Abstract
Positive matrix factorization (PMF) was used to elucidate sources of fine particulate material (PM 2.5 ) for a study conducted during July 2003 in Rubidoux, CA. One-h averaged semi-continuous measurements were made with a suite of instruments to provide PM 2.5 mass and chemical composition data. Total PM 2.5 mass concentrations (nonvolatile plus semi-volatile) were measured with a R&P filter dynamic measurement system (FDMS) and a conventional TEOM monitor was used to measure nonvolatile mass concentrations. Semi-volatile material (SVM) was calculated as the FDMS minus the TEOM determined PM 2.5 mass. PM 2.5 chemical species monitors included a R&P 5400 carbon monitor, an Anderson Aethalometer and a R&P 8400N nitrate monitor. Gas phase data including CO, NO 2 , NO x , and O 3 were also collected during the sampling period. Two distinct PMF analysis were performed. In analysis 1, the TEOM was excluded from the analysis and in analysis 2, the SVM was excluded from the analysis. PMF2 was able to identify six factors from the data set and factors corresponding to both primary and secondary sources were identified. Factors were attributed to being primarily from automobile, diesel emissions, secondary nitrate formation, a secondary photochemical associated source, organic emissions and primary emissions. Good agreement was observed between the PMF predicted mass and the FDMS measured mass for both analyses.
INTRODUCTION
Human health endpoints associated with exposure to airborne particulate matter (PM) include increased mortality and morbidity from respiratory and cardiopulmonary disease (CitationPope and Dockery 2006). These effects are observed with exposure to concentrations substantially below the U.S. PM10 (particulate matter with aerodynamic diameter less than 10 μ m) ambient air quality standard. The exacerbation of observed health problems is believed to be associated more closely with exposure to fine particles (PM2.5, particulate matter with aerodynamic diameter less than 2.5 μ m), especially those generated by combustion, than exposure to coarse particles. As a result, the U.S. Environmental Protection Agency has promulgated revised standards for PM, which establishes annual and 24-h PM2.5 standards. The EPA's decision to revise the PM National Ambient Air Quality Standards (NAAQS) has sparked renewed interest in the ability to accurately measure fine PM and to improve source characterization methods. Identification of the sources of fine particles responsible for the epidemiologically identified health effects would significantly aid in the implementation of the new PM2.5 standard (CitationThurston et al. 2005; CitationMar et al. 2006).
PM2.5 in the atmosphere is not composed of a single pollutant but consists of both stable and semi-volatile species. Stable species in the atmosphere include trace metals (including toxic, crustal, and transition metals), elemental carbon (EC), nonvolatile organic material (NVOM), and sulfate. Semi-volatile material (SVM) exists in dynamic equilibrium between the gas and particle phase and includes ammonium nitrate (NH4NO3) and low molecular weight organic species.
Source receptor models have commonly been used to deduce source contributions to measured PM2.5 mass (CitationZheng et al. 2004; Eatough et al. (in press); CitationKim et al. 2003). A recently developed positive matrix factorization program, PMF2 (CitationPaatero 1997), allows for source determination using a least squares approach to solve the factor analysis problem. Solutions are forced to be nonnegative and estimates in the measurement error are used as weighting parameters for individual data points. A linear combination of a source matrix and an error estimate matrix is employed.
Semi-continuous monitoring data have been shown to greatly improve the power of receptor models to determine sources because the data include information on diurnal changes in the atmosphere. Such variation assist in the factor analysis identification of sources (or atmospheric processes) which vary diurnally (CitationGrover et al. 2006). In this study, PMF2 was used to deduce source contributions from a sampling campaign conducted in Rubidoux, CA during July 2003. Semi-continuous measurements (1-h average) were made using an FDMS TEOM (total fine particulate mass), a conventional TEOM (nonvolatile fine particulate mass), an R&P 5400 Carbon monitor (elemental and organic carbon), an Aethalometer (black carbon), and an R&P 8400N nitrate monitor. Hourly average CO, NO x , and NO gas phase data were also available. This article discusses the application of PMF2 to 1-h averaged data, the PMF2 results obtained, and the interpretation of the resulting factor profiles and contributions.
DATA COLLECTION AND ANALYSIS
Data collection was conducted at the South Coast Air Quality Monitoring District (SCAQMD) sampling site in Rubidoux, CA during July 2003 and has been extensively described elsewhere (CitationGrover et al. 2005). Rubidoux is located at the eastern end of the Los Angles basin. PM2.5 originating from the Los Angeles and other urban areas in the South Coast Basin is transported across the basin during periods of high photochemical activity. Stable inversions were frequent during the sampling period, resulting in high concentrations of secondary particulate organic material (OM) and nitrate. Furthermore, several ammonia sources exist near the end of the basin prior to the Rubidoux sampling site abetting the formation of NH4NO3, much of which is semi-volatile in nature. These conditions resulted in a good opportunity to study an urban aerosol with high concentrations of both non-volatile material (NVM) and SVM (CitationGrover et al. 2005).
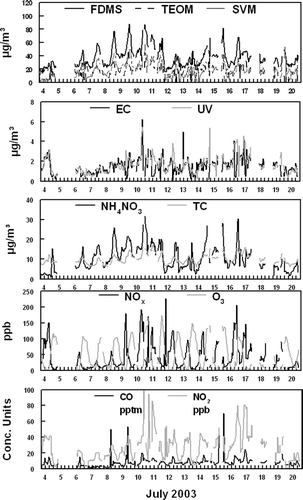
One-hour semi-continuous measurements were made throughout the study period of 4–21 August 2005 with instruments to measure both PM2.5 mass, PM2.5 chemical species, and gas phase species with concentrations shown in . Total PM2.5 mass, including nonvolatile and semi-volatile mass, was measured using a R&P FDMS (Filter Dynamic Measurement System) TEOM (Tapered Element Oscillating Microbalance) monitor. Incoming sample air passes through a Nafion dryer, then either directly to the mass sensor operated at 30°C (Base mode), or first through a chilled filter and then to the 30°C mass sensor (Reference mode). Only the derived total PM2.5 was used from this instrument. Recent studies indicate that the FDMS monitor accurately measures total PM2.5 mass (CitationGrover et al. 2007; CitationGrover et al. 2006). A conventional TEOM monitor, operated at 50°C, was also employed. Because the TX40 filter is heated on the conventional TEOM monitor, this technique only measures nonvolatile mass (CitationMignacca and Stubbs 1999; CitationLong et al. 2003; CitationEatough et al. 2003; CitationGrover et al. 2005). A R&P 5400 Carbon monitor was used to measure atmospheric carbonaceous material concentrations, which measures organic carbon (OC), EC, and total carbon (TC). Due to missing data and problems associated with the instrument during sampling, only TC data were used in the PMF analysis. EC concentrations were measured by an Anderson Aethalometer. The Aethalometer also provided an absorption measurement at 350 nm, referred to as UV, which has been claimed to be a good marker for diesel emissions (CitationHansen et al. 2002). Nitrate concentrations were monitored with a R&P 8400N nitrate monitor. Due to excess ammonia in the Riverside area, nitrate was assumed to be present as NH4NO3. Because the FDMS monitor measures total PM2.5 concentration and the TEOM monitor measures only non-volatile PM2.5 concentrations, SVM concentrations can be determined as the R&P FDMS measurement minus the R&P TEOM monitor concentrations. The calculated SVM concentrations were occasionally slightly negative (5 of the 326 data points), but never by more than 3σ for the uncertainty in the FDMS and TEOM data (CitationGrover et al. 2005). Gas phase data including, carbon monoxide (CO), ozone (O3), nitrogen dioxide (NO2), and nitrogen oxides (NO + NO2 = NOx), were measured by the SCAQMD at the Rubidoux, CA site.
FIG. 1 Semi-continuous monitor data including measurements of PM2.5 mass, chemical species, and gas phase species.
In the treatment of data for this study, SVM was not measured directly but was inferred as the difference between two different measurement techniques (FDMS-TEOM), as mentioned previously. Because SVM is not measured independently, including SVM and its parent data in the same PMF analysis may bias the receptor models results. In other words, a receptor model looking for correlations between input data will automatically be biased if one metric is calculated from other input data because a relationship exists between the inferred metric and its parent data. However, both non-volatile mass and SVM are important components of urban aerosols and therefore, will play an important role in the ability of PMF to deduce sources. Therefore for this study, the input data were treated in two distinct PMF analyses.
In the first analysis, the TEOM mass was excluded and the SVM calculated as FDMS-TEOM was included. The disadvantage to this analysis is that no measurement of nonvolatile mass is included in the analysis and must be inferred when sources were deduced from factors obtained in the PMF analysis. In the second analysis, the SVM was excluded and the TEOM mass was included. The disadvantage to this analysis is that no measurement of SVM is included in the PMF analysis but must be inferred from semi-volatile species (i.e., ammonium nitrate and OM) when sources are deduced from the factors obtained from the PMF analysis. Analyses were attempted using both the TEOM and SVM results. However, reasonable results could not be obtained, presumably because of the interdependence of the 3 mass variables.
RECEPTOR MODEL ANALYSIS
PMF2 and the algorithm used in the analysis has been previously described (CitationPaatero 1997). In PMF2, the results are constrained so that factor contributions cannot be negative for any species.
One of the unique advantages to PMF is the ability to handle missing and below detection limit data. The uncertainty in each measurement can be adjusted to account for aberrations in the data set. In this study, error uncertainty estimates were chosen similar to those previously outlined by CitationPolissar et al. (1998). For all validated data where the concentrations were above the limit of detection (LOD), error estimates were assigned as the measurement error plus 1/3 the limit of detection (LOD). In the few instances when the measurement was below the LOD, the concentration values were set to one half the LOD and the error was estimated as 5/6 the LOD. Missing values in the data set were accounted for by taking the geometric mean of the hour preceding and following the missing data point. The error estimates were then set to 4 times the geometric mean value. Following periods of high concentrations of SVM, TEOM mass measurements can be negative due to excessive loss of SVM off the collection filter (CitationMignacca and Stubbs 1999; CitationEatough et al. 2003). For these time periods, the negative concentration value was retained and the error estimate was set to four times the absolute value of the negative mass measurement. In this study, SVM concentrations were obtained by the difference between the FDMS TEOM and the conventional TEOM PM2.5 mass results. Therefore, the error estimate was performed as mentioned above using the highest LOD of the two measurement techniques. For time periods when SVM concentrations were negative, the negative concentration was used and the error estimate was set to four times the absolute value of the negative concentration or 5/6 the LOD, whichever was higher.
In performing PMF, the number of factors to be identified is defined by the user. However, a higher order solution does not necessarily contain the same factors as a lower order solution. Experimentation with the number of factors is performed until the most reasonable results are obtained (i.e., the results describe the data and are meaningful). In this study, the robust mode (CitationPaatero 1997) was used in which data were down weighted if the standard deviation was greater than four times the error estimate.
Ten species (FDMS TEOM, TEOM or SVM, NH4NO3, TC, EC, UV, CO, NOx, NO2, and O3) were used in the study and six factors were identified for both analysis 1 and analysis 2. An eleventh species, NO, was available but since it was a combination of the NOx and NO2 data did not provide any atmospheric relevance and actually hindered the identification of factors. This seems reasonable since NOx is expected to be a measure of combustion emission and NO2 of atmospheric conversion processes. Therefore, adding species to the analysis that do not provide useful information in the identification of factors does not necessarily assist the power of the analysis to define factors. The time period from the evening of July 4 through July 5 was intentionally removed from the analysis due to a fourth of July fireworks display and resulting fire near the sampling site (CitationGrover et al. 2005).
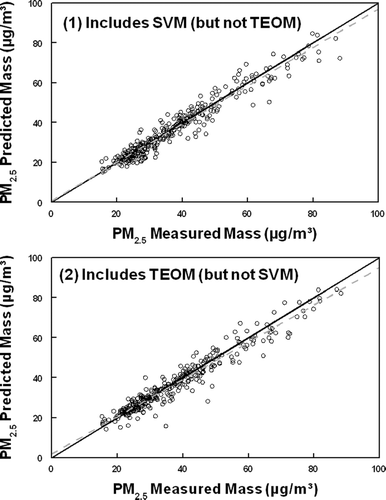
Rotational ambiguity (Paatero 1977), which can plague this type of factor analysis, can be restrained by applying an FPEAK value to test for the effect of rotation (Paatero, 1977). In this case, an FPEAK value of zero resulted in the most meaningful results. An evaluation of the quality of the fitted data can be obtained by comparing the degrees of freedom (i.e., the number of data points) with the calculated value of Q (the error function). If a reasonable fit is obtained, the calculated value of Q should be approximately equal to the degrees of freedom. Deviation from the theoretical value suggests that the errors in the model are not well defined. For this study, the number of degrees of freedom was 3586 and the resultant Q values (error function, Paatero 1977) were 1424 and 1432 for analysis 1 (includes SVM) and analysis 2 (includes TEOM), respectively. A second check on the “fit” of the solution can be made by comparing the sum of the factor contributions to the measured mass, to verify that the measured mass is well defined by the calculated sources. In this case, the sum of the factor contributions was in excellent agreement with the measured PM2.5 mass as shown in for both PMF analysis. Linear regression analysis for analysis 1 (includes SVM) resulted in a zero intercept slope of 0.982 ± 0.005 (R2 = 0.937, n = 326) and a calculated slope of 0.956 ± 0.014 (R2 = 0.936 and b = 1.1 ± 3.7). For analysis 2 (includes TEOM), linear regression analysis resulted in a zero intercept slope of 0.976 ± 0.005 (R2 = 0.931, n = 326) and a calculated slope of 0.928 ± 0.014 (R2 = 0.933 and b = 2.1 ± 3.7).
FIG. 2 Comparison of PM2.5 total mass (nonvolatile plus semi-volatile) measured by the FDMS and the PM2.5 predicted mass by PMF2 for both analysis 1 and analysis 2.
Source profiles for the six identified factors are shown in for analysis 1 (includes SVM) and for analysis 2 (includes TEOM) The source identification, as detailed below, is also given. and identify the specific chemical species contributions of the identified factors for analysis 1 and analysis 2, respectively. In source apportionment an a priori knowledge of chemical markers, that can be attributed to a particular source, is needed to identify sources most likely associated with each factor. Relevant time patterns were also used in this study to aid in the identification of the sources. For the two distinct analysis, four of the factors showed good consistencies between the source profiles and the source contributions. However, the source contributions for two factors did show some inconsistencies. The source contributions assigned for both analyses are shown in .
FIG. 3 Source profiles deduced by PMF2 for analysis 1 (includes SVM) from the semi-continuous monitoring data collected during July 2003 in Rubidoux, CA. Grams of species mass per gram of factor mass are given on the left y-axis for the particulate species (hatch marked) and the ratio of gas-phase species (ppm for CO and ppb for the other gases) per μ g of factor mass (shaded marked) are given on the right y axis. The ratio of SVM to PM2.5 mass for Source 5 is 1.38. The associated source is given in (), see text.
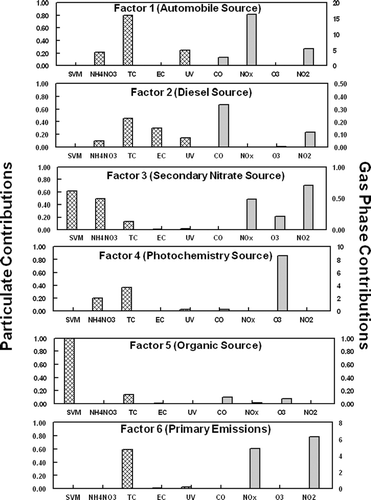
FIG. 4 Source profiles deduced by PMF2 for analysis 2 (includes TEOM) from the semi-continuous monitoring data collected during July 2003 in Rubidoux, CA. Grams of species mass per gram of factor mass are given on the left y-axis for the particulate species (hatch marked) and the ratio of gas-phase species (ppm for CO and ppb for the other gases) per μ g of factor mass (shaded marked) are given on the right y axis. The ratio of TEOM to PM2.5 for Sources 1 and 6 are 1.66 and 1.04, respectively. The associated source for each factor is given in (), see text.
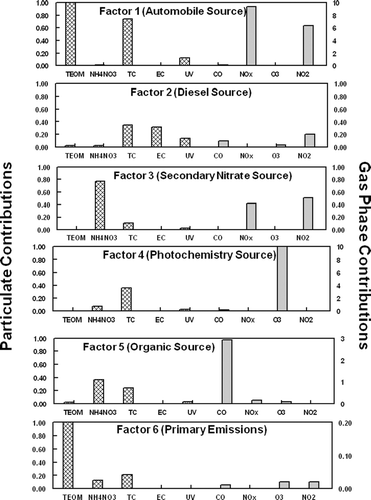
FIG. 5 Source contributions from the six sources obtained at the SCAQMD Rubidoux, CA sampling site for both analysis 1 (includes SVM) and analysis 2 (includes TEOM).
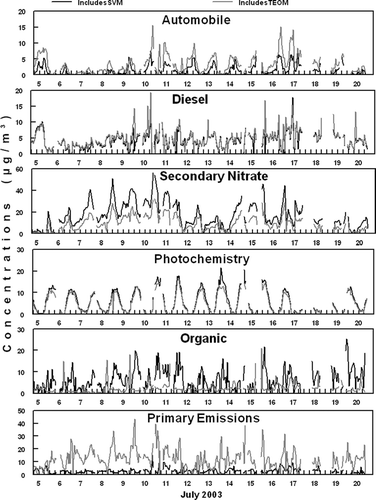
TABLE 1 Source concentration profiles for the six identified sources with the contribution of the respective chemical species to the source mass for analysis 1 (includes SVM)
TABLE 2 Source concentration profiles for the six identified sources with the contribution of the respective chemical species to the source mass for analysis 2 (includes TEOM)
Mobile source emissions are often a large fraction of urban aerosols. Rubidoux is often impacted by weekday morning rush hour traffic as commuters head toward the Los Angeles area. Organic material, NO2, and CO have been shown to be good markers of automobile emissions (Eatough et al. submitted; CitationLong et al. 2002; CitationGrover et al. 2006). For analysis 1 (includes SVM), a large fraction of the TC, CO, NO2, and NOx was attributed to factor 1 as shown in . For analysis 2 (includes TEOM) NO2, and NOx were also a large faction of factor 1, but significantly less CO was attributed to this source in analysis 2. The source contributions for both PMF analysis show similar diurnal patterns with peak concentrations during early morning rush hour time periods. This is consistent with morning traffic patterns and meteorology at the sampling site (). Factor 1 for both analysis was therefore, identified as local automobile emissions. One would expect that local automobile emissions would contain a large fraction of primary species as was observed in analysis 2 when the TEOM measured mass was included in the PMF analysis.
Factor 2 for both analysis was identified as local diesel emissions. Diesel emissions are rich in EC concentrations. The majority of the EC and UV measured by the Aethalometer was attributed to this source as shown in for analysis 1 and for analysis 2. Both EC and UV have been shown to be good markers for diesel emissions (CitationHansen et al. 2002). Of the six sources identified, factor 2 in both analyses contains the majority of the EC. However, UV is divided between factors 1 and 2 in both analyses. The time patterns observed in factor 2 correlate well with local diesel traffic patterns () and good agreement was observed between the source contributions for both PMF analysis, as shown in . Diesel emissions were observed throughout the daytime sampling periods and did not exhibit a discernable daily diurnal pattern. Heavy-duty diesel trucks are not constrained to rush hour time periods and tend to be on the road during most daytime periods. Furthermore, higher concentrations were observed during weekday time periods compared with weekend time periods, as would be expected.
In analysis 1 (includes SVM), factor 3 was dominated by SVM. The majority of the NH4NO3, a semi-volatile species, measured during this study was also attributed to factor 3. The NH4NO3 in the factor was slightly smaller than the total SVM. When SVM was not included as a metric in the PMF analysis (analysis 2), factor 3 was dominated by NH4NO3. Furthermore for both analysis, this factor contained a substantial amount of the NO2, a precursor to nitrate formation. The source contributions () for both analyses show similar time-series patterns with increased concentrations during mid-day time periods. A similar time pattern is observed in NH4NO3 concentrations observed during the study, as shown in . HYSLIT generated back trajectories (CitationNOAA 2002) during the study time period indicated that ground level air was dominated by coastal winds from the Los Angeles area to the Rubidoux sampling site. These coastal winds were most dominant during mid-day to afternoon time periods when PM2.5 concentrations were the highest. Nitric acid is formed by the reaction of NO2 with the OH radical during periods of high photochemical activity and then transported by coastal winds across the Los Angeles basin. Excess ammonia in the region reacts with nitric acid to form NH4NO3. Factor 3 was therefore identified as a secondary nitrate source for both PMF analyses. A weekday-weekend pattern is observed in this factor and NH4NO3 data, with decreased concentrations observed during weekend time periods. As expected, NO2 emissions are more intense during the week propagating the formation of nitric acid and subsequent formation of NH4NO3 in the Rubidoux area.
The formation of tropospheric O3 is a secondary process due to photochemical reactions that promote the formation of O3. Peak concentrations of O3 were observed during mid-day time periods () during this study when photochemical activity was the highest. Many complex reactions involving volatile organic species (VOCs) and NOx also contribute to the formation of O3. Consequently, O3 concentrations are geographically variable. Local O3 concentrations would therefore be expected to correspond with local photochemical activity but would not be expected to correlate well with secondary pollutants that resulted from transport mechanisms. In both PMF analyses essentially all of the O3 observed during the study was attributed to factor 4, as shown in and . Time patterns correspond well with local O3 concentrations, with peak concentration periods observed during mid-day time periods (), and consistency is observed between the source contributions in both analyses. Factor 4 can therefore be attributed to local photochemical formation of secondary species in the aerosol and is labeled a local photochemistry source. The composition of the secondary aerosol is dominated by carbonaceous material in both analysis ( and ).
SVM consists of both NH4NO3 and low molecular weight organic species, the majority of which are secondary (CitationEatough et al. 2003). Reaction pathways for the formation of secondary organic aerosols are not well understood but are thought to be initiated by O3 and the OH radical. Increased concentrations of secondary organic species in the Los Angeles basin have been observed during high O3 concentration episodes (CitationTurpin and Hunzickler 1995; CitationTurpin et al. 1991). In analysis 1, when the TEOM was excluded from the analysis, but a metric of SVM was included, PMF was able to separate semi-volatile nitrate and semi-volatile organic material (SVOM) into two separate factors in this study. In analysis 1, factor 5 contained a large fraction of the SVM but no NH4NO3 (). Time patterns for factor 5 exhibit increased concentrations during daytime periods when photochemical activity was the greatest (). This is consistent with the observation that SVOM is secondary and may be formed by reactions induced by photochemical processes. In analysis 1, factor 5 was therefore defined as a secondary organic source. In analysis 2, when SVM was not included in the PMF analysis, the source contribution for factor 5 does not resemble the one obtained for analysis 1, as shown in . Because SVM was not included as a metric in analysis 2, PMF was not able to distinctly determine a source that can be related to SVOM. Factor 5 in analysis 2 was associated with small factions of TC, CO, and NO2. Some similarities are observed in the source contributions with automobile emissions, and this factor seems to be an organic source that may be partially related to automobile emissions. The fact the PMF was not able to distinctly determine a SVOM source in analysis 2 when no metric for SVM was put into the model is not unexpected.
The identified source contribution for factor 6, when SVM is excluded from the PMF analysis (analysis 2), shows similar, but not identical, time patterns as the factor identified as secondary nitrate. This factor is highly associated with TEOM, which only measures nonvolatile species. Because the identified time pattern of factor 6 in analysis 2 correlates well with the transport of PM2.5 across the basin, the dominant source associated with this factor was identified as primary transported mass, i.e., PM2.5 that is transported by coastal winds but is nonvolatile in nature. Several stable fine-particle aerosol species exist that would be expected to be transported with coastal winds including, ammonium sulfate, non-volatile organic species, and crustal elements. However, this factor is not related to NOx emissions that would be expected to be associated with transported material across the Los Angeles Basin.
In analysis 1, when TEOM measured mass is excluded from the analysis, the factor 6 identified source contribution does not exhibit similar time patterns to factor 6 identified in analysis 2. However, this factor is associated with TC and NO2, both of which can be related to primary, nonvolatile emissions. Factor 6 in analysis 1 was therefore, identified as a primary emission source that is most likely related to organic emissions associated with conversion of NO emissions to NO2 during transport across the Los Angeles Basin. The source identified as a secondary nitrate source was also highly associated with NOx and NO2. The fact that factor 6 in analysis 1 does not exhibit a time pattern similar to secondary nitrate formation may suggest that not including a metric of nonvolatile mass (TEOM measured mass) inhibits the ability of PMF to distinctly identify a source related to these primary emissions.
DISCUSSION AND CONCLUSIONS
The inconsistencies in the source contributions for the two different analysis for factors identified as organic emissions and primary emissions warrants some discussion. It is understandable that the PMF analysis is limited by the input data, and that identification of sources is contingent upon the metrics used in the analysis. This is evident in the inconsistencies in the source contributions identified in factors 5 and 6 between the two PMF analysis.
The percent contributions to the total PM2.5 predicted mass for all of the sources for both analysis 1 and 2 are shown in . When no metric for primary emissions is included in the analysis (analysis 1), the amount of mass identified as secondary (e.g., factors 3 and 4) is much larger then when SVM is excluded from the analysis (analysis 2). In analysis 2, when a metric for SVM was not included in the analysis, the identification of a secondary organic source is not possible. In this case, more mass is shifted to the automobile source, and the organic source resembles, but is not identical, to an automobile source contribution.
FIG. 6 Percent contributions to the total PM2.5 predicted mass for all of the sources for both analysis 1 and 2.
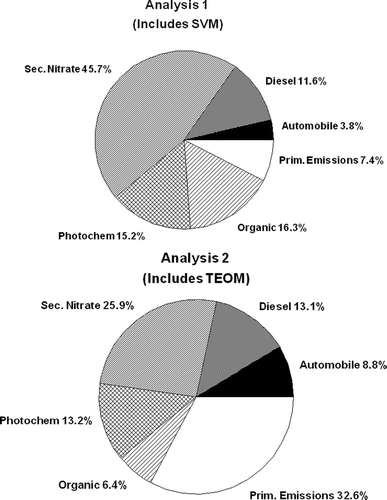
When the metric included in the analysis is well defined, general consistency is observed between the two PMF analysis as was observed with the factors identified as diesel, secondary nitrate, and photochemistry. The percent contributions of these sources to the total mass are relatively consistent between the two analyses, and the metrics used in the identification of these factors were all well defined and consistent in the two distinct PMF analyses. Therefore, the ability of PMF to identify sources is conditional upon the input data. However, the inconsistencies in the two analyses are consistent with what would be expected from the differences in the input data. In other words, when a metric for SVM is included in the analysis and nonvolatile mass is excluded (analysis 1), more of the PM2.5 mass is identified as secondary. When no metric for SVM is included in the analysis but nonvolatile mass is included (analysis 2), more of the mass is shifted into primary sources. In both of the PMF analyses, however, the predicted mass was in good agreement with the FDMS measured mass.
Since no statistically relevant differences were observed between the two analyses, expected atmospheric processes were used to determine the better of the two solutions. Since general consistency in time patterns was observed for four of the factors, focus was emphasized on the factors that exhibited inconsistencies. For the factor identified as organic emissions (factor 5) no diurnal pattern was observed in analysis 2 (includes TEOM). However, analysis 1 (includes SVM) showed a distinct diurnal pattern that would be expected to be associated with secondary organic formation. Since semi-volatile organic species were a major faction of the aerosol measured in Rubidoux during the sampling campaign (CitationGrover et al. 2005), one would expect to observe a source related to SVOM.
Although, when the TEOM is included in the analysis, the time pattern for the factor identified as primary emissions is similar to time pattern of secondary nitrate, this factor is not related to NOx and NO2, as would be expected. When using the SVM in the analysis, primary transport does not show as distinct a similarity with the secondary nitrate source. However, this factor is highly associated with NOx and NO2 and appears to be more related to atmospheric processes.
As mentioned previously, when SVM is used in the analysis, more mass is included in the factor identified as organic emissions. When TEOM mass is used in the analysis, more mass is included in the automobile emissions source. Looking at the mass balance of the solutions shown in and , it is observed that the SVM in analysis 1 is approximately 40% greater than the FDMS measured mass for the factor identified as organic emissions. However in analysis 2, for the factor identified as automobile emissions, the TEOM measured mass is approximately 66% greater than the FDMS measured mass. The mass balance is generally good for all other metrics in the various factors for both analysis. These various observations lead the authors to believe that the PMF analysis that includes SVM as a metric appears to be more related to atmospheric processes and is therefore, the more reasonable solution.
The advantage of using semi-continuous sampler data in the application of source apportionment is evident in this study. Urban aerosols are often impacted by short-term pollution episodes that cannot be temporally resolved using integrated samplers. One-h averaged data applied to source apportionment models may increase the power of the model to predict sources that exhibit diurnal short-term episodes. Furthermore, using only 10 species we were able to identify six sources over a short study time period.
An attractive alternate approach to the apportionment of organic material in fine particulate material is the use of specific organic marker compound data (CitationLin et al. 2007). Apportionment using CMB approaches with organic marker data have been reported for the Los Angeles Basin (CitationSchauer 1996, Citation2002). The earlier of thee two studies only presented annual average apportionment results (CitationSchauer 1996) and is, therefore, more uncertain for comparison with the results reported here. The study did, however, find that organic material in gasoline emissions, tire wear debris (which would be included in the automotive and diesel sources in this study), and diesel emission (in increasing order) accounted for about 40% of the fine particulate organic material on an annual basis at Rubidoux. The related Automobile, Diesel, and Transported Primary Emission sources identified here contributed a comparable 48% of the total C based on the data in . CitationSchauer et al. (1996) reported that organic material from diesel emissions was significantly greater than that from automobile emissions, as was also seen in the present study. Also consistent with the CitationSchauer et al. study (1996), the concentration of other organic material (seen here as the secondary organic material in the secondary sources) was comparable to the primary organic material. A major species seen in the Schauer et al study Citation(1996), but not identified here is emissions from meat cooking, which accounted for about 20% of the total fine particulate organic material. A more recent study using organic marker compounds (CitationSchauer et al. 2002) focused on summer photochemical events but did not include measurements at Rubidoux. The more related data to the present study were probably data from Azusa, 50 km WNE of Rubidoux. In this study, vehicle related organic material account for about 30% of the total with a reduced importance of meat cooking contributions. Diesel and gasoline related organic emissions were comparable. About 60% of the organic material was not identified, and presumed to be secondary. Both these studies are consistent with the results reported here.
Although PMF was able to identify six factors that could be related to both primary and secondary sources in Rubidoux during the study period, it is not reasonable to assume that all sources were identified. The factors determined by PMF do not contain all emission sources in the region, e.g., there was no data to identify meat cooking emissions. However, the dominant or major sources of PM2.5 in Rubidoux during the sampling period were identified as factors in the PMF analyses and the source contributions explained the total PM2.5 mass measured by the FDMS monitor.
Acknowledgments
The sampling campaign conducted in Rubidoux, CA was supported by the U.S. Environmental Protection Agency (EPA) through its Office of Research and Development under contract 3C-R044-NAEX with Brigham Young University. Special thanks is given to the SCAQMD for help during the sampling and for supplying the gas-phase species data. The help of Jeff Ambs of Rupprecht and Patashnick and Russell W. Long of the U.S. EPA during sampling is also gratefully acknowledged.
REFERENCES
- Eatough , D. J. , Long , R. W. , Modey , W. K. and Eatough , N. L. 2003 . Semi-Volatile Secondary Organic Aerosol in Urban Atmospheres: Meeting a Measurement Challenge . Atmos. Environ. , 37 : 1277 – 1292 .
- Eatough , D. J. , Anderson , R. A. , Martello , D. V. , Modey , W. K. , Davidson , C. L. , Pekney , N. J. and Mangelson , N. F. Apportionment of Ambient Primary and Secondary PM2.5 During a 2001 Summer Intensive Study at the CMU Supersite and NETL Pittsburgh Site Using PMF2, in press
- Grover , B. D. , Kleinman , M. A. , Eatough , N. L. , Eatough , D. J. , Hopke , P. K. , Long , R. W. , Wilson , W. E. , Meyer , M. B. and Ambs , J. L. 2005 . Measurement of Total PM2.5 Mass (Nonvolatile plus Semi-Volatile) with the FDMS TEOM Monitor . J. Geophysical Research , : 110 D07S03
- Grover , B. D. , Carter , C. B. , Kleinman , M. A. , Richards , J. S. , Eatough , N. L. , Eatough , D. J. , Dasgupta , P. K. , Al-Horr , R. and Ullah Rahmat , S. M. 2006 . Monitoring and Source Apportionment of Fine Particulate Matter at Lindon, Utah . Aerosol Sci. Technol , 40 : 941 – 951 .
- Grover , B. D. , Eatough , N. L. , Woolwine , W. R. , Cannon , J. P. , Eatough , D. J. and Long , R. W. 2007 . Semi-Continuous Mass Closure of the Major Components of Fine Particulate Matter in Riverside, CA . Atmos. Environ. , in press
- Hansen , A. D. A. 2002 . The Aethalometer Operating Manual http://www.mageesci.com/Aethalometer_book_2002.06.5.pdf accessed September 16, 2002
- NOAA . 2002 . http://www.arl.noaa.gov/ready/hysplit4.html accessed September 19, 2005
- Kim , E , Hopke , P. K. and Edgerton , E. S. 2003 . Source Identification of Atlanta Aerosol by Positive Matrix Factorization . J. of Air & Waste Manage. Assoc. , 53 : 731 – 739 .
- Lin , L. L. , Lee , M. L. and Eatough , D. J. 2007 . Recent Advances in Detection of Organic Compound Markers in Fine Particulate Matter and Their Use for Source Apportionment . Atmos. Environ. , submitted
- Long , R. W. , Smith , R. , Smith , S. , Eatough , N. L. , Mangelson , N. F. and Eatough , D. J. 2002 . Sources of Fine Particulate Material Along the Wasatch Front . Energy and Fuels , 16 : 282 – 293 .
- Long , R. W. , Eatough , N. L. , Mangelson , N. F. , Thompson , W. , Fiet , K. , Smith , S. , Smith , R. and Eatough , D. J. 2003 . The Measurement of PM2.5, Including Semi-Volatile Components, in the EMPACT Program: Results from the Salt Lake City Study and Implications for Public Awareness, Health Effects, and Control Strategies . Atmos. Environ. , 37 : 4407 – 4417 .
- Mar , T. F. , Kazuhiko , I. , Koenig , J. Q. , Larson , T. V. , Eatough , D. J. , Henry , R. C. , Kim , E. , Laden , F. , Lall , R. , Neas , L. , Stolzel , M. , Paatero , P. , Hopke , P. K. and Thurston , G. D. 2006 . PM source Apportionment and Health Effects. 3. Investigation of Inter-method Variations in Associations between Estimated Source Contributions of PM2.5 and Daily Mortality in Phoenix, AZ . J. Exposure Analy. Environ. Epidemiology , 16 : 311 – 320 .
- Mignacca , D. and Stubbs , K. 1999 . Effects of Equilibration temperature on PM10 Concentrations from the TEOM Method in the Lower Fraser Valley . J. Air & Waste Manage. Assoc. , 49 : 1250 – 1254 .
- Paatero , P. 1997 . Least Squares Formulation of Robust, Non-negative Factor Analysis . Chemomet. Intell. Lab. Syst. , 37 : 13 – 35 .
- Polissar , A. V. , Hopke , P. K. , Malm , W. C. and Sisler , J. F. 1998 . Atmospheric Aerosol over Alaska 2. Elemental Composition and Sources . J. Geophys. Research , 103 : 19045 – 10057 .
- Pope , C. A. III and Dockery , D. W. 2006 . Health Effects of Fine Particulate Air Pollution: Lines that Connect . J. of Air & Waste Manage. Assoc. , 56 : 709 – 742 .
- Schauer , J. J. , Rogge , W. F. , Hildemann , L. M. , Mazurek , M. A. , Cass , G. R. and Simoneit , B. R. T. 1996 . Source Apportionment of Airborne Particulate Matter Using Organic Compounds as Tracers . Atmos. Environ. , 30 : 3837 – 3855 .
- Schauer , J. J. , Fraser , M. P. , Cass , G. R. and Simoneit , B. R. T. 2002 . Source Reconciliation of Atmospheric Gas-Phase and Particulate-Phase Pollutants During a Severe Photochemical Smog Episode . Environ. Sci. Technol. , 36 : 3806 – 14 .
- Thurston , G. D. , Mar , T. , Christensen , W. F. , Eatough , D. J. , Henry , R. C. , Kim , E. , Laden , F. , Hall , R. , Larson , T. V. , Liu , H. , Neas , L. , Pinto , J. , Stolzel , M. , Shuh , H. and Hopke , P. K. 2005 . Workgroup Report: Workshop on Source Apportionment of Particulate Matter Health Effects-Intercomparison of Results and Implications . Environ. Health Perspect. , : 1768 – 1774 . December 113: Number 12
- Turpin , B. J. and Huntzicker , J. J. 1995 . Identification of Secondary Organic Aerosol Episodes and the Quantization of Primary and Secondary Aerosol Concentrations during SCAQS . Atmos. Environ. , 29 : 3527 – 3544 .
- Turpin , B. J. , Huntzicker , J. J. , Larson , S. M. and Cass , G. R. 1991 . Los Angeles Summer Midday Particulate Carbon: Primary and Secondary Aerosol . Environ. Sci. Technol. , 25 : 1788 – 1793 .
- U.S. Environmental Protection Agency . 2002 . Air Quality Criteria for Particulate Matter , Research Triangle Park, NC : Environmental Protection Agency . EPA/600/P-99/002aC
- Watson , J. G. and Chow , J. C. 2004 . Receptor Models for Air Quality Management . Environ. Management , : 15 – 23 .
- Zheng , L. , Hopke , P. K. , Husain , L. , Qureshi , S. , Dutkiewicz , V. A. , Schwab , J. J. , Drewnick , F. and Demerjian , K. L. 2004 . Sources of Fine Particle Composition in New York City . Atmos. Environ , 38 : 6521 – 6529 .