Eatough , D. J. 2006 . The Measurement of Fine-Particulate Semivolatile Material in Urban Aerosols . J. Air & Waste Manage. Assoc. , 56 : 384 – 397 .
- Zappoli , S. , Andracchio , A. , Fuzzi , S. , Facchini , M. C. , Gelencser , A. , Kiss , G. , Krivacsy , Z. , Molnar , A. , Meszaros , E. , Hansson , H. C. , Rosman , K. and Zebuhr , Y. 1999 . Inorganic, Organic and Macromolecular Components of Fine Aerosol in Different Areas of Europe in Relation to Their Water Solubility . Atmos. Environ. , 33 : 2733 – 2743 .
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Allan , J. D. , Coe , H. , Ulbrich , I. , Alfarra , M. R. , Takami , A. , Middlebrook , A. M. , Sun , Y. L. , Dzepina , K. , Dunlea , E. , Docherty , K. , DeCarlo , P. F. , Salcedo , D. , Onasch , T. , Jayne , J. T. , Miyoshi , T. , Shimono , A. , Hatakeyama , S. , Takegawa , N. , Kondo , Y. , Schneider , J. , Drewnick , F. , Borrmann , S. , Weimer , S. , Demerjian , K. , Williams , P. , Bower , K. , Bahreini , R. , Cottrell , L. , Griffin , R. J. , Rautiainen , J. , Sun , J. Y. , Zhang , Y. M. and Worsnop , D. R. 2007 . Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes . Geophys. Res. Lett. , : 32
- Zheng , M. , Cass , G. R. , Schauer , J. J. and Edgerton , E. S. 2002 . Source Apportionment of PM2.5 in the Southeastern United States Using Solvent-Extractable Organic Compounds as Tracers . Environ. Sci. Technol. , 36 : 2361 – 2371 .