Abstract
Both short- and long-term exposure to particulate matter (PM) air pollution have been demonstrated to cause increases in cardiovascular disease, cancer, and respiratory disorders. Although the specific mechanisms by which exposure to PM cause these affects are unclear, significant evidence has accumulated to suggest that PM exposure leads to increased inflammation as the result of excessive production of reactive oxygen species (ROS) in critical cell types. In order to better understand how real-world PM exposure causes adverse health effects, there is a need to efficiently integrate metrics of PM toxicity into large scale air monitoring and health effects/epidemiology studies. Here we describe a rapid, inexpensive, method that can be employed to assess the potential of sub-mg masses of PM to generate oxidative stress in alveolar macrophage cells. Importantly, the approach is compatible with routine daily PM sampling programs such as those administered by EPA (Speciation trends network (STN), IMPROVE network, PM2.5 mass monitoring network), allowing for multiple samples to be assessed simultaneously with low volumes and brief exposure periods. We apply the method to a set of water extracts of daily PM2.5 samples (25–350 μ g PM mass) collected in the Denver-Metro area. Variations in the magnitude of the ROS response observed between the samples were only partially explained by differences in mass loading, with the highest levels of ROS being observed in samples collected during the summer months. This assay provides a very useful tool that can be coupled with detailed chemical analysis and statistical models to work towards the goal of attributing PM toxicity to specific real-world chemical sources.
1. INTRODUCTION
Growing epidemiological evidence has demonstrated a significant increase in cardiovascular and respiratory diseases, cancer, and mortality after both short- and long-term exposure to ambient particulate matter (PM) air pollution (CitationHong et al. 2007; CitationSorenson et al. 2003; CitationSamet et al. 2000; CitationBrunekreef et al. 2002). The respiratory effects of PM exposure are thought to be mediated by increased airway inflammation and include exacerbation of preexisting diseases such as asthma and chronic obstructive pulmonary disease (COPD) (CitationLi et al. 2008; CitationPope and Dockery 2006; CitationLi et al. 2003). The mechanisms underlying the associations with cardiovascular disease and cancer are less clear, though a pro-inflammatory response is also implicated (CitationPope et al. 2004; CitationLippmann and Schlesinger 2000). Although most studies to-date have focused on the association between PM mass and adverse health affects (CitationSamet et al. 2000), it is also thought that the chemical composition of PM is important in driving its inflammatory and toxicological effects (CitationSchwarze et. al. 2006; CitationLippmann et al. 2006; CitationBecker et al. 2005). However, the biological mechanism(s) by which PM exposure causes pro-inflammatory effects, and the sensitivity of inflammation and subsequent toxicity to variations in PM composition remain to be fully understood.
An increase in the abundance of reactive oxygen species (ROS), and resulting oxidative stress, has been hypothesized to play a direct role in the pulmonary inflammation that can occur after PM exposure (CitationCastro and Freeman 2001; CitationDonaldson et al. 2003; CitationTao et al. 2003). ROS encompass many chemical species including the oxygen and hydroxyl radicals, as well as other reactive forms of O2 such as hydrogen peroxide and singlet O2 (CitationHalliwell and Cross 1994). ROS is constantly formed in the human body as the natural consequence of aerobic metabolism, and is integral for maintaining tissue oxygen homeostasis. Generation of ROS and the activity of antioxidant and radical scavenger defenses appear more or less balanced in vivo (CitationHalliwell and Cross 1994). Oxidative stress results when ROS concentrations exceed the capacity of the antioxidant systems. The ability of PM to generate oxidative stress is associated with two general mechanisms: the primary oxidant-generating properties, and the ability to stimulate cellular generation of ROS (CitationHuang et al. 2003; CitationFach et al. 2002; CitationImrich et al. 2000; CitationImrich et al. 1999; CitationGoldsmith et al. 1997).
PM-associated ROS generation is the focus of much attention in on-going efforts to understand the mechanisms of the observed adverse health effects associated with PM (CitationSoukup et al. 2000; CitationCho et al. 2005; CitationKunzli et al. 2006; CitationVenkatachari et al. 2007). Particle type, size, and surface area are all important factors mediating particle-antioxidant interactions in the airways (CitationZielinski et al. 1999). Studies using diesel exhaust particles as model PM have been carried out in cultured cells, whole animals, and humans and have provided strong evidence that PM exposure results in increased formation of ROS in important pulmonary cell types (CitationKumagai et al. 2002; CitationLi et al. 2003). The increase in ROS is hypothesized to be driven by redox-reactions of soluble PM components such as transition metals (CitationCiapetti et al. 1998; CitationPrahalad et al. 1999) or organic compounds (e.g., quinines; CitationSquadrito et al. 2001; CitationCho et al. 2005), or by analogous reactions on particle surfaces. Increased ROS levels can result in direct DNA damage (CitationRisom et al. 2005) and, critically, have also been demonstrated to activate redox-sensitive signaling pathways such as the NFκ B and mitogen-activated protein kinase (MAPK) cascades which result in the induction of pro-inflammatory mediators such as TNF-α, IL-6, and IL-8 (CitationTakizawa et al. 1999; CitationNg et al. 1998; Xio et al. 2003; CitationPourazar et al. 2005). The increased expression of inflammatory mediators is thought to relate directly to decreased lung function and exacerbation of diseases such as asthma and COPD.
A variety of methods, in both cell-free and cell-based systems have been used to examine the oxidative stress activity of PM. In cell-free constructs (CitationKunzli et al. 2006), biochemical methods such as the consumption of dithiothreitol (DTT) (CitationLi et al. 2003; CitationCho et al. 2005) or the oxidation of deoxyribose as measured with thiobarbituric acid (TBA) (CitationFrampton et al. 1999), have been used to assess ROS generation. In addition redox sensitive fluorescent probes such as dichlorofluorescin (DCFH) have also been used in a cell-free manner (CitationSee et al. 2007; CitationVenkatachari et al. 2007). These cell-free methods can be used in a high-throughput screening approach and detect redox cycling compounds that may be present on PM. However, these methods are constrained in several key areas: (1) the DTT and TBA approaches are typically less sensitive than the fluorescent-probes and therefore significant masses of PM may be required, (2) reaction kinetics with certain probes are slow, and a catalyst may be required, and (3) the physiological relevance is limited in that they do not take into account the additional biological response occurring within a PM-exposed cell. Cell-based methods (CitationSoukup et al. 2000) likely provide a more comprehensive assessment of the ROS activity of the PM as they take into account both the capacity of the PM itself (or PM extract) to directly produce ROS and also the ability to stimulate cellular generation of ROS. Fluorescent probes such as luminal (CitationBecker et al. 1996), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and dihydrorhodamine 123 (CitationBecker et al. 2005) have all been used to study the oxidative generating capacity of PM in a variety of pulmonary cell types. The broad spectrum ROS probe, DCFH-DA, which is responsive to most common reactive oxygen species, including the hydroxyl radical, peroxide, superoxide radical, and peroxynitrite (CitationSchoonen et al. 2006; CitationCarranza and Pantano 2003) was applied in the study reported here. DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) is a non-fluorescent, membrane permeable compound and upon entering a cell it is de-acetylated by cellular house-keeping esterases yielding 2′,7′-dichlorodihydrofluorescein (DCFH). The non-fluorescent DCFH can then be converted by ROS within the cell into the highly fluorescent 2′7′-dichlorofluorescein (DCH) which can be easily monitored using a fluorescence plate reader, or by flow cytommetry (CitationBecker et al. 2005; CitationImrich and Kobzik 1997). The non-specificity of the DCFH probe was valued in the context of our application, as we were looking for a comprehensive assessment of total ROS activity of the PM.
Because of their location on the inner epithelial surface of the lung, alveolar macrophages are directly exposed to inhaled environmental insults and act as a principal line of defense against pulmonary injury. They act to clean and sterilize the alveoli by ingesting inhaled particles, subsequently being eliminated along the mucociliary pathway (CitationBowden 1984). As part of the immunologic system of the lung, alveolar macrophages also play an important signaling role in promoting pulmonary inflammation in response to environmental exposures (CitationIles and Forman 2002). Thus alveolar macrophages represent an important potential cellular target for PM toxicity.
In order to better understand how real-world PM exposure causes adverse health effects, there is a need to efficiently integrate metrics of PM toxicity into large scale air monitoring and health effects/epidemiology studies. This will require that large numbers of daily PM samples be evaluated for toxicity in order to document temporal and spatial trends. This presents a significant throughput and analytical challenge as samples with very limited PM masses will need to be analyzed. We have developed a rapid, inexpensive cell-based method that is performed in rat alveolar macrophages and uses DCFH-DA as the redox-sensitive fluorescent probe. We demonstrate that this method can be employed to assess the ROS activity of atmospheric PM, specifically focusing on the contribution of water extractable constituents. Importantly, we demonstrate that this assay is effective on less than 100 micrograms of PM.
The water extractable components of particulate matter, which include colloidal components and insoluble particles that pass through a 0.2 μ m pore size filter, are an important and reproducibly sampled component of PM that contributes to ROS generation. Many studies have demonstrated that soluble PM components, especially metals, are potent initiators of ROS, key drivers in redox-driven ROS generation mechanisms (CitationArredouani et al. 2005), and that a large fraction of the oxidant stress of PM may be attributable to soluble species (Imrich et al. Citation2007; CitationSeagrave et al. 2006; CitationGeng et al. 2006; CitationGoldsmith et al. 1998; CitationCiapetti et al. 1998).
The complexities of the chemical composition of atmospheric PM make it very difficult with current tools to definitively attribute ROS activity to specific PM components or mechanisms (CitationSoukup et al. 2000). However, by greatly facilitating the routine use of an ROS activity measurement, and providing a quantitative measure of a well-defined fraction of the particulate matter induced ROS generation, this assay provides a tool to address this goal. The assay can be coupled with chemical analysis, epidemiology studies, and statistical approaches to establish linkages between source contributions, specific chemical species, ROS, and adverse human health outcomes.
2. METHODS
2.1. ROS Bioassay
2.1.1. Rat Alveolar Macrophages
Experiments were performed with the rat alveolar cell line, NR8383, obtained from the American Type Culture Collection (ATCC). This highly responsive alveolar macrophage cell line exhibits all the characteristics of normal primary macrophage cells, responding to appropriate microbial, particulate or soluble stimuli with phagocytosis and killing. The cells display oxidative burst and secrete many cytokines (e.g., IL-1, TNF-beta, IL-6). Importantly, the NR8383 cells express a functional mannose receptor (CitationLane et al. 1998), an attribute not present in other macrophage cell lines such as HL60, U937, and RAW264. The mannose receptor is an important phagocytic and endocytic receptor critical for immune response and host defense. The attributes of this cell line have been exploited in numerous studies of the health impacts of environmental particles (CitationRiley et al. 2005; CitationGazin et al. 2004; CitationFach et al. 2002; CitationLane et al. 1998; CitationLong et al. 1997). Per ATCC protocol, cultures are maintained by transferring floating cells to additional flasks—the floating cells are functionally normal. Upon reseeding, a significant fraction of the cells will attach, but the normal floating cells are harvested for use. NR8383 cells were maintained in Hams F12 medium containing 2 mM L-glutamine supplemented with 1.176 g/L sodium bicarbonate and 15% heat inactivated fetal bovine serum. Cells were cultured at 37°C in a humidified 5% CO2 incubator and maintained by transferring non-adherent cells to new flasks weekly. Cultures were set up to contain a floating cell concentration of approximately 4 × 105 cells mL− 1 of media.
2.1.2. Preparation of Filters for ROS Bioassay
Typically each PM-laden filter (or filter blank) was sectioned in-half (using a ceramic blade), with one half used for chemical analysis and the other half for the ROS bioassay (refer to section 2.3 for details of sample collection). The water-soluble components were extracted from the PM by agitating each filter half in 900 μ L of Type I purified water in 1.5 mL polypropylene microcentrifuge tubes (pre-cleaned with 1% HCl) at room temperature and in the dark. After an overnight (typically 16 h) extraction, the filters were removed, samples centrifuged at 6600 RPM for approximately 1 min, and the supernatant filtered through a 0.22 μ m polypropylene filter. Method blanks were prepared by processing Type 1 water through the complete extraction/filtration protocol just described. Filter blanks, collected and prepared as described in section 2.3, were treated identically to that of actual samples.
Buffered PM extract solutions were prepared for the cell exposures/ROS bioassay by adding a 10× concentrated Salts Glucose Medium (10× SGM: 500 mM HEPES, 1M NaCl, 50 mM KCl, 20 mM CaCl2, 50 mM dextrose, pH 7.2, CitationKlein et al. 2002) to the filter extracts to achieve a 1× SGM final concentration. Untreated controls were set-up in a similar manner by making a 1× SGM solution in sterile Type 1 water. For concentration-response studies, leach samples were diluted with 10× SGM and Type 1 water to generate 66% and 33% dilutions of the original extract, and evaluated in the bioassay along with the undiluted samples. SGM was chosen as the medium to buffer the extract solutions because previous results demonstrated that the cells remained viable in this medium and it is a simple medium that allows for chemical speciation determination and accurate exposure assessment.
2.1.3. Exposure of Alveolar Macrophages to PM Extracts and Detection of ROS
NR8383 cells were harvested by collecting the non-adherent fraction of cells. Cell concentrations were determined using a Coulter EPICS XL flow cytometer (Beckman Coulter, Miami, FL) using standardized Flow-Count Fluorospheres (Beckman Coulter) at a known concentration. Cells were gently concentrated by centrifugation at 750 RPM for 5 min, the culture medium removed, and replaced with the SGM to generate a cell suspension of 1,000 cells/μL. A 15 mM stock solution of 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma) was prepared in N,N′-dimethyl formamide (Sigma) and stored under nitrogen at −20°C for up to six months. Just prior to use DCFH-DA was diluted 10 fold in 1× SGM.
One hundred μ L of the macrophage cell suspensions were dispensed into each well of the 96 well plate (i.e., 100,000 cells/well) and then incubated at 37°C for 2 h. Approximately 15 min before the end of the incubation period, the diluted DCFH-DA solution was added to each prepared sample extract to achieve a final concentration of 15 μ M DCFH-DA. After the incubation period, during which time > 98% of the cells settled and adhered to the well bottom, the SGM was pipetted off and immediately replaced with 100 μL of sample extract or control sample. The fluorescence intensity in each well was determined at 450 ± 50 excitation and 530 ± 25 emission using a CytoFlour II automated fluorescence plate reader (PerSeptive Biosystems) after the exposure period (typically 2.5h). For each exposure experiment several untreated and method blank controls were included. Un-opsonized zymosan was included as a positive control by exposing cells to a 1× SGM solution containing 0.125 mg/ml zymosan. Each sample/dilution was run in triplicate (i.e., 3 wells each). All data is reported as the increase in fluorescence observed in treated samples compared to the fluorescence observed in untreated controls.
Zymosan (a β -1,3 polysaccharide of D-glucose) was chosen as the primary positive control because it is recognized (binds) by TLR-2 receptors on macrophage cells, activating a strong immuno-chemical response.
2.2. Cyto-Toxicity Assay
The viability (i.e., overall cell health/toxicity) of the macrophage cells was evaluated by assessing cell membrane integrity (CitationOkayama et al. 2006; CitationRiley et al. 2005). The Lactate Dehydrogenase (LDH) assay, which probes leakage of LDH from the cell, was employed (LDH-Cytotoxicity Assay kit, BioVision). Macrophages were seeded into a 96-well plate and allowed to adhere overnight. The following day the culture medium was removed and replaced with the sample extract exposure solutions made in SGM medium as described in section 2.1.2. Cells were allowed to incubate for 7 h, after which the medium was removed and assessed for LDH activity as described by manufacturer's instructions. Untreated cells incubated in SGM were included as controls. In all sample exposures, the assay results were not significantly different than controls, indicating viable cells, and no overt toxicity from the samples. The incubation time of the LDH assay was necessarily longer than that used in the ROS assay to provide the requisite sensitivity. Therefore, outcomes are worst case, and given the negative results at 7 h, we would not expect toxicity at the ROS endpoint time of 2.5 h.
2.3. Collection of PM2.5 Samples
Daily PM2.5 samples [nominal: 27 m3 of air sampled at 18.75 L min1] were collected for one year in the metro area of Denver, CO in 2003 on parallel-configured pre-cleaned (acid-rinsed) 47 mm diameter Teflon (Teflo®) and quartz filters. A sub-set of ∼ 50 filters from this larger study were used for the ROS bioassay method development and application demonstration. Teflon filters were tared and re-weighed on a robotic microbalance to a sensitivity of 1 μ g. The median PM mass of the PM2.5 samples used in this study was 133 μ g [average = 151 ± 65 μg, minimum = 24, maximum = 348 μ g]. Field blanks (Teflon filters transported to the field site, loaded into filter holders, and then unloaded) were collected at a frequency of 15% of sample filters, and treated identically to sample filters.
2.4. Chemical Analysis
Total organic and element carbon (OC/EC) were quantified from the quartz fiber filters using the NOISH thermal-optical method (CitationNIOSH 1996). The Teflon filters (either whole filter or 1/2 filters where the ROS bioassay was performed) were extracted with 20 mL of high purity MQ water in capped polypropylene vials on a shaker table. Extracts were filtered through 0.22 μm polypropylene filters and then analyzed for water soluble organic carbon (WSOC) using a high-temperature combustion method (CitationWangersky 1993), and a suite of 47 major and trace elements using magnetic-sector Inductively-Coupled Plasma Mass Spectrometry (HR-ICPMS; Finnigan Element 2). The HR-ICPMS was interfaced to a quartz cyclonic spray-chamber fitted with an ESI low-flow (80 μL min− 1) Teflon micro-concentric nebulizer. All water extracts were stabilized (2%) with Optima grade 16N HNO3. Sample filters were extracted and analyzed in batches of 40 samples, with the following QA/QC samples incorporated within each batch: unfiltered and filtered method blanks, extraction spike recoveries, sample spike recoveries, analytical and sample replicates, isotope and resolution checks, and a set of check blanks (CCB's) and calibration verification checks (CCV's). Instrumental detection limits (3-sigma) were in the range of 0.01 to 2 ng L−1 (equivalent to 0.01–2 pg m−3 of air) for most trace elements and in the range of 5 to 50 ng L−1 (5–50 pg m−3 of air) for major elements. Extraction spike recoveries at the 40 and 80 ng level (equivalent to 2 and 4 μ g L− 1) were, with only minor exceptions, all within our acceptance window (85–115%). Sample spike recoveries were also well within our acceptance window (85–115%).
3. RESULTS AND DISCUSSION
3.1. Method Optimization
3.1.1. Time Course of ROS Response
The optimum exposure time of the samples to the DCFH-DA-loaded macrophage cells in-terms of noise (S/N) was evaluated in experiments where the fluorescence signal was followed as a function of time (response measured every 30 min over a 180-min period; ). These time-course studies were performed for both our positive control (Zymosan) and several real-world samples (Denver-area PM2.5). As shown in , the method blanks, after a lag of ∼40–60 min exhibited an essentially flat response at 20–30% increase in signal over untreated controls. We believe the slight increase in signal above untreated controls observed in this experiment to be an anomaly as it was not typically observed in the method blank exposures performed for all other experimental runs (see ). The Zymosan samples exhibited a rapid increase in fluorescence reaching a stable maximum (400% of control) at 90 min and holding there for the remaining evaluation period. The PM2.5 extracts clearly exhibited a contrasting time course response, with fluorescence increasing more slowly and reaching a maximum (250–350% of control) at 150 min. All three real-world samples displayed similar profiles. We chose 150 min for routine measurements—a stable region for the positive control, and within the optimum time-frame for the real-world samples.
FIG. 1 Time course of the ROS response. The time course of increased fluorescence was determined in DCFH-DA loaded NR8383 cells exposed to extracts prepared from 3 separate PM 2.5 loaded filters, method blank leachate or 0.125 mg/ml zymosan control. Fluorescence was measured every 30 min and data is represented as the fold increase in fluorescence over that observed in the untreated control at each time point. Values represent mean ± standard deviation of n = 3. PM masses on filters 31A, 37A, and 40A were 273 μ g, 188 μ g, and 249 μ g, respectively.
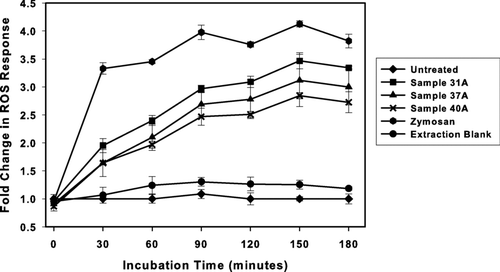
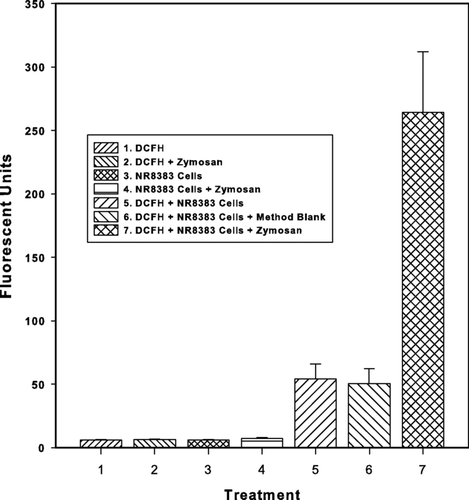
FIG. 3 Specificity and reproducibility of the NR8383 ROS assay. Fluorescence was measured in wells containing the individual reagents of the assay and also the reagents in various combinations. Treatments included DCFH-DA alone, DCFH-DA + zymosan, NR8383 cells alone, NR8383 cells + zymosan, and NR8383 cells loaded with DCFH-DA. Reproducibility of the ROS response was demonstrated by incubation of DCFH-DA loaded NR8383 cells with either method blank leachate (MB) or zymosan (Zym) over a series of separate experiments. Values represent mean ± standard deviation. For blank and specificity results, n = 3 wells of cells. For detection limits n = 16 separate exposures, 3 wells of cells/exposure. For zymosan exposure n = 10 separate exposures, 3 wells of cells/exposure.
Nearly without exception the blank-corrected zymosan responses of the dilution series (33%, 66%, 100%; data not shown) are, if not linear, at least substantially increasing with lower dilution. Also, we have run the zymosan at significantly higher exposure concentrations (400 μ g/mL) at the 100 K cells per well loading and have observed a nearly proportional increase in ROS response. Thus we do not believe we are operating under saturation (either substrate or instrument) conditions.
3.1.2. Sensitivity to Cell Numbers
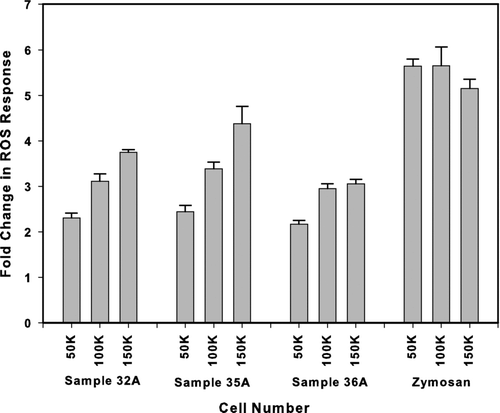
The influence of macrophage cell numbers on ROS response was evaluated with three different PM2.5 sample extracts and positive control (Zymosan). Three cell levels (50,000, 100,000, 150,000 DCFH-DA loaded cells per well) were chosen based upon earlier experimentation and literature reviews. Replicate sub-samples of PM2.5 extracts and Zymosan were each exposed at the three cell levels and fluorescence determined after 150 min (). Ideally, to limit concerns about substrate limitation, one would prefer to run the assay under conditions where ROS response is relatively invariant with cell number and also within the linear range of the response “curve.” These experiments indicated that at the 100,000 cells per well level, we typically achieve that criterion. The ROS response in the 100,000 cells per well treatments of two of the three filter extracts and the Zymosan samples were not significantly different than the 150,000 cells per well treatments (). Given these data and our goal of minimizing blank response, as well as the need to maximize use of available macrophage cells, we chose 100,000 cells as the standard well loading. A discussion of linearity and dilution series follows in section 3.7.
FIG. 2 Effect of cell number on ROS response. Extracts from three separate PM2.5 loaded filters or 0.125 mg/ml zymosan control were incubated with 50,000, 100,000, or 150,000 DCFH-DA loaded cells and the fluorescence read after 2.5 h. Data is represented as the fold increase in fluorescence over the respective untreated control. Values represent mean ± standard deviation of n = 3.
Several studies (CitationOhmann and Babiuk 1984) have shown that culture conditions, in particular whether cells are suspended or adherent, may impact macrophage in-vitro activity. To minimize the influence of this variable, we exclusively selected suspended cells for the exposure experiments, as per ATCC recommended culturing and harvesting protocols.
3.2. Blanks and Detection Levels
3.2.1. Blanks and Specificity
A series of experiments were conducted to isolate blank components and evaluate the specificity of the ROS response. A summary of data outcomes is presented in where fluorescent response is plotted against treatment type. Treatments with the fluoro-probe (DCFH-DA) alone or fluoro-probe + Zymosan elicited no detectable fluorescence, thereby demonstrating the lack of auto-fluorescence (the acetate group is not cleaved under baseline assay conditions). The fact that no response was observed from the DCFH-DA + Zymosan treatments clearly indicates that the ROS-activity of this agent operates via sensitization of the macrophages. Similarly, fluorescent response was insignificant in treatments with the NR8383 macrophage cells alone or with NR8383 cells + Zymosan—the macrophage cells have minimal intrinsic fluorescence. The ROS response of untreated controls (i.e., DCFH-DA loaded macrophage cells) and method blanks (i.e., Type 1 water carried through the complete filter extraction and assay protocol and exposed to DCFH-DA loaded macrophage cells) were not significantly different (), indicating that the overall method is ROS neutral and that the baseline fluorescence is almost entirely controlled by endogenous ROS generation in the macrophage cells. Variation in this native ROS production will determine the method detection levels (refer to 3.2.2). All other blank components are insignificant.
3.2.2. Detection Limits
Control samples and method blanks run with the Denver area field samples were used to generate detection limit metrics. These were routine QC samples run over a 5-month period on 15 separate dates (not a dedicated detection limit study) and therefore represent conservative, real world application, estimates of method quantification limits. Untreated blanks were not significantly different from method blanks and therefore the 3 samples of each, run on each of 15 dates, were used to generate within batch and between batch estimates of variability. The average between batch blank (n = 15) was 53 fluorescence units (FU) (27 μ g equivalents of Zymosan), ranging from 34.8 to 68.5 FU, with a standard deviation of 12.3 FU (6.3 μ g equivalents of Zymosan). The average (n = 15) within batch (n = 6) blank standard deviation was 2.6 ± 0.75 FU (1.3 ± 0.4 μ g equivalents of Zymosan). A 3-sigma detection limit (DL) (based upon the within batch method blanks) is therefore ∼ 4 μ g equivalents of Zymosan. A DL estimated from the uncertainty in the variation in method blanks between batches is actually much smaller (1.2 μ g).
Given the typical volume of air sampled (13.5 m3 for 1/2 filter) and median PM mass collected (150 μ g) in this study, the 4 μ g Zymosan equivalent DL equates to an air concentration DL of 0.3 μ g m− 3 Zymosan equivalent, and a mass normalized DL of 26 μ g Zymosan equivalent/mg of PM.
3.2.3. Filter Type Response
The ROS blank of three other common aerosol sampling substrates (Zefluor® Teflon filters, quartz fiber filters (QFF), and Teflon-coated glass fiber filters (Pallflex®)) were evaluated in addition to the standard Teflo® filters. As was observed with the Teflo® filters, method blanks incorporating these filters were not significantly different than untreated controls (data not shown), therefore the method is broadly unrestrictive in the choice of PM sampling substrates.
3.3. Sensitivity and Signal/Noise
Zymosan positive controls run on 11 separate dates (3 replicates per date) over the time period outlined in section 3.2.2, averaged 224 ± 41 FU. Normalizing these data to Zymosan concentration (nominally 114 μ g mL−1) resulted in an average response slope of 1.96 ± 0.36 FU/μ g Zymosan. The average daily signal to noise (S/N) ratio at the nominal positive control concentration (114 μ g mL−1) = 32 (range 18 to 56). This S/N level provides ample detection power to probe the ROS activity of nearly all daily PM samples. To generate an ROS fluorescence signal of twice the noise (i.e., S/N = 2), 8 μ g of Zymosan would be required.
In a separate experiment we compared the ROS activity of Zymosan to that of a water extract of diesel particulates. Diesel exhaust particulates (DEP, NIST SRM 1650a) were extracted with high purity water (523 μ g mL−1) and processed in an identical manner to that of the Zymosan. The mass normalized ROS activity of the DEP extract (0.187 ± 0.008 FU/μ g) was significantly less than the Zymosan (1.57 ± 0.11 FU/μ g).
3.4. Precision
3.4.1. Within and Between Batches
We use the responses of the positive control (Zymosan) to provide estimates of intra- and inter-batch precision. The within batch standard deviation of triplicate samples of Zymosan ranged from 2.0 to 25.4 FU (n = 11, mean = 11.3 FU = ∼6 μg Zymosan) producing a relative standard deviation (RSD) of 0.5 to 8.5% (mean = 4.0%). As noted in section 3.3, Zymosan positive controls, run on 11 separate dates (3 replicates per date) over the 5-month evaluation period averaged 224 ± 41 FU. This represents an external (inter batch) RSD of 18.3% ().
3.4.2. Sectioned Filter
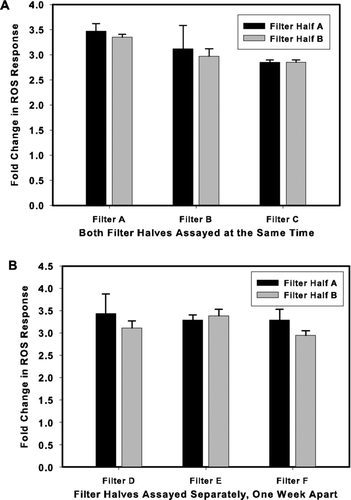
To provide an assessment of the overall precision of the method (including sample filter sectioning) several real-world filter samples (Denver-area PM2.5) were sectioned in half and each section independently extracted and assayed. The results of experiments where the extraction and ROS assay on paired filter halves were run concurrently are shown in . The relative percent difference (RPD) in ROS activity between filter halves were 3%, 6%, and 1.5% (none significantly different) for the 3-paired/sectioned filters—on average 3.5% overall RPD.
FIG. 4 Method Reproducibilty and Extract Stability. PM2.5 loaded filters were cut in half and each half extracted and assayed independently in order to determine the reproducibility of the method. (a) Both filter half A and filter half B were extracted and assayed concurrently. (b) Filter half A and filter half B were extracted simultaneously. Leachate from filter half A was assayed immediately and leachate from filter half B was stored at 4°C for 1 week before being assayed. Data is represented as the fold increase in fluorescence over the untreated control. Values represent mean ± standard deviation of n = 3. Experimental means for each filter were compared using a Student's T-test assuming equal variances. Homogeneity of variance was evaluated using the F-test. No significant difference between filter halves was found for any of the filters.
3.5. Extract Stability
The stability (ROS activity) of the PM extract was evaluated in an experiment similar to that described above. Three Denver area PM2.5 samples were sectioned in half and each section simultaneously extracted. However, unlike in section 3.4.2 where all extracts were assayed simultaneously and soon after extraction, the extract from one half filter was assayed for ROS immediately, and the extract from the other half was stored at 4°C for 1 week before assaying. The outcome of this experiment is summarized in . In all three test cases the ROS activities of the stored extracts were not significantly different from that measured immediately. The relative stability of the extracts eliminates a potential source of variability in the method, providing further support of method robustness and suitability for scale-up and application in routine monitoring studies.
3.6. Spike Recovery
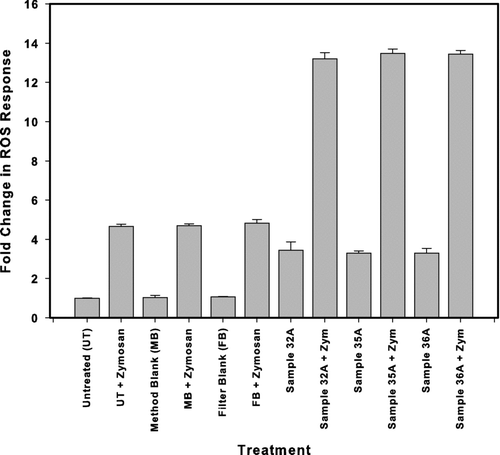
A study was conducted to test whether an ROS active agent (Zymosan) could be quantitatively recovered from control solutions AND ambient filter extracts. The primary focus of this series of experiments was to determine whether ROS activity was suppressed (i.e., poor recovery) in filter extracts. Known and identical amounts of Zymosan were spiked into (a) untreated controls, (b) method blanks, (c) blank filters, and (d) extracts of three ambient Denver filters. A summary of the outcomes, which only in-part, met expectations are presented in . Recovery of Zymosan from our blanks/controls was quantitative, however, the recovery of Zymosan from the filter extracts was much larger (3–4 fold) than anticipated. All three filter extracts gave very similar outcomes. These results suggest a synergistic or sensitization reaction, geometrically enhancing the ROS production. While beyond the scope of this study, clearly, additional effort should be directed at further elucidating the nature of this response.
FIG. 5 Effect of co-exposure to PM2.5 leaches and zymosan on ROS response. DCFH-DA-loaded NR8383 cells were left untreated or incubated with either method blank extract, extract prepared from a blank filter not loaded with PM2.5 material, or extracts from three separate PM2.5 loaded filters. Cells were exposed to either leachate alone or co-exposed to both the leachate and 0.125 mg/ml zymosan and the fluorescence measured in each well after 2.5 hr incubation. Data is represented as the fold increase in fluorescence over the untreated control. Values represent mean ± standard deviation of n = 3.
3.7. Linearity & Dynamic Range
The Zymosan response at the 125 μ g mL−1 concentration typically induced an ROS response greater than that of samples of ambient PM. Dilution series (100%, 66%, 33%) of the Zymosan exhibited the expected concentration–ROS response relationship, confirming linearity, and indicating that ambient samples should not be impacted by saturation artifacts. The dynamic range of the method therefore appeared to be adequate to address a seasonally diverse assemblage of PM from the Denver area. The greatest mass of ambient PM extracted was 348 μ g [range 24 to 348 μ g]. Routine use of dilution series will accommodate exceptionally active samples without the need for adjustment of filter fractions. In most, but not all cases, the dilution series (100%, 66%, 33%) also exhibited the expected concentration–ROS response relationship, signifying that we were working in the linear range and avoiding saturation. A non-linear response was observed in the dilution series from nine of the 46 Denver samples. The LDH assay indicated that toxicity was not present, so the non-linearity in these selected samples must be attributed to other (unknown) factors. Routine use of dilution series will accommodate exceptionally active samples without the need for adjustment of filter fractions.
3.8. Application to Denver Area PM2.5 Samples
Cell toxicity (membrane integrity), as probed by the LDH assay, was not observed in any of the Denver filter extracts. Therefore variations in the amount of ROS production were likely not caused by differences in cell viability. This finding is important in that cell toxicity would complicate interpretation of the ROS-assay, making sample comparisons more difficult. This further supports the use of the NR8383 cell line as a robust platform from which ROS activity can be evaluated.
Of the 46 Denver area PM2.5 samples selected for evaluation, only five exhibited non-detectable ROS activity. PM masses of three of the five samples were < 88 μ g and four of the five below detection samples were from Fall collections (October–December). Interestingly, 15 of the 20 samples with the greatest ROS activity were from summer collections (June–August), while none of the 25 least active samples were from this period. Variations in the magnitude of the ROS response observed between samples can only partially be explained by differences in the PM mass loading () of the filters (i.e., exposure mass) or soluble organic carbon (). This suggests that the minor constituents (and likely source) of the PM2.5 are also important in mediating this response (e.g., iron, ), and therefore may be important factors in explaining how PM2.5 exposure leads to adverse health effects. It's interesting to note that the average (n = 46) mass normalized ROS activity of the ambient Denver PM (0.37 ± 0.28 FU/μ g; range BDL to 1.3) is nearly double that of the NIST DEP. Continuing efforts are underway to establish linkages between toxicity and specific chemical species using both statistical and micro-separation approaches.
FIG. 6 Correlation of ROS response with PM mass and soluble organic carbon and iron. Aqueous extracts were prepared from daily PM2.5 samples collected in the Denver-Metro area. The ROS generated by the filter extracts is regressed against (a) total PM mass on the filters; (b) soluble organic carbon on the filters; and (c) soluble Fe on the filters. Data for the ROS response was normalized to the zymosan control for the respective run in order to control for minor run-to-run variability. Values represent mean of n = 3 for each filter extract (100% data [i.e., no extract dilution] shown).
![FIG. 6 Correlation of ROS response with PM mass and soluble organic carbon and iron. Aqueous extracts were prepared from daily PM2.5 samples collected in the Denver-Metro area. The ROS generated by the filter extracts is regressed against (a) total PM mass on the filters; (b) soluble organic carbon on the filters; and (c) soluble Fe on the filters. Data for the ROS response was normalized to the zymosan control for the respective run in order to control for minor run-to-run variability. Values represent mean of n = 3 for each filter extract (100% data [i.e., no extract dilution] shown).](/cms/asset/ad7dcaf0-dd94-4425-907b-c2fe19315dd2/uast_a_336548_o_f0006g.gif)
4. SUMMARY
Alveolar macrophages (AM) are an ideal model target for assessment of aerosol toxicity, as the cells are recruited to the lungs as a principal line of defense against pulmonary insult, including that from PM. Additionally, ROS activity is generally recognized as a primary mode of cellular damage and toxicity—thus development of a macrophage-based ROS assay for coupling with epidemiological studies is clearly defensible. The advantages in using a highly responsive immortalized macrophage cell line over bronchial-lavaged cells are obvious. Though potentially marginally less sensitive to oxidative stress than other cell types in-terms of ROS generation (CitationOhmann and Babiuk 1984), the ROS response of (AM) is more uniform and predictable than with other cell lines (e.g., PMNs and bronchial epithelial cells). Importantly, AM can be stimulated with non-opsomized particles/toxins, thus should be responsive to a wide range of environmental contaminants. The NR8383 AM cell line, while maintaining its sensitivity to oxidative stress (i.e., ROS generation) imposed by PM extracts, does not appear to be substantially damaged/killed by those extracts. Maintenance of the NR8383 line is exceptionally undemanding, aided by the floating-cell growth habit. Because it more closely replicates the immune response of mature primary macrophages than other macrophage cell lines, the NR8383 line may represent the best current macrophage for studying various aspects of macrophage function (CitationLane et al. 1998).
The bioassay protocol promulgated in this manuscript exhibits several attributes that make it a good candidate for implementation in the context of large scale air monitoring and epidemiological studies. Sensitivity, or more precisely, signal-to-noise (S/N) is a key metric, as the full power of statistical source and component modeling of toxicity is realized only when non-detects are minimized. In the context of medium-volume daily PM samples, this necessitates working with PM masses in the sub-500 μ g range or less, especially if parallel detailed chemistry is to be performed. We demonstrate that method blanks exhibit low (3-sigma detection limit is ∼ 4 μ g equivalents of Zymosan) and reproducible response and that S/N is adequate to quantify ROS activity in PM masses of < 100 micrograms. The NR8383 cell line is relatively easy to manage, and importantly, exhibits excellent within (< 5% RSD) and between (< 15% RSD) batch reproducibility of ROS generation. Overall method precision, including filter sectioning and extraction, is also good. Though our study was necessarily limited in the geographic distribution of PM sampled, the dynamic range of the method appeared to be adequate to address a seasonally diverse assemblage of PM. Routine use of dilution series will accommodate exceptionally active samples without the need for adjustment of filter fractions, and will assist in the identification of cellular toxicity. Further study should be directed at refining the optimal working range.
Another method attribute with relevance to scale-up and broader implementation is throughput and suitability for automation. In this regard the proposed method exhibits several positive features. On the front-end, extractions are simple, use only small (< 1 mL) liquid volumes, and can be performed in microfuge tubes. Large numbers of samples can be simultaneously processed. Substituting centrifugation for the filtration step would further improve efficiency. On the back-end, exposures and fluorescence measurements are carried-out in wells of 96-well plates with an automated plate reader—a system with inherently high throughput. While in certain configurations flow-cytometry may provide greater sensitivity with the DCFH probe, the disadvantages—lower throughput, higher cost, and more limited automation potential—are significant, and the plate reader approach, in our implementation, provided adequate sensitivity. The 120–150 min exposure period, while not insignificant is very workable, and multiple plates can be set-up as the extracts were demonstrated to be stable for extended periods. While the high sensitivity of the method is due in large part to the small filter extraction volume (0.9 mL), this volume does not in any substantive way limit the implementation of QA/QC protocols, procedures especially critical in the context of large scale implementation. Because we apply 96-well plate technology, only a fraction (100–150 μ L) of the 900 μ L extract is used in a given well. Thus sufficient volume remains for multiple replicates, dilution series, and spike studies. As demonstrated, the extraction volume is also sufficient for parallel bioassays (e.g., LDH cytotoxicity). Lab supplies are standard and implementation costs relatively low. We expect that the method described would have the necessary throughput to process large numbers of samples and provide the novel opportunity to evaluate, in high resolution, temporal and spatial trends in aerosol oxidative stress capacity. As with all bioassay procedures, maintenance of the organism/cell is critical, and a potentially throughput limiting step. The NR8383 AM cell line has proved to be especially robust in this regard, and with appropriate care, we expect that culturing can be scaled to provide the requisite cell numbers.
We are incorporating metrics of ROS activity as probed by this assay into receptor models of aerosol source—thereby allowing us to identify and rank the relative ROS activity of various aerosol sources. Follow-up studies should attempt to identify and confirm the active agents in these sources. These studies would benefit from measurements of oxidative stress (e.g., HO-1, and GSH/GSSG ratios) and inflammation (e.g., a suite of cytokines) markers, and phagocytosis impairment.
Finally, though not a major focus of this article, an important method development criterion was the design of a speciation-compatible multi-use extraction protocol. It is expected that a simplified exposure medium will facilitate examination of biochemical controls on bioavailability and ROS activity. A significant effort was devoted to evaluating a series of exposure media that would support healthy and robust AM cells, yet minimize or eliminate the use of ligand-rich organic media components on which cells are traditionally supported. The SGM substantially met this goal and supported optimal AM health over the exposure period. While not totally eliminating organic functional groups, the stability constants of the remaining organic buffer (HEPES) and glucose, for trace metals, are low. Additionally the inorganic matrix is simple and well-defined, again supportive of both direct (e.g., electrochemical) chemical and speciation modeling.
REFERENCES
- Arredouani , M. S. , Palecanda , A. , Koziel , H. , Huang , Y.-C. , Imrich , A. , Sulahian , T. H. , Ning , Y. , Yang , Z. , Pikkarainen , T. , Sankala , M. , Vargas , S. O. , Takeya , M. , Tryggvason , K. and Kobzik , L. 2005 . MARCO is the Major Binding Receptor for Unopsonized Particles and Bacteria on Human Alveolar Macrophages . J. Immunology , : 6058 – 6064 .
- Becker , S. , Soukup , J. M. , Gilmour , M. I. and Devlin , R. B. 1996 . Stimulation of Human and Rat Alveolar Macrophages by Urban Air PartiCulates: Effects on Oxidant Radical Generation and Cytokine Production . Toxicol. Appl. Pharmacol , 141 : 637 – 648 .
- Becker , S. , Dailey , L. A. , Soukup , J. M. , Grambow , S. C. , Devlin , R. B. and Huang , Y. T. 2005 . Seasonal Variations in Air Pollution Particle-Induced Inflammatory Mediator Release and Oxidative Stress . Environ. Health Perspect , 113 : 1032 – 1038 .
- Bowden , D. H. 1984 . The Alveolar Macrophage . Environ. Health Perspect , 55 : 327 – 341 .
- Brunekreef , B. and Holgate , S. T. 2002 . Air Pollution and Health . Lancet , 360 : 1233 – 1242 .
- Carranza , S. E. and Pantano , P. 2003 . Fluorescence Microsopy and Flow Cytofluorometry of Reactive Oxygen Species . Applied Spectroscopy Reviews , 38 : 245 – 261 .
- Castro , L. and Freeman , B. 2001 . Reactive Oxygen Species in Human Health and Disease . Nutrition , 17 ( 2 ) : 161 – 165 .
- Ciapetti , G. , Granchi , D. , Verri , E. , Savarino , L. , Cenni , E. , Savioli , F. and Pizzoferrato , A. 1998 . Fluorescent Microplate Assay for the Respiratory Burst of PMNs Challenged in Vitro with Orthopedic Metals . J. Biomed. Mater. Res , 41 : 455 – 460 .
- Cho , A. K. , Sioutas , C. , Miguel , A. H. , Kumagai , Y. , Schmitz , D. A. , Singh , M. , Eiguren-Fernandez , A. and Froines , J. R. 2005 . Redox Activity of Airborne Particulate Matter at Different Sites in The Los Angeles Basin . Environ. Res , 99 : 40 – 47 .
- Donaldson , K. , Stone , V. , Borm , P. J. A. , Jimenez , L. A. , Gilmour , P. S. , Schins , R. P. F. , Knaapen , A. M. , Rahman , I. , Fauz , S. P. , Brown , D. M. and MacNee , W. 2003 . Oxidative Stress and Calcium Signaling in the Adverse Effects of Environmental Particles (PM10) . Free Radical Biol. Med , 34 : 1369 – 1382 .
- Fach , E. , Waldman , W. J. , Williams , M. , Long , J. , Meister , R. K. and Dutta , P. K. 2002 . Analysis of the Biological and Chemical Reactivity of Zeolite-based Aluminosilicate Fibers and Particulates . Environ Health Perspect. , 110 : 1087 – 1096 .
- Frampton , M. W. , Ghio , A. J. , Samet , J. M. , Carson , J. L. , Carter , J. D. and Devlin , R. B. 1999 . Effects of Aqueous Extracts of PM10 filters from Utah Valley on Human Airway Epithelial Cells . Am. J. Physiol , 277 : L960 – L967 .
- Geng , H. , Meng , Z. Q. and Zhang , Q. X. 2006 . In Vitro responses of Rat Alveolar Macrophages to Particle Suspensions and Water-Soluble Components of Dust Storm PM2.5 . Toxicology In Vitro , 20 : 575 – 584 .
- Goldsmith , C.-A. W. , Imrich , A. , Danaee , H. , Ning , Y. and Kobzik , L. 1998 . Analysis of Air Pollution Particulate-Mediated Oxidant Stress in Alveolar Macrophages . J. Toxicol. Environ. Health Part A , 54 : 529 – 545 .
- Goldsmith , C.-A. , Frevert , C. , Imrich , A. , Sioutas , C. and Kobzik , L. 1997 . Alveolar Macrophage Interaction with Air Pollution Particulates . Environ Health Perspect. , 105 ( Suppl 5 ) : 1191 – 1195 .
- Gazin , V. , Kerdine , S. , Grillon , G. , Pallardy , M. and Raoul , H. 2004 . Uranium Induces TNF Alpha Secetion and MAPK Activation in a Rat Alveolar Macrophage Cell Line . Toxicol. Appl. Pharmacol , 194 : 49 – 59 .
- Halliwell , B. and Cross , C. E. 1994 . Oxygen-Derived Species—Their Relation to Human-Disease and Environmental-Stress . Environ. Health Perspect , 102 : 5 – 12 .
- Hong , Y. , Hwang , S. , Kim , J. H. , Lee , K. , Lee , H. , Lee , K. , Yu , S. and Kim , D. 2007 . Metals in Particulate Pollutants Affect Peak Expiratory Flow in Schoolchildren . Environ. Health Perspect , 115 : 430 – 434 .
- Huang , S.-L. , Hsu , M.-K. and Chan , C.-C. 2003 . Effects of Submicrometer Particle Compositions on Cytokine Production and Lipid Peroxidation of Human Bronchial Epithelial Cells . Environ Health Perspect. , 111 : 478 – 482 .
- Iles , K. E. and Forman , H. J. 2002 . Macrophage Signaling and Respiratory Burst . Immunologic Research , : 95 – 105 .
- Imrich , A. , Ning , Y. , Lawrence , J. , Coull , B. , Gitin , E. , Knutson , M. and Kobzik , L. 2007 . Alveolar Macrophage Cytokine Response to Air Pollution Particles: Oxidant Mechanisms . Toxicol. Appl. Pharmacol , 218 : 256 – 264 .
- Imrich , A. , Ning , Y. and Kobzik , L. 2000 . Insoluble Components of Concentrated Air Particles Mediate Alveolar Macrophage Responses in Vitro . Toxicol. Appl. Pharmacol , 167 : 140 – 150 .
- Imrich , A. , Ning , Y. and Kobzik , L. 1999 . Intracellular Oxidant Production and Cytokine Responses in Lung Macrophages: Evaluation of Fluorescent Probes . J. Leukoc. Biol. , 65 : 499 – 507 .
- Imrich , A. and Kobzik , L. 1997 . “ Flow Cytometric Analysis of Macrophage Oxidative Metabolism using DCFH ” . In Protocols for Flow Cytometry , Edited by: Jaroszeski , M. and Heller , R. Volume 91 , 97 – 108 . Totowa, NJ : Humana Press .
- Klein , C. B. , Su , L. , Bowser , D. and Leszczynska , J. 2002 . Chromate-Induced Epimutations in Mammalian Cells . Environ. Health Perspect. , 110 ( suppl 5 ) : 739 – 743 .
- Kumagai , Y. , Koide , S. , Taguchi , K. , Endo , A. , Nakai , Y. , Yoshikawa , T. and Shimojo , N. 2002 . Oxidation of Proximal Protein Sulfhydryls by Phenanthraquinone, A Component of Diesel Exhaust Particles . Chem. Res. Tox , 15 ( 4 ) : 483 – 489 .
- Kunzli , N. , Mudway , I. S. , Gotschi , T. , Shi , T. M. , Kelly , F. J. , Cook , S. , Burney , P. , Forsberg , B. , Gauderman , J. W. , Hazenkamp , M. E. , Heinrich , J. , Jarvis , D. , Norback , D. , Payo-Losa , F. , Poli , A. , Sunyer , J. and Borm , P. J. A. 2006 . Comparison of Oxidative Properties, Light Absorbance, and Total and Elemental Mass Concentration of Ambient PM2.5 Collected at 20 European Sites . Environ. Health Perspect. , 114 ( 5 ) : 684 – 690 .
- Lane , K. B. , Egan , B. , Vick , S. , Abdolrasulna , R. and Shepherd , V. L. 1998 . Characterization of a Rat Alveolar Macrophage Cell Line That Expresses a Functional Mannose Receptor . J. Leukocyte Biol , 64 : 345 – 350 .
- Li , N. , Xia , T. and Nel , A. E. 2008 . The Role of Oxidative Stress in Ambient Particulate Matter-Induced Lung Diseases and its Implications in the Toxicity of Engineered Nanoparticles . Free Rad. Biol. Med. , 44 : 1689 – 1699 .
- Li , N. , Hao , M. , Phalen , R. F. , Hinds , W. C. and Nel , A. E. 2003 . Particulate Air Pollutants and Asthma. A Paradigm for the Role of Oxidative Stress in PM-induced Adverse Health Effects . Clin. Immunol , 109 ( 3 ) : 250 – 65 .
- Lippmann , M. , Ito , K. , Hwang , J. S. , Maciejczyk , P. and Chen , L. C. 2006 . Cardiovascular Effects of Nickel in Ambient Air . Environ. Health Perspect , 114 : 1662 – 1669 .
- Lippmann , M. and Schlesinger , R. B. 2000 . Toxicological Bases for the Setting of Health-Related Air Pollution Standards . Ann. Rev. Pub. Health , 21 : 309 – 333 .
- Long , J. F. , Dutta , P. K. and Hogg , B. D. 1997 . Fluorescence Imaging of Reactive Oxygen Metabolites Generated in Single Macrophage Cells (NR8383) Upon Phagocytosis of Natural Zeolite (Erionite) Fibers . Environ. Health Perspect. , 105 : 706 – 711 .
- NOISH . 1996 . NIOSH Manual of Analytical Methods , Cincinnati, OH : National Institute of Occupational Safety and Health .
- Ng , D. , Kokot , N. , Hiura , T. , Faris , M. , Saxon , A. and Nel , A. 1998 . Macrophage Activation by Polycyclic Aromatic Hydrocarbons: Evidence for the Involvement of Stress-Activated Protein-Kinases, Activator Protein-1, and Antioxidant Response Elements . J. Immunol. , 161 : 942 – 951 .
- Ohmann , H. B. and Babiuk , L. A. 1984 . In Vitro Generation of Hydrogen Peroxide and of Superoxide Anion by Bovine Polymorphonuclear Neutrophilic Granulocytes, Blood Monocytes, and Alveolar Macrophages . Inflamation , 8 ( 3 ) : 251 – 275 .
- Okayama , Y. , Kuwahara , M. , Suzuki , A. K. and Tsubone , H. 2006 . Role of Reactive Oxygen Species on Diesel Exhaust Particle-Induced Cyctotoxicity in Rat Cardiac Myocytes . J. Toxicol. Environ. Health Part A , 69 : 1699 – 1710 .
- Pope , C. A. and Dockery , D. W. 2006 . Health Effects of Fine Particulate Air Pollution: Lines That Connect . J. Air & Waste Man. Assoc , 56 : 709 – 742 .
- Pope , C. A. , Burnett , R. T. , Thurston , G. D. , Thun , M. J. , Calle , E. E. , Krewski , D. and Godleski , J. J. 2004 . Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution—Epidemiological Evidence of General Pathophysiological Pathways of Disease . Circulation , 109 ( 1 ) : 71 – 77 .
- Pourazar , J. , Mudway , I. S. , Samet , J. M. , Helleday , R. , Blomberg , A. , Wilson , S. J. , Frew , A. J. , Kelly , F. J. and Sandstrom , T. 2005 . Diesel Exhaust Activates Redox-Sensitive Transcription Factors and Kinases in Human Airways . Am. J. Physiol. Lung Cell. Mol. Physiol , 289 ( 5 ) : L724 – L730 .
- Prahalad , A. K. , Soukup , J. M. , Inmon , J. , Willis , R. , Ghio , A. J. , Becker , S. and Gallagher , J. E. 1999 . Ambient Air Particles: Effects on Cellular Oxidant Radical Generation in Relation to Particulate Elemental Chemistry . Toxicol. Appl. Pharmacol. , 158 ( 2 ) : 81 – 91 .
- Riley , M. R. , Boesewetter , D. E. , Turner , R. A. , Kim , A. M. , Collier , J. M. and Hamilton , A. 2005 . Comparison of the Sensitivity of Three Lung Derived Cell Lines to Metals From Combustion Derived Particulate Matter . Toxicology In Vitro , 19 : 411 – 419 .
- Risom , L. , Moller , P. and Loft , S. 2005 . Oxidative Stress-Induced DNA Damage by Particulate Air Pollution . Mutat. Res , 592 : 119 – 137 .
- Samet , J. M. , Dominici , F. , Curriero , F. C. , Coursac , I. and Zeger , S. L. 2000 . Fine Particulate Air Pollution and Mortality in 20 U.S. cities 1987–1994 . N. Engl. J. Med. , 343 : 1742 – 1749 .
- Schoonen , M. A. A. , Cohn , C. A. , Roemer , E. , Laffers , R. , Simon , S. R. and O'Riordan , T . 2006 . Mineral-Induced Formation of Reactive Oxygen Species . Reviews in Mineralogy & Geochemistry , 64 : 179 – 221 .
- Schwarze , P. E. , Ovrevik , J. , Lag , M. , Refsnes , M. , Nafstad , P. , Hetland , R. B. and Dybing , E. 2006 . Particulate Matter Properties and Health Effects: Consistency of Epidemiological and Toxicological Studies . Human & Exper. Toxicol. , 25 : 559 – 579 .
- Seagrave , J.-C. , McDonald , J. D. , Bedrick , E. , Edgerton , E. S. , Gigliotti , A. P. , Jansen , J. J. , Ke , L. , Naeher , L. P. , Seilkop , S. K. , Zheng , M. and Mauderly , J. L. 2006 . Lung Toxicity of Ambient Particulate Matter from Southeastern U.S. Sites with Different Contributing Sources: Relationships Between Composition and Effects . Environ Health Perspect. , 114 : 1387 – 1393 .
- See , S. W. , Wang , Y. H. and Balasubramanian , R. 2007 . Contrasting Reactive Oxygen Species and Transition Metal Concentrations in Combutsion Aerosols . Environ. Res , 103 : 317 – 324 .
- Sorensen , M. , Autrup , H. , Moller , P. , Hertel , O. , Jensen , S. S. , Vinzents , P. , Knudsen , L. E. and Loft , S . 2003 . Linking Exposure to Environmental Pollutants with Biological Effects . Mutat. Res , 544 : 255 – 271 .
- Soukup , J. M. , Ghio , A. J. and Becker , S. 2000 . Soluble Components of Utah Valley Particulate Pollution Alter Alveolar Macrophage Function in Vivo and in Vitro . Inhal. Toxicol , 12 ( 5 ) : 401 – 414 .
- Squadrito , G. L. , Cueto , R. , Dellinger , B. and Pryor , W. A. 2001 . Quinoid Redox Cycling as a Mechanism for Sustained Free Radical Generation by Inhaled Airborne Particulate Matter . Free Radical Biol. Med , 31 ( 9 ) : 1132 – 1138 .
- Takizawa , H. , Ohtoshi , T. , Kawasaki , S. , Kohyama , T. , Desaki , M. , Kasama , T. , Kobayashi , K. , Nakahara , K. , Yamamoto , K. , Matsushima , K. and Kudoh , S. 1999 . Diesel Exhaust Particles Induce NF-kappa B Activation in Human Bronchial Epithelial Cells in Vitro: Importance in Cytokine Transcription . J. Immunol , 162 : 1405 – 1411 .
- Tao , F. , Gonzalez-Flecha , G. and Kobzik , L. 2003 . Reactive Oxygen Species in Pulmonary Inflammation by Ambient Particles . Free Radical Biol. Med , 35 : 327 – 340 .
- Venkatachari , P. , Hopke , P. K. , Brune , W. H. , Ren , X. R. , Lesher , R. , Mao , J. Q. and Mitchel , M. 2007 . Characterization of Wintertime Reactive Oxygen Species Concentrations in Flushing, New York . Aerosol Sci. Technol. , 41 ( 2 ) : 97 – 111 .
- Wangersky , P. J. 1993 . Dissolved Organic-Carbon Methods—A Critical-Review . Marine Chemistry , 41 ( 1–3 ) : 61 – 74 .
- Xiao , G. G , Wang , M. , Li , N. , Loo , J. A. and Nel , A. E. 2003 . Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line . J. Biol. Chem , 278 : 50781 – 50790 .
- Zielinski , H. , Mudway , I. S. , Berube , K. A. , Murphy , S. , Richards , R. and Kelly , F J. 1999 . Modeling the Interactions of Particulates With Epithelial Lining Fluid Antioxidants . American Journal of Physiology—Lung Cellular and Molecular Physiology , 277 : L719 – L726 .