Abstract
Previous studies have found significant quantities of oxidative species associated with airborne particulate matter. Although oxidative stress is thought to be an important part of the mechanism by which particles produce adverse health effects, the lack of a suitable method to measure ROS on a routine basis has resulted in no work being undertaken to assess the effects of particle-bound ROS on health. In order to fill this need, an automated monitor for the continuous sampling of ambient aerosol and the measurement of concentrations of ROS on the sampled aerosol was developed. Potential methods to quantify ROS were compared in order to arrive at a suitable method to automate. The dichlorofluorescein (DCFH) fluorescence method was found to be the most non-specific, general indicator of particle-bound oxidants. Hence it was deemed the best suited method for the automated monitor. An integrated sampling-analysis system was designed and constructed based on collection of atmospheric particles in an aqueous slurry, and subsequent detection of the ROS concentration of the slurry using the DCFH fluorescence method. The results of the lab-scale investigation of the ROS sampling-analysis system suggested that the prototype continuous system was capable of detecting particle-bound ROS, and accounting for short-term variabilities in the same. The instrument was found to be capable of detecting nanomolar equivalent concentrations of ROS.
INTRODUCTION
Because of their long lifetimes in the ambient air and respiratory deposition characteristics, over the last decade, fine particles (PM2.5) have been linked to a range of respiratory and cardiovascular health problems. Numerous epidemiological studies conducted over this period have shown that PM2.5 is correlated with severe health effects, including enhanced mortality (CitationBernstein et al. 2004 and references therein). A central hypothetical mechanism of how particles affect human health involves the generation of reactive oxygen species (ROS) at target sites in the lung. ROS has been defined to include families of oxygen-centered or related free radicals, ions, and molecules. The free radical family includes hydroxyl, hydroperoxyl, and organic peroxy radicals. Ions such as the superoxide, hypochlorite, and peroxynitrite ions, and molecules such as hydrogen peroxide, organic and inorganic peroxides also come under the umbrella of “Reactive Oxygen Species.” Much of the attention has focused on the formation of ROS in situ after particle deposition in the respiratory tract generally through the interaction with transition metal ions (CitationStohs et al. 1997), organic hydrocarbons, such as polycyclic aromatic hydrocarbons and quinones (CitationSquadrito et al. 2001), and ultrafine particle surfaces (CitationLi et al. 2003). However, recent work has shown that ROS is present in the atmosphere on respirable particles to which we are exposed (CitationHung and Wang 2001; CitationHasson and Paulson 2003; CitationVenkatachari et al. 2005, Citation2007). The hypothesis that the ROS present on particles could cause the same kind of systemic dysfunction as endogenously generated ROS has clear merit and represents a fundamental issue for further investigation.
The lack of a suitable method to measure ROS on a routine basis has resulted in no work being undertaken to assess the effects of particle-bound ROS on health. The Aerosol Research and Inhalation Epidemiology Study (ARIES), an extensive air quality characterization study investigating air quality parameters and human health, in Atlanta, GA, measured all of the hypothesized causative components of the air pollution mixture except for peroxides. They cite the lack of an automated method as the reason for its omission from their suite of measurements. Increasing interest in ROS as a significant component of particle toxicity has been recognized by the Environmental Protection Agency (EPA), and they are routinely measuring ROS in their Concentrated Ambient Particle (CAP) samples to which animal models are being exposed. Manual methods are available for measuring ROS (CitationVenkatachari et al. 2005; CitationMudway et al. 2004; CitationHung and Wang 2001), involving filter collection for a reasonable period of time, and subsequent analyses of the filter extract.
However, a drawback of this method is that the chemical analysis of the collected particles is usually conducted after the aerosol is sampled on filters or other sampling devices. Short-lived oxidative species associated with the aerosol, including hydrogen peroxide, organic peroxides and free radicals, may be much more biochemically active than the components measured days or weeks later. This loss leads to possibly significant underestimation of the ambient particle-bound ROS concentrations. To combat this problem, the analysis must be done in the field immediately after collection, making the operation extremely labor-intensive, difficult to implement, and impracticable for an extended sampling period (for e.g., 24 hours) that is typical of most sampling programs. The difficulty of obtaining timely and accurate measurements is a stumbling block for both the research and regulatory communities. As a result, there is a need for an automated instrument that can fulfill the requirements of both these interest groups.
The following are among the expected benefits of developing instrumentation for the automated sampling and chemical analysis of ROS: (1) The instrument would provide information on the distribution of ROS as a function of location, time of day, and day of year; (2) It would aid the understanding of the evolution of ambient particle compositions vis-á-vis ROS, as they are transported from their sources to the receptor site, subsequently helping apportion ROS to individual sources. Accomplishing this would also help group and rank the PM sources based on health outcomes; (3) Sufficient measurements would permit the statistical evaluation of the role of ambient ROS in the induction of adverse health effects as well as the assessment of the overall oxidative capacity of PM as a predictor for their toxicological effects; and (4) there is widespread concurrence on the need for science-based regulations, and a composition-specific standard for particulate matter concentrations. An automated instrument for the measurement of ROS could conceivably be used to determine compliance in such a scenario.
In atmospheric applications, the requisite sensitivity involved in the trace determinations of atmospheric oxidants limits the choice to photo-luminescent and spectrophotometric methods. Several ROS quantification methods have been used and described in the literature. CitationCho et al. (2005) have developed an assay for PM redox activity, utilizing the reduction of oxygen by dithiothreitol (DTT) that serves as an electron source. CitationHasson and Paulson (2003) used the peroxidase enzyme catalyzed reaction of hydroperoxides with p-hydroxyphenylacetic acid (POHPAA) to produce a dimer that fluoresces strongly, under alkaline conditions at excitation and emission wavelengths of 320 and 400 nm, respectively, to quantify aerosol-bourne ROS in urban air. A fluorescence technique based on the oxidation of deacetylated (via NaOH) 2′-7′-dichlorodihydrofluorescein diacetate (DCFH) to its fluorescent product, 2′-7′-dichlorodihydrofluorescein (DCF), with excitation and emission wavelengths of 485 and 530 nm, respectively, in a phosphate buffer containing the radical electron acceptor, horseradish peroxidase (CitationHung and Wang 2001; CitationVenkatachari et al. 2005, Citation2007) has also been used in prior studies to quantify particle-bound ROS in the ambient atmosphere. The development of an automated ROS monitor involves the selection of the best available analytical approach to quantify the ROS, and the subsequent integration of the best analytical approach to a suitable sampling system. The development of the integrated sampling-analysis system configuration, and laboratory validation of the performance of the ROS monitor will be presented.
EXPERIMENTAL SECTION
Comparison of Potential Analytical Approaches
Each of aforementioned ROS measurement methods will respond to different oxidants and have different sensitivities and problems with respect to automating the procedures. Initially, it is not known which ROS species are important for health or environmental effects. A general, non-specific measurement of any oxidative species that has the capability to oxidize the antioxidant lining of the human respiratory tract and lead to damage to the underlying tissue needs to be measured by the ROS monitor. With this in mind, the DTT, POPHAA, and DCFH methods for detecting ROS were evaluated and compared in terms of their strengths, limitations, and ease of automation. The reactivities of the three methods to the various oxidative groups likely to be found in ambient aerosol (namely hydroperoxides, organic peroxides, alkylperoxyl radicals, and redox active organics), and to interferences in terms of positive artifacts from other common functional groups that could also be present in the organic fraction of the aerosol (namely, acids, aldehydes, ketones, and alcohols), were compared. The range of concentrations explored with the surrogate compounds for these functional groups was 0.1–10 μ M.
Compounds were chosen to perform a comparison of the three analytical approaches for quantifying particle-bound ROS (see ). They were selected such that it would be possible to compare the reactivities of the three methods to the various oxidative groups likely to be found in ambient aerosol, namely hydroperoxides, organic peroxides, alkylperoxyl radicals, and redox active organics. The reactivities of the three methods to interferences in terms of positive artifacts from other common functional groups that could also be present in the organic fraction of the aerosol, namely, acids, aldehydes, ketones, and alcohols, were also compared. The surrogate compounds representing the various functional groups are detailed in . Tert-butyl hydroperoxide (98%, TBHP), di-tert butyl peroxide (≥ 95%, DTBP), phenanthroquinone (99+%, P-Q), cis-pinonic acid (98%, CPA), and 4-hydroxy 3-methoxy cinnamaldehyde (98%, HMCA) were purchased from Sigma Aldrich (St. Louis, MO). These chemicals, along with sodium hypochlorite (4–6%, Fisher, Fair Lawn, NJ), and 2,2′-azobis (2-amidinopropane) dihydrochloride (Calbiochem, San Diego, CA, AAPH) were used without further purification. 2,2-azobis (2- amidinopropane) dihydrochloride is an azo-initiator that forms alkyl radicals as a result of thermal decomposition, and these alkyl radicals can react with molecular oxygen to give alkylperoxyl radicals (CitationDamiani et al. 2000).
TABLE 1 Tested functional groups and surrogate test compounds
summarizes the results of the responses of the analytical systems to the surrogate ROS species. It was observed that the DCFH fluorogenic probe was the most responsive method in that it reacted unselectively with the oxidant species, while not being affected as much as the other two methods by the positive interference compounds. The non-specificity of DCFH towards the oxidative species occurs largely due to the ease of abstraction of the hydrogen atom located at the 9′ position of the DCFH molecule, considered the central carbon atom of a triphenylmethane. However, DCFH was prone to autooxidation, and a consequent spontaneous increase in fluorescence on exposure to light. The POPHAA and DTT assays were found to be too specific in their responses to be used to quantify total ROS concentrations. The POPHAA fluorescence technique was found to be reliable, and free from interferences such as autooxidation on exposure to light or air. However, a positive method response from the POPHAA assay was seen only from strongly oxidative species such as hydrogen peroxide, thus creating the possibility of underestimating the ambient particle-bound ROS concentrations. DTT is a strong oxidizing agent, and has a tendency towards autooxidation in air, and hence care would have to be taken to account for this with the blank or conduct the experiment in an inert atmosphere. The DTT assay was seen to respond to redox active organic compounds such as phenanthroquinone, although the response was not linear and that would make it difficult to automate. Moreover, it did not respond to any other oxidative groups that fell under the umbrella of ROS. Hence, the DCFH fluorescence technique was determined to be the method of choice for use in the automated ROS monitoring configuration.
TABLE 2 Responses of the various ROS measurement agents with the surrogate compounds
Design and Construction of the Integrated ROS Sampling-Analysis System
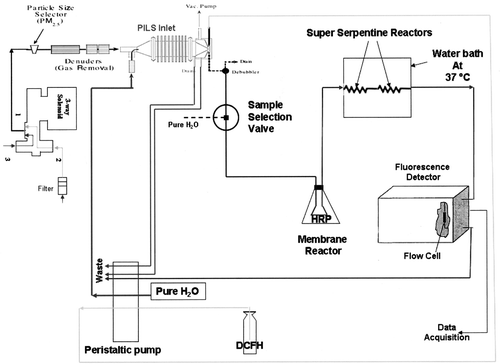
A schematic diagram of the integrated ROS sampling-analysis system is shown in. Previous studies have shown that condensational growth can be effectively used to grow small particles into large droplets that can be easily separated from an airstream. Several devices, often referred to as steam collection devices, use this technique of growing aerosol particles into droplets in a supersaturated environment of water vapor have been developed (CitationSimon and Dasgupta 1995; CitationKhylstov et al. 1995; CitationZellweger et al. 1999; CitationKidwell and Ondov 2001). The particle-into-liquid sampler (PILS) (CitationWeber et al. 2001; CitationOrsini et al. 2003) was chosen for use in our system because it was inlet system could be purchased (Metrohm). The PILS operating principle is to mix an ambient aerosol sample flow (∼ 1 m3/hr) with a smaller, turbulent flow of 100°C steam (∼ 1.5 ml/min), in order to produce a solution containing the aerosol species sampled at a certain moment in time. Rapid adiabatic cooling of the warmer steam by the cooler ambient sample flow produces a high supersaturation of water vapor in which particles grow into droplets large enough (droplet diameter > 1μ m) to be collected by impaction on a quartz impaction surface. The dichlorofluorescein (DCFH, 1μ M, 0.40 ml/min, Sigma Aldrich, St. Louis, MO) flow is pumped by a 8-channel peristaltic pump (Model 205CA, Watson Marlow, Wilmington, MA), and introduced at the top of the impaction surface, transports the impacted liquid droplets to an exit tube at the base of the impactor. The droplets are collected to produce a continuous liquid flow for online analysis for ROS in solution.
In any type of continuous flow analysis system, detector noise increases with increasing number of pumped flow channels and increases the limit of detection (LOD) due to flow noise and mixing inhomogeneities. As a result, it was decided to introduce the horseradish peroxidase (HRP) enzyme through an active membrane reactor (CitationHwang and Dasgupta 1986). The active membrane reactor is made of a 1.5 cm length of porous PTFE membrane tubing (mean pore size 25 μ m, 1.3 mm i.d., 1 mm wall, GlobalFIA Inc., Fox Island, WA) filled with a 1 mm diameter PTFE filament. The sample PTFE flow tubes are inserted into the terminal ends of the membrane tube. The connection is leakproof as is since a higher external pressure exists during operation. The membrane assembly is completely inserted in a solution of HRP (10 units/ml, Sigma Aldrich, St. Louis, MO) in 0.2 M phosphate buffer solution (pH = 7.2), contained in a screw-capped high density polyethylene bottle. The enzyme is introduced into the main flow stream through the porous membrane walls by applying pneumatic pressure. The sample flow tubes connected to the membrane enter/exit through a silicone rubber stopper, while a third hole admits a tube supplying the pressurizing gas. Compressed, clean house air is admitted to the reactor bottle via a single stage pressure regulator. The parameters governing the reagent introduction rate include membrane pore size, tortuosity, surface porosity, thickness, available surface area, and transmembrane pressure. For any given reagent concentration and membrane material, the reagent introduction rate can be controlled by varying the length of the membrane and transmembrane pressure. Optimum peroxidase reagent introduction was achieved by regulating the transmembrane pressure at ∼ 8 psi (CitationHwang and Dasgupta 1986).
In order to mix the sample with the DCFH slurry and HRP in the flowing stream and allow a finite length of time for the reaction to be complete enough to make sensitive measurements, delay coils and superserpentine reactors (GlobalFIA, Fox Island, WA) were employed with a view of minimizing axial dispersion and the pressure drop due to the reaction conduit while maximizing mixing with minimum dilution and peak broadening. Two superserpentine reactors of lengths 1 m and 0.5 m were used in series along with the delay coil to provide a residence time of ∼ 60 sec. The reactors were maintained in a 37°C water bath to provide optimum reaction conditions. The instrument used in the flow analysis system to detect fluorescence was the Kratos FS970 Spectrofluoromonitor (Kratos Analytical, Ramsey, NJ). An excitation monochromator as well as a prefilter were used to provide a sharp cutoff excitation wavelength of 485 nm. The fluorescence leaving the flow cell, through which the analyte solution flows, is transmitted via a quartz reflector to an emission filter with a cut-off wavelength of 530 nm that is used to eliminate any light of the excitation frequency.
The sampling-flow analysis system was set so as to have sample and blank measurement pathways by means of a three-way solenoid (ASCO valves, Florham Park, NJ), equipped with a timer (Omron, Schaumburg, IL), shown in . The air to be sampled is filtered before entering the system to assess contributions from sources other than the aerosol species being sampled, such as gas-phase species that escape capture by the manganese dioxide impregnated denuder employed before the PILS inlet to remove gas-phase oxidants, and by the components of the analysis system such as the water being used and autofluorescence occurring within the system. The sample and blank cycles were run for 6 minutes each with 1 minute of the sample/blank slurry going to waste via the sample selection valve shown in (6-port valve, Valco Instruments, Houston, TX), while the analysis system is purged with MilliQ water via the sample selection valve in between the two cycles to eliminate effects of one cycle on the next.
RESULTS AND DISCUSSION
Calibration of the System
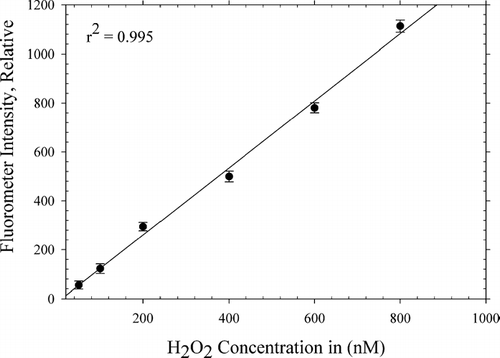
Calibration of the instrument was performed with standard H2O2 solutions of concentrations ranging from 50 to 800 nM, prepared by serial dilutions of a 30% stock solution of H2O2 (Sigma Aldrich, Milwaukee, WI), with water serving as a blank. The standard H2O2 solutions and DCFH were introduced and mixed in a mixing tee prior to flow entering the 6 port valve in order to provide approximately the same reaction time as that for the measurement system. shows the blank-subtracted linear response curve obtained for the H2O2 solutions. The instrument was found to be linear (r2 = 0.995) over a large dynamic range of H2O2 concentrations typical of the equivalent ROS concentrations observed in prior studies (CitationVenkatachari et al. 2005, Citation2007). The linearity can be improved by increasing the reaction time. However, this increase also leads to undesirable effects such as greater dispersion and lower throughput rates. The precision at levels above 200 nM was typically < 5% relative standard deviation. At lower levels, the relative standard deviation increases, primarily due to the omnipresent and variable water blank. Although the blank values varied over time, consecutive blank values were within one standard deviation of each other, and hence were not a factor affecting the accuracy of the analysis. During the course of the calibrations, laboratory reagent water (> 18.2 MΩ) was found to contain anywhere between 5–35 nM H2O2. In order to eliminate the variability in the water blank, the water was treated with manganese dioxide to remove any residual peroxide in the reagent water. However, the water was found to return to a steady-state value in the aforementioned range, due to the presence of dissolved oxygen in the water resulting in significantly high blanks at low nominal levels of H2O2. The reappearance of the H2O2 signal is usually accelerated by visible and long-wavelength UV radiation (CitationHwang and Dasgupta 1986). To minimize variability arising from this phenomenon, as well as to prevent photo-oxidation of the fluorescent probe (DCFH), flow lines were masked with aluminum foil.
Laboratory Testing of the Integrated ROS Sampling-Analysis System
In order to characterize and validate the performance of the developed ROS monitor on a lab-scale under controlled conditions, the ROS generator developed at Clarkson University, and described in detail elsewhere (CitationVenkatachari and Hopke 2007a) was used. In brief, the ROS generation system employed the chemistry of the α -pinene-ozone chemical reaction in a flow reactor system. The characterization of the particle-phase products formed in the flow reactor revealed the presence of peroxidic groups in the particle phase (CitationVenkatachari and Hopke 2007b). Since hydroperoxides and organic peroxides are expected to be important atmospheric constituents of ROS (CitationZiemann 2002; CitationDocherty et al. 2005), this system was deemed to be an ideal laboratory scale system for the testing of the ROS monitor. The flow rate through the flow reactor was varied from 14 to 25 LPM, thus varying the residence time for the particles in the ROS generator. The output of the generator was routed to the PILS inlet through the 3-way solenoid, and measurements were made by the ROS monitor. The 8-channel pump transported water to the steam generator at a flow rate of 1.5 mL/min through a packed reactor (PTFE, 6 cm active length, 3.2 mm i.d., glass wool plugs at each end) containing granular MnO2 (Matheson, East Rutherford, NJ) (CitationHwang and Dasgupta 1986). The purpose of this reactor is to remove any residual peroxide in the reagent water supplied to the steam generator.
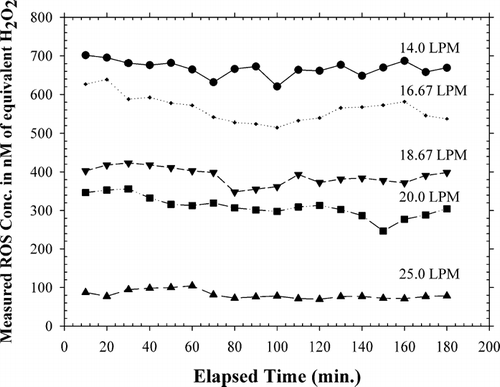
shows the concentration-time plot of the measurements. As can be seen, 5 different residence times were employed by varying the flow rate through the flow reactor from 14 to 25 LPM. provides a perspective on the variation in observed concentration with residence time in the flow reactor with a box and whisker plot detailing the statistical parameters of the observed ROS concentrations for different residence times in the flow reactor. In this figure, the boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. It can be seen that the ROS concentrations measured by the ROS monitor increase with increasing the residence time, with the lowest observed average ROS concentrations occurring at the residence time of 18.5 sec (81.1 nM or 1.3 nanomoles/m3 of flow sampled) and the highest observed average ROS concentrations occurring at the residence time of 33 sec (668.1 nM or 19.1 nanomoles/m3 of flow sampled). These results indicate that the increased time for gas phase and particle phase reactions as well as gas-particle conversion processes (nucleation and condensation) lead to the formation of increasing levels of particle-bound ROS. Based on the characterization performed on the ROS generator, it can be surmised that increasing amounts of particle-phase peroxides are formed with the increase in residence time in the flow reactor. (reproduced from CitationVenkatachari and Hopke 2007a) depicts the results of measurements using the manual method on 1-hr, 2-hr, and 3-hr integrated samples collected from the ROS Generator. Comparing the results of the continuous method with the results of the manual method employed on the ROS generator (CitationVenkatachari and Hopke 2007a), it is observed, on the basis of the one-hour integrated average ROS concentrations, that the manual method (7.56 ± 1.16 nanomoles or 314.4 nM) underestimates the ROS by approximately 25% compared to the continuous method (9.92 ± 0.45 nanomoles or 412.5 nM). This underestimation could be attributed to the fact that the continuous method accounts for the short-lived reactive oxidative species that decay before they can be analyzed by the manual method after collection.
FIG. 4 Box and Whisker plot of variation in the measured ROS concentrations indicated by the ROS Monitor with residence time in the flow reactor.
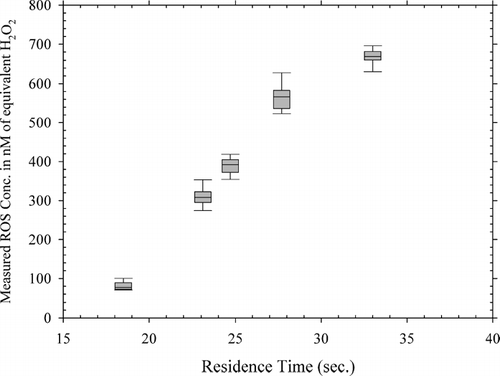
TABLE 3 Measured ROS concentrations (in nanomoles of equivalent H2O2) for different sampling intervals at 0.3 M α -pinene-hexane, ∼ 2.4 ppm ozone, and sampling flow rate of 18 LPM (reproduced from CitationVenkatachari and Hopke 2007a)
The results of the lab-scale investigation of the ROS sampling-analysis system with the ROS generator suggest that the prototype continuous system is capable of detecting particle-bound ROS, and accounting for short-term variabilities in the same. It is a simple and robust instrument that can provide rapid quantitative measurements of the particle-bound ROS. Sampling artifacts associated with filter techniques are minimized since particles are rapidly stabilized by formation of water drops collected into a flowing liquid that is analyzed in real time. The instrument is capable of detecting nanomolar concentrations of ROS. However, a caveat to this claim is that relatively high blanks are present at such low concentrations due to the H2O2 present in the reagent water. It is proposed to test the ROS sampling-analysis system in field tests to establish the utility of the instrument.
CONCLUSIONS
The lack of a suitable method to measure ROS on a routine basis has resulted in no work being undertaken to assess the effects of particle-bound ROS on health effects. An automated method to sample and measure ROS on particles could conceivably be useful to both the research and regulatory communities. Potential analytical approaches for the automated measurement of particle-bound ROS were evaluated and inter-compared in terms of their strengths, limitations, and ease of automation. The dichlorofluorescein (DCFH) fluorogenic probe was the most responsive in that it reacted unselectively with all the reactive species, while not being affected as much as the other methods by the positive interference compounds. An automated sampling-analysis system based on the collection of particles in the ambient air by the process of condensational growth followed by impaction, and the subsequent analysis of the aqueous slurry, in which the particles are dissolved, for ROS using DCFH fluorescence was developed. The results of the lab-scale investigation of the ROS sampling-analysis system with the ROS generator suggested that the prototype continuous system was capable of detecting particle-bound ROS, and accounting for short-term variabilities in the same. It is proposed to test the ROS sampling-analysis system in field tests to establish the utility of the instrument.
Acknowledgments
This work was supported by U.S. Environmental Protection Agency's Science to Achieve Results (STAR) Program through a subcontract from the University of Rochester PM and Health Center Grant RD832415 and by the Syracuse Center of Excellence CARTI project award, which is supported by a grant from the U.S. Environmental Protection Agency [Award No: X832325010]. Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency, it has not been subjected to the Agency's required peer and policy review and therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Notes
a 2,2′-azobis (2-aminopropane) dihydrochloride, sonicated and incubated with DCFH.
REFERENCES
- Bernstein , J. A. , Alexis , N. , Baines , C. , Bernstein , I. L. , Nel , A. E. , Peden , D. , Diaz-Sanchez , D. , Tarlo , S. M. and Williams , P. B. 2004 . Health Effects of Air Pollution . J. Allergy Clin. Immunol. , 114 : 1116
- Cho , A. K. , Sioutas , C. , Miguel , A. H. , Kumagai , Y. , Schmitz , D. A. , Singh , M. , Eiguren-Fernandez , A. and Froines , J. R. 2005 . Redox Activity of Airborne Particulate Matter At Different Sites in the Los Angeles Basin . Environ. Res. , 99 : 40 – 47 .
- Damiani , E. , Kalinska , B. , Canapa , A. , Canestrari , S. , Wozniak , M. , Olmo , E. and Greci , L. 2000 . The Effects of Nitroxide Radicals on Oxidative DNA Damage . Free Rad. Bio. Med. , 28 : 1257 – 1265 .
- Docherty , K. S. , Wu , W. , Lim , Y. B. and Ziemann , P. J. 2005 . Contributions of Organic Peroxides To Secondary Aerosol Formed From Reactions of Monoterpenes with O3 . Environ. Sci. Technol. , 39 : 4049 – 4059 .
- Hasson , A. S. and Paulson , S. E. 2003 . An Investigation of the Relationship Between Gas-Phase and Aerosol-Bourne Hydroperoxides in Urban Air . J. Aerosol Sci. , 34 : 459 – 468 .
- Hung , H.-F. and Wang , C.-S. 2001 . Experimental Determination of Reactive Oxygen Species in Taipei Aerosols . J. Aerosol Sci. , 32 : 1201 – 1211 .
- Hwang , H. and Dasgupta , P. K. 1986 . Fluorometric Flow Injection Determination of Aqueous Peroxides At Nanomolar Level Using Membrane Reactors . Anal. Chem. , 58 : 1521 – 1524 .
- Khylstov , A. , Wyers , G. P. and Slanina , J. 1995 . The Steam-Jet Aerosol Collector . Atmos. Environ. , 29 : 2229 – 2234 .
- Kidwell , C. B. and Ondov , J. M. 2001 . Development and Evaluation of a Prototype System for Collecting Sub-Hourly Ambient Aerosol for Chemical Analysis . Aerosol Sci. Technol. , 35 : 596 – 601 .
- Li , N. , Sioutas , C. , Cho , A. , Schmitz , D. , Misra , C. , Sempf , J. , Wang , M. , Oberley , J. , Froines , J. and Nel , A. 2003 . Ultrafine Particulate Pollutants Induce Oxidative Stress and Mitochondrial Damage . Environ. Health Persp. , 111 ( 4 ) : 455 – 460 .
- Mudway , I. S. , Stenfors , N. , Duggan , S. T. , Roxborough , H. , Zielinski , H. , Marklund , S. L. , Blomberg , A. , Frew , A. J. , Sandstrom , T. and Kelly , F. J. 2004 . An In Vitro In Vivo Investigation of the Effects of Diesel Exhaust on Human Airway Lining Fluid Antioxidants . Archives Biochem. Biophys. , 423 : 200 – 212 .
- Orsini , D. A. , Ma , Y. , Sullivan , A. , Sierau , B. , Baumann , K. and Weber , R. J. 2003 . Refinements to the Particle-Into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition . Atmos. Environ. , 37 : 1243 – 1259 .
- Simon , P. K. and Dasgupta , P. K. 1995 . Continuous Automated Measurement of the Soluble Fraction of Atmospheric Particulate Matter . Anal. Chem. , 67 : 71 – 78 .
- Squadrito , G. L. , Cueto , R. , Dellinger , B. and Pryor , W. A. 2001 . Quinoid Redox Cycling as a Mechanism for Sustained Free Radical Generation by Inhaled Airborne Particulate Matter . Free Rad. Bio. Med. , 31 ( 9 ) : 1132 – 1138 .
- Stohs , S. J. , Bagchi , D. and Bagchi , M. 1997 . Toxicity of Trace Elements in Tobacco Smoke . Inhal. Toxicol. , 9 : 867 – 890 .
- Venkatachari , P. , Hopke , P. K. , Grover , B. D. and Eatough , D. J. 2005 . Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols . J. Atmos. Chem. , 50 : 49 – 58 .
- Venkatachari , P. , Hopke , P. K. , Brune , W. H. , Ren , X. , Lesher , R. , Mao , J. and Mitchell , M. 2007 . Characterization of Wintertime Reactive Oxygen Species Concentrations in Flushing, New York . Aerosol Sci. Technol. , 41 : 97 – 111 .
- Venkatachari , P. and Hopke , P. K. 2007a . Development and Evaluation of A Particle-Bound Reactive Oxygen Species Generator . J. Aerosol Sci. , Accepted
- Venkatachari , P. and Hopke , P. K. 2007b . Characterization of Products for Med in the Reaction of Ozone with α-Pinene: Case for Organic Peroxides, Under Review
- Weber , R. J. , Orsini , D. , Daun , Y. , Lee , Y.-N. , Klotz , P. J. and Brechtel , F. 2001 . A Particle-Into-Liquid Collector for Rapid Measurement of Aerosol Bulk Composition . Aerosol Sci. Technol. , 35 : 718 – 727 .
- Zellweger , C. , Ammann , M. , Hofer , P. and Baltensperger , U. 1999 . NO y Speciation with a Combined Wet Effluent Diffusion Denuder-Aerosol Collecter Coupled to Ion Chromatography . Atmos. Environ. , 33 : 1131 – 1140 .
- Ziemann , P. J. 2002 . Evidence for Low Volatility Diacyl Peroxides as a Nucleating Agent and Major Component of Aerosol for Med From Reactions of O3 with Cyclohexene and Homologous Compounds . J. Phys. Chem. A , 106 : 4390 – 4402 .