Abstract
Six nucleation parameterizations are tested using the Dynamic Model for Aerosol Nucleation (DMAN). Weak, moderate, and strong nucleation events together with days without nucleation from the Pittsburgh Air Quality Study (PAQS) were selected for the evaluation. The ability of the different theories to reproduce the occurrence or lack of a nucleation event was evaluated. The ternary sulfuric acid-ammonia-water theory was the only one that was successful for all tests. Sensitivity tests of the remaining parameterizations suggest that increasing or decreasing the corresponding nucleation rates does not change the overall performance of the parameterizations when both the nucleation and non-nucleation days are included in the tests.
1. INTRODUCTION
New particles are introduced in the atmosphere by direct emission from a variety of sources and in-situ formation (nucleation). The relative contributions of these two pathways to aerosol number concentrations are not well understood. Most new particles are nanoparticles, defined here as particles smaller than 0.1 μ m. Nanoparticles may be more harmful to the human body than fine particles (0.1–1 μ m), due to their large surface area per unit mass (CitationDonaldson et al. 1998, Citation2002). When newly formed particles grow to the size of cloud condensation nuclei (CCN), they can become cloud droplets (CitationAdams and Seinfeld 2003). Cloud reflectivity and lifetime is affected by changes in CCN concentration, perturbing the energy balance of the planet (indirect effect of aerosols on climate) (CitationTwomey 1974; CitationAlbrecht 1989).
Several nucleation theories have been proposed in order to explain in-situ particle formation. These include sulfuric acid-water binary nucleation (CitationLaaksonen 1999; CitationVehkamäki et al. 2002), nucleation of organic vapors (CitationMarti et al. 1997; CitationZhang et al. 2004), ion-induced nucleation (CitationLaakso et al. 2002 and references therein), halogen-oxide nucleation (CitationHoffmann et al. 2001), and ternary nucleation (CitationCoffman and Hegg 1995; CitationKorhonen et al. 1999; CitationKulmala et al. 2002; CitationNapari et al. 2002). Significant uncertainties arise from the lack of understanding of the identity of the species involved in the formation and growth of these nuclei (CitationKulmala et al. 2004). Field measurements (CitationEisele and McMurry 1997; CitationWeber et al. 1998, Citation1999; CitationJanson et al. 2001) and model simulations (CitationKerminen et al. 2001; CitationKulmala et al. 2000; CitationPirjola and Kulmala 2001; CitationAnttila and Kerminen 2003) have indicated that condensation of sulfuric acid alone is often not enough to grow these nuclei to detectable sizes because fresh particles have a short lifetime due to coagulation with larger particles. To account for the growth of the fresh nuclei, condensation of organic species (CitationKerminen et al. 2000; CitationAnttila and Kerminen 2003), heterogeneous reactions (CitationZhang and Wexler 2002), and ion-enhanced condensation (CitationLaakso et al. 2002) have been proposed.
There has been only limited evaluation of multiple nucleation theories against extensive observational datasets. CitationLucas and Akimoto (2006) explored the implications of several nucleation theories for tropospheric nucleation rates but did not compare their predictions to nucleation observations. Most studies that utilize observations focus on a single nucleation theory (CitationKulmala 1998; CitationLovejoy et al. 2004; CitationYu 2006a) and evaluate it for a few days when nucleation events took place. There has been little effort to evaluate nucleation theories in days when no nucleation was observed.
We evaluate six different nucleation parameterizations: (1) the ternary NH3-H2SO4-H2O nucleation parameterization of CitationNapari et al. (2002), (2) the binary H2SO4-H2O parameterization of CitationVehkamäki et al. (2002), (3) the binary H2SO4-H2O parameterization of the CitationJaecker-Voirol and Mirabel (1989) theory by CitationRussell et al. (1994), (4) the semi-empirical first order in sulfuric acid concentration expression proposed by CitationSpracklen et al. (2006), (5) the ion-induced nucleation parameterization of CitationModgil et al. (2005), and (6) the barrierless rate expression of CitationClement and Ford (1999).
We first summarize in section 2 the available measurements from the Pittsburgh Air Quality Study. A brief description of the six different nucleation theories and corresponding parameterizations is given in section 3. In section 4, we describe the aerosol dynamics model used in the simulations. Finally, the results of the simulations and their sensitivities to the parameters in the corresponding theories are presented in section 5.
2. AEROSOL AND GAS-PHASE MEASUREMENTS
During the Pittsburgh Air Quality Study (PAQS), ambient air sampling was conducted from July 2001 to September 2002 (CitationWittig et al. 2004). The measurements collected included PM10, PM2.5, aerosol size distribution, and chemical composition, aerosol physical and optical properties, single particle composition, gaseous species concentrations, and meteorology.
The SO2 concentration was monitored continuously and 10 min average values are used here. The ambient aerosol number size distribution (3 nm to 10 μ m) was monitored using two Scanning Mobility Particle Sizers (SMPS) and an Aerodynamic Particle Sizer (APS) every 15 min (CitationStanier et al. 2004a,Citationb). Temperature and relative humidity were measured every 15 min (CitationWittig et al. 2004) and are used as inputs to the simulation.
NH3(g) is estimated from measurements of total NH3 (PM2.5 ammonium + gas-phase NH3) and measured particulate sulfate and nitrate. A thermodynamic model (GFEMN) (CitationAnsari and Pandis 1999) was used to calculate the partitioning of the total available ammonia and nitric acid in their gas and particulate components. The model was evaluated against the PAQS aerosol measurements by CitationTakahama et al. (2004) and was found to reproduce the aerosol nitrate measurements within experimental error.
CitationStanier et al. (2004b) measured aerosol size distributions at two sampling sites. The main site is located in a park next to the Carnegie Mellon University campus, and another site was located in Florence, PA, 38 km upwind of the main site. Despite Florence being upwind of Pittsburgh most of the time, nucleation took place almost always at both locations during the same days and practically at the same time. In days when there was no nucleation activity in one of the sites, there was no nucleation in the other either. CitationStanier et al. (2004a) concluded that nucleation happens over a wide area (at least a hundred kilometers). This suggests that a box model is a suitable tool for the simulation of regional scale nucleation events in this area.
For the purpose of this study, we define four different types of days as (1) non-nucleation days, (2) weak nucleation days, (3) moderate nucleation days, and (4) strong nucleation days based on the rate of change of the number concentration of particles larger than 10 nm (dN10/dt). Strong events have dN10/dt > 15,000 cm−3 hr−1, moderate events from 4,000 to 15,000, and weak events between 2,000 and 4,000 cm−3 hr−1. No new particle formation refers to a dN10/dt that was not distinguishable from the natural variability in nuclei mode particle concentrations due to local primary sources. Usually the noise threshold was about 2,000 cm−3 hr−1. From the available one year of PAQS measurements, we selected days that had complete datasets (especially estimated gas-phase ammonia concentrations) and span the complete range of nucleation behavior. The days selected as non-nucleation days are July 4, July 7, July 19, Aug. 15, Aug. 28, and Oct. 1, 2001. These were all sunny days where nucleation was probable based on the high production rates of sulfuric acid and the relatively low aerosol surface area. For weak nucleation, July 13, 2001 is selected. July 2, 2001 and July 15, 2001 are selected as moderate nucleation days. For strong nucleation, July 27, 2001 is chosen. These days had all regional nucleation events.
3. NUCLEATION THEORIES
3.1. Ternary NH3-H2SO4-H2O Nucleation
CitationKoorhonen et al. (1999) calculated the Gibbs free energy of formation of a cluster considering three components: water, sulfuric acid, and ammonia. Finding the saddle point of the Gibbs free energy surface they calculated numerically the composition of the critical nucleus. Once the composition was known, the nucleation rate was calculated based on rigorous nucleation kinetics and thermodynamically consistent version of the classical model (CitationTrinkaus 1983). The equilibrium distribution of clusters was obtained from the self-consistent equilibrium distribution (CitationNoppel 2000).
This nucleation rate was parameterized by CitationNapari et al. (2002) using four variables: temperature, relative humidity, H2SO4 concentration, and NH3 mixing ratio. The parameterization is valid for temperatures 240–300 K, relative humidities 5–95%, sulfuric acid concentrations 104–109 molecules cm−3, ammonia mixing ratios 0.1–100 ppt, and nucleation rates 10−5–106 cm−3 s−1. When the NH3 is less than 0.1 ppt the binary sulfuric acid-water parameterization of CitationVehkamäki et al. (2002) is used, while for values of NH3 exceeding 100 ppt, the rate at 100 ppt is used.
The predictions of this parameterization are several orders of magnitude higher than the laboratory measurements for this system (CitationBall et al. 1999). CitationYu (2006b) has developed a kinetic ternary nucleation theory based on these laboratory data. This work suggests that ammonia does not increase significantly the nucleation rate of the sulfuric acid system so it will not be discussed independently.
3.2. Binary Nucleation—CitationJaecker Voirol and Mirabel (1989)
CitationJaecker Voirol and Mirabel (1989) applied a correction to the classical free energy of cluster formation, taking into account the influence of hydrate formation in the gas phase.
The nucleation rate was parameterized by CitationRussell et al. (1994) for relative humidity (RH) from 20 to 100% and of temperature (−50 to 100°C) using the following equation:
3.3. Binary nucleation—CitationVehkamäki et al. (2002)
Similar to CitationJaecker Voirol and Mirabel (1989), this theory considers hydrates in the vapor phase. These small clusters of hydrates have negative formation energy and hinder nucleation by stabilizing the vapor. The number concentrations of i-hydrates are calculated using the equilibrium constants for successive additions of water molecules to an acid molecule. CitationNoppel et al. (2002) provide the details of this classical binary nucleation theory.
The CitationVehkamäki et al. (2002) parameterization is valid for the temperature range 230–300 K, relative humidities 0.01–100% and total sulfuric acid concentrations 104–1011 cm−3. While CitationJaecker Voirol and Mirabel (1989) used the capillarity assumption, CitationNoppel et al. (2002) considered ab-initio structures of small water-sulfuric acid clusters and experimental results to derive equilibrium constants for hydrate formation. CitationNoppel et al. (2002) used the rigorous nucleation kinetics and thermodynamically consistent version of the classical model (CitationTrinkhaus 1983) to calculate the nucleation rate. These factors result in a lower nucleation rate than the one predicted by the original CitationJaecker Voirol and Mirabel (1989) theory without any scaling factors.
3.4. Semi-Empirical First Order Nucleation
According to observations made in Hyytiälä, the particle formation rate in the boundary layer appears to be proportional to sulfuric acid to the power 1 to 2 (CitationSihto et al. 2006). CitationKulmala et al. (2006) compared three different types of nucleation theories: activation, kinetic and thermodynamic. Rates based on the activation, kinetic and thermodynamic approaches are proportional to the power of 1, 2, and 3 of sulfuric acid concentration, respectively. Activation theory is used in this work, and the equation for the nucleation rate is:
3.5. Barrierless Nucleation
If all clusters are stable, one does not need to consider a free energy barrier in the nucleation rate calculations. For example, when there is high enough ammonia, the clusters may be stabilized as ammonium bisulfate. Extending CitationLushnikov and Kulmala (1995), CitationClement and Ford (1999) presented a barrierless nucleation theory. They proposed a nucleation rate expression:
3.6. Ion Induced Nucleation—CitationModgil et al. (2005)
CitationLovejoy et al. (2004) developed a kinetic ion induced nucleation model for sulfuric acid and water nucleation. The uncertainties related to thermodynamic properties of small ionic and neutral clusters were reduced by using the measured value for the binding of H2SO4 and H2O, connecting the measured small cluster thermodynamics to the bulk liquid drop, and use of the kinetic model which treated neutral and ionic clusters explicitly. CitationModgil et al. (2005) developed a parameterization of this ion-induced nucleation theory that is valid for temperature range 190–300 K, relative humidities 0.05–0.95, number concentration of H2SO4 (105–108 cm−3), preexisting aerosol surface area (2-100 μ m2cm− 3), and ion source rate (1–50 cm−3 s−1).
The ion source rate is affected by both radioactive decay of radon and galactic cosmic rays (CitationLaakso et al. 2004; CitationLucas and Akimoto 2006). It increases as one moves from the equator to high latitudes because of the orientation and shape of the Earth's magnetic field, which results in a stronger deflection of the galactic cosmic rays along the magnetic equator compared to the magnetic poles (CitationHensen and van der Hage 1994). An average ionization rate is around 10 ion pairs cm−3 s−1 at a height of 1 m from the ground in continental areas (CitationIsrael 1970, Citation1973; CitationChalmers 1967). CitationLaakso et al. (2004) calculated average ion production rates as 2.6 ion pairs cm−3 s−1 from aerosol size distribution-measurements, and 4.5 ion pairs cm−3 s−1 based on external radiation and radon measurements in a boreal forest. CitationModgil et al. (2005) evaluated that the ion induced nucleation rate is not much affected by the ion source rate above 260 K. An ion source rate of 2 ion pairs cm−3 s−1 is selected as the base case value here (CitationTurco et al. 1998; CitationYu and Turco 2001) and the sensitivity of the results to this value will be investigated.
4. DYNAMIC MODEL FOR AEROSOL NUCLEATION
The Dynamic Model for Aerosol Nucleation (DMAN) (CitationJung et al. 2006) simulates nucleation, coagulation, and condensation/evaporation for a multicomponent aerosol population assuming that the aerosol is internally mixed. The model is based on the TwO-Moment Aerosol Sectional (TOMAS) algorithm (CitationAdams et al. 2002) tracking both mass and number concentrations in each size section simultaneously. The aerosol size distribution is described with 41 size sections with the lowest boundary at 3.75× 10− 25 kg dry aerosol mass per particle. That corresponds to 0.8 nm dry diameter assuming a density of 1.4 g cm−3. Each successive boundary has double the mass of the previous one. The largest bin corresponds to about 10 μ m.
DMAN allows its user to select the nucleation parameterization that will be used in the simulations. Choices include the ternary NH3-H2SO4-H2O nucleation parameterization of CitationNapari et al. (2002), the binary H2SO4-H2O parameterization of CitationVehkamäki et al. (2002), the binary parameterization of CitationJaecker-Voirol and Mirabel (1989) multiplied by a 107 nucleation “tuner” (CitationRussell et al. 1994), the semiempirical first order in sulfuric acid concentration expression proposed by CitationSpracklen et al. (2006), the ion-induced nucleation parameterization of CitationModgil et al. (2005), and the barrierless rate expression of CitationClement and Ford (1999). In all cases the fresh nuclei are placed in the first DMAN size section (0.8–1 nm). The results of the model are relatively insensitive to this simplifying assumption (CitationCapaldo et al. 1999). Additional details about DMAN can be found in CitationJung et al. (2006).
DMAN is used here in a box-model framework following the approach of CitationGaydos et al. (2005). The inputs to DMAN are NH3, OH, SO2, T, and RH, and the initial size distribution. SO2, T, RH, and initial distribution were measured directly while NH3 and OH were calculated based on measurements (see CitationGaydos et al. [2005] for details). The OH concentration was estimated based on the measured UV intensity each day (temporal profile) and the predictions of a 3-D Chemical Transport Model for the area (CitationKarydis et al. 2007). The sensitivity of the results to the estimated OH will be discussed in a subsequent section. The outputs of DMAN are number and mass concentrations of particles as a function of time. The model does not consider in this application the direct emission of nanoparticles from vehicle and other sources.
5. RESULTS
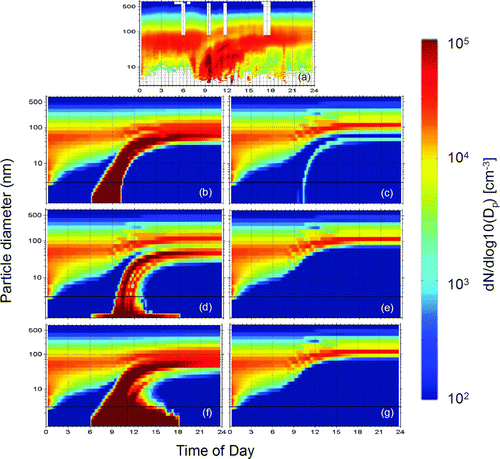
The predictions of DMAN using six different nucleation parameterizations for July 15 (moderate nucleation) are shown in . Three of the parameterizations (ternary NH3-H2SO4-H2O, the empirical nucleation theory of CitationSpracklen et al. (2006), and the CitationClement and Ford (1999) expression for barrierless nucleation) reproduce the occurrence of the event. The CitationRussell et al. (1994) parameterization predicts a very weak nucleation event that lasts only a few minutes. The other two theories predict no nucleation on this day.
FIG. 1 Evolution of the aerosol number distribution for July 15, 2001 in Pittsburgh, Pennsylvania: (a) observations, (b) ternary NH3-H2SO4-H2O (CitationNapari et al. 2002), (c) binary H2SO4-H2O (CitationRussell et al. 1994), (d) binary H2SO4-H2O (CitationVehkamäki et al. 2002), (e) first order in sulfuric acid (CitationSpracklen et al. 2006), (f) barrierless nucleation (CitationClement and Ford 1999), and (g) ion-induced (CitationModgil et al. 2005) parameterizations. Particle number distribution (z-axis) is plotted against time of day (x-axis) and particle diameter (y-axis). The predicted diameter range extends to 0.8 nm while the measured to only 3 nm. A black line in the predicted diameter range indicates the measurement threshold, 3 nm. Eastern Standard Time (EST) is used.
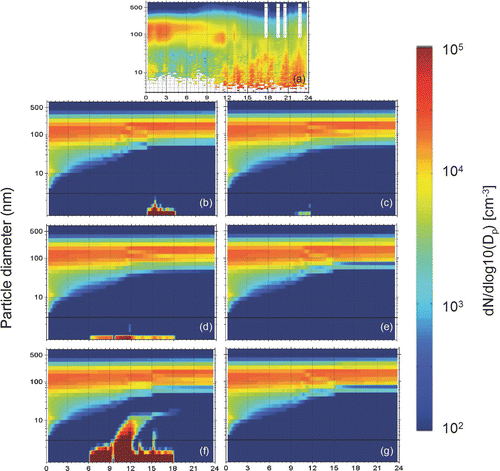
For a non-nucleation day, July 4, 2001, five parameterizations predict no in-situ formation of detectable particles, but the barrierless nucleation theory predicts a strong nucleation event and formation of 15,000 cm− 3 new particles above 10 nm (). The ternary parameterization, the binary parameterization of CitationRussell et al. (1994), and the first order nucleation of CitationSpracklen et al. (2006) predict formation of nuclei but no particles grow to detectable size (3 nm); therefore, their predictions are in agreement with the observations. These results suggest that days when no formation of particles was observed are important for the evaluation of nucleation theories that may tend to overestimate the frequency of nucleation.
FIG. 2 Evolution of the aerosol number distribution for July 4, 2001 in Pittsburgh, Pennsylvania for: (a) observations, (b) ternary NH3-H2SO4-H2O (CitationNapari et al. 2002), (c) binary H2SO4-H2O (CitationRussell et al. 1994), (d) binary H2SO4-H2O (CitationVehkamäki et al. 2002), (e) first order in sulfuric acid (CitationSpracklen et al. 2006), (f) barrierless nucleation (CitationClement and Ford 1999), and (g) ion-induced (CitationModgil et al. 2005) parameterizations. Particle number concentration (z-axis) is plotted against time of day (x-axis) and particle diameter (y-axis). The predicted diameter range extends to 0.8 nm while the measured to only 3 nm. A black line in the predicted diameter range indicates the measurement threshold, 3 nm. Eastern Standard Time (EST) is used.
For non-nucleation days, all theories do not predict nucleation on July 19, 2001 (). For July 4 and Aug. 28, 2001, all nucleation theories do not predict new formation of particles except the barrierless nucleation. For July 7, 2001, both the first order nucleation theory and the barrierless nucleation predict nucleation while other theories reproduce the lack of an event. For Aug. 15 and Oct. 1, 2001, the binary nucleation of CitationJaecker-Voirol and Mirabel (1989) also predicts the formation of the small particles together with the 1 st order and the barrierless nucleation. For a weak nucleation day, July 13, 2001, the ternary, 1 st order, and barrierless nucleation theories predict nucleation. For the two moderate nucleation days, July 2 and July 15, 2001, ternary, the binary of CitationRussell et al. (1994), 1 st order, and the barrierless nucleation predict nucleation events, but the other two parameterizations fail to reproduce the observed event. For a strong nucleation day, July 27, 2001, ternary, 1 st order, and barrierless nucleation theories predict correctly.
TABLE 1 Summary of DMAN predictions with different nucleation parameterizations
shows a summary of the results of the six different nucleation parameterizations. While ternary nucleation predicts the occurrence or lack of an event for all ten simulation days correctly, the number of correct predictions of the other five nucleation theories are between 5 and 7. Considering the fact that random guesses would result on average in 5 correct predictions, the predictions of the other five nucleation theories in Pittsburgh are little better than random guesses.
5.1. Sensitivity Tests
5.1.1. Scaling of Nucleation Rate
Given uncertainties in the nucleation rates predicted by any nucleation theory, it is worth considering whether a given nucleation parameterization might perform better with a scaling factor applied to the nucleation rate. This section investigates to what extent the predictions of the five poorly performing parameterizations may be improved.
The binary parameterization of CitationRussell et al. (1994) does not predict nucleation on two days, July 13 and July 27, 2001 when nucleation happens, and predicts nucleation on two days, Aug. 15 and Oct. 1, 2001 when nucleation did not occur. Increasing the nucleation tuner to 1010 increases the duration of the nucleation events. So the predicted nucleation bursts become more similar to observation for the two average nucleation days, July 2 and July 15 (not shown). However, it also results in small nucleation bursts on July 7 and August 28 when nucleation was not observed. Overall, the number of days correctly predicted is reduced from 6 to 5. Decreasing the nucleation tuner to 104 results in lack of nucleation in all days ().
TABLE 2 Sensitivity tests of DMAN predictions with different nucleation parameterizations
The binary nucleation parameterization of CitationVehkamäki et al. (2002) did not reproduce any observed nucleation event. Multiplying the rate by a correction factor of 1010 results in one predicted event. However, this event occurs on Oct. 1, 2001, a day when nucleation was not observed. Increasing the rate by a factor of 1015, one more nucleation day, July 2, 2001, is predicted correctly at this time. The number of days correctly predicted becomes six after this correction. However, nucleation is still not predicted for the other three nucleation days.
The empirical nucleation theory used by CitationSpracklen et al. (2006) predicts nucleation events when nucleation was not observed on three days: July 7, Aug. 15, and Oct. 1, 2001. The rate constant, k, was reduced to 0.5 × 10−6s−1 the value used in CitationKulmala et al. (2006). The strong nucleation event day of July 27, 2001 was not reproduced anymore decreasing the correct predictions from 7 to 6. However, despite the decrease of the k value, the parameterization continues to predict nucleation on those three days when it was not observed. The k value was then further reduced to a value of 0.1 × 10−7 s−1. This “turned off” nucleation on Aug. 15, 2001 which used to be predicted incorrectly, and the number of correct predictions increased to 7, the same as the base case.
The theoretical barrierless nucleation by CitationClement and Ford (1999) predicts nucleation events on 5 days when no nucleation was observed. We scaled this nucleation rate by a factor of 10−3. This change improved the model performance on July 4, reproducing the lack of nucleation. The duration of nucleation was reduced for the four non-nucleation days, July 7, Aug. 15, Aug. 28, and Oct. 1, 2001 but it was not turned off. We reduced the nucleation factor further to 10−6, with no change of the number of correct predictions. This change improved the model performance for Aug. 28 but also “turned off” incorrectly nucleation on July 27.
The ion induced nucleation parameterization by CitationModgil et al. (2005) did not predict any nucleation event with an ion production rate of 2 ion pairs cm−3 s−1 in the base case scenario. We varied the ion production rate in the 2–50 ion pairs cm−3 s−1 range, but still the model failed to reproduce any of the observed events. The ion-induced nucleation theory did not predict any significant nucleation events in a 3D global modeling application by CitationLucas and Akimoto (2006). This is also consistent with the results of field studies (CitationEisele et al. 2006) suggesting that ion-induced nucleation plays a limited role in the lower troposphere. However, the modeling work of CitationYu (2006a) has suggested that ion induced nucleation can take place in the lower atmosphere under favorable conditions.
5.1.2. Sensitivity to Estimated OH
The estimated OH concentrations following the approach of CitationGaydos et al. (2005) introduce significant uncertainties to the simulated H2SO4 concentration in the model. To investigate their effect we have repeated all simulations by increasing and decreasing the OH concentration by a factor of two. The DMAN results for the binary nucleation of CitationVehkamäki et al. (2002), and the ion induced nucleation parameterization of CitationModgil et al. (2005) were not affected by these OH changes. No nucleation events were predicted in any of the days in all tests. The results for the remaining four parameterizations are summarized in . Overall, the changes of the OH caused at most change in the model performance in one of the 10 days.
TABLE 3 Sensitivity tests of DMAN predictions to the OH concentration
For the ternary nucleation parameterization reducing the OH by a factor of two still resulted in ten correct predictions. On the other hand, the doubling of the OH resulted in one incorrect prediction of a nucleation event on a day (July 7) where no nucleation bursts were observed. This insensitivity is consistent with the conclusions of CitationGaydos et al. (2005) about the importance of ammonia as the limiting reactant for nucleation during the summer in that area.
6. CONCLUSIONS
The only parameterization that reproduced the observations on all ten days was the ternary expression of CitationNapari et al. (2002). The barrierless expression of CitationClement and Ford (1999) predicted nucleation events on most of the days, while the ion-induced nucleation expression and the binary H2SO4-H2O parameterization of CitationVehkamäki et al. (2002) predicted that there should have been no nucleation events. The binary CitationRussell et al. (1994) parameterization (after its rate increase by 107) reproduces 2 out of the 4 events, but also predicts nucleation in 2 out of the 6 non-event days. The semi-empirical expression of CitationSpracklen et al. (2006), based to a large extent on measurements in the boreal forest, performs well on 7 out of 10 days in this sulfur-rich relatively polluted environment. However, it also predicts three nucleation events on days when there was no nucleation. Ion-induced nucleation of CitationModgil et al. (2005) does not predict any nucleation with the ion production rate of 2 ion pairs cm−3 s−1. The ion-induced nucleation day of CitationModgil et al. (2005) does not predict any nucleation for the all days simulated even with ion production rate of 50 ion-pairs cm−3 s−1. Sensitivity analysis shows that scaling the rates by a constant nucleation factor does not improve their performance significantly. The uniform scaling results in better performance on some days but these gains are offset by deteriorating performance on others. The performance of the various parameterizations is also relatively insensitive to changes of the estimated OH concentration within a factor of two. This robustness suggests that it is important in similar evaluation studies to consider the performance of the various theories on days when nucleation did not take place.
This work was supported by NSF ATM-0336296 and the European Commission through the project EUCAARI. The authors thank C. O. Stanier for his help with the selection of the events to be modeled.
Notes
1Capitals are used if the model reproduced the observations and lowercase if it failed to capture the occurrence or lack of a nucleation event.
1Capitals are used if the model reproduced the observations and lowercase if it failed to capture the occurrence or lack of a nucleation event.
1Capitals are used if the model reproduced the observations and lowercase if it failed to capture the occurrence or lack of a nucleation event.
REFERENCES
- Ansari , A. S. and Pandis , S. N. 1999 . Prediction of Multicomponent Inorganic Atmospheric Aerosol Behavior . Atmos. Environ. , 33 : 745 – 757 .
- Adams , P. J. and Seinfeld , J. H. 2002 . Predicting Global Aerosol Size Distributions in General Circulation Models . J. Geophys. Res. , 107 : 4370
- Adams , P. J. and Seinfeld , J. H. 2003 . Disproportionate Impact of Particulate Emissions on Global Cloud Condensation and Nuclei Condensations . Geophys. Res. Lett. , 30 : 1239 – 1243 .
- Albrecht , B. A. 1989 . Aerosols, Cloud Microphysics, and Fractional Cloudiness . Science , 245 : 1227 – 1230 .
- Anttila , T. and Kerminen , V.-M. 2003 . Condensational Growth of Atmospheric Nuclei by Organic Vapours . J. Aerosol Sci. , 34 : 41 – 61 .
- Ball , S. M. , Hanson , D. R. , Eisele , F. L. and McMurry , P. H. 1999 . Laboratory Studies of Particle Nucleation, Initial Results for H2SO4, H2O, and NH3 Vapor . J. Geophys. Res. , 104 : 23709 – 23718 .
- Capaldo , K. P. , Kasibhatla , P. and Pandis , S. N. 1999 . Is Aerosol Production within the Remote Marine Boundary Layer Sufficient to Maintain Observed Concentrations? . J. Geophys. Res. , 104 : 3483 – 3500 .
- Chalmers , J. 1967 . Atmospheric Electricity , Oxford, London : Pergamon Press .
- Clement , C. F. and Ford , I. J. 1999 . Gas-to-Particle Conversion in the Atmosphere, II. Analytical Models of Nucleation Bursts . Atmos. Environ. , 33 : 489 – 499 .
- Coffman , D. J. and Hegg , D A. 1995 . A Preliminary Study of the Effect of Ammonia on Particle Nucleation in the Marine Boundary Layer . J Geophys. Res. , 100 : 7147 – 7160 .
- Donaldson , K. , Brown , D. , Clouter , A. , Duffin , R , MacNee , W. , Renwick , L. , Tran , L. and Stone , V. 2002 . The Pulmonary Toxicology of Ultrafine Particles . J. Aerosol Med. , 15 : 213 – 220 .
- Donaldson , K. , Li , X. Y. and MacNee , W. 1998 . Ultrafine (Nanometre) Particle Mediated Lung Injury . J. Aerosol Sci. , 29 : 553 – 560 .
- Eisele , F. L. , Lovejoy , E. R. , Kosciuch , E. , Moore , K. F. , Mauldin , R. L. III , Smith , J. N. , McMurry , P. H. and Iida , K. 2006 . Negative Atmospheric Ions and Their Potential Role in Ion-Induced Nucleation . J. Geophys. Res. , 111 : D04305
- Eisele , F. L. and McMurry , P H. 1997 . Recent Progress in Understanding Particle Nucleation and Growth . The Royal Society , 352 : 191 – 201 .
- Gaydos , T. M. , Stanier , C O. and Pandis , S. N. 2005 . Modeling of In Situ Ultrafine Atmospheric Particle Formation in the Eastern United States . J. Geophys. Res. , 110 : D07S12
- Hensen , A. and van der Hage , J. C. H. 1994 . Parameterization of Cosmic Radiation at Sea Level . J. Geophys. Res. , 99 : 10693 – 10695 .
- Hoffmann , T. , O'Dowd , C. D. and Seinfeld , J. H. 2001 . Iodine Oxide Homogeneous Nucleation, An Explanation for Coastal New Particle Production . Geophys. Res. Lett. , 28 : 1949 – 1952 .
- Israel , H. 1970 . Atmospheric electricity , Vol 1 , Jerusalem : Israel Program for Scientific Translations .
- Israel , H. 1973 . Atmospheric electricity , Vol 2 , Jerusalem : Israel Program for Scientific Translations .
- Jaecker-Voirol , A. and Mirabel , P. 1989 . Heteromolecular Nucleation in the Sulfuric Acid-Water System . Atmos. Environ. , 23 : 2053 – 2057 .
- Janson , R. , Rosman , K. , Karlsson , A. and Hansson , H.-C. 2001 . Biogenic Emissions and Gaseous Precursors to Forest Aerosols . Tellus Ser. B. , 53 : 423 – 440 .
- Jung , J. , Adams , P. J. and Pandis , S. N. 2006 . Simulating the Size Distribution and Chemical Composition of Ultrafine Particles During Nucleation Events . Atmos. Environ. , 40 : 2248 – 2259 .
- Karydis , V. A. , Tsimpidi , A. P. and Pandis , S. N. 2007 . Evaluation of a Three-Dimensional Chemical Transport Model (PMCAMx) in the Eastern United States for all Four Seasons . J. Geophys. Res , 112 : D14211 doi,10.1029/2006JD007890
- Kerminen , V.-M. , Pirjola , L. and Kulmala , M. 2001 . How Significantly does Coagulational Scavenging Limit Atmospheric Particle Production? . J. Geophys. Res. , 106 ( 24 ) : 119 – 24125 .
- Kerminen , V.-M. , Virkkula , A. , Hillamo , R. , Wexler , A. S. and Kulmala , M. 2000 . Secondary Organics and Atmospheric Cloud Condensation Nuclei Production . J. Geophys. Res. , 105 : 9255 – 9264 .
- Korhonen , P. , Laaksonen , A. , Viisanen , Y. , McGraw , R. and Seinfeld , J. H. 1999 . Ternary Nucleation of H2SO4, NH3, and H2O in the Atmosphere . J. Geophys. Res. , 104 : 26349 – 26353 .
- Kulmala , M. , Laaksonen , A. and Pirjola , L. 1998 . Parameterizations for Sulfuric Acid/Water Nucleation Rates . J. Geophys. Res. , 103 : 8301 – 8307 .
- Kulmala , M. , Pirjola , L. and Mäkelä , J. M. 2000 . Stable Sulphate Clusters as a Source of New Atmospheric Particles . Nature , 404 : 66 – 69 .
- Kulmala , M. , Korhonen , P. , Napari , I. , Karlsson , A. , Berresheim , H. and O'Dowd , C. D. 2002 . Aerosol Formation During PARFORCE, Ternary Nucleation of H2SO4, NH3, and H2O . J. Geophys. Res. , 107 : 8111
- Kulmala , M. , Kerminen , V.-M. , Anttila , T. , Laaksonen , A. and O'Dowd , C. D. 2004 . Organic Aerosol Formation via Sulfate Cluster Activation . J. Geophys. Res. , 109 : D04205
- Kulmala , M. , Lehtinen , K. E. J. and Laaksonen , A. 2006 . Cluster Activation Theory as an Explanation of the Linear Dependence between Formation Rate of 3 nm Particles and Sulphuric Acid Concentration . Atmos. Chem. Phys. , 6 : 787 – 793 .
- Laakso , L. , Mäkelä , J. M. , Pirjola , L. and Kulmala , M. 2002 . Model Studies on Ion–Induced Nucleation in the Atmosphere . J. Geophys. Res. , 107 : 4427
- Laakso , L. , Petäjä , T. , Lehtinen , K. E. J. , Kulmala , M , Paatero , J. , H[otilde]rrak , U. , Tammet , H. and Joutsensaari , J. 2004 . Ion Production Rate in a Boreal Forest Based on Ion, Particle and Radiation Measurements . Atmos. Chem. Phys. , 4 : 1933 – 1943 .
- Laaksonen , A. , McGraw , R. and Vehkamäki , H. 1999 . Liquid-Drop Formalism and Free-Energy Surface in Binary Homogeneous Nucleation Theory . J. Chem. Phys. , 111 : 2019 – 2027 .
- Lovejoy , E. R. , Curtius , J. and Froyd , K. D. 2004 . Atmospheric Ion-Induced Nucleation of Sulfuric Acid and Water . J. Geophys. Res. , 109 : D08204
- Lucas , D. D. and Akimoto , H. 2006 . Evaluating Aerosol Nucleation Parameterizations in a Global Atmospheric Model . Geophys. Res. Lett. , 33 : L10808
- Lushnikov , A. A. and Kulmala , M . 1995 . Source-Enhanced Condensation in Monocom–Ponent Disperse Systems . Phys. Rev. E. , 52 : 1658 – 1668 .
- Marti , J. J. , Weber , R. J. , McMurry , P. H. , Eisele , F. , Tanner , D. and Jefferson , A. 1997 . New Particle Formation at a Remote Continental Site, Assessing the Contributions of SO2 and Organic Precursors . J. Geophys. Res. , 102 : 6331 – 6339 .
- Modgil , M. S. , Kumar , S. , Tripathi , S. N. and Lovejoy , E. R. 2005 . A Parameterization of Ion-Induced Nucleation of Sulphuric Acid and Water for Atmospheric Conditions . J. Geophys. Res. , 110 : D19205
- Napari , I. , Noppel , M. , Vehkamäki , H. and Kulmala , M. 2002 . Parameterization of Ternary Nucleation Rates for H2SO4-NH3-H2O Vapors . J. Geophys. Res. , 107 : 4381 – 4386 .
- Noppel , M. 2000 . Self-Consistent Binary Cluster Size Distributions of Sulfuric Acid-Water System . Nucleation and Atmospheric Aerosols. 15th International Conference . 2000 , Melville, New York. Edited by: Hale , B. and Kulmala , M. pp. 339 – 342 .
- Noppel , M. , Vehkamäki , H. and Kulmala , M. 2002 . An Improved Model for Hydrate Formation in Sulfuric Acid-Water Nucleation . J. Chem. Phys. , 116 : 218 – 228 .
- Pirjola , L. and Kulmala , M. 2001 . Development of Particle Size and Composition Distributions with a Novel Aerosol Dynamics Model . Tellus Ser. B. , 53 : 491 – 509 .
- Raes , F. , Saltelli , A. and Van Dingenen , R. 1992 . Modelling Formation and Growth of H2SO4-H2O Aerosols, Uncertainty Analysis and Experimental Evaluation . J. Aerosol Sci. , 23 : 759 – 771 .
- Riipinen , I. , Sihto , S.-L. , Kulmala , M. , Arnold , F. , Dal Maso , M. , Birmili , W. , Saarnio , K. , Teinilä , K. , Kerminen , V.-M. , Laaksonen , A. and Lehtinen , K. E. J. 2007 . Connections between Atmospheric Sulphuric Acid and New Particle Formation During QUEST III–IV Campaigns in Heidelberg and Hyytiälä . Atmos. Chem. Phys. , 7 : 1899 – 1914 .
- Russell , L. M. , Pandis , S. N. and Seinfeld , J. H. 1994 . Aerosol Production and Growth in the Marine Boundary Layer . J. Geophys. Res. , 99 ( 20 ) : 989 – 21,003 .
- Sihto , S.-L. , Kulmala , M. , Kerminen , V.-M. , Dal Maso , M. , Petäjä , T. , Riipinen , I. , Korhonen , H. , Arnold , F. , Janson , R. , Boy , M. , Laaksonen , A. and Lehtinen , K. E. J. 2006 . Atmospheric Sulphuric Acid and Aerosol Formation, Implications from Atmospheric Measurements for Nucleation and Early Growth Mechanisms . Atmos. Chem. Phys. Diss. , 6 : 3845 – 3882 .
- Spracklen , D. V. , Carslaw , K. S. , Kulmala , M. , Kerminen , V.-M. , Mann , G. W. and Sihto , S.-L. 2006 . The Contribution of Boundary Layer Nucleation Events to Total Particle Concentrations on Regional and Global Scales . Atmos. Chem. Phys. , 6 : 5631 – 5648 .
- Stanier , C. O. , Khlystov , A. Y. and Pandis , S. N. 2004a . Ambient Aerosol Size Distributions and Number Concentrations Measured During the Pittsburgh Air Quality Study (PAQS) . Atmos. Environ. , 38 : 3275 – 3284 .
- Stanier , C. O. , Khlystov , A. Y. and Pandis , S. N. 2004b . Nucleation Events During the Pittsburgh Air Quality Study, Description and Relation to Key Meteorological, Gas Phase, and Aerosol Parameters . Aerosol Sci. Technol. , 38 : 253 – 264 .
- Takahama , S. , Wittig , A E. , Vayenas , D. V. , Davidson , C. I. and Pandis , S. N. 2004 . Modeling the Diurnal Variation of Nitrate During the Pittsburgh Air Quality Study . J. Geophys. Res. , 109 : D16S06
- Trinkhaus , H. 1983 . Theory of the Nucleation of Multicomponent Precipitates . Phys. Rev. B. , 27 : 7372 – 7378 .
- Turco , R. P. , Zhao , J.-X. and Yu , F. 1998 . A New Source of Tropospheric Aerosols, Ion–Ion Recombination . Geophys. Res. Lett. , 25 : 635 – 638 .
- Twomey , S. 1974 . Pollution and the Planetary Albedo . Atmos. Environ. , 8 : 1251 – 1256 .
- Vehkamäki , H. , Kulmala , M. , Napari , I. , Lehtinen , K. E. J. , Timmreck , C. , Noppel , M. and Laaksonen , A. 2002 . An Improved Parameterization for Sulfuric Acid-Water Nucleation Rates for Tropospheric and Stratospheric Conditions . J. Geophys. Res. , 107 : 4622 – 4632 .
- Weber , R. J. , McMurry , P. H. , Mauldin , R. L. III , Tanner , D. J. , Eisele , F. L. , Clarke , A. D. and Kapustin , V. N. 1999 . New Particle Formation in the Remote Troposphere, A Comparison of Observations at Various Sites . Geophys. Res. Lett. , 26 : 307 – 310 .
- Weber , R. J. , McMurry , P. H. , Mauldin , L. , Tanner , D. J. , Eisele , F. L. , Brechtel , F. J. , Kreidenweis , S. M. , Kok , G. L. , Schillawski , R. D. and Baumgardner , D. 1998 . A Study of New Particle Formation and Growth Involving Biogenic and Trace Gas Species Measured During ACE 1 . J. Geophys. Res. , 103 : 16385 – 16396 .
- Wittig , A. E. , Anderson , N. , Khlystov , A. Y. , Pandis , S. N. , Davidson , C. and Robinson , A. 2004 . Pittsburgh Air Quality Study Overview . Atmos. Environ. , 38 : 3107 – 3125 .
- Yu , F. 2006a . From Molecular Clusters to Nanoparticles, Second-Generation Ion-Mediated Nucleation Model . Atmos. Chem. Phys. , 6 : 5193 – 5211 .
- Yu , F. 2006b . Effect of Ammonia on New Particle Formation, A Kinetic H2SO4-H2O-NH3 Nucleation Model Constrained by Laboratory Measurements . J. Geophys. Res. , 111 : D01204
- Yu , F. and Turco , R. P. 2001 . From Molecular Clusters to Nanoparticles, Role of Ambient Ionization in Tropospheric Aerosol Formation . J. Geophys. Res. , 106 : 4797 – 4814 .
- Zhang , K. M. and Wexler , A. S. 2002 . A Hypothesis for Growth of Fresh Atmospheric Nuclei . J. Geophys. Res. , 107 : 4577
- Zhang , R. , Suh , I. , Zhao , J. , Zhang , D. , Fortner , E. C. , Tie , X. , Molina , L. T. and Molina , M. J. 2004 . Atmospheric New Particle Formation Enhanced by Organic Acids . Science , 304 : 1487 – 1490 .