Abstract
Quinones are reactive organic compounds known to initiate reactions associated with a host of toxicological events. Their presence in different atmospheres has been demonstrated although their sources remain uncertain. As a result of their reactivity and instability during chemical analysis, only a limited number of studies have reported on atmospheric concentrations of quinones in ambient air. Furthermore, besides the limited information on quinones associated with particulate matter, no previous studies have quantified vapor-phase quinones. We report vapor- and particle-phase concentrations of 1,2- and 1,4-naphthoquinones (1,2-NQ, 1,4-NQ), 9,10-phenanthraquinone (9,10-PQ), and 9,10-anthraquinone (9,10-AQ), measured over a 5-year period in Southern California. The results showed that vapor-phase concentrations of the target quinones were in general higher than those in the particle-phase. Vapor-phase concentrations ranged from 80 pg/m 3 for the AQ to 1747 pg/m 3 for the 1,4-NQ, and the particle-phase concentrations between 13 pg/m 3 for the 1,2-NQ and 250 pg/m 3 for 9,10-AQ. The target quinones were found to be distributed between vapor- and particle-phase, with the exception of 9,10-PQ found only in the particle-phase. The differences observed in the concentrations among sites and seasons suggest different source contributions; source sites were dominated by primary sources, while downwind locations showed a high contribution from photochemical activity.
INTRODUCTION
Quinones are toxicologically important components of air pollution (CitationEndo et al. 2007; CitationLame et al. 2003; CitationMatsunaga et al. 2008). They have been found in ambient particulate matter (PM) (CitationCho et al. 2004; CitationKishikawa et al. 2004); automotive exhaust emissions (CitationJakober et al. 2007; CitationValavanidis et al. 2006), and wood smoke particles (CitationFine et al. 2001). In addition to combustion sources, several controlled experiments have shown that quinones may also be formed by photochemical reactions of their parent polycyclic aromatic hydrocarbons (PAHs) with NO3, ozone, and OH free-radicals (CitationLane et al. 1996; CitationWang et al. 2007). Based on their specific vapor-pressures and the observed differences among the four quinones under study, it is expected to find these compounds distributed between the gas and particle phases. Gas-phase quinones may include the highly volatile and reactive 1,2- and 1,4-naphthoquinones (1,2-NQ and 1,4-NQ), while higher molecular weight quinones such as 9,10-phenenthraquinone (PQ) and 9,10-anthraquinone (AQ) are expected to be found mostly in the particle-phase. However previously reported measurements have referred only to quinones associated with PM (CitationAlbinet et al. 2007; CitationAllen et al. 1997; CitationChung et al. 2006; CitationSienra 2006), and to our knowledge no information regarding ambient gas-phase concentrations is available in the open literature.
Quinones and their reduction products, semiquinones and hydroquinones, are of toxicological interest because of their ability to generate reactive oxygen species, which can elicit changes in the redox status of cells through their ability to carry out electron transfer reactions, and to form covalent bonds with tissue macromolecules to inactive cellular proteins (CitationCadenas et al. 1992; CitationHenry and Wallace 1996; CitationMonks and Lau 1992; CitationObrien 1991; CitationTaguchi et al. 2007). All these properties can have significant adverse effects in pulmonary cells (Iwamoto et al. 2007; CitationTaguchi et al. 2007).
Quinones such as 1,2- and 1,4-NQ and 1,4-benzoquinone (1,4-BQ) also possess electrophilic properties. For example, 1,2-NQ has been shown to inactivate protein tyrosine phosphatase 1B by covalent bond formation with a cysteine thiol (Iwamoto et al. 2007) and 1,4-BQ forms covalent bonds with glyceraldehyde-3-phosphate dehydrogenase, an enzyme responsible for a key step in glycolysis (CitationRodriguez et al. 2005). The toxicology of phenanthrene quinones has been well documented (CitationBolton et al. 2000; CitationHenry and Wallace 1996), but extensive studies related with measurements of their atmospheric levels, phase distribution, and the adverse health effects associated with exposure to these compounds have not been performed. This lack of information may have arisen from difficulties in the sampling as quinones are more susceptible to sampling artifacts than their parent PAHs. Direct determination of low molecular weight quinones by GC/MS is prone to thermal decomposition of the target compounds rendering insufficient sensitivity to quantify their atmospheric concentrations (usually on the order of tenths of ng/m3). A new analytical approach, developed in the last few years, is now available offering the opportunity to better characterize the atmospheric level and properties of these low MW compounds (CitationCho et al. 2004; CitationJakober et al. 2007).
Recently CitationLu et al. (Lu et al. 2005) applied a mathematical model to estimate the distribution of 1,4-naphthoquinone, in both the gaseous- and the particle-phase in Southern California. These estimates were based on the predicted naphthalene levels and its photochemical reactions to yield 1,4-NQ. The model predicted that 1,4-naphthoquinone is concentrated inland (east of the Los Angeles Basin) along the mountain slopes where air parcels arrive from the coastal region to the west. This is in agreement with several chamber studies which measured the formation rate of quinones as a result of photochemical transformations of their parent polycyclic aromatic hydrocarbons (CitationLane et al. 1996; CitationMihele et al. 2002; CitationWang et al. 2007). However, the predicted concentrations by either modeling or smog-chamber studies could not be validated by comparison with actual atmospheric measurements. Therefore, measurements of atmospheric levels, especially in the gas-phase, are of paramount importance.
This article describes the results of an analysis of the vapor- and particle-phase distributions of four quinones in the Southern California atmosphere, collected over a 5-year period in 12 sites around Southern California. The main goals of this study were to evaluate the seasonal and spatial variation of 1,2-NQ, 1,4-NQ, PQ, and AQ concentrations, and to characterize the quinone distribution between particle- and gas-phase in the atmosphere. These data are necessary to try and establish possible association of the target species with adverse health effects.
EXPERIMENTAL
Sampling
As part of a multi-year chronic respiratory health study of Southern California schoolchildren (CitationPeters et al. 1999a, Citation1999b) samples were collected at 12 different sites over a 5-year period. These sites, as part of the Children's Health Study, were geographically distributed over two hundred kilometers, extending from coastal central California through coastal Los Angeles to high mountain areas located to the west in Riverside and San Bernardino counties, and south into eastern San Diego County. San Dimas, Upland, Mira Loma, and Riverside are located 40–100 km downwind and east of downtown Los Angeles. These areas are influenced by upwind emissions enriched with secondary photochemical pollutants during transport. Lompoc, Santa Maria, and Atascadero are communities located in counties several hundred km northwest of Los Angeles in more rural regions. Lancaster is a populated area separated from Los Angeles by the San Gabriel Mountains, while Lake Arrowhead and Alpine are rural communities located at higher altitudes (). Detailed information of the sites, sampling periods, and conditions can be found elsewhere (CitationEiguren-Fernandez et al. 2004; CitationEiguren-Fernandez et al. 2007a). Briefly, Tisch Model 1202 samplers (Tisch, Cleves, Ohio) were deployed on roofs of the school buildings to collect vapor-phase and PM2.5 samples. Field samples were collected every eighth-day over 24 h periods (midnight to midnight) at a flow rate of 113 L/min. XAD-4 resin (20 gr) and quartz fiber filters (10 cm diameter) were used to separately collect gas-phase and PM2.5 samples. Particle- and vapor-phase samples were collected over a 5-year period, with a total of 21 samples collected at each study site (seven samples were collected during each season).
Sample Extraction
Details of the extraction procedure are described elsewhere (CitationEiguren-Fernandez et al. 2004; CitationEiguren-Fernandez et al. 2007a). During the first two years of the study, the extraction of the vapor-phase, collected using XAD-4 resin and a polyurethane foam (PUF), was done using a soxhlet unit, with dichloromethane as extractant, over 24 h. When the extract was reduced to dryness before the quinone assay, a viscous residue was obtained which interfered with the quinone measurement. As a result of this problem the XAD-4/PUF sandwich was substituted with 20 gr of XAD-4 (CitationEiguren-Fernandez et al. 2007a). The new system was tested for possible losses of quinones during collection, but recovery results showed that 20 gr of XAD-4 were enough to collect 99% of the target quinones present in the vapor-phase. This change not only allowed the identification of the quinones in the vapor-phase but also facilitated the extraction process.
Briefly, vapor- and particle-phase samples were extracted by sonication for three periods of 8 min with dichloromethane:acetonitrile (2:1 v/v) followed by filtration through 0.45 μm nylon filters (CitationEiguren-Fernandez and Miguel 2003). An aliquot of the extract was taken for PAH quantification; the rest was assayed for quinones.
Quinone Measurement
The target quinones were separated and quantified following the procedure of CitationCho et al. (Cho et al. 2004) which transforms the target quinones into their diacetyl derivatives for measurement by GC/MS. In the procedure, sample extracts were evaporated under nitrogen to ∼50 μL. A portion of zinc (∼100 mg) and 200 μL acetic anhydride were added and the capped tubes were heated at 80°C for 15 min. Samples were then cooled to room temperature, and an additional similar portion of zinc added. The tubes were capped again and heated for an additional 15 min. The reaction was quenched by the addition of 0.5 mL water and 3.0 mL of pentane. The samples were mixed and centrifuged at 2,000 rpm for 10 min. The pentane layer was concentrated by evaporation to dryness and the sample reconstituted in 80 μL of dry acetonitrile; a 1 μL aliquot was injected into the GC-MS system.
The GC-MS system consisted of an Agilent (HP) 6890 Plus GC System equipped with a 6890 series injector and interfaced to a 5973 Mass Selective Detector (MSD). An HP-5MS capillary column (0.25 mm id, 0.25 μM film thickness, 30 m) was used to separate the acetylated derivatives. Initial column temperature was maintained at 100°C for 4.0 min then ramped to 280°C at a rate of 5°C/min. Column flow was 1.0 mL/min. The limit of detection (in ng) for each quinone after derivatization following the method described are 0.3 for 1,2-NQ, 0.4 for 1,4-NQ, 0.2 for PQ, and 4.8 for AQ.
Deuterium-labeled internal standards 1,2-napthoquinone-d6, 1,4-naphthoquinone-d6, 9,10-anthraquinone-d8, 9,10-phenan-thraquinone-d8 were prepared from the hydrocarbon precursors using published synthetic procedures for the target quinones (CitationBraude and Fawcett 1953; CitationKrohn et al. 1990; CitationOyster and Adkins 1921; CitationUnderwood and Walsh 1943). Calibration curves (from 0 to 250 ng) were obtained for quinone standards and internal standards following the acetylation procedure.
RESULTS AND DISCUSSION
Spatial Distribution of Quinone Concentrations in Southern California
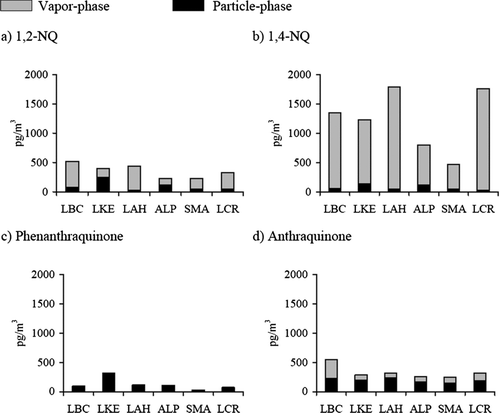
Significant levels of all four quinones were found in both vapor- and particle-phase. Annual average vapor-phase concentrations were calculated using all samples (n = 21) collected at each site over a one year period, which included different seasons and atmospheric conditions. Maximum and minimum values reflected the concentration range observed for the different sampling seasons at each location; maximum concentrations were normally observed in colder season, while minimum concentrations were measured in warmer seasons (). Due to extraction problems results for vapor-phase quinone concentrations are not available for six of the sampling sites (Atascadero, Lompoc, Upland, Mira Loma, San Dimas, and Riverside). Quinone concentrations found in the remaining sites varied from 87.1 ± 49.1 pg/m3 for AQ at Lake Elsinore to 1747 ± 1638 pg/m3 for 1,4-NQ at Lake Arrowhead (). These concentrations were in general significantly higher than the ones measured in the particle-phase, which ranged from 5.8 ± 12.8 pg/m3 for the 1,2-NQ, to 311 ± 449 pg/m3 for the PQ in Lompoc and Lake Elsinore, respectively. Among these quinones, at all sites, vapor-phase 1,4-NQ levels were the highest. High concentrations of all quinones were observed at Lake Elsinore, the furthest site downwind of the predominant wind trajectory in the Los Angeles (LA) Basin. These results suggest that the formation of secondary quinones by photochemical reactions during transport is an important contributor to the atmospheric burden of these species. A recent study conducted in the LA Basin has showed that secondary formation during transport plays an important role in the overall particle-phase PQ concentrations (CitationEiguren-Fernandez et al. 2007b). Alpine, a site located south of the LA Basin, presented high concentrations, which may indicate that the atmospheric and meteorological conditions of the area have a significant impact on these quinone levels. High levels of other chemicals present in the atmosphere such as NO3, ozone, and OH radicals may be involved in secondary formation of quinones at this site (CitationWang et al. 2007). Atmospheric concentrations of quinones were low for rural areas, especially those located north of the LA Basin. Concentration differences between urban and rural areas have been previously reported (CitationAlbinet et al. 2007; CitationYeo et al. 2003); in general, the higher concentrations observed in urban areas suggested an important contribution of primary sources of both quinones and parent compounds in these areas. Except for 9,10-AQ, the annual average concentrations varied among the sites, indicating a spatial variability that has to be taken in account when conducting human exposure studies.
TABLE 1 Annual average (n = 21) ±STDEV, (minimum and maximum) concentrations (pg/m3) for vapor- and particle-phase quinones
During this study, we observed that the annual average concentration of particle-phase AQ were very similar for all sites, suggesting that artifacts may have occurred during sampling of AQ using quartz fiber filters.
It is important to note that with the exception of 9,10-PQ, which is found only in the particle-phase, the other quinones are found in both phases (). The quinone distribution does not seem to be only a result of partitioning because the ratios vary with the site. The naphthoquinones, which are more volatile, are mainly in the vapor-phase, while 9,10-AQ showed a relatively consistent distribution between both phases.
Several studies have reported an important effect of season in partitioning for other ambient species (CitationBae et al. 2002; CitationGriffin et al. 2003), thus we may expect to observe similar effects on quinones as ambient conditions such as temperature and inversion layer height change during the year.
As previously mentioned, the distribution of 1,4-NQ in the Los Angeles Basin was estimated using a mathematical model based on naphthalene concentrations and its photooxidation reaction rates (CitationLu et al. 2005). The model predicted that spatial distributions of 1,4-NQ are generally similar in summer and winter, but ambient concentrations reported in the present study indicated that season is an important factor, specially for the 1,4-NQ which is highly affected by photochemistry. This discrepancy between model predictions and the actual ambient concentrations of quinones suggests, as stated by Lu et al. that additional field and laboratory research is needed to improve the performance of mathematical models developed to characterize naphthoquinone concentrations, distributions and exposures in the Southern California environment.
Seasonal Variability
Atmospheric quinones are emitted as combustion products of fossil fuels (CitationValavanidis et al. 2006) and are also formed in the atmosphere by photochemical reactions of their parent polycyclic aromatic hydrocarbons (PAHs) (CitationEiguren-Fernandez et al. 2007b; CitationHolt et al. 2005; CitationSasaki et al. 1997; CitationWang et al. 2007). Considering the two sources, it is reasonable to expect variability in quinone levels over different seasons. During the summer, when photochemical activity is expected to be highest, levels of quinones should be generally higher than during colder seasons; however, as a result of lower inversion layer during the winter, at sites with no significant photochemical activity, quinone levels would be expected to be higher, especially those associated with the particle-phase. Previous studies evaluating the effect of temperature on particle-phase concentrations (CitationEiguren-Fernandez et al. 2004; CitationYeo et al. 2003) found a negative correlation between temperature and particle-phase PAH concentrations for primary emitted PAHs; similar results may be expected for quinones.
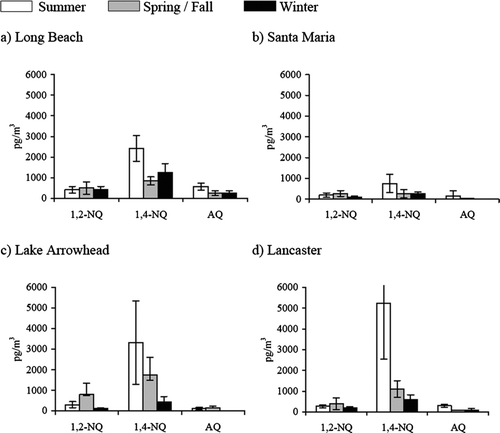
The study presented in this article was conducted over a 5-year period, collecting samples at three different seasons, grouped as summer, spring/fall and winter, at each site, depending on the average temperature during each sampling period (). The results from this study showed that photochemistry is a major contributor to quinone formation during the summer, although its contribution differs with compound and site ( and ). Sites such as Long Beach and Santa Maria did not show a significant effect of photochemistry on the target vapor-phase quinones, although slightly higher concentrations are observed during the summer ( and ). On the other hand, downwind sites such as Lake Arrowhead and Lancaster presented much higher concentrations during the summer and spring/fall seasons which may have resulted from higher photochemical effects on quinone formation ( and ). The contribution of photochemistry is clear for 1,4-NQ which showed a considerable increase in its levels at all study sites during the seasons with high photochemical activity.
TABLE 2 Average temperatures (°C) for each sampling period (season) and site
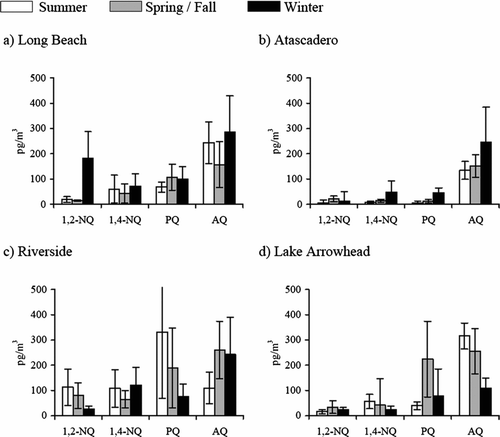
Particle-phase quinones showed again two different patterns with season, supporting the hypothesis that photochemistry is an important contributor of quinones formation in certain locations (). At Long Beach (), a site highly impacted by primary sources, particle-phase quinone concentrations increased with decreasing average seasonal temperature; highest concentrations were observed in winter when temperatures were the coldest. Similar behavior was observed at other sites, especially rural areas such as Atascadero () and Lompoc. Previous studies showed that Atascadero is a site with high levels of PAHs emitted by primary sources (CitationEiguren-Fernandez et al. 2004), which suggests a similar contribution of primary emissions to the observed quinone levels. Sites located downwind in the Basin ( and ) showed higher levels during the summer season suggesting a more important contribution of photochemistry, resulting from transformation processes occurring during transport.
CONCLUSIONS
The results from this study showed an important spatial variability in atmospheric quinone levels in Southern California. Quinones were found in both vapor- and particle-phase with low molecular weight distributed mainly in the vapor-phase and accounting for a significant portion of the total quinone levels.
Primary sources were major contributors in areas highly impacted by vehicular emissions such as Long Beach and Atascadero, while areas located downwind showed contributions from both primary and secondary sources.
Season is an important factor when studying ambient levels of quinones and assessing human exposure to these compounds; our results from the sites affected by major sources indicate that ambient temperature play an important role in the particle-phase quinone concentrations, while at receptor site areas, photochemistry during summer and spring may be the major contributor to the total quinone burden.
Although this study clearly suggests the role of both primary sources and photochemistry on the ambient quinone concentrations, further studies are needed in order to more accurately assess the specific contribution of each parameter at each one of the study sites.
Acknowledgments
The authors thank the respective school districts and schools participating in the Children's Health Study (California Air Resources Board Contract A033-186) for their cooperation and use of facilities. This research was supported by the Southern California Particle Center and Supersite (USEPA Grants #R827352-01-0 and CR-82805901). Additional investigator support was provided by a NIEHS Center Grant (SP30ES07048-02). Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency, it has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
REFERENCES
- Albinet , A. , Leoz-Garziandia , E. , Budzinski , H. and ViIlenave , E. 2007 . Polycyclic Aromatic Hydrocarbons (PAHs), Nitrated PAHs and Oxygenated PAHs in Ambient Air of the Marseilles Area (South of France): Concentrations and Sources . Sci. Total Environ. , 384 ( 1–3 ) : 280 – 292 .
- Allen , J. , Dookeran , N. , Taghizadeh , K. , Lafleur , A. , Smith , K. and Sarofim , A. 1997 . Measurement of Oxygenated Polycyclic Aromatic Hydrocarbons Associated with a Size-Segregated Urban Aerosol . Environ. Sci. Technol. , 31 ( 7 ) : 2064 – 2070 .
- Bae , S. Y. , Yi , S. M. and Kim , Y. P. 2002 . Temporal and Spatial Variations of the Particle Size Distribution of PAHs and Their Dry Deposition Fluxes in Korea . Atmos. Environ. , 36 ( 35 ) : 5491 – 5500 .
- Bolton , J. L. , Trush , M. A. , Penning , T. M. , Dryhurst , G. and Monks , T. J. 2000 . Role of Quinones in Toxicology . Chemical Research in Toxicology , 13 ( 3 ) : 135 – 160 .
- Braude , E. A. and Fawcett , J. S. 1953 . 1,4-Naphthoquinone . Organic Syntheses , 33 : 50 – 51 .
- Cadenas , E. , Hochstein , P. and Ernster , L. 1992 . Prooxidant and Antioxidant Functions of Quinones and Quinone Reductases in Mammalian-Cells . Advances in Enzymology and Related Areas of Molecular Biology , 65 : 97 – 146 .
- Cho , A. K. , Di Stefano , E. , You , Y. , Rodriguez , C. E. , Schmitz , D. A. , Kumagai , Y. , Miguel , A. H. , Eiguren-Fernandez , A. , Kobayashi , T. , Avol , E. and Froines , J. R. 2004 . Determination of Four Quinones in Diesel Exhaust Particles, SRM 1649a, an Atmospheric PM2.5 . Aerosol Sci. Technol. , 38 : 68 – 81 .
- Chung , M. Y. , Lazaro , R. A. , Lim , D. , Jackson , J. , Lyon , J. , Rendulic , D. and Hasson , A. S. 2006 . Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts . Environ. Sci. Technol. , 40 ( 16 ) : 4880 – 4886 .
- Eiguren-Fernandez , A. and Miguel , A. H. 2003 . Determination of Semivolatile and Particulate Polycyclic Aromatic Hydrocarbons in srm 1649a and PM2.5 Samples by HPLC-Fluorescence . Polycyclic Aromatic Compounds , 23 ( 2 ) : 193 – 205 .
- Eiguren-Fernandez , A. , Miguel , A. H. , Froines , J. R. , Thurairatnam , S. and Avol , E. L. 2004 . Seasonal and Spatial Variation of Polycyclic Aromatic Hydrocarbons in Vapor-Phase and PM 2.5 in Southern California Urban and Rural Communities . Aerosol Sci. Technol. , 38 ( 5 ) : 447 – 455 .
- Eiguren-Fernandez , A. , Avol , E. L. , Thurairatnam , S. , Hakami , M. , Froines , J. R. and Miguel , A. H. 2007a . Seasonal Influence on Vapor- and Particle-Phase Polycyclic Aromatic Hydrocarbon Concentrations in School Communities Located in Southern California . Aerosol Sci. Technol. , 41 ( 4 ) : 438 – 446 .
- Eiguren-Fernandez , A. , Miguel , A. H. , Lu , R. , Purvis , K. , Grant , B. , Mayo , P. , Di Stefano , E. , Cho , A. K. and Froines , J. R. 2007b . Atmospheric Formation of 9,10-Phenanthraquinone in the Los Angeles Air Basin . Atmospheric Environment , submitted for publication
- Endo , A. , Sumi , D. and Kumagai , Y. 2007 . 1,2-naphthoquinone Disrupts the Function of cAMP Response Element-Binding Protein Through Covalent Modification . Biochemical and Biophysical Research Communications , 361 ( 1 ) : 243 – 248 .
- Fine , P. M. , Cass , G. R. and Simoneit , B. R. T. 2001 . Chemical Characterization of Fine Particle Emissions from Fireplace Combustion of Woods Grown in the Northeastern United States . Environ. Sci. Technol. , 35 ( 13 ) : 2665 – 2675 .
- Griffin , R. J. , Nguyen , K. , Dabdub , D. and Seinfeld , J. H. 2003 . A Coupled Hydrophobic-Hydrophilic Model for Predicting Secondary Organic Aerosol Formation . J. Atmospheric Chemistry , 44 ( 2 ) : 171 – 190 .
- Henry , T. R. and Wallace , K. B. 1996 . Differential Mechanisms of Cell Killing by Redox Cycling and Arylating Quinones . Archives of Toxicology , 70 ( 8 ) : 482 – 489 .
- Holt , J. , Hothem , S. , Howerton , H. , Larson , R. and Sanford , R. 2005 . 9,10-phenanthrenequinone Photoautocatalyzes its Formation from Phenanthrene, and Inhibits Biodegradation of Naphthalene . J. Environmental Quality , 34 ( 2 ) : 462 – 468 .
- Iwamoto , N. , Sumi , D. T. I. , Uchida , K. , Cho , A. K. , Froines , J. R. and Kumagai , Y. 2008 . Chemical Knockdown of Protein Tyrosine Phosphatase 1B by 1,2-naphthoquinone through Covalent Modification Causes Persistent Transactivation of Epidermal Growth Factor Receptor . J. Biol. Chem. , in press
- Jakober , C. A. , Riddle , S. G. , Robert , M. A. , Destaillats , H. , Charles , M. J. , Green , P. G. and Kleeman , M. J. 2007 . Quinone Emissions from Gasoline and Diesel Motor Vehicles . Environ. Sci. Technol. , 41 ( 13 ) : 4548 – 4554 .
- Kishikawa , N. , Wada , M. , Ohba , Y. , Nakashima , K. and Kuroda , N. 2004 . Highly Sensitive and Selective Determination of 9,10-phenanthrenequinone in Airborne Particulates Using High-Performance Liquid Chromatography with Pre-Column Derivatization and Fluorescence Detection . J. Chromatography A , 1057 ( 1–2 ) : 83 – 88 .
- Krohn , K. , Rieger , H. and Bruggmann , K. 1990 . Transition-Metal Catalyzed Oxidations. 4. Improved Method for the Oxidation of 1-Naphthols and 2-Naphthols to 1,2-Napthoquinones . Synthesis-Stuttgart , 12 : 1141 – 1143 .
- Lame , M. W. , Jones , A. D. , Wilson , D. W. and Segall , H. J. 2003 . Protein Targets of 1,4-benzoquinone and 1,4-naphthoquinone in Human Bronchial Epithelial Cells . Proteomics , 3 ( 4 ) : 479 – 495 .
- Lane , D. A. , Fielder , S. S. , Townsend , S. J. , Bunce , N. J. , Zhu , J. , Liu , L. , Wiens , B. and Pond , P. 1996 . Atmospheric Photochemistry of Naphthalene: A Practical and Theoretical Approach . Polycyclic Aromatic Compounds , 9 ( 1–4 ) : 53 – 59 .
- Lu , R. , Wu , J. , Turco , R. P. , Winer , A. M. , Atkinson , R. , Arey , J. , Paulson , S. E. , Lurmann , F. W. , Miguel , A. H. and Eiguren-Fernandez , A. 2005 . Naphthalene Distributions and Human Exposure in a Southern California . Atmos. Environ. , 39 ( 3 ) : 489 – 507 .
- Matsunaga , T. , Kamiya , T. , Sumi , D. , Kumagai , Y. , Kalyanaraman , B. and Hara , A. 2008 . L-Xylulose Reductase is Involved in 9,10-Phenanthrenequinone-Induced Apoptosis in Human T Lymphoma Cells . Free Radical Biology and Medicine , 44 ( 6 ) : 1191 – 1202 .
- Mihele , C. M. , Wiebe , H. A. and Lane , D. A. 2002 . Particle Formation and Gas/Particle Partition Measurements of the Products of the Naphthalene-OH Radical Reaction in a Smog Chamber . Polycyclic Aromatic Compounds , 22 ( 3–4 ) : 729 – 736 .
- Monks , T. J. and Lau , S. S. 1992 . Toxicology of Quinone-Thioethers . Crit. Rev. Toxicol. , 22 ( 5–6 ) : 243 – 270 .
- Obrien , P. J. 1991 . Molecular Mechanisms of Quinone Cytotoxicity . Chemico-Biological Interactions , 80 ( 1 ) : 1 – 41 .
- Oyster , L. and Adkins , H. 1921 . The Preparation of 9(10)-Phenanthridone from Phenanthrene . J. Amer. Chem. Soc. , 43 : 208 – 210 .
- Peters , J. M. , Avol , E. , Navidi , W. , London , S. J. , Gauderman , W. J. , Lurmann , F. , Linn , W. S. , Margolis , H. , Rappaport , E. , Gong , H. and Thomas , D. C. 1999a . A Study of Twelve Southern California Communities with Differing Levels and Types of Air Pollution—I. Prevalence of Respiratory Morbidity . Amer. J. Respir. Crit. Care Med. , 159 ( 3 ) : 760 – 767 .
- Peters , J. M. , Avol , E. , Gauderman , W. J. , Linn , W. S. , Navidi , W. , London , S. J. , Margolis , H. , Rappaport , E. , Vora , H. , Gong , H. and Thomas , D. C. 1999b . A Study of Twelve Southern California Communities with Differing Levels and Types of Air Pollution—II. Effects on Pulmonary Function . Amer. J. Respir. Crit. Care Med. , 159 ( 3 ) : 768 – 775 .
- Rodriguez , C. E. , Fukuto , J. M. , Taguchi , K. , Froines , J. and Cho , A. K. 2005 . The Interactions of 9,10-phenanthrenequinone with Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), A Potential Site for Toxic Actions . Chemico-Biological Interactions , 155 ( 1–2 ) : 97 – 110 .
- Sasaki , J. , Aschmann , S. M. , Kwok , E. S. C. , Atkinson , R. and Arey , J. 1997 . Products of the Gas-Phase OH and NO3 Radical-Initiated Reactions of Naphthalene . Environ. Sci. Technol. , 31 ( 11 ) : 3173 – 3179 .
- Sienra , M. D. 2006 . Oxygenated Polycyclic Aromatic Hydrocarbons in Urban Air Particulate Matter . Atmos. Environ. , 40 ( 13 ) : 2374 – 2384 .
- Taguchi , K. , Fujii , S. , Yamano , S. , Cho , A. K. , Kamisuki , S. , Nakai , Y. , Sugawara , F. , Froines , J. R. and Kumagai , Y. 2007 . An Approach to Evaluate Two-Electron Reduction of 9,10-phenanthraquinone and Redox Activity of the Hydroquinone Associated with Oxidative Stress . Free Radical Biology and Medicine , 43 ( 5 ) : 789 – 799 .
- Underwood , H. W. J. and Walsh , W. L. 1943 . Organic Syntheses Collective , Volume II. , 553 – 554 . New York : John Wiley & Sons .
- Valavanidis , A. , Fiotakis , K. , Vlahogianni , T. , Papadimitriou , V. and Pantikaki , V. 2006 . Determination of Selective Quinones and Quinoid Radicals in Airborne Particulate Matter and Vehicular Exhaust Particles . Environ. Chem. , 3 ( 2 ) : 118 – 123 .
- Wang , L. , Atkinson , R. and Arey , J. 2007 . Formation of 9,10-phenanthrenequinone by Atmospheric Gas-Phase Reactions of Phenanthrene . Atmos. Environ. , 41 ( 10 ) : 2025 – 2035 .
- Yeo , H. G. , Choi , M. , Chun , M. Y. and Sunwoo , Y. 2003 . Gas/Particle Concentrations and Partitioning of PCBs in the Atmosphere of Korea . Atmos. Environ. , 37 ( 25 ) : 3561 – 3570 .