Abstract
We present details on the design, construction, and calibration of a radial differential mobility analyzer suitable for sizing aerosol nanoparticles with mobility diameters as small as one nanometer. Design improvements targeted decreasing the residence time with a concomitant reduction in particle agglomeration and diffusional losses to walls. Calibration was performed using aerosolized monodisperse molecular ions. Instrument resolution was found to increase with applied voltage as V 1/2 , indicating that diffusion is broadening the transfer function and reaching a value of ∼7 for the largest molecular ion measured (mobility diameter of 1.47 nm). The mobilities of three molecular ions in the tetra-alkyl ammonium halide homologous series are also reported.
1. INTRODUCTION
Nanometer-sized aerosol particles are encountered extensively in research ranging from atmospheric science to nanotechnology. These particles form in the atmosphere through homogeneous nucleation of products of photochemical reactions. Originating as small clusters, these molecular-sized particles grow by condensation and coagulation. Nanoparticle synthesis in aerosol reactors proceeds similarly via homogenous nucleation and subsequent growth. For these and numerous other applications, measurements of the particle size distribution provide important insights into the growth dynamics of the aerosol. Furthermore, the ability to measure the size of nanomaterials is critical for applications in nanotechnology because optical, electrical, and magnetic properties at the nanoscale are size dependent.
The preferred instrument for measuring submicron aerosol particle size has been the differential mobility analyzer (DMA) (CitationKnutson and Whitby 1975; CitationFlagan 1998; CitationMcMurry 2000). This instrument classifies charged particles according to their electrophoretic migration velocity in an electric field applied across a laminar, particle-free sheath flow, usually in the channel between coaxial cylindrical electrodes (cylindrical DMA). Charged particles are transmitted when their migration velocity is near a value determined by instrument design as well as sheath flow rate, Qsh . The ratio of the migration velocity, v m, to the applied electric field, E, is called the particle mobility, ZP , which is an inverse function of particle mobility diameter, Dp . This dependency implies that the smaller a charged particle is, the larger is its mobility and, thus, a smaller electric field will be required to reach the transmitted migration velocity of a given instrument (vm =ZpE). Particles with a particular ZP will be maximally transmitted by properly setting E or, equivalently, the voltage V applied across the instrument electrodes.
Inherently, DMAs can resolve small differences in particle mobility. When diffusion is not important, the best attainable mobility resolution depends on the ratio of the volumetric flow rate of the sheath gas to that of the aerosol, Qsh /Qa (CitationStolzenburg 1988; CitationFlagan 1999). This resolution is degraded by Brownian diffusion, which becomes important when the electrostatic potential energy of the migrating particle is small compared to its thermal energy. In this diffusion-limited regime, the theoretical resolution scales with V 1/2, which limits resolution for small particles.
To achieve high resolution classification of small particles requires making the residence time in the classification region small or correspondingly making the transmitted vm large. When this condition is met, a high mobility particle (small particle size) can be classified at a high applied voltage. A short classification region is a straightforward way to reduce particle residence time (CitationRosell-Llompart et al. 1996; CitationChen et al. 1998). Further improvements can be achieved by combining a short column with a large sheath flow rate (Citationde Juan and de la Mora 1998; CitationRosser and de la Mora 2005; CitationMartínez-Lozano and de la Mora 2006).
In contrast to the cylindrical DMA, CitationZhang et al. (1995) and CitationFissan et al. (1998) demonstrated a radial DMA (RDMA) design, where the sheath gas flows radially inward between two flat electrodes (disks). The aerosol was introduced near the edge of the “primary” electrode and the charged particles were allowed to migrate towards the “counter” electrode in a uniform electric field. The combined flow was subsequently split unevenly between two outflow ports in the center of the electrodes. A small flow (classified flow, Qs ) was directed through the counter-electrode (across from the aerosol inlet annular slot) for further transmission to a particle detector; the remaining flow (excess flow, Qe ) exited through the center of the primary electrode. The initial RDMA was capable of classifying particles larger than 5 nm in diameter. In order to detect smaller particles, the migration velocity of those particles that are transmitted through the classifier must be increased. However, unlike in the cylindrical DMA design, the particle migration velocity of the original radial DMA design can not be arbitrarily increased by increasing the sheath gas flow rate. A larger sheath flow leads to a correspondingly larger excess flow, which may cause turbulence in the excess outlet port. Turbulence generally degrades the resolution of the instrument and must be avoided. Alternatively, a reduction in particle residence time can be accomplished by using a smaller sizing region and this approach was chosen for the new RDMA design.
In this article, we report on the design, construction, and calibration of a RDMA, capable of classifying particles with sizes between 1 and 13 nm. As compared to the earlier RDMA design, the modified version reduces the residence time, first, by decreasing the distance between the aerosol inlet and sampling outlet, and second, by modestly increasing the sheath flow rate, while still avoiding turbulence. Instrument calibration is performed by employing gas ions as monodisperse particles in the 1 to 2 nm size range.
2. EXPERIMENTAL METHOD
2.1. Instrument Design
We seek to design an RDMA capable of nanoparticle classification down to one nanometer for use as an in-situ diagnostic in nanoparticle synthesis reactors (CitationSankaran et al. 2005). De la Mora and coworkers (Citationde Juan and de la Mora 1998; CitationMartinez-Lozano and de la Mora 2006; CitationRosser and de la Mora, 2005; CitationUde and de la Mora 2005) have demonstrated high resolution sizing in the one nanometer range by operating a short-column cylindrical DMA at very high flow rates (i.e., 500–2200 L min-1). Such operation is impractical when instrument portability or power consumption is important or when the sheath gas must be of high purity to avoid trace impurity contamination. In such applications, the instrument must operate at a low sheath flow rate (∼10 L min−1).
In an RDMA, the mobility of particles transmitted with the highest efficiency is given by (CitationZhang et al. 1995):
We are concerned with classification of particles in the free molecular limit, for which the electrical mobility of a singly charged particle is:
While similar to the original RDMA (CitationZhang et al. 1995), the new design (termed “nano-RDMA” in the discussion that follows) incorporates several key changes. The first and most significant difference is the radius (labeled Ri in ) of the circular slit where the aerosol is introduced that has been reduced from 50 mm to 7.5 mm. Secondly, the RDMA of (CitationZhang et al. 1995) achieved a resolution in the high voltage limit that was lower than that predicted for non-diffusive particles. It was later found (unpublished) that this was due to a combination of: (1) imperfect concentricity between the knife edge and the inner surface that forms the annular aerosol entrance slot, and (2) a less-than-ideal pressure drop through that slot. In the present design, mounting of the knife-edge ring has been modified to ensure concentricity, while permitting adjustment of the aerosol inlet gap height with high precision. The knife edge (labeled in ) and inner electrode are machined from 304 stainless steel (SS) to have a slip-fit tolerance on the radius, thereby ensuring concentricity and eliminating any gap through which the aerosol can leak. Precision shims were used to set and optimize the aerosol inlet gap. A shim thickness of 0.44 mm was found to give a minimum in the line-width of the ion mobility distribution. Smaller gaps resulted in loss of signal that was attributed to diffusional losses in the inlet gap.
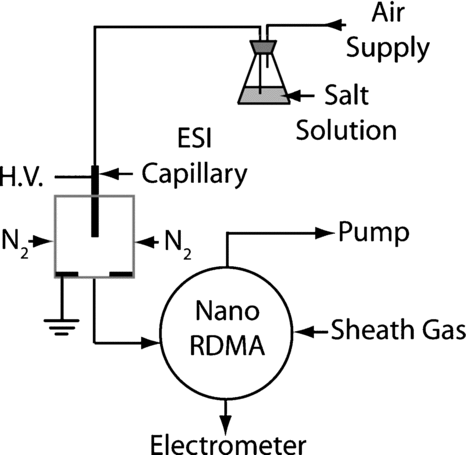
FIG. 1 Simplified schematic of the nano-RDMA operation illustrating the direction of the sheath gas flow (Qsh ), the aerosol inlet (Qa ), the sampled outlet (Qs ), and the excess flow outlet (Qe ).
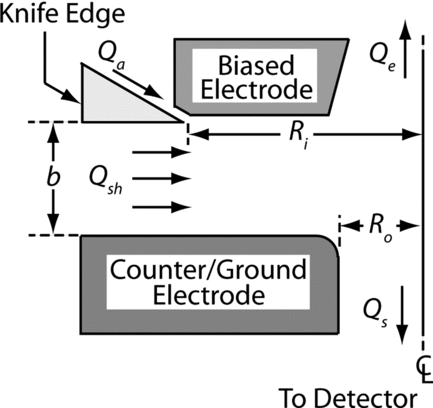
FIG. 2 Detailed schematic of the nano-RDMA. The materials used for construction are noted in the text. Filled circles represent O-Rings. The inset depicts the slip fit tolerances and the knife edge in more detail as well as the location where precision shim disks are placed to create a gap between the chamfer and the knife edge through which the aerosol leaves the racetrack and enters the sizing portion of the device.
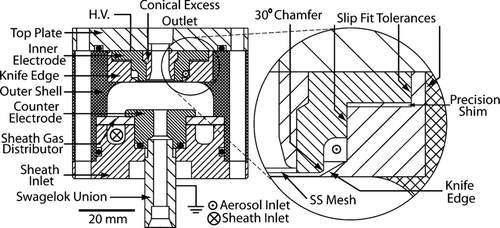
As in the original RDMA design, the aerosol is introduced tangentially into a continuous annular channel (so-called “race track”) between the pieces defining the inlet. A high voltage bias is applied to the aerosol entrance electrode; electrical contact is made through a screw (not shown), which is inserted through the top plate. Opposite this biased electrode is the counter electrode that is grounded through a Swagelok union. The classified aerosol exits through a port in the center of the counter electrode.
The remaining pieces were made of white Delrin (except as noted). These include, the outer shell, the top plate, the sheath gas inlet, the conical excess outlet, the inlet extension (not pictured) and a sheath gas distributor (made of porous polyethylene sheet with an average pore diameter of 25 μm). These pieces are scaled versions of parts from the original design with two exceptions:
| 1. | The conical excess outlet was designed to fit inside the inner electrode from the top rather than being press-fit from the bottom, as in the original RDMA. This piece is made of Delrin (dielectric). Computational fluid dynamics simulations performed using COMSOL MultiphysicsTM indicated that the dielectric surface distorts the uniformity of the electric field within the classifying region. Field distortion was avoided by affixing a thin, metallic wire mesh to the bottom of the conical port in electrical contact with the inner electrode. Operation of the RDMA with the conductive mesh yielded higher particle transmission efficiency than observed in preliminary experiments without the mesh, thus confirming the simulation prediction. All data presented herein were obtained with the mesh in place. | ||||
| 2. | In the first design of the nano-RDMA, the aerosol was introduced directly into the race-track through a grounded Swagelok fitting attached to the outer shell. The strong electric field produced between this fitting and the biased upper electrode caused particle loss to walls before entering the classification region (CitationKousaka et al. 1986). To avoid this problem, the Swagelok fitting was moved away from the top electrode by incorporating a 20 mm long Delrin tube. The additional inlet length is expected to result in particle loss to the walls by diffusion. Two alternative approaches were also considered. First, the inlet tubing could be biased to the same potential as the upper electrode. For safety reasons, this option was not implemented. The second alternative was to maintain the upper electrode at ground potential and to bias the bottom electrode. This approach runs into the same issue it was attempting to fix and is less desirable since particle loss would occur in a section where the particles have already been classified. | ||||
2.2. Instrument Calibration
The performance of the new RDMA design was evaluated by using molecular ions (tetra-alkyl ammonium halides) based on the work and reported mobilities of Ude and Citationde la Mora (2005). The molecular ions were produced by electrospraying solutions of the analyte species. The liquid delivery apparatus consisted of an Erlenmeyer flask (125 mL) fitted with a stopper through which two pieces of plastic tubing were inserted as depicted in . One tube was connected to a regulated air supply with fine pressure control (±0.1 psi). The other tube was immersed into the liquid and was connected to a stainless-steel capillary tube (O.D. ∼ 1.6 mm and I.D. 127 μm) that served as the electrospray nozzle. The nozzle was biased to 3.5 kV with respect to a fitting (cross) into which the capillary tube was inserted through a threaded Delrin rod for electric isolation. Equal nitrogen flows (0.6 L min−1 total flow) introduced through the side arms of the cross carry the electrosprayed ions through the outlet port. The electrospray nozzle and aerosol outlet were positioned vertically to prevent dripping that might cause an electrical short.
The calibration standards were ions of tetra-alkyl ammonium salts (tetra-propyl ammonium iodide (TPropylAI), tetra-butyl ammonium iodide (TButylAI), and tetra-heptyl ammonium bromide (THeptylAB)). Solutions were prepared that contained 0.47, 0.27, and 0.20 mmol/L of these analytes, respectively, in 1-propanol (Sigma Aldrich, 99.7% purity); the analyte concentrations are one-hundredth of those used in the work of CitationUde and de la Mora (2005). The substantial dilution ensured that mostly monomeric tetra-alkyl ammonium ions (R4N+, A) and “dimers” ((R4NX)R4N+, A2B) were produced, thereby allowing unambiguous peak assignment without the need for coupling the RDMA to a mass spectrometer. Additional solutions with tetra-ethyl ammonium iodide (TEthylAI), tetra-pentyl ammonium bromide (TPentylAB) and tetra-hexyl ammonium bromide (THexylAB) in 1-propanol at concentrations of 0.20 mmol/L were employed to produce ions with similar mobilities. For TEthylAI (as well as tetra-methyl ammonium iodide), the crystals did not dissolve completely in 1-propanol and the actual dissolved concentration was unknown. The mobilities of the latter three salts have not been previously measured by DMA.
A sheath flow rate of 10 L min−1 was chosen for the calibration experiments. The combined sheath flow and aerosol inlet flow are split so that the excess flow rate is 10 L min−1 and the sampled flow rate is 0.6 L min−1 (i.e., Qsh = Qe and Qa = Qs ). While the choice of aerosol flow rate was fixed to mimic the operating conditions of a nanoparticle synthesis reactor, the sheath flow rate of 10 L min−1 was found to produce the narrowest distributions of the molecular ions. The device should be capable of operating at higher aerosol flow rates, but additional optimization of the aerosol inlet gap might be necessary.
Molecular ion concentration was calculated from the current output of a home-made faraday cup electrometer as a function of voltage applied to RDMA electrodes. A computer with a 16-bit analog output channel was used to remotely program the voltage output of a 10 kV power supply (Acopian model P010HD3). The RDMA was operated in stepping mode, which consisted of applying a fixed voltage and waiting 5 s at each voltage before the recording the average current over a 1 s measurement interval. While shorter waiting times (as short as 2 s) produced similar distributions, all measurements were done at 5 s waiting times to ensure steady state operation.
3. RESULTS
From Equation (Equation1), the product Zp *V is observed to depend on the DMA geometry and sheath flow rate. Based on this equation, the theoretical Zp *V was calculated to be 109.8 cm2/s. Fitting the concentration distributions of the monomers and dimers for the previously reported salts, the experimental value was found to be 125.61 ± 0.93 cm2/s. Using this value, each voltage is converted to an inverse mobility (1/Zp ) and the distribution for each tetra-alkyl ammonium salt ion is shown in . The mobility distributions for the monomer and dimer are fitted well with two separate Gaussian distributions. As shown in , the measured mean inverse mobilities match the previously reported values over the entire range.
FIG. 4 Inverse mobility distributions of (a) TPropylAI, (b) TButylAI, and (c) THeptylAB. The long dash curve fits the A+ (i.e., R4N+) distribution and the short dash curve fits the A2B+ distribution. The mobility values plotted are scaled values.
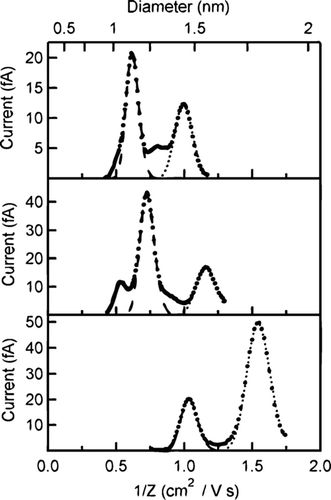
FIG. 5 Compilation of the geometric mean mobilities obtained from fitting size distributions plotted versus the mobility found previously (CitationUde and de la Mora 2005). The empty symbols are for the monomer and the filled symbols are for the dimer.
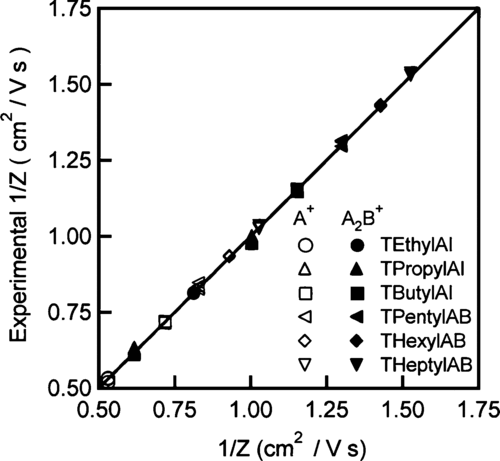
The experimental value for Zp *V is 14.4% higher than theoretical estimate based on Equation (Equation1). The difference is partially due to non-uniformities in the electric field that were not taken into account in the derivation of Equation (Equation1), which therefore requires a higher voltage to classify ions of a known ZP . The deviation occurs as the ions migrate towards the axis of the device. Here, the holes in the electrodes for the excess and sample flow outlet ports decrease the effective field (E = V/b). Although the excess flow outlet port is covered with a SS mesh, the field is nonetheless decreased. Also, the model assumes a radial source for the aerosol. In the actual device, the aerosol enters the classification region through a finite gap in the upper electrode between the knife edge (a) and the inner electrode (b), thus introducing uncertainty in the estimation of the radial distance. This is particularly problematic for the nano-RDMA since the gap is comparable in size to the radial distance to the knife edge. Hence, the mobility of the particles transmitted through the nano-RDMA is estimated using an empirical calibration factor, i.e.,
As with the previous salt solutions, DMA scans of electrosprayed solutions of TEthylAI, TPentylAB, and THexylAB revealed new peaks in the mobility spectra with reasonable inverse mobility values, plotted in . The measured inverse mobility distributions of these compounds are presented in . As before, each individual peak was fit with a Gaussian distribution. The mean inverse mobility found for each ion is listed in along with the values for the calibration standards.
FIG. 6 Inverse mobility distributions of (a) TEthylAI, (b) TPentylAB, and (c) THexylAB. The long dash curve fits the A+ (i.e., R4N+) distribution and the short dash curve fits the A2B+ distribution. The mobility values plotted are scaled values.
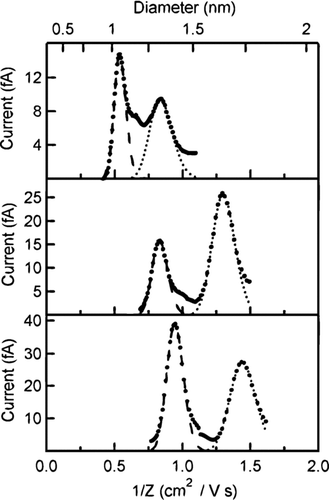
TABLE 1 Previously determined and experimentally measured inverse mobilities for each of the different tetra-alkyl ammonium salts as well as the calculated resolution
4. DISCUSSION
The second figure of merit for size measurements is the instrument mobility resolution, which is defined as the ratio of the mean particle mobility over the full-width at half-maximum of the mobility distribution (CitationFlagan 1999):
Measurements made using mono-mobility ions enables direct determination of the mobility resolution from the widths of the mobility peaks seen in and . Experimentally, the instrument resolution was calculated for each molecular ion monomer and dimer from the mobility distribution fits found previously and plotted in . Over the range of voltages used, the resolution of the monomeric molecular ion (A+) is found to increase with voltage with approximately the same slope as that predicted in the diffusive (high mobility/small particle) limit. The observed resolution is clearly smaller than predicted when assuming uniform parallel flow and uniform electrical fields. Empirically for the nano-RDMA, we find G mig = 17.3 ± 1.1; this deviates from the simplistic model estimate of G mig = 10.5. One possible explanation for the observed resolution being lower than theoretically predicted is the possibility of a recirculation bubble near the stagnation point on the axis of the instrument (Citationde la Mora 2002). Regardless of the cause of deviation, the nano-RDMA is observed to operate in the diffusive limit for the range of mobilities of the molecular ions measured in this study. Yet, the instrument clearly demonstrates resolving ability for particle mobilities that were not even measurable with the original RDMA.
FIG. 7 Comparison of the theoretically calculated and experimentally measured resolution. The empty symbols correspond to the monomer whereas the filled symbols are for the dimer. The solid line corresponds to the fit of the experimental data while the dashed lines are the calculated theoretical limits in the diffusion regime (short dash) and in the absence of the effects of diffusion.
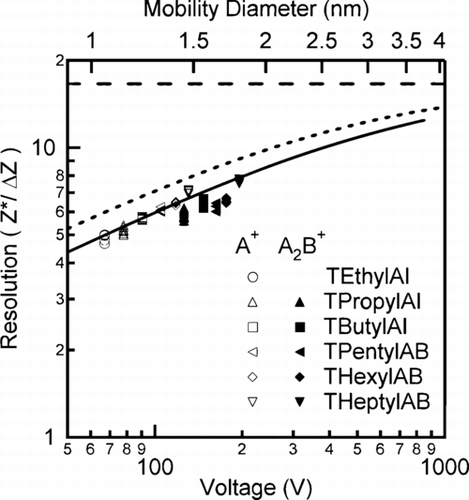
For the dimer molecular ions ((AB)A+), the measured resolution falls below that of the monomer ions, but they appear to follow the same V 1/2 power law. The data preclude the possibility that the resolution begins to degrade as the voltage increases since the THeptylAB monomer resolution follows the V 1/2 trend of the monomers and is measured at nearly the same voltage as the TPropylAI dimer. While not rigorously tested, the observed difference in the trends of the resolution for the monomers and the dimers could be due to the non-spherical nature of the dimer. As observed for significantly larger particles (CitationZelenyuk et al. 2006) classified at low voltages (i.e., <2000 V), the dimers tend to migrate through the DMA with an orientation that approaches random. Since the orientation of the dimer can affect the drag force, the dimers will be transmitted over a broader range of voltages leading to a lower effective mobility.
The results presented above have been discussed in terms of inverse mobility, but this quantity is hard to visualize. The real-space analog of inverse mobility is diameter. In the free-molecular limit, the mobility diameter is larger than the physical particle diameter by 0.3 to 0.4 nm (CitationTammet 1995). The observed difference between the two diameters corresponds to the radius of the background gas molecules. In terms of mobility diameter, particle sizes as small as 1.03 nm (TEthylAI monomer ion) were sized with a standard geometric deviation (σ g ) of 1.05 (Resolution of ∼4.7). Resolution increased with diameter reaching a value of ∼7 at 1.47 nm (monomer ion of THeptylAB), which corresponds to a σ g of 1.035. In other words, this instrument can differentiate a particle with a diameter of 1.0 nm from a particle with a diameter 1.1 nm. More importantly, this instrument has the necessary resolving power to distinguish growth by vapor deposition from that by coagulation down to a mobility diameter of 1 nm, since the experimentally observed σ g is much smaller than the self-preserving distribution value of about 1.3 associated with coagulation. Beyond measuring the size of molecular ions, the instrument can be utilized for larger ions. Based upon the empirical calibration constant determined from measuring molecular ions, the upper size limit for the sheath flow rate investigated here is calculated to be 13 nm. To characterize the instrument response for particles larger than 1.8 nm will require additional measurements using the tandem DMA technique or larger molecular ions.
5. SUMMARY
We described a nano-RDMA that enables the classification of molecular ions with mobility diameters in the range of 1 to 1.8 nm. The instrument was calibrated using monomer ions. Non-uniformities in flow and electric field emanating from the very short classification region required empirical corrections to the predicted mobility response and resolution from which an upper limit for size measurement was calculated to be 13 nm. While the nano-RDMA operates in the diffusive limit for these molecular ions, its resolving power is, nonetheless, substantial and should enable investigation of aerosol or nanoparticle growth dynamics at mobility diameters as small as 1 nm.
Acknowledgments
This work was partially supported by the Jacobs Institute for Molecular Engineering and Medicine at Caltech. An NSF graduate fellowship to NAB is also acknowledged.
Notes
REFERENCES
- Chen , D. R. , Pui , D. Y. H. , Hummes , D. , Fissan , H. , Quant , F. R. and Sem , G. J. 1998 . Design and Evaluation of a Nanometer Aerosol Differential Mobility Analyzer (Nano-DMA) . J. Aerosol Sci. , 29 : 497 – 509 .
- de Juan , L. and de la Mora , J. F. 1998 . High Resolution Size Analysis of Nanoparticles and Ions Running a Vienna DMA of Near Optimal Length at Reynolds Numbers Up to 5000 . J. Aerosol Sci. , 29 : 617 – 626 .
- de la Mora , J. F. 2002 . Diffusion Broadening in Converging Differential Mobility Analyzers . J. Aerosol Sci. , 33 : 411 – 437 .
- Fissan , H. , Pöcher , A. , Neumann , S. , Boulaud , D. and Pourprix , M. 1998 . Analytical and Empirical Transfer Functions of a Simplified Spectromètre De Mobilité Electrique Circulaire (SMEC) for Nano Particles . J. Aerosol Sci. , 29 : 289 – 293 .
- Flagan , R. C. 1998 . History of Electrical Aerosol Measurements . Aerosol Sci. Technol. , 28 : 301 – 380 .
- Flagan , R. C. 1999 . On Differential Mobility Analyzer Resolution . Aerosol Sci. Technol. , 30 : 556 – 570 .
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications . J. Aerosol Sci , 6 : 443 – 451 .
- Kousaka , Y. , Okuyama , K. , Adachi , M. and Mimura , T. 1986 . Effect of Brownian Diffusion on Electrical Classification of Ultrafine Aerosol Particles in Differential Mobility Analyzer . J. Chemical Engineer. Japan , 19 : 401 – 407 .
- Martínez-Lozano , P. and de la Mora , J. F. 2006 . Resolution Improvements of a Nano-DMA Operating Transonically . J. Aerosol Sci. , 37 : 500 – 512 .
- McMurry , P. H. 2000 . A Review of Atmospheric Aerosol Measurements . Atmos. Environ. , 34 : 1959 – 1999 .
- Rosell-Llompart , J. , Loscertales , I. G. , Bingham , D. and de la Mora , J. F. 1996 . Sizing Nanoparticles and Ions with a Short Differential Mobility Analyzer . J. Aerosol Sci. , 27 : 695 – 719 .
- Rosser , S. and de la Mora , J. F. 2005 . Vienna-Type DMA of High Resolution and High Flow Rate . Aerosol Sci. Technol. , 39 : 1191 – 1200 .
- Sankaran , R. M. , Holunga , D. , Flagan , R. C. and Giapis , K. P. 2005 . Synthesis of Blue Luminescent Si Nanoparticles Using Atmospheric-Pressure Microdischarges . Nano Lett. , 5 : 531 – 535 .
- Stolzenburg , M. R. 1988 . An Ultrafine Aerosol Size Distribution Measuring System , University of Minnesota . dissertation
- Tammet , H. 1995 . Size and Mobility of Nanometer Particles, Clusters and Ions . J. Aerosol Sci. , 26 : 459 – 475 .
- Ude , S. and de la Mora , J. F. 2005 . Molecular Monodisperse Mobility and Mass Standards from Electrosprays of Tetra-Alkyl Ammonium Halides . J. Aerosol Sci. , 36 : 1224 – 1237 .
- Zelenyuk , A. , Cai , Y. and Imre , D. 2006 . From Agglomerates of Spheres to Irregularly Shaped Particles: Determination of Dynamic Shape Factors from Measurements of Mobility and Vacuum Aerodynamic Diameters . Aerosol Sci. Technol. , 40 : 197 – 217 .
- Zhang , S. H. , Akutsu , Y. , Russell , L. M. , Flagan , R. C. and Seinfeld , J. H. 1995 . Radial Differential Mobility Analyzer . Aerosol Sci. Technol. , 23 : 357 – 372 .