Abstract
This article demonstrates the feasibility of scaling-up the technique for particle size selection in the gas phase based on differential mobility analysis. Nano-DMAs used to select the particle size in processes for the synthesis of nanomaterials in the laboratory operate at aerosol flow rates of a few liters per minute. A new DMA capable of classifying nanoparticles of up to 30 nm in size at aerosol flow rates as high as 100 l· min −1 will be presented (HF-DMA). A major advantage of the HF-DMA over current nano-DMAs covering the same particle size is that its resolution is almost unaffected by Brownian diffusion for particles as small as 3 nm. Monodisperse nanoparticles in the 5 to 25 nm size range have been produced at flow rates of up to 90 l· min −1 . The spread in particle size and the particle number concentration were studied with respect to their dependency on the flow rates in the HF-DMA. The measurements reflect the behavior predicted by the theory. The HF-DMA makes it possible to deliver nanoparticles of a well-defined size at yields two orders of magnitude higher than with current nano-DMAs.
1. INTRODUCTION
The first attempts to enhance the aerosol flow rate in differential mobility classifiers (DMAs) were made in the 1960s (CitationFlagan 1998). The problem then was to design a mobility analyzer capable of operating at sufficiently high aerosol flow rates to achieve measurable signals of particles having a mobility of as low as 2· 10−4 cm2· V−1· s−1 which were present in the atmosphere in concentrations below the detection limit of the electrometers existing at that time. Originally, differential mobility classifiers consisted of two parallel plate electrodes and the aerosol sample was introduced at the midpoint between the electrodes through a narrow tube, which resulted in too low aerosol flow rates. To increase the aerosol sampling rate to 12 l· min−1, CitationLanger et al. (1964) developed the first cylindrical mobility analyzer, into which the aerosol was drawn through a cylindrical annulus located roughly midway between the two cylindrical electrodes. The major problems associated with this instrument were the difficulty of matching the potential and the associated deposition at the tip of the aerosol inlet. These problems were overcome later in the Whitby Aerosol Analyzer (WAA) by CitationWhitby and Clark (1966), in which the aerosol was introduced in an annulus near the outer electrode of a cylindrical condenser. This instrument had an inner diameter of 149 mm and an active length of 762 mm, and was capable of laminar flow operation at aerosol flow rates of up to 70 l· min−1. The WAA represented a breakthrough, since it was the first mobility analyzer to be refined and robust enough for commercial production (TSI Inc., Minneapolis, MN, USA; Model 3000). The TSI 3000 WAA weighed nearly 182 kg and occupied a volume of 0.9 m3; as a result of which the instrument had little potential for mobile applications. Nonetheless, both the prototype and the commercial instrument were used in a number of aerosol studies, such as the study of the physico-chemical properties of the atmospheric aerosols in the Los Angeles basin (CitationWhitby et al. 1972).
A new DMA capable of operating at aerosol flow rates as high as 100 l· min−1 is introduced in this article (HF-DMA). The motivation behind it, however, is quite different from the one which motivated the WAA described above. The HF-DMA is designed to increase the yield in delivering monodisperse nanoparticles (a population of particles having a size distribution with a geometric standard deviation σ g of less than 1.1).
Nano-DMAs are DMAs intended to classify nanoparticles (singly charged objects with a mobility equivalent diameter in the range between 1 and 100 nm). A number of nano-DMAs, both commercial instruments and research prototypes, are currently used in laboratories all over the world to investigate the influence of particle size on nanoparticle behavior and properties as well as on the functional properties of nanomaterials obtained by deposition of nanoparticles from the gas phase. Current nano-DMAs capable of classifying nanoparticles in the range of 3 to 100 nm attain a best resolution of about 20 for particles larger than 10 nm. For smaller particles, both resolution and particle transmission decrease with decreasing particle size and become unacceptable below 3 nm. Nano-DMAs typically deliver monodisperse nanoparticles in the mentioned size interval with number concentrations of up to 105 cm−3 at flow rates of a few liters per minute. On account of the low flow rates and the low particle concentrations, experiments using monodisperse aerosols are limited to small-scale laboratory setups, whereas in many chemical engineering studies larger flow rates are desirable. Examples are filter testing, experiments involving turbulent flow, and aerosol behavior at pilot-plant scale. Meanwhile, assuming that the nanoparticles have a diameter of 20 nm, a density of 104 kg· m−3 and a number concentration of 105 cm−3 in a gas at a flow rate of 1 l· min−1, the mass rate is 0.25 μ g· h−1. When these nanoparticles are deposited onto substrates for further characterization of the deposits as functional materials, for example gas sensors (CitationJoshi and Kruis 2006), the characteristic time to attain the amount of material or the deposit thickness required by the characterization techniques is in the order of a few days.
The HF-DMA represents the first step in scaling-up the technique for particle size selection based on electrical mobility analysis. The nominal aerosol and sheath flow rates are 100 and 1000 l· min−1, respectively. In this article, the criteria applied to the design of the HF-DMA are reviewed and the resolution of this new instrument is discussed in comparison with existing nano-DMAs on the basis of DMA theory. The transfer function of the HF-DMA was measured in experiments with monomobile ions, which revealed acceptable resolution and transmission values even for species with a mobility equivalent diameter as small as 1–2 nm. In addition, the capability of the HF-DMA to deliver monodisperse nanoparticles at flow rates of up to 90 l· min−1 was proven in experiments in which a radioactive ionizer was used to charge aerosols of tin oxide (SnO).
2. DESIGN CONSIDERATIONS FOR SCALING-UP DMAs FOR NANOPARTICLES
This section summarizes the basic aspects that need to be taken into account when designing DMAs for size classification of nanoparticles. These considerations have served as the basis for the design of the DMA for size selection of nanoparticles at high gas flow rates (HF-DMA), which is the objective of this study. Since the HF-DMA has cylindrical electrodes, we will refer to DMAs of this geometry in the following.
Resolution
The transfer function of the DMA depicts the probability that a particle of mobility Z that enters the working region through the aerosol inlet slit leaves that region through the aerosol outlet slit. CitationKnutson and Whitby (1975) found that the transfer function of the cylindrical DMA operating at an aerosol flow rate q, a sheath gas flow rate Q, and an applied voltage V has the shape of a triangle centered at a critical mobility Z*
However, the transfer function of the DMA deviates from that described above for very small particles on account of Brownian diffusion. When decreasing the particle size the peak of the triangle lowers (particles are lost by diffusion to the walls) and broadens (the particles follow random trajectories around a mean trajectory). CitationStolzenburg (1988) derived an analytical expression of the transfer function of the DMA which takes into account the Brownian motion of the particles. This is a bell-shaped function in which mean value E and standard deviation S are given by
The resolution of the DMA therefore depends upon two parameters, β and σ, which account for the effect of the aerosol flow rate and particle diffusion in the transfer function respectively. For non-diffusive large particles, the resolution is equal to β −1 = Q/q. In practice, a minimum aerosol flow rate q min is necessary to overcome particle losses in the conduits through which the aerosol enters and exits the DMA and to obtain an acceptable signal at the aerosol outlet, measurable with a detector such as a Condensation Particle Counter (CPC) or an electrometer. The theory of the DMA described previously assumes that the flow is stationary and laminar. DMAs operate in such flow conditions up to a value of the sheath flow rate Q max beyond which the flow in the working region becomes unsteady or turbulent; this occurs at flow Reynolds numbers typically in the order of a few thousands. The best resolution attainable with the DMA is then given by the ratio Q max/q min.
For diffusive small particles, the resolution of the DMA is determined by the diffusion broadening factor σ. This is a function of the Peclet number Pe, which is the product of the flow Reynolds number and the particle Schmidt number, Pe = ReSc. The Reynolds number of the flow in the working region of a cylindrical DMA has the form
After substituting Equation (Equation11) in Equation (Equation7) and this in Equation (Equation6), the following expression of σ is obtained
Rosell et al. (1993, 1996) investigated the resolution of the DMA at the limit of large Reynolds numbers, when the condition β 2 ≪ σ2 ≪ 1 is satisfied and Equation (Equation9) can be approximated as follows
We assume for simplicity that the flow in the working region is uniform; then I(γ) = 1/2(1+ γ), and substituting Equation (Equation14) in Equation (Equation12), a value of the length of the working region is found at which the resolution shows a maximum. The optimal length L* and the corresponding resolution R* are given by
The above results have important implications with regard to the design of DMAs for size classification of nanoparticles or nano-DMAs. On one hand, the resolution is maximal when the length of the working region is equal to the optimal length, which is a function of the radii of the electrodes. On the other hand, the resolution increases with the Reynolds number of the flow in the working region. Thus, as shown in Equation (Equation10), the resolution can be improved further by reducing the radii of the electrodes and/or increasing the sheath flow rate. The challenge then consists in running the DMA in laminar flow conditions at high Reynolds numbers.
Another important parameter is the maximum operating voltage V max of the DMA. The minimum mobility Z min of the particles that can be classified with the maximum resolution is given by Equation (Equation1), as follows
Reasons for Deviation from Ideal Behavior
A number of hypotheses are implicit in the theory of the DMA described previously. On one hand, it is assumed that the inner surfaces are perfectly polished and that the electrodes are perfectly centered. In reality, however, a certain degree of imperfection is expected depending upon the tolerances and the precision of the machines and tools used to manufacture and assemble the DMA. Moreover, the flow is considered to be stationary, laminar, and axisymmetric from the inlet to the outlet. Flow instabilities, turbulence, and asymmetries appear commonly in different regions of the DMA. On account of all these undesirable features, the flow and the electric field in the working region do not behave as described by the theory and the ideal resolution, given by Equations (3) and (9) for non-diffusive and diffusive particles is never achieved. A number of measurements are implemented to avoid or at least minimize non-idealities in DMAs.
Firstly, the correct selection of the width of the aerosol slit δ is of great importance, and an inadequate choice can lead to poor resolution for a variety of reasons. On the one hand, δ must be such that the mean velocity of the particles at the slit u =q/(2π r i δ) is larger than the particle electrical velocity u e = ZE, with Z given by Equation (Equation1), E = V/[ln(r 2/r 1)r i ] and i equal to 1 (outlet slit) or 2 (inlet slit); then particles are dominantly driven by the fluid velocity u. Otherwise, phenomena such as the penetration and the distortion of the electric field in the vicinity of the slits enter into the picture, and their impact on resolution is difficult to assess (CitationRosell et al. 1996). These effects are minimal for values of the flow to electric velocity ratio larger than unity, u/u e = (q/Q) · (L/δ) < 1}. On the other hand, the aerosol slit should not be so narrow as to form a wall jet moving faster than the sheath gas, since this would destabilize the mixing layer between the aerosol and the sheath flows (CitationFernández de la Mora et al. 2007, CitationChen and Pui 1997). Both requirements are usually met in conventional DMAs operating at values of the aerosol to sheath flow rate ratio q/Q of about 0.1 and using a ratio of L/δ ∼ 100.
The inlet and the outlet regions of the sheath gas are also optimized. A particle filter followed by one or more screens is allocated upstream of the working region in order to damp the turbulence of the sheath gas at the entrance to the DMA and to distribute this uniformly over the flow cross-section. Moreover, the geometry of the exhaust region of the sheath gas tends to be quite intricate and a major source of flow asymmetries, unsteadiness and turbulence. It has been demonstrated that pressure fluctuations in the turbulent exhaust region of the DMA propagate as acoustic waves upstream, making the flow in the classification region unsteady and reducing DMA resolution (CitationMartínez-Lozano and Fernández de la Mora 2005a).
Characteristics of Existing Nano-DMAs
Several DMAs for size classification of particles in the nanometric range are currently available on the market (TSI Inc., MN, USA; NanoEngineering Co., FL, USA; Grimm Aerosol Technik GmbH and Co. KG, Ainring, Germany). Among them, the Nano-DMA commercialized by TSI (Model 3085) is the most used all over the world. The radii of the electrodes r 1 and r 2 are 9 and 19 mm respectively, and the length of the working region L is 50 mm. The Nano-DMA operates at aerosol flow rates in the range of 0.3 to 2 l· min−1, sheath flow rates of up to 20 l· min−1 (Re < 250), and voltages of up to a 10 kV. The Nano-DMA thus classifies nanoparticles in the range of 3 to 50 nm in diameter with a typical resolution of 10 for particles above 10 nm. Below 10 nm, the resolution and the transmission diminish with decreasing particle size; for particles of less than 3 nm, both the resolving power and the transmission efficiency of the Nano-DMA are relatively poor (CitationChen et al. 1998).
In the last few years Fernández de la Mora and co-workers have developed several prototypes of supercritical DMAs (DMAs running laminarly at very high Reynolds numbers; CitationFernández de la Mora, 2003), which exhibit much improved resolution and transmission below 3 nm than the TSI Nano-DMA. In these DMAs the flow remains laminar at Reynolds numbers up to 65000, attaining resolving powers as large as 100 for ions of mobility equivalent diameter of about 1 nm. These instruments are characterized by their small dimensions and by their aerodynamic designs aimed at maximizing the flow capacity achievable with a given pump (CitationMartínez-Lozano and Fernández de la Mora 2005b; CitationRamiro et al. 2005; CitationRamiro and Rivero 2007), delaying the transition to turbulent flow (CitationFernández de la Mora 2003; CitationRosser and Fernández de la Mora 2005), and reducing particle electrophoretic losses (CitationLabowsky and Fernández de la Mora 2006; CitationMartínez-Lozano et al. 2006). Supercritical DMAs are conceived for classifying species in the order of a few nanometers size (macro-molecules, clusters, proteins, etc.) with high resolution (R ∼ 100). Because of the short classification lengths (L ∼ 5–18 mm) and the large sheath flow rates (Q ∼ 1000–4000 l· min−1), these DMAs cannot classify larger particles (up to 16 nm in the Labowsky DMA). A recent prototype (NanorangerTM, NanoEngineering Co., FL, USA) based on the Rosser DMA (CitationFernández de la Mora et al. 2007) includes an enlarged classification column (L ∼ 100 mm) covering the full nanometric range (1–100 nm) with excellent resolution down to 1 nm (R ∼ 25) (CitationFernández de la Mora and Attoui 2007).
3. DESIGN OF A DMA RUNNING AT AEROSOL FLOW RATES OF UP TO 100 l· < eqid17 > −1
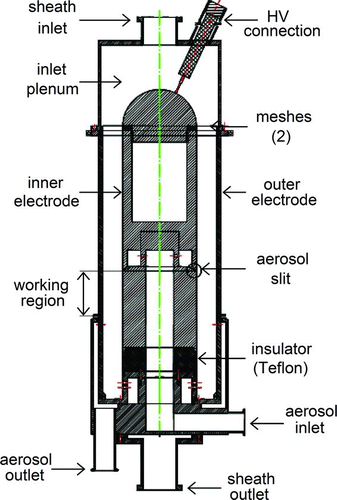
This section deals with the design features of the DMA for size selection of nanoparticles at aerosol flow rates up to 100 l· min−1 (HF-DMA) object of this work. The HF-DMA is made of aluminum; all its parts were manufactured by using precision machines (tolerance of a hundredth of mm) except for the outer electrode, a commercial cylinder having the highest precision available in the market (out-of-roundness tolerance of tenths of mm). shows a drawing of the HF-DMA. The sheath gas flows axially from the top to the bottom and the flow is asymmetric in both the inlet plenum and the exhaust region. The hemispherical top end of the inner electrode is surrounded by two meshes mounted on a rigid structure that keeps the inner electrode in place and helps center the electrodes. The meshes are made of polyester and are identical; the fraction of open area is 34.8% and the wire diameter is 70 μ m (Sefar Holding AG, Freibach, Switzerland; Model SEFAR® PET 1000 54–70 W). They also serve to damp the turbulence of the flow upstream and to spread the sheath gas uniformly over the flow cross-section. The conduits through which the aerosol and the sheath gas enter and leave the HF-DMA were optimized in order to prevent undesirable flow features and to minimize flow pressure drop and particle deposition.
The HF-DMA has been designed to select nanoparticles in the size range of 3 to 30 nm at a nominal flow rate of 100 l· min−1 with a resolution comparable to the resolution of the TSI Nano-DMA for non-diffusive particles (R = 10). Equation [3] shows that a sheath flow rate Q equal to 10 times the aerosol flow rate q is required to meet that condition. Thus the HF-DMA operates at nominal sheath flow rate of 1000 l· min−1. In pipes, the flow remains laminar up to Reynolds numbers of about 2000. We assumed this value for the Reynolds number of the flow in the classification region of the HF-DMA, Re = (Q + q)/π v g (r 2 + r 1), when running at the nominal aerosol and sheath flow rates. After substituting Re (2000), Q (1000 l· min−1), q (100 l· min−1), and ν g (1.5· 10−5 m2· s−1), a value of 195 mm is obtained for r 1 + r 2.
We estimated 1 mm to be the optimal value for the width of the aerosol inlet slit δ when the HF-DMA operates at the desired resolution (R = Q/q = 10). The length of the working region was then fixed at 100 mm to meet δ /L = 10−2. Moreover, we chose a maximum operating voltage V max of 35 kV, for which HV supplies, connections and cables are available on the market. The radii of the electrodes were determined on the basis of the machining capabilities of the manufacturer (University of Duisburg-Essen), the condition r 1+ r 2 = 195 mm above, and taking into account that the distance between the electrodes r 2−r 1 must be large enough to prevent electrical breakdown at the maximum applied voltage V max. The final choice was r 1 = 78 mm and r 2 = 120 mm. The minimum electrical mobility Z min of the particles that can be classified with the HF-DMA at an aerosol flow rate of 100 l· min−1 and a resolution of 10 is given by Equation (Equation18). After substituting Q max (1000 l· min−1), V max(35 kV), and Λ (0.232 m−1), a value of Z min of 3.26· 10−3 cm2· V−1· s−1 is obtained, which corresponds to particles of 26 nm in diameter with a single elementary charge. Larger particles can be classified with a resolution of 10 at lower flow rates on condition that a ratio Q/q of 10 is maintained (e.g., Q/q = 300/30 for particles of 50 nm in diameter).
The performance of the HF-DMA in terms of resolution is discussed on the basis of the theory of DMA described in section 2 and in comparison to the TSI Nano-DMA. We chose the TSI Nano-DMA as a reference because it covers the particle size range in question (3–30 nm) and because numerical and experimental data on the performance of this instrument are available (CitationChen and Pui 1997; CitationChen et al. 1998). By contrast, the supercritical DMAs are not designed to classify particles larger than a few nanometers because of the short classification region (L < 18 mm) and the large sheath flow rate (Q > 1000 l· min−1). For classifying nanoparticles of 30 nm with supercritical DMAs the sheath flow rate must be reduced in two orders of magnitude (the mobility of spheres of diameters 3 and 30 nm are, respectively, 2.3· 10−1 and 2.5· 10−3 cm2· V−1· s−1), so that the DMAs would run at Reynolds numbers below 1000. The performance of supercritical DMAs operating at such low Reynolds numbers has not been investigated and needs further assessment. Another aspect to take into account when operating supercritical DMAs at the high aerosol flow rates q of interest here (100 l· min−1) is the large Reynolds number, then the high pressure drop of the flow in the small conduits through which the aerosol is injected (extracted) into (from) the DMA. In particular, the slits (δ ≤ 0.1 mm) through which the aerosol enters and exits the classification zone should be widened to minimize the flow pressure drop and prevent choked flow. Both the authors (Fernández de la Mora and Attoui) and the company that commercializes (Nano Engineering Co., FL, USA) the NanoRanger DMA claim that this instrument is capable to deliver with excellent resolution nanoparticles of up to 100 nm at aerosol flow rates as large as 100 l· min−1. Nonetheless, results on the performance of the NanoRanger at such large nanoparticles and aerosol flow rates have not been reported up to date. Only results on the resolution of the DMA for ions (mobility equivalent diameter about 1.44 nm) with aerosol flow rates of a few liters per minute were presented in a conference (CitationFernández de la Mora and Attoui 2007).
The transfer functions of the Nano-DMA and the HF-DMA calculated according to Stolzenburg are displayed in for particles of 5 nm and a ratio Q/q of 10; the triangular transfer function is also depicted. In addition, shows the theoretical resolution of the two DMAs given by Equation (Equation9) for particles smaller than 10 nm and the same flow rates as in . The curves tend asymptotically to the limiting value corresponding to the non-diffusive case (R = 10). Negligible diffusion broadening is observed for particles as small as 3 nm when operating the HF-DMA at the highest sheath flow rate (1000 l· min−1). Even at sheath flow rates far from the nominal rate (100 l· min−1), the HF-DMA performs better than the Nano-DMA.
FIG. 2 Transfer function of the Nano-DMA and of the HF-DMA for particles of 5 nm according to CitationStolzenburg (1988).
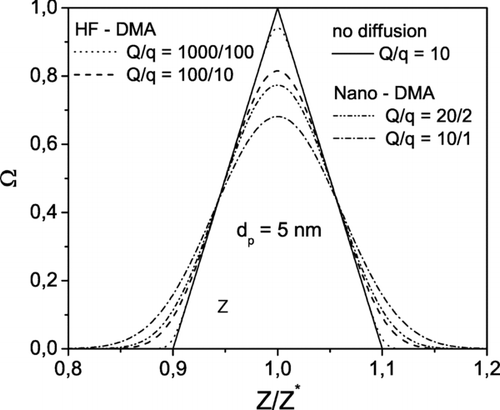
FIG. 3 Resolution of the Nano-DMA and of the HF-DMA vs. particle size according to CitationStolzenburg (1988).
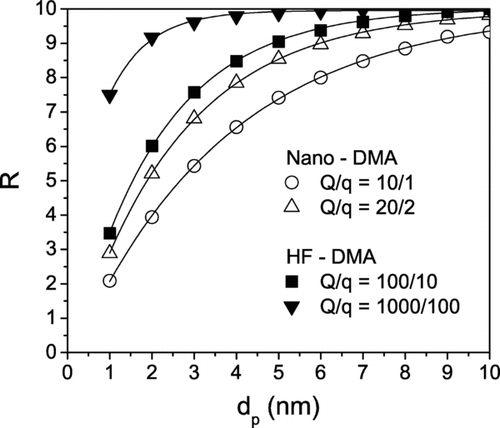
There are two main reasons for the improved resolution of the HF-DMA compared with the Nano-DMA. Firstly, the flow Reynolds number in the HF-DMA is up to 8 times that in the Nano-DMA, enhancing resolution by a factor of 3, as derived from Equation (Equation16). At the same time, the ratio of the length of the working region to the optimal length b = L/L* (L* = r 2− r 1) is 2.5 and 5 in the HF-DMA and the Nano-DMA, respectively, which results in a further enhancement of resolution by a factor of √2, as shown in Equation (Equation17).
4. EXPERIMENTAL METHODS
In the light of the theory described in section 2, the HF-DMA exhibits improved resolving power compared to conventional DMAs used for size selection of nanoparticles at room temperature and atmospheric pressure in the laboratory, like the TSI Nano-DMA. Nonetheless, there are some major sources of imperfections related to the assembly of the HF-DMA. One difficulty is holding the inner and outer electrodes in fully upright position, since both parts undergo sideways tilt; so that some misalignment of the electrodes is inevitable. Small DMAs can be held in a lathe and, while the instrument is spinning, the eccentricity of the electrodes is measured with a comparator and corrected by carefully tapping the electrodes. This cannot be done with the HF-DMA because of the large size of the electrodes. The aerosol enters the classification region through an annular slit of 15.6 cm in diameter and a width that is equal to the separation distance between the central and the lower parts of the inner electrode. This distance is adjusted by varying the elevation of the central part in relation to the lower one, whose position is fixed. The method, however, causes some tilt in the central part of the inner electrode, so that the width of the annular slit is not perfectly uniform along the circumferential direction. Other aspects are the asymmetries in the sheath inlet and outlet regions and the ability of the screens to favor the transition from a highly inhomogeneous non-stationary turbulent flow in the upper plenum to a stationary laminar flow in the working region. It is observed that, at a flow rate of 1000 l· min−1, the Reynolds number of the flow in the pipe through which the sheath gas enters the HF-DMA is nearly 35,000 and it reduces down to 2000 in the classification region.
Non-idealities are therefore expected in the HF-DMA, the impact of which on resolution is unknown a priori. The performance of the HF-DMA was assessed in two series of experiments. Firstly, we measured the transfer function with a source of ions of well-defined electrical mobility. Secondly, the HF-DMA was used to select nanoparticles of a given size from a polydisperse aerosol source. In general, DMAs are characterized by both resolution and transmission efficiency as a function of particle size. In this study, particle transmission could not be measured because of the unavailability of a source of monodisperse particles at the high flow rates at which the HF-DMA operates. The ion measurements, however, provide some insight into the performance of the HF-DMA in this respect.
Transfer Function
The standard technique for measuring the transfer function of the DMA, called Tandem DMA (TDMA), uses a configuration of two DMAs in series (CitationRader and McMurry 1986). The first DMA is used to separate particles of a given mobility from a polymobile aerosol source and the mobility distribution of the selected particles is measured with the second DMA. When the transfer function of the first DMA is known, the transfer function of the second DMA is derived from the mobility distribution measurements using deconvolution algorithms (CitationStratmann et al. 1997; CitationLi et al. 2006). This technique could not be applied for measuring the transfer function of the HF-DMA since only one unit of this prototype was available and no other existing DMA is capable of running at the high aerosol flow rates required here. As an alternative, a source of ions of well-defined mobility can be used, which makes it possible to directly measure the transfer function of the DMA. To do this, we used ions of tetraheptyl ammonium (THA) obtained by electrospraying (CitationFenn et al. 1989) a dilute alcohol solution of tetraheptyl ammonium bromide (THABr) into dry air. THABr is a neutral species, which upon dissociation leads to two kinds of ions THA+ and Br–. The polarity of the electrospray source was positive. The mobilities of the monomer and the dimer ions of THA in air are 0.96 and 0.67 cm2· V−1· s−1, respectively, with corresponding mobility equivalent diameters of 1.44 and 1.73 nm (CitationGamero-Castaño and Fernández de la Mora 2000).
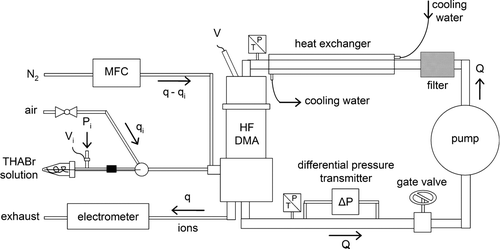
The experimental set-up used to measure the transfer function of the HF-DMA is shown in . The electrospray unit consists of a vial containing a sample of a solution of THABr in ethanol and a capillary having a tapered tip with an orifice, to which a high voltage is applied. At the outlet of the spray tip aerosol forms, which is a mist of small charged droplets about a few microns across. A stream of air is used to help nebulize the liquid and evaporate the neutral solvent in the droplets. As the solvent evaporates, the droplet size becomes so small that the charge density becomes high enough (above the Rayleigh limit) to cause the droplet to undergo a coulombic explosion, splitting into a collection of smaller droplets in which the process is repeated. The continuation of this process may eventually result in the formation of an ion containing a single analyte molecule. The molecule retains some of its droplet's charge to become a free ion as the last of the solvent vaporizes. Ions can also be evaporated under the high electric field conditions present on the surface of very small droplets. Both ion formation mechanisms, charge residue and field-evaporation, act together, each doing part of the ionization job. CitationGamero-Castaño and Fernández de la Mora (2000) claim that ions of diameter below a threshold are produced mostly by field-evaporation, which was possibly the case of the THA ions in our experiments.
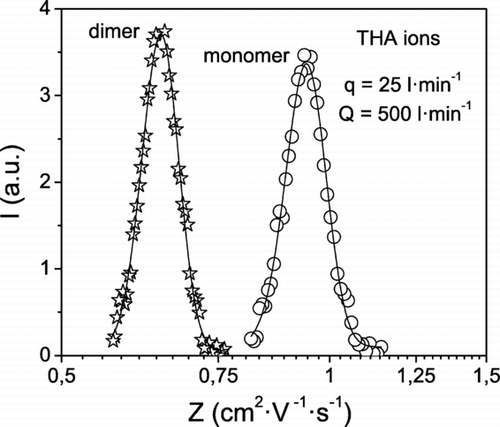
The flow of ions is mixed with a stream of nitrogen and then enters the HF-DMA. Inside the HF-DMA ions are carried by the sheath gas, which circulates in a closed-loop with the help of a pump. At the outlet of the HF-DMA, the ion current is measured as a function of the applied voltage V by means of an electrometer (Lazcano Inc., Madrid, Spain). Electrophoretic losses occur mainly in the conduit through which the ions leave the DMA, on account of the voltage jump between the inner electrode and the ion exhaust pipe, which is grounded. In the experiments, a minimum ion flow rate q of 25 l· min−1 was necessary to obtain a detectable signal, while the sheath flow rate Q was ten times q and larger. The mobility distribution of the THA ions is obtained by converting V into Z by using Equation (Equation1). shows the mobility spectrum of THA ions measured with the HF-DMA operating at ion and sheath flow rates of 25 and 500 l· min−1, respectively. Measured ion currents indicate that an appreciable amount of ions are transmitted through the HF-DMA; hence, one would expect low losses of nanoparticles.
Monodisperse Nanoparticles
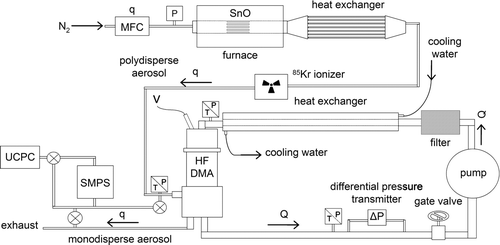
displays a layout of the facility that was built to test the capability of the HF-DMA to select nanoparticles of a well-defined size. It consists of a three-zone tube furnace having an inner diameter of 15 cm and a length of 1.27 m (Carbolite, Hope Valley, UK; Model GZF 12), where a powder of tin oxide (Acros Organics, New Jersey, NJ, USA; Tin(II) Oxide 98+%) is evaporated at intermediate temperatures (700–900°C) and carried away in a stream of nitrogen at flow rates up to 100 l· min−1 (Bronkhorst High-Tech BV, AK Ruurlo, Netherlands; Model EL-FLOW). Downstream the furnace the gas becomes supersaturated in SnO vapor. This nucleates and forms small clusters which grow further by way of vapor condensation and coalescence. The result is a polydisperse aerosol containing nanoparticles of a wide range of sizes is obtained.
An ionizer containing a radioactive source of 85Kr (10 mCi) is used to charge the particles of the polydisperse aerosol. When passing though a radioactive ionizer, particles are charged by diffusion of the ions of both polarities (±) in the gas to the surface of the particles; thus the particles attain a bipolar charge distribution. Both the charging efficiency and the charging level of the particles depend upon the N i t product, where N i is the concentration of ions in the gas and t is the mean residence time of the aerosol in the ionizer. In current radioactive chargers, the N i t product reaches values higher than a critical value above which steady-state charging conditions are met. The particles then attain a well-known equilibrium charge distribution, which has been both predicted theoretically (CitationGunn 1954; CitationKeefe et al. 1959; CitationNatanson, 1960; CitationFuchs 1963; CitationFilippov, 1993; CitationMatsoukas, 1994) and measured experimentally (CitationHussin et al. 1983; CitationPorstendörfer et al. 1984; CitationAdachi et al. 1985; CitationWiedensohler, 1988; CitationReischl et al. 1996; CitationAlonso et al. 1997). In the equilibrium, particles of sizes less than 20 nm are singly charged and the proportion of particles of 50 nm carrying more than one charge is nearly 3%. Multiple charging is not desirable since, as Equation (Equation1) shows, particles of different sizes and different charge states may have the same electrical mobility and, then, are classified simultaneously with the DMA. In this case, the aerosol that leaves the DMA is not monodisperse. The 85Kr charger used in this study was designed for charging aerosols at flow rates of up to 10 l· min−1 to steady-state. Since higher aerosol flow rates were used in our experiments, aerosols were not charged to equilibrium and particles of sizes below 50 nm carried mostly a single charge.
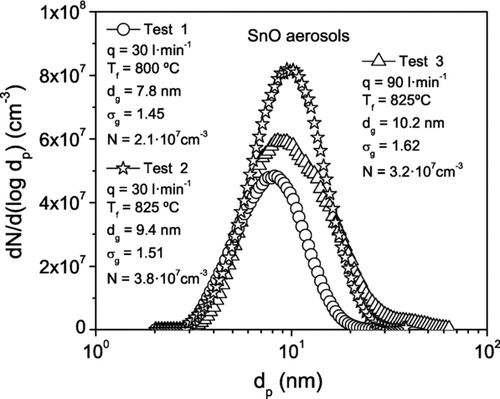
A sample (1.5 l· min−1) of the aerosol is sucked from the main line and carried to the SMPS (TSI 3080) with the Nano-DMA (TSI 3085) followed by the UCPC (TSI 3025) to measure the particle size distribution of the polydisperse aerosol upstream of the HF-DMA. The SMPS provides the main parameters of the distribution: the total particle number concentration N, the geometric mean diameter d g , and the geometric standard deviation σ g . The particle size distribution of the aerosol depends upon the operating conditions, mainly the temperature and flow rate of the carrier gas, in the furnace. In general, for a given flow rate, both the particle concentration N and the mean geometric diameter d g increase with temperature; while for a given temperature N and d g decrease with increasing gas flow rate. The particle size distribution of the polydisperse aerosols that entered the HF-DMA in experiments with different operating conditions in the furnace are plotted in .
FIG. 7 Particle size distribution of polydisperse aerosols of tin oxide measured by the SMPS (TSI 3080) at the entrance to the HF-DMA with the furnace operating at the indicated temperatures T f and flow rates q. The total particle number concentration N, the geometric mean diameter d g , and the geometric standard deviation σ g are given by the SMPS.
For given aerosol q and sheath Q flow rates, the voltage applied to the HF-DMA (Fug Elektronik GmbH, Rosenheim, Germany; Model HCN35-35000) is scanned and the particle size distribution of the selected aerosol is measured as a function of V with the SMPS-UCPC system at the aerosol outlet. Heat exchangers were installed in the facility to cool down both the aerosol and the sheath gas before they enter the HF-DMA. This must operate isothermally at room temperature in order to minimize the effects of particle diffusion and of thermal gradients in the gas on its resolution.
Sheath Gas Recirculation Line
Special attention was paid to the recirculation line of the sheath gas in this study. The main component is a roots pump, an oil-free and no-leak pump able to supply large volume flow rates (Leybolds Vacuum GmbH, Köln, Germany; Model RUVAC WA 501). The pumping speed is controlled by a manually operated gate valve (VAT Vacuum Valves AG, Haag, Switzerland). At the outlet of the pump, the sheath gas flows radially through a ULPA filter (99.9995%) and, then, is cooled down by passing through two water-circulated heat exchangers of 0.8 and 2 m in length in series. A sensor measures the temperature of the sheath gas at the inlet of the HF-DMA. Downstream of the HF-DMA, a differential pressure transmitter (Furness Controls Ltd., Sussex, UK; Model 352) measures the flow pressure drop in a straight section having a nominal diameter of 63 mm and a length of 0.5 m. Sensors are allocated to measure the temperature and the pressure of the flow at the section entrance. The sheath gas flow rate Q is then derived from these data using standard correlations of the pressure drop in pipe flows (CitationG&P Engineering Software, 2005). Vacuum connections and flanges are used in all the parts of the line to prevent gas leakages. A low pressure of 10−3 mbar is attained by means of a vacuum pump, indicating the tight sealing of the HF-DMA and the sheath recirculation line. Finally, flexible joints were installed at both ends of the roots pump in order to minimize the effect of vibrations on the resolution of the HF-DMA.
5. RESULTS AND DISCUSSION
Transfer Function
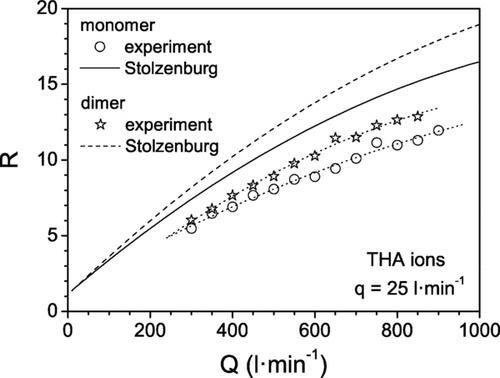
In the experiments with the THA ions, the HF-DMA was operated at a fixed ion flow rate of 25 l· min−1 and variable sheath flow rate of between 300 and 900 l· min−1. For a given value of Q, the applied voltage V was scanned and the current of ions leaving the HF-DMA was measured on-line at the outlet. In this way the transfer function of the HF-DMA for the THA ions was obtained. shows the transfer function of the monomer ion correspondent to different values of the sheath flow rate. Firstly, it is observed that the signal decreases when the sheath flow rate is increased. This result is consistent with the theory according to which, for a given value of q, the height and the width and consequently the area of the transfer function diminish when Q is increased. The peaks fit rather well with Gaussian functions, for which the FWHH and the standard deviation S are related by the expression FWHH = (8ln2) 1/2 S. Then the resolution of the HF-DMA is estimated as R = FHWW−1. The resolution values obtained experimentally and the theoretical values calculated with Equation (Equation9) are compared in for both monomer and dimer ions. In reality, the resolution of the HF-DMA is about 25% less than predicted by the theory. Deviations from the ideal resolution have also been observed in current DMAs and are attributed mainly to imperfections in the centering of the electrodes and/or to flow instabilities and asymmetries in the exhaust line of the sheath gas propagating upstream into the working region. As explained already, these non-ideal features are expected to be amplified in the HF-DMA, which has operating flow rates two orders of magnitude larger than conventional DMAs. Nonetheless, the HF-DMA is able to classify ions of mobility equivalent diameters between 1 and 2 nm with a resolution higher than 10, as shown in .
FIG. 8 Transfer function of the HF-DMA for the THA monomer ion measured experimentally at the indicated ion q and sheath Q flow rates.
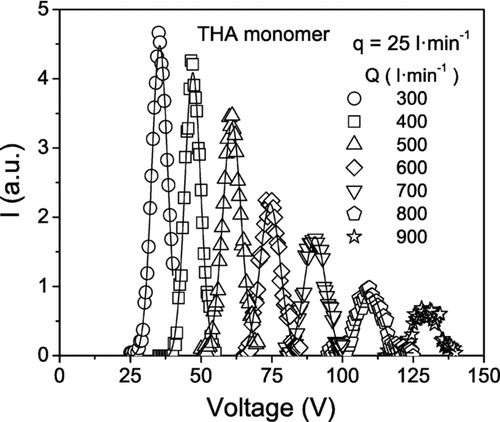
FIG. 9 Resolution of HF-DMA for THA ions vs. sheath flow rate. Experimental results and theoretical predictions (CitationStolzenburg 1988).
Monodisperse Nanoparticles
In the experiments with SnO aerosols the procedure was the same as in the experiments with the ions. For given values of the aerosol and the sheath flow rates, the applied voltage was increased progressively from zero to 20 kV and the particle size distribution of the aerosols selected with the HF-DMA was measured at the outlet using the system consisting of the SMPS followed by the UCPC. For each value of V, the SMPS provides the particle number concentration N, the geometric mean diameter d g , and the geometric standard deviation σ g of the particle size distribution.
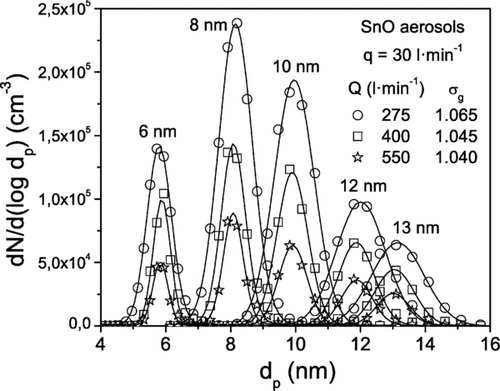
Several experimental series were carried out. A series consists of a number of experiments in which the aerosol flow rate is fixed to a value q and the sheath flow rate Q is varied between 200 and 1200 l· min−1. The particle size distributions of various aerosols selected with the HF-DMA are plotted in . They were obtained with the HF-DMA running at an aerosol flow rate of 30 l· min−1 and three different values of Q. The particle number concentration N is given by the area under the curves, while the geometric mean diameter d g and the geometric standard deviation σ g as given by the SMPS are shown in the figure. As explained in relation to , the signal of the UCPC reduces with increasing sheath flow rate.
FIG. 10 Particle size distributions of tin oxide aerosols measured with the SMPS (TSI 3080) at the outlet of the HF-DMA, when operating at the indicated aerosol q and sheath Q flow rates. The values of the geometric standard deviation σ g are given by the SMPS.
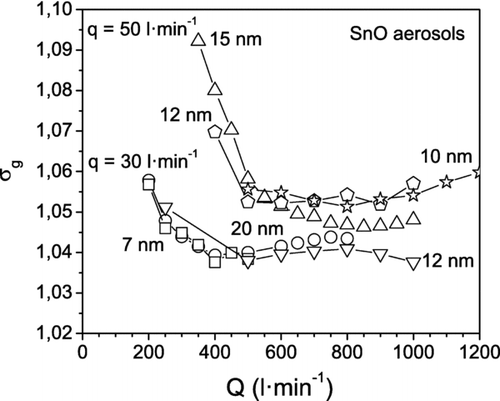
The particle number concentration and the geometric standard deviation of aerosols with different particle mean sizes are displayed as a function of the sheath flow rate in and , respectively. It can be seen that N decreases linearly when increasing Q. Moreover, σ g decreases with increasing Q at low sheath flow rates and then reaches a roughly constant value. The same behavior has been observed in current DMAs when the centering of the electrodes is poor or the aerosol inlet slit is too thick, etc., these features can limit the resolution of the instrument. This is not the case for the HF-DMA, whose resolution increases continuously with the sheath flow rate, as shown in . We attribute the behavior in to the Nano-DMA that was used to measure the particle size distribution of the aerosol downstream of the HF-DMA. The Nano-DMA operated at aerosol and sheath flow rates of 1.5 and 15 l· min−1, respectively, in all the experiments. The resolution of the Nano-DMA remained constant: 10 for particles larger than 10 nm, and lower than 10 for smaller particles (see ). Contrarily, for given values of the particle size and of the aerosol flow rate q, the resolution of the HF-DMA increased with Q in the experiments. At low values of the sheath flow rate, when the ratio Q/q is less than 10, the resolution of the HF-DMA is lower than the resolution of the Nano-DMA, and the contrary is true for values of Q/q above 10. In the latter case, the Nano-DMA operating at the mentioned sheath to aerosol flow rate ratio is not able to resolve the mobility distribution of the particles that leave the HF-DMA further, which narrows progressively with increasing sheath flow rate. The geometric standard deviation σ g of the particle size distribution measured with the SMPS reaches a limiting value, as observed in . Lower values of σ g were obtained at an aerosol flow rate q of 30 l· min−1 than at 50 l· min−1. The explanation is that the σ g of the size distribution of the particles selected with the HF-DMA decreases when the resolution R is increased, which in turn increases when the sheath to aerosol flow rate ratio Q/q is increased.
FIG. 11 Particle number concentration N of tin oxide aerosols measured with the UCPC (TSI 3025) at the outlet of the HF-DMA, when operating at the indicated aerosol q and sheath Q flow rates.
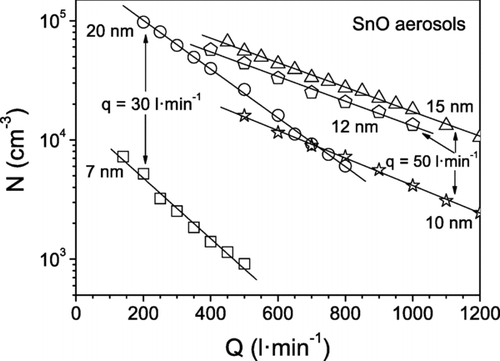
FIG. 12 Geometric standard deviation σ g of tin oxide aerosols measured with the SMPS (TSI 3080) at the outlet of the HF-DMA, when operating at the indicated aerosol q and sheath Q flow rates.
displays the normalized particle size distribution of aerosols with particle mean sizes in the range of 5 to 25 nm selected with the HF-DMA operating at different aerosol and sheath flow rate of 30 and 375 l· min−1 and 90 and 1100 l· min−1. The large pressure drop in the aerosol injection line prevented the MFC to attain the maximum aerosol flow rate of 100 l· min−1 that was the target of this work. One important result is that the geometric standard deviation of the distribution is almost independent of the particle size; in the two cases, a value of σ g of almost 1.05 was obtained for all particle sizes. This is consistent with the theoretical predictions shown in ; for particle sizes above 5 nm, the resolution of the HF-DMA is practically unaffected by diffusion broadening, even at the lowest sheath flow rate (100 l· min−1). Then the resolution is determined mainly by the sheath to aerosol flow rate ratio Q/q, the values of which were 12.5 (375/30) and 12.2 (1100/90). This explains the similar value of σ g obtained in the two experiments. It is noticeable however that, because of the lower resolving power of the TSI Nano-DMA compared with the HF-DMA, the actual geometric standard deviation σ g of the particle size distribution is likely smaller than that given by the SMPS (1.05).
FIG. 13 Normalized particle size distributions of tin oxide aerosols measured with the SMPS (TSI 3080) at the outlet of the HF-DMA, when operating at the indicated aerosol q and sheath Q flow rates. A geometric standard deviation σ g of almost 1.05 was measured with the SMPS for all particle sizes in the two series of experiments.
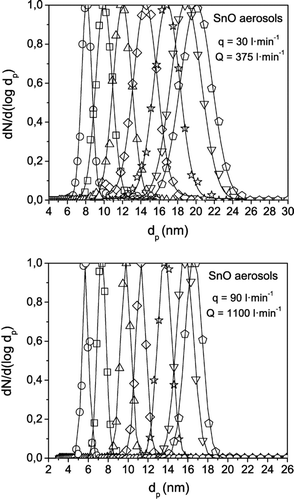
Finally, for most practical applications an aerosol is considered to be monodisperse when the geometric standard deviation of the particle size distribution measured with the SMPS is inferior to 1.1. It was observed that the particle size distributions of the aerosols selected with the HF-DMA showed values of σ g well below 1.1 in most of the experiments (see ).
6. CONCLUSIONS
A new DMA (HF-DMA) has been developed that is capable of delivering monodisperse nanoparticles of up to 30 nm in size at an aerosol flow rate of 90 l· min−1 and number concentrations of between 104 and 105 cm−3. In this way, the yield of monodisperse nanoparticles in gas phase has been enhanced by two orders of magnitude with respect to other existing nano-DMAs. This study demonstrates the feasibility of large-scale DMAs to handle aerosol flow rates orders of magnitude higher than small-scale DMAs used in the laboratory without any loss of performance. This encouraging result opens the door to the application of large DMAs, such as the HF-DMA and even larger, in the production of nanoparticulate materials using a well-defined particle size.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the Collaborative Research Centre on Nanoparticles from the gas phase: formation, structure and properties (SFB-445). Dr. E. Hontañón was supported by Ministerio de Educación y Ciencia (MEC) of Spain. Dr. P. Martínez-Lozano is acknowledged for assisting in measuring the transfer function of the HF-DMA.
REFERENCES
- Adachi , M. , Kousaka , Y. and Okuyama , K. 1985 . Unipolar and Bipolar Diffusion Charging of Ultrafine Aerosol Particles . J. Aerosol Sci , 16 : 109 – 123 .
- Alonso , M. , Kousaka , Y. , Nomura , T. , Hashimoto , N. and Hashimoto , T. 1997 . Bipolar Charging and Neutralization of Nanometer-Sized Aerosol Particles . J. Aerosol Sci. , 28 : 1479 – 1490 .
- Chen , D.-R. and Pui , D. Y. H. 1997 . Numerical Modeling of the Performance of Differential Mobility Analyzers for Nanometer Aerosol Measurements . J. Aerosol Sci. , 28 : 985 – 1004 .
- Chen , D.-R. , Pui , D. Y. H. , Hummes , D. , Fissan , H. , Quant , F. R. and Sem , G. J. 1998 . Design and Evaluation of a Nanometer Aerosol Differential Mobility Analyzer (Nano-DMA) . J. Aerosol Sci. , 29 : 497 – 509 .
- Fenn , J. B. , Mann , M. , Meng , C. K. , Wong , S. F. and Whitehouse , C. M. 1989 . Electrospray Ionization for Mass Spectrometry of Large Biomolecules . Science , 246 : 64 – 71 .
- Fernández , de la Mora J. 2003 . Diffusion Broadening in Converging Differential Mobility Analyzers . J. Aerosol Sci. , 33 : 411 – 437 .
- Fernández , de la Mora J. and Attoui , M. 2007 . A DMA Covering the 1–100 nm Particle Size Range with High Resolution Down to 1 nm European Aerosol Conference EAC 2007, Salzburg (Austria), Abstract T02A029.
- Fernández , de la Mora J. , Labowsky , M. , Schmitt , J. J. and Neilson , W. 2007 . Method and Apparatus to Increase the Resolution and Widen the Range of Differential Mobility Analyzers (DMAs) US Patent 7,161,143 B2 (01/09/2007)
- Filippov , A. V. 1993 . Charging of Aerosols in the Transition Regime . J. Aerosol Sci. , 24 : 423 – 436 .
- Flagan , R. C. 1998 . History of Electrical Aerosol Measurements . Aerosol Sci. Technol. , 28 : 301 – 380 .
- Flagan , R. C. 1999 . On Differential Mobility Analyzer Resolution . Aerosol Sci. Technol. , 30 : 556 – 570 .
- Fuchs , N. A. 1963 . On the Stationary Charge Distribution on Aerosol Particles in a Bipolar Ionic Atmosphere . Pure and Applied Physics. , 56 : 185 – 193 .
- Gamero-Castaño , M. and Fernández , de la Mora J. 2000 . Mechanisms of Electrospray Ionization of Singly and Multiply Charged Salt Clusters . Analytica Chimica Acta , 406 : 67 – 91 .
- Gunn , R. 1954 . Diffusion Charging of Atmospheric Droplets by Ions and the Resulting Combination Coefficients . J. Meteorol. , 11 : 339 – 347 .
- Hussin , A. , Scheibel , H. G. , Becker , K. H. and Porstendörfer , J. 1983 . Bipolar Diffusion Charging of Aerosol Particles I: Experimental Results within the Diameter Range 4–30 nm . J. Aerosol Sci. , 14 : 671 – 677 .
- Joshi , R. K. and Kruis , F. E. 2006 . Influence of Ag Particle Size on Ethanol Sensing of SnO1.8:Ag Nanoparticle Films: A Method to Develop ppb–Level Gas Sensors . Appl. Phys. Lett. , 89 : 153116
- Keefe , D. , Nolan , P. J. and Rich , T. A. 1959 . Charge Equilibrium in Aerosols According to the Boltzmann Law. . Proceedings of the Royal Irish Academy , 60A : 27
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electrical Mobility: Apparatus, Theory and Applications . . J. Aerosol Sci. , 6 : 443 – 451 .
- Labowsky , M. and Fernández , de la Mora J. 2006 . Novel Ion Mobility Analyzers and Filters . J. Aerosol Sci. , 37 : 340 – 362 .
- Langer , G. , Pierrard , J. and Yamate , G. 1964 . Further Development of an Electrostatic Classifier for Submicron Airborne Particles . Intl. J. Air and Water Poll. , 8 : 167 – 176 .
- Li , W. , Li , L. and Chen , D.-R. 2006 . Technical Note: A New Deconvolution Scheme for the Retrieval of True DMA Transfer Function from Tandem DMA Data . Aerosol. Sci. Technol. , 40 : 1052 – 1057 .
- Martínez-Lozano , P. and Fernández , de la Mora J. 2005a . Effect of Acoustic Radiation on DMA Resolution . Aerosol Sci. Technol. , 39 : 866 – 870 .
- Martínez-Lozano , P. and Fernández , de la Mora J. 2005b . Resolution Improvements of a Nano-DMA Operating Transonically . J. Aerosol Sci , 37 : 500 – 512 .
- Martínez-Lozano , P. , Labowsky , M. and Fernández , de la Mora J. 2006 . Experimental Tests of a Nano-DMA With No Voltage Change between Aerosol Inlet and Outlet Slits . J. Aerosol Sci. , 37 : 1629 – 1642 .
- Matsoukas , T. 1994 . Charge Distributions in Bipolar Charging . J. Aerosol Sci. , 25 : 599 – 609 .
- NanoRanger . 2007 . FL, , USA : NanoEngineering Co. . http://www.nanoengineeringcorp.com
- Natanson , G. L. 1960 . On the Theory of the Charging of Microscopic Aerosol Particles as a Result of Capture of Gas Ions . Soviet Phy.—Tech. Phy. , 5 : 538 – 551 .
- PipeDrop . 2005 . G&P Engineering Software . http://www.gpengineeringsoft.com
- Porstendörfer , J. , Hussin , A. , Scheibel , H. G. and Becker , K. H. 1984 . Bipolar Diffusion Charging of Aerosol Particles II: Influence of the Concentration Ratio of Positive and Negative Ions on the Bipolar Charge Distribution . J. Aerosol Sci. , 15 : 47 – 56 .
- Rader , D. J. and McMurry , P. H. 1986 . Application of the Tandem Differential Mobility Analyzer to Studies of Droplet Growth or Evaporation . J. Aerosol Sci. , 17 : 771 – 787 .
- Ramiro , E. , Sánchez , M. , Hontañón , E. and Martínez-Lozano , P. 2005 . A High Resolution DMA of Plane Geometry. Abstracts of the European Aerosol Conference EAC 2005 , 9 Belgium : Ghent .
- Ramiro , E. and Rivero , A. 2007 . Wide Range Differential Mobility Analyzer (DMA) with Very High Resolution International Patent WO 2007/020303 A1 (22/02/2007).
- Reischl , G. P. , Mäkela , J. M. , Karch , R. and Necid , J. 1996 . Bipolar Charging of Ultrafine Particles in the Size Range Below 10 nm . J. Aerosol Sci. , 27 : 931 – 949 .
- Rosell-Llompart , J. and Fernández , de la Mora J. 1993 . “ Minimization of the Diffusive Broadening of Ultrafine Particles in Differential Mobility Analyzers ” . In Synthesis and Characterization of Ultrafine Particles , Edited by: Marijnissen , J. and Pratsinis , S. 109 – 114 . Delft : Delft University Press .
- Rosell , J. , Loscertales , I. G. , Bingham , D. and Fernández , de la Mora J. 1996 . Sizing Nanoparticles and Ions with a Short Differential Mobility Analyzer . J. Aerosol Sci. , 27 : 695 – 719 .
- Rosser , S. and Fernández , de la Mora J. 2005 . Vienna-type DMA of High Resolution and High Flow Rate . Aerosol Sci. Technol. , 39 : 1191 – 1200 .
- Stolzenburg , M. R. 1988 . An Ultrafine Aerosol Size Distribution Measuring System. PhD thesis , MN, , USA : Department of Mechanical Engineering, University of Minnesota .
- Stratmann , F. , Kauffeldt , T. , Hummes , D. and Fissan , H. 1997 . Differential Electrical Mobility Analysis: A Theoretical Study . Aerosol Sci. Technol. , 26 : 368 – 383 .
- Whitby , K. T. and Clark , W. E. 1966 . Electrical Aerosol Particle Counting and Size Distribution Measuring System for the 0.015 to 1 μ m Size Range . Tellus. , 18 : 573 – 586 .
- Whitby , K. T. , Liu , B. Y. H. , Husar , R. B. and Barsic , N. J. 1972 . The Minnesota Aerosol Analyzing System used in the Los Angeles Smog Project . J. Coll. Interface Sci. , 39 : 136 – 164 .
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range . J. Aerosol Sci. , 19 : 387 – 389 .
- Zhang , S. H. and Flagan , R. C. 1996 . Resolution of the Radial Differential Mobility Analyzer for Ultrafine Particles . J. Aerosol Sci , 27 : 1179 – 1200 .