Abstract
In order to model accurately the size and number of atmospheric particles, it is necessary to predict aerosol nucleation rates. However, the explicit prediction of the sulfuric acid vapor concentration may become computationally intensive when nucleation and condensation are simultaneously occurring. In this article, we develop and test a computationally efficient solution to the problem of solving for the sulfuric acid vapor concentration. Rather than explicitly solving the differential equation for the temporal profile of sulfuric acid vapor, we assume that the sulfuric acid vapor is at the concentration in steady state with its source (oxidation of SO2) and sinks (condensation and nucleation); this is known as the Pseudo-Steady-State Approximation (PSSA). Two versions of a box model with online size-resolved aerosol microphysics were developed to test the PSSA; (1) a “benchmark model” that solves explicitly for the sulfuric acid vapor concentration, and (2) a “PSSA model” that uses the PSSA. A wide array of atmospheric conditions was used to compare the benchmark and PSSA models. The mean difference in the total number of particles in the two models with diameters larger than 10 nm was only 1.8% and 1.1% in lower troposphere simulations after 2 and 6 hours, and 3.8% and 2.3% in the upper troposphere simulations after 2 and 6 h. The PSSA model was faster in 97% of the tests, more than ten times faster in 91% of the points, and more than 100 times faster in 69% of the tests.
1. INTRODUCTION
Atmospheric aerosols affect human health (CitationSpeizer 1989) and the energy balance of the earth (CitationForster et al. 2007). Aerosol nucleation (in situ particle formation) is, along with primary emissions, one of the two dominant ways particles enter the atmosphere. In order to understand accurately anthropogenic changes in atmospheric aerosol, nucleation rates must be properly constrained. Several nucleation mechanisms have been proposed that may have global atmospheric influences on aerosol concentrations, such as binary (H2SO4-H2O) nucleation (CitationVehkamäki et al. 2002; CitationJaecker-Voirol and Mirabel 1989; CitationKulmala et al. 1998), ternary (H2SO4-NH3-H2O) nucleation (CitationYu 2006; CitationKulmala et al. 2002; CitationNapari et al. 2002; CitationAnttila et al. 2005) and ion-induced nucleation (CitationModgil et al. 2005; CitationLovejoy et al. 2004). Each of these nucleation mechanisms depends greatly on the sulfuric acid (H2SO4) vapor concentration. Nucleation rates predicted by these mechanisms vary greatly both in magnitude and in location (CitationLucas and Akimoto 2006; CitationJung et al. 2008).
A recent development in global and regional 3-D atmospheric aerosol simulations is the inclusion of on-line size-resolved aerosol microphysics modules that predict the number, size and composition of particles (CitationPierce et al. 2007; CitationLiu et al. 2005; CitationJacobson 2001; CitationPierce and Adams 2006; CitationAdams and Seinfeld 2002; CitationJung et al. 2008; CitationSpracklen et al. 2005; CitationStier et al. 2005). Prediction of the number, size and composition of particles is necessary for understanding the climate effects of aerosols. While aerosol nucleation did not need to be considered in simulations that modeled only aerosol mass, it must be included in size-resolved microphysical models to predict accurately the aerosol size distribution. Although nucleation has been explored in some 3-D models (CitationSpracklen et al. 2006; CitationLucas and Akimoto 2006), the explicit calculation of nucleation can greatly increase computation time.
The underlying reason for the computational burden of nucleation is the sulfuric acid vapor lifetime in the vapor phase. Sulfuric acid vapor is formed when gaseous sulfur dioxide (SO2) is oxidized. Sulfuric acid vapor has a very low vapor pressure and will condense onto existing particles or participate in nucleating new particles on times scales less than one second or up to several hours in the troposphere. When there is a high aerosol surface area for condensation (e.g., polluted regions), the time scale is on the shorter end of this range. Because the nucleation rates generally depend strongly on the sulfuric acid vapor concentration, sulfuric acid concentrations must be simulated accurately in order to predict the number of new particles formed. It follows that to predict numerically and accurately the evolving atmospheric sulfuric acid concentration, the model time steps for nucleation and condensation must be considerably shorter than the lifetime of sulfuric acid vapor. These time steps may be significantly shorter than the time steps required to resolve other aerosol processes (e.g., coagulation, deposition, emissions) and thus the inclusion of nucleation may slow down the model appreciably. An accurate approximation of the sulfuric acid vapor concentration that reduces the computation time for condensation and nucleation can be of great benefit in 3-D aerosol microphysics models.
The pseudo-steady-state approximation (PSSA) is commonly used in chemical kinetics to formulate an algebraic solution for the concentration of short-lived species that depends on the concentrations of longer lived species. It reduces the computational “stiffness” of systems where species have very different lifetimes. In chemical kinetics, the sink for these short lived species is chemical reactions; however, the approach is equally valid when the sink of a species is the transition to another phase (e.g., as in condensation or nucleation).
The PSSA for sulfuric acid has been used to approximate the concentration of ambient sulfuric acid vapor from measured estimates of the sulfuric acid vapor sources and sinks (CitationStanier et al. 2004; CitationWeber et al. 1997; CitationGong et al. 2008). CitationBoy et al. (2005) compared this technique to direct measurements of the sulfuric acid concentration from a chemical ionization mass spectrometer and found generally good agreement between the two techniques. Several recent papers have applied this technique predictive models of aerosol microphysics and may potentially increase computational efficiency during nucleation events (CitationPierce and Adams 2008a, Citationb; CitationChang et al. 2008).
In this article we will:
-
Derive the pseudo-steady-state approximation (PSSA) for sulfuric acid vapor.
-
Evaluate the error of the PSSA in prediction of the sulfuric acid vapor concentration and the total number of particles over the time scales of a nucleation event.
-
Evaluate the change in computational burden when using the PSSA for sulfuric acid vapor.
The following section describes the PSSA for sulfuric acid vapor. Section 3 describes a box model used to evaluate this approximation. The results of the PSSA evaluation are shown in Section 4, and the conclusions follow in Section 5.
2. GAS-PHASE SULFURIC ACID DYNAMICS AND THE PSSA
The concentration of sulfuric acid vapor in the atmosphere is governed by the following differential equation:
In this equation, [H 2 SO 4] is the concentration of sulfuric acid vapor, P H2SO4 is the chemical production rate of sulfuric acid vapor, CS is the condensation sink or the first-order condensational loss rate constant for sulfuric acid vapor, Jnuc is the nucleation rate and Mnuc is the amount of sulfuric acid in each critical cluster. P H2SO4 is determined by the reaction rate between sulfur dioxide and the OH radical. The condensation sink, CS, is given by
Sulfuric acid vapor is at its steady-state concentration when [H 2 SO 4] is not changing with time because the source and sink terms for [H 2 SO 4] are balanced:
In Equation (Equation3), [H 2 SO 4] ss , J nuc − ss and M nuc − ss are the steady-state values for the sulfuric acid vapor concentration, nucleation rate and the amount of sulfuric acid in the critical cluster. If the system is at steady state for a given P H2SO4 and CS, one may then solve for the steady-state sulfuric acid vapor concentration, [H 2 SO 4] ss as well as J nuc − ss and M nuc − ss , which are functions of [H 2 SO 4]. Because Jnuc and Mnuc often have a complicated dependence on [H 2 SO 4], the solution may need to be done numerically using some variation of bisection and/or Newton's methods. If a numerical solution of Equation (Equation3) is necessary, the will be an additional computational burden of applying the PSSA. This will be evaluated later in this article.
The pseudo-steady-state approximation (PSSA) is the assumption that sulfuric acid vapor is at the steady-state concentration even when the source and sink terms in Equation (Equation3) are changing with time and may not exactly balance each other. The time scale for sulfuric acid vapor to reach its steady-state concentration, τ ss , is as follows.
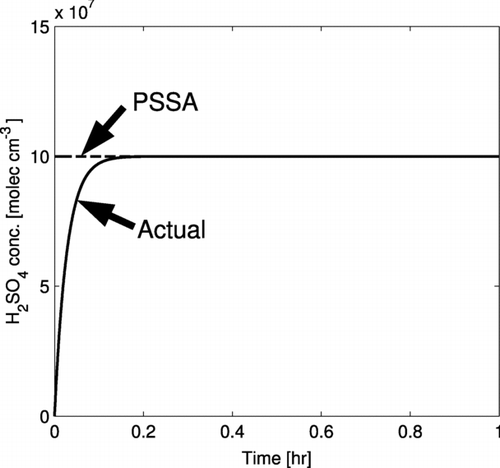
shows an example of the PSSA for sulfuric acid vapor. In this example, the condensation sink is 36 hr−1, the nucleation rate is zero, and the production rate initially undergoes a step change from zero to 106 molecules cm−3 s−1. These values are maintained at constant values throughout the hour. In the actual profile of the sulfuric acid vapor concentration, the steady-state concentration of sulfuric acid is reached in about 10 minutes. In the PSSA, this steady-state concentration is assumed to hold even during this initial adjustment period. Similar to this example, there are clearly times where [H 2 SO 4] in portions of the atmosphere will not be at its steady-state concentration (e.g., because of a sudden change in the chemical production rate of sulfuric acid or sudden removal of CS). We will assess in this article if errors from the PSSA for [H 2 SO 4] generates large errors in the predicted new particle number concentrations.
FIG. 1 Demonstration of how the pseudo-steady state approximation (PSSA) resembles the actual H2SO4 concentration profile. The condensation sink is a fixed value of 36 hr–1, the nucleation rate is zero, and the production rate is changed from zero to 106 molecules cm–3 s–1 at t = 0. In the actual profile, the steady-state concentration is reached in about 10 minutes. The PSSA calculates this steady-state concentration and uses it as the concentration for the entire time step.
Because [H 2 SO 4] ss is a function of the sulfuric acid vapor sources and sinks, it is necessary to recalculate [H 2 SO 4] ss whenever the values of P H2SO4 or CS change (or also when Jnuc and Mnuc change if the changes are due to changes in temperature, RH, ammonia or ion production rate). Often in 3-D atmospheric models, P H2SO4, temperature and RH (and the ion production rate if applicable) are calculated at the beginning of a time step and are treated as constant for the duration of a master time step. In these cases, [H 2 SO 4] ss should be calculated at the beginning of the master time step and changes only in CS (and ammonia if applicable) govern if and when [H 2 SO 4] ss must be recalculated within this master time step. A simple solution to the recalculation problem is to allow CS to change up to a certain threshold (e.g., 1% or 10%) before [H 2 SO 4] ss is recalculated. An example of where the PSSA time steps are required to be short may be at the beginning of nucleation events. During the time between when the nucleation event starts and when a steady-state nucleation mode has developed, CS may be changing quickly. This will be explored further when we look at the computational demands of the PSSA.
A solution to the fast changes in CS at the beginning of a nucleation event is to use approximations that assume a steady-state nucleation mode to determine the rate that new particles grow to larger sizes (e.g., 3, 10, or 20 nm) (CitationKerminen et al. 2004). The inputs to the CitationKerminen et al. (2004) parameterization are the critical cluster nucleation rate and size as well as the condensation sink and growth rate. When using these approximations, Equation (Equation2) becomes:
3. BOX MODEL DESCRIPTION
We will evaluate the ability of the PSSA to predict correctly the sulfuric acid vapor concentration and the total number of particles over a wide array of atmospheric conditions (discussed in the subsequent section) using two versions of a box model. One version explicitly calculates the sulfuric acid vapor concentration and the other uses the PSSA. In both versions of the box model, the aerosol size distribution is predicted as a function of time using the TwO-Moment Aerosol Sectional (TOMAS) microphysics model (CitationAdams and Seinfeld 2002). The size distribution evolves through coagulation, condensation of sulfuric acid and nucleation of new particles. The size distribution spans particle dry diameters of 0.6 nm to 10 μm using 44 geometrically spaced size bins, each representing a doubling of dry particle mass. Each simulation begins with an initial aerosol size distribution (described in next section) and runs for a total of 72 h.
Aerosol sulfate, ammonium and water are included in the model. For simplicity, the aerosols are assumed to be composed of ammonium sulfate (i.e., two molecules of ammonia condense for every molecule of sulfuric acid) regardless of the ammonia concentration used to predict the nucleation rate. Water is assumed to be in the aerosol phase at equilibrium at the relative humidity for the simulations specified in the next section using the parameterization of (CitationTang and Munkelwitz 1994). The ammonia vapor concentration is held constant at values described in the next section. Condensing sulfuric acid vapor is assumed to have an accommodation coefficient value of 0.65 (CitationPöschl et al. 1998). An accommodation coefficient of 1 could not be ruled out by CitationPöschl et al. (1998), and more recent experiments have shown this to be the case (CitationHanson 2005); however, the will not greatly affect our assessment of the applicability and benefits of the PSSA.
Two nucleation parameterizations are used in the box model. For simulations with ammonia vapor below 1 pptv, the binary nucleation mechanism of CitationVehkamäki et al. (2002) is used. When ammonia vapor is present at concentrations above 1 pptv, the ternary nucleation mechanism of CitationNapari et al. (2002) is used. The nucleation rates predicted by CitationNapari et al. (2002) have been shown to be unrealistically high (CitationMerikanto et al. 2007; CitationAnttila et al. 2005). However, the goal of these simulations is to test the PSSA across a wide array of nucleation rates and other atmospheric conditions and not to test the accuracy of the nucleation parameterizations themselves. In fact, use of a wide range of nucleation rates provides a stringent test of the PSSA.
The two variations of the box model are as follows:
-
The first version is the “benchmark model.” This model does not make the PSSA; rather it solves Equation (Equation1) explicitly by taking forward Euler time steps. Condensation, nucleation, coagulation and sulfuric acid production are all solved successively using operator splitting. The overall aerosol time step is 6 minutes. An adaptive sub-time step for the chemical production of sulfuric acid vapor, condensation of sulfuric acid and nucleation of new particles is used to ensure that none of the aforementioned processes change the gas-phase sulfuric acid concentration by more than 1% during the time step. During these adaptive time steps, the chemical production of sulfuric acid vapor is performed first followed by condensation and finally nucleation. Coagulation is performed at the conclusion of the total 6 minutes of production/condensation/nucleation.
-
The second version of the model is the “PSSA model.” This model assumes that Equation (Equation4) is valid all of the time and solves for the steady-state concentration of sulfuric acid vapor and the steady-state nucleation rate. The operator splitting in the “PSSA model” is different from the “benchmark model”; condensation, nucleation and sulfuric acid production are solved simultaneously, while coagulation remains a split operation. In order to determine the steady-state concentration of sulfuric acid vapor, Equation (Equation3) must be solved numerically (find the value of [H 2 SO 4] where the left hand side equals zero). To find this solution we use the robust method of bisection (other methods that may be faster, such as Newton's method will occasionally fail without modification). To perform bisection, we begin by guessing an upper and lower bound of sulfuric acid concentrations. The initial upper and lower bounds of sulfuric acid vapor concentration are set to the upper and lower limit of sulfuric acid concentration given by the nucleation parameterizations (using [H 2 SO 4] values outside of this range may cause the parameterizations to have large errors and possibly be unstable). The value of the left hand side of Equation (Equation3) is evaluated using these two bounding values. If the left hand side is less than zero for the lower limit guess of [H 2 SO 4], then the PSSA sulfuric acid vapor concentration is too low for nucleation to occur, and all sulfuric acid generated is condensed. If the left hand side is greater than zero for the upper limit guess of [H 2 SO 4], then the PSSA sulfuric acid vapor concentration is too high for the nucleation parameterizations (this, however, did not occur in any of our tests). If the left hand side is greater than zero for the lower limit guess and less than zero for the upper limit guess, then we begin bisection. We then choose a new guess for [H 2 SO 4] that is geometric mean of the lower and upper bounds and evaluate Equation (Equation3) with this new guess. If the left hand size of Equation (Equation3) is less than zero, the new guess for [H 2 SO 4] becomes our new upper bound. If it is greater than zero, it becomes the new lower bound. Using the updated set of bounds, we again find a new guess for [H 2 SO 4] and repeat. Convergence onto [H 2 SO 4] ss occurs to 5 significant figures in about 20 iterations. The overall aerosol time step is 6 minutes. There also is a sub-time step for the PSSA (which includes vapor production, condensation and nucleation) to ensure that CS does not change by more than 1% during this condensation/nucleation time step. Coagulation is performed at the conclusion of the total of 6 minutes of production/condensation/nucleation.
For purposes of comparing the accuracy and computation time between the two models, we have attempted to design the adaptive time steps in the two models to similar specifications. For the PSSA model, an error in CS of 1% would cause an error in the prediction of [H 2 SO 4] ss on the order of 1%. This is similar to the benchmark model where [H 2 SO 4] is allowed to fluctuate by 1%.
4. PSSA EVALUATION
4.1. Test Simulations
shows the input variables for the various tests of PSSA sulfuric acid vapor. There is a set of tests for cold lower tropospheric conditions as well as a set of tests for upper tropospheric conditions each with associated meteorological conditions. The cold conditions for the lower troposphere generated faster nucleation rates to more stringently test the PSSA. Within each of these locations is a set of values for the gas-phase ammonia concentrations, sulfuric acid vapor generation rate and the initial number of particles. Every permutation of these values is tested for a total of 100 simulations for both the benchmark and PSSA models for the lower tropospheric conditions and 48 simulations for the upper tropospheric case. The ammonia concentrations span the range of no ammonia to 100 pptv, the maximum ammonia concentration allowed in the ternary nucleation parameterization of CitationNapari et al. (2002). With the two nucleation parameterizations used here, nucleation rates increase greatly with increases in ammonia concentrations. The gas-phase sulfuric acid vapor production rates span four orders of magnitude. The particle concentration is initialized into a single lognormal mode with a number median diameter at 100 nm and a σ g of 2. The condensation sink values corresponding to these initial number distributions with a mass accommodation coefficient of 0.65 are also included in . The aerosol size distribution and condensation sink will evolve with time in the model due to ongoing aerosol microphysical processes.
TABLE 1 Test conditions for the benchmark and PSSA models
The initial sulfuric acid vapor concentration in the benchmark model is zero. The initial sulfuric acid vapor concentration for the PSSA is the steady-state sulfuric acid vapor concentrations for the initial conditions. In these cases, the actual sulfuric acid concentrations in the benchmark model will be initially be far from the steady-state sulfuric acid concentration. If the PSSA is to predict accurately the sulfuric acid vapor concentration and the correct number of particles generated by nucleation, the benchmark model will need to approach the steady-state sulfuric acid concentration rather quickly. This provides a very stringent test for the PSSA because in the atmosphere, production rate of sulfuric acid vapor often changes gradually. The PSSA should generally perform better in 3-D atmospheric models, where the diurnal cycle of chemistry is simulated. Each test simulation lasts 72 h.
4.2. Prediction of Sulfuric Acid Vapor Concentration
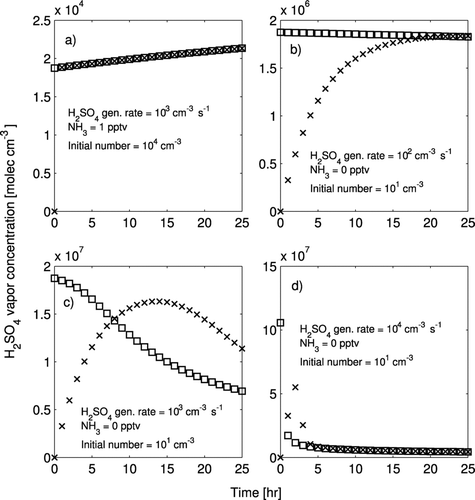
In this subsection, we evaluate the ability of the PSSA model to predict accurately the sulfuric acid vapor concentrations. To illustrate the potential profiles of the sulfuric acid vapor concentration, four examples of the sulfuric acid vapor timeseries are shown in . In panel (a), the condensation sink is high, so within one hour the benchmark model is predicting the same sulfuric acid vapor concentration as the PSSA model. Behavior similar to panel a dominated the tests; however, different behaviors were seen in several tests with very low initial condensation sinks. In panel (b), the initial condensation sink is very low, the sulfuric acid vapor production rate is very low and no ammonia is present. No nucleation occurs in the test corresponding to panel (b). For the initial value of the condensation sink, the time scale to reach steady state is 5 hours, so it takes about a day for the two models to converge fully. Panel (c) has the same low condensation sink as in panel (b); however, the sulfuric acid concentration is high enough that nucleation occurs at the initial steady-state concentration. In the PSSA model, nucleation occurs from the onset of the simulation and the sulfuric acid vapor concentration drops as the condensation sink increases. The benchmark model requires time to build up enough sulfuric acid vapor for nucleation to occur, so the condensation sink remains low. Because of this lag in the condensation sink, the sulfuric acid vapor concentration is higher in the benchmark model after 8 h. The behavior in panel (d) is similar to that of panel (c). The conditions are the same except for the sulfuric acid vapor production rate, which is larger than in panel (c). In panel (d), the benchmark model begins nucleating new particles sooner and the sulfuric acid vapor concentrations converge within 5 h.
FIG. 2 H2SO4 concentration profiles as a function of time on the first day of simulation for four of the test cases in the lower troposphere. x's represent the hourly results from the benchmark model. Squares represent the hourly results from the PSSA model. The conditions for each panel are given in the figure. Please note that the y-axis scale is different in each panel.
A comparison of the sulfuric acid vapor concentrations predicted by the benchmark and PSSA models 2 or 6 h into all of the tests is shown in . Red squares show simulations where nucleation was predicted by benchmark model within the first 2 or 6 h. A nucleation occurrence is defined here as an increase in the total number of particles larger than 10 nm by at least 0.1%. The behavior of most of the tests are similar to that in , and the two model versions have converged within the first 2 h. The outlier data points have behavior similar to one of –. shows statistics on the performance of the PSSA model relative to the benchmark model. The overall agreement improves between 2 and 6 h because the benchmark model has more time to approach the steady-state sulfuric acid concentration. In simulations where nucleation occurred, the condensation sink increases, and this helps the benchmark model reach the steady-state sulfuric acid vapor concentration more quickly. On average, the two models agree within 1% when nucleation occurred in both models. For simulations where nucleation did not occur, the errors are larger on average, particularly in the upper troposphere case. This occurred in tests where the initial CS is low and remains low when nucleation does not occur, and the benchmark model does not reach steady-state in these time scales. The errors are higher in the upper troposphere case because there are cases where the initial number of particles is 1 cm−3 corresponding to an initial time scale to reach steady state of 50 h (10 times longer than the longest time scale in the lower troposphere tests).
FIG. 3 Comparison of H2SO4 concentrations from the PSSA model with those from the benchmark model. The solid line is the 1:1 line and the dashed lines are 2:1 and 1:2 ratio lines. Circles represent simulations in which new particle formation above 10 nm was not observed, and squares represent simulations in which new particle formation above 10 nm was observed in the benchmark model. The results for the lower tropospheric simulations after 2 h of simulation are in (a) and after 6 h of simulation are in (b). The results for the upper tropospheric simulations after 2 h of simulation are in (c) and after 6 h of simulation are in (d).
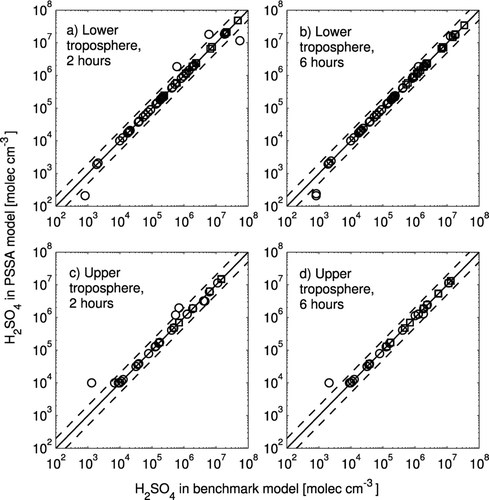
TABLE 2 Mean percent error for H2SO4 concentrations in the PSSA model versus the benchmark model. “Nucleation” refers to simulations in which the number of particles larger than 10 nm increased in the benchmark model during the testing time period (2 or 6 h)
From these tests, we can conclude that, on time scales of typical atmospheric nucleation events (several hours), the PSSA can predict the sulfuric acid vapor concentration with reasonable accuracy. However, errors in the prediction of the sulfuric acid vapor concentration shortly after a change in sulfuric acid production rate may lead to incorrect predictions of the total number of particles generated. Furthermore, due to the generally strong non-linear dependence of the nucleation rate on the sulfuric acid vapor concentration, there may be errors in the number of particles nucleated that are much larger than the errors in the sulfuric acid vapor concentration itself. For these reasons, we will evaluate the ability of the PSSA model to predict the total particle number concentrations in the next subsection.
4.3. Prediction of CN
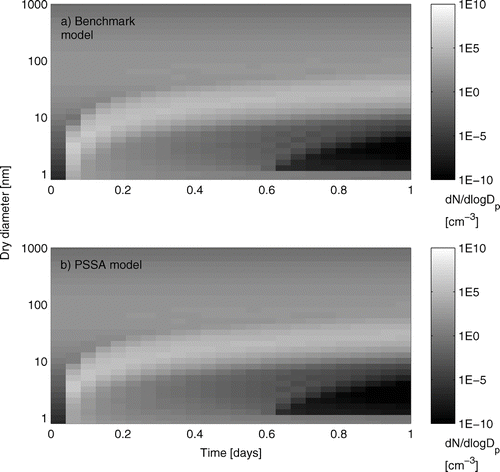
illustrates an example of the evolving aerosol size distribution in the two model versions for a single test case. The time resolution of this figure is 1 h. In this example, the sulfuric acid vapor production rate is 105 cm–3 s–1, the ammonia concentration is 0 pptv, the initial number concentration is 100 cm–3, and the two models qualitatively predict the same behavior for the two size distributions. To compare quantitatively the aerosol predictions between the two model versions, we evaluate the ability of the PSSA model to predict the total number of condensation nuclei (CN10, i.e., particles with diameters larger than 10 nm).
FIG. 4 Example of evolution of the aerosol size distribution in the benchmark and PSSA models. The sulfuric acid vapor production rate is 105 molecules cm–3 s–1. The ammonia concentration is 0 pptv. The initial number concentration of particles is 100 cm–3. The time resolution for this figure is 1 h.
A comparison of the CN10 concentrations predicted by the benchmark and PSSA models for all of the tests is shown in . Panel a shows the results two hours into the lower troposphere tests, panel b shows the results six hours into the lower troposphere tests, panel c shows the results two hours into the upper troposphere tests, and panel d shows the results six hours into the upper troposphere tests. As with , red squares show simulations where nucleation was predicted by benchmark model within the first 2 or 6 h. There appears to be fewer blue circles in than ; however, this is due to the blue circles overlapping near the initial CN10 concentrations. In these cases without nucleation, the number of particles changes only through coagulation. shows statistics on the performance of the PSSA model relative to the benchmark model. As with the comparison of sulfuric acid vapor concentrations, the agreement of the two model versions improves between 2 h and 6 h as the benchmark model moves towards a steady-state concentration and premature nucleation in PSSA coagulations away. Contrary to sulfuric acid concentrations, the comparison of CN10 concentrations agree better when no nucleation is occurring than when nucleation is occurring. This is because when no nucleation is occurring, only coagulation changes the CN10 concentrations. When nucleation is occurring the models agree within about 10% on average for both the lower and upper troposphere at 2 and 6 h.
FIG. 5 Comparison of CN10 from the PSSA model with the CN10 from the benchmark model. The solid line is the 1:1 line and the dashed lines are 2:1 and 1:2 ratio lines. Circles represent simulations in which new particle formation above 10 nm was not observed, and squares represent simulations in which new particle formation above 10 nm was observed in the benchmark model. The results for the lower tropospheric simulations after 2 h of simulation are in (a) and after 6 h of simulation are in (b). The results for the upper tropospheric simulations after 2 h of simulation are in (c) and after 6 h of simulation are in (d).
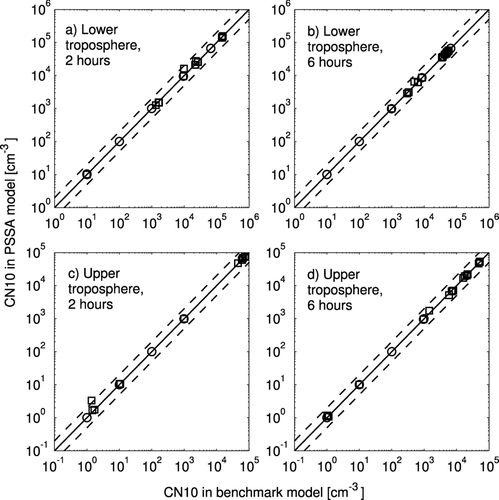
TABLE 3 Mean percent error for CN10 concentrations in the PSSA model versus the benchmark model. “Nucleation” refers to simulations in which the number of particles above 10 nm increased in the benchmark model during the testing time period (2 or 6 h)
These tests show that the particles formed from typical nucleation events of several hours can be approximated well using the PSSA.
4.4. Computation Time Comparison
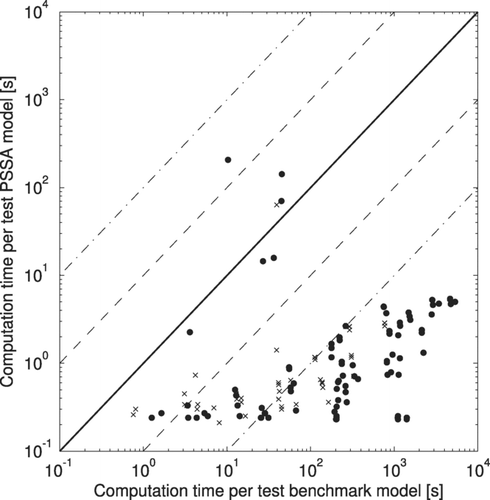
The major benefit of using the PSSA is the potential for large decreases in computation time. compares the total computation time to run each test using both the benchmark model and the PSSA model. It is clear from that there is much variability in the computation time in both the benchmark model and the PSSA models; however, the PSSA model is faster in 97% of the tests, more than ten times faster in 91% of the tests, and more than 100 times faster in 69% of the tests. When the condensation sink is at its highest values in the tests (2·103 hr–1), the benchmark model must take timesteps of ∼0.01 s in order for the sulfuric acid vapor concentration to not change by more than 1% during condensation. This greatly slows down the benchmark model. At these high condensation sink values, the time scale for changes in the condensation sink tends to be long, allowing the PSSA model to take full 6-minute time steps for condensation/nucleation. The data points in which the computation time for the PSSA model is either comparable to or longer than the benchmark model occur when the condensation sink changes steadily throughout the simulation forcing the PSSA model to take short adaptive production/condensation/nucleation time steps. In chemical transport models (CTMs), these periods of steady condensation sink increase from a low value may occur after rain events have removed a large fraction of the aerosol.
FIG. 6 Comparison of the total computation time for each test between the PSSA model and the benchmark model. Dots are lower tropospheric simulations and x's are upper tropospheric simulations. Dashed lines represent a factor of 10 difference in computation time. Dash dot lines represent a factor of 100 difference in computation time.
In the benchmark model, we have used forward Euler steps to solve for the sulfuric acid vapor concentration in the benchmark model. Higher-order methods such as the fourth-order Runge-Kutta method give potentially faster solutions for a similar accuracy; however, it is not likely to speed up the model by a factor of 100 for the same accuracy.
The computational benefits of the PSSA may be even greater when the master aerosol operator time step is greater. This would allow the production/condensation/nucleation time steps to be longer during periods when condensation sinks are not changing. The limiting reason for the short aerosol operator time steps in this model is the coagulation time scale of the nuclei sized particles. For accuracy, coagulation needed to be calculated every several minutes. By using a parameterization of the nucleation mode gap, such as CitationKerminen et al. (2004), the microphysics of the nucleation mode particles are not explicitly simulated. This allows for longer aerosol operator time steps because the smallest predicted particles in the model will have larger sizes (e.g., 3 or 10 nm), and longer coagulation time scales.
5. CONCLUSIONS
In this article, we have derived the pseudo-steady-state approximation (PSSA) for sulfuric acid vapor, applied it to the competition between condensation and nucleation, and tested it in a box model with size-resolved aerosol microphysics. Two versions of the box model were developed to test the PSSA; (1) the “benchmark model” used short forward Euler steps to solve explicitly for the sulfuric acid vapor concentration, and (2) the “PSSA model” uses the PSSA to determine the sulfuric acid vapor concentration. We found that the error from using the PSSA in predicting the sulfuric acid vapor concentration and the number of new particles formed during the time scale of a typical atmospheric nucleation event was very small. The mean error in the sulfuric acid vapor concentration from all simulations was 8.4% and 4.0% in the lower troposphere after 2 and 6 h, respectively, and 23% and 9.5% in the upper troposphere, after 2 and 6 h. For simulations in which nucleation occurred, the error in sulfuric acid vapor concentration was always less than 1%. The mean error in the total number of particles (CN10) was 1.8% and 1.1% in the lower troposphere after 2 and 6 h, and 3.8% and 2.3% in the upper troposphere after 2 and 6 h. These estimates are an upper limit for the error in using the PSSA because in these tests we assumed a step change in the sulfuric acid production rate. In many cases in the atmosphere, the sulfuric acid production rate will change gradually allowing the PSSA to be more accurate.
There is a large computational benefit to using the PSSA. The PSSA model was faster in 97% of the tests, more than 10 times faster in 91% of the points, and more than 100 times faster in 69% of the tests. We recommend the use of the PSSA in 3-D CTMs for decreasing the computation time in calculating the sulfuric acid vapor concentration during normal tropospheric conditions.
Acknowledgments
This research was supported by the Environmental Protection Agency (EPA) through the Science to Achieve Results (STAR) Graduate Fellowship (91668201–0) as well as by the National Aeronautics and Space Administration (NASA Award NNG04GE86G).
REFERENCES
- Adams , P. J. and Seinfeld , J. H. 2002 . Predicting Global Aerosol Size Distributions in General Circulation Models . J. Geophys. Res. , 107 : 4370 doi: 10.1029/2001JD001010
- Anttila , T. , Vehkamaki , H. , Napari , I. and Kulmala , M. 2005 . Effect of Ammonium Bisulphate Formation on Atmospheric Water-Sulphuric Acid-Ammonia Nucleation . Boreal Environment Research , 10 : 511 – 523 .
- Boy , M. , Kulmala , M. , Ruuskanen , T. M. , Pihlatie , M. , Reissell , A. , Aalto , P. P. , Keronen , P. , Dal Maso , M. , Hellen , H. , Hakola , H. , Jansson , R. , Hanke , M. and Arnold , F. 2005 . Sulphuric Acid Closure and Contribution to Nucleation Mode Particle Growth . Atmos. Chem. Phys. , 5 : 863 – 878 .
- Chang , L.-S. , Schwartz , S. E. , McGraw , R. and Lewis , E. R. 2008 . Sensitivity of Aerosol Properties to New Particle Formation Mechanism and to Primary Emissions in a Continentalscale Chemical Transport Model, Submitted to Journal of Geophysical Research . (in press)
- Forster , P. , Ramaswamy , V. , Artaxo , P. , Berntsen , T. , Betts , R. , Fahey , D. W. , Haywood , J. , Lean , J. , Lowe , D. C. , Myhre , G. , Nganga , J. , Prinn , R. , Raga , G. , Schulz , M. , Dorland , R. and Van . 2007 . “ Changes in Atmospheric Constituents and in Radiative Forcing ” . In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M. and Miller , H. L. Cambridge, , UK and New York : Cambridge University Press .
- Gong , Y. G. , Su , H. , Cheng , Y. F. , Liu , F. , Wu , Z. J. , Hu , M. , Zeng , L. M. and Zhang , Y. H. 2008 . Analysis on Concentration and Source Rate of Precursor Vapors Participating in Particle Formation and Growth at Xinken in the Pearl River Delta of China . Adv. Atmos. Sci. , 25 : 427 – 436 .
- Hanson , D. R. 2005 . Mass Accommodation of H2SO4 and CH3SO3H on Water-Sulfuric Acid Solutions from 6% to 97% RH . J. Phys. Chem. A , 109 : 6919 – 6927 .
- Jacobson , M. Z. 2001 . Global Direct Radiative Forcing due to Multicomponent Anthropogenic and Natural Aerosols . J. Geophys. Res. , 106 : 1551 – 1568 .
- Jaecker-Voirol , A. and Mirabel , P. 1989 . Heteromolecular Nucleation in the Sulfuric Acid-Water System . Atmos. Environ. , 23 : 2053 – 2057 .
- Jung , J. G. , Adams , P. J. and Pandis , S. N. 2008 . Evaluation of Nucleation Theories in a Sulfur-Rich Environment . Atmos. Env. , 45 ( 7 ) : 495 – 504 .
- Kerminen , V. M. , Anttila , T. , Lehtinen , K. E. J. and Kulmala , M. 2004 . Parameterization for Atmospheric New-Particle Formation: Application to a System Involving Sulfuric Acid and Condensable Water-Soluble Organic Vapors . Aerosol Sci. Technol. , 38 : 1001 – 1008 .
- Kulmala , M. , Korhonen , P. , Napari , I. , Karlsson , A. , Berresheim , H. and O'Dowd , C. D. 2002 . Aerosol Formation During PARFORCE: Ternary Nucleation of H2SO4, NH3, and H2O . J. Geophys. Res. , 107 : 8111 doi: 10.1029/2001JD000900
- Kulmala , M. , Laaksonen , A. and Pirjola , L. 1998 . Parameterizations for Sulfuric Acid/Water Nucleation Rates . J. Geophys. Res. , 103 : 8301 – 8307 .
- Liu , X. H. , Penner , J. E. and Herzog , M. 2005 . Global Modeling of Aerosol Dynamics: Model Description, Evaluation, and Interactions between Sulfate and Nonsulfate Aerosols . J. Geophys. Res. , 110
- Lovejoy , E. R. , Curtius , J. and Froyd , K. D. 2004 . Atmospheric Ion-Induced Nucleation of Sulfuric Acid and Water . J. Geophys. Res. , 109 : D08204 doi: 10.1029/2003JD004460
- Lucas , D. D. and Akimoto , H. 2006 . Evaluating Aerosol Nucleation Parameterizations in a Global Atmospheric Model . Geophys. Res. Lett. , 33 : L10808 doi: 10.1029/2006GL025672
- Merikanto , J. , Napari , I. , Vehkamaki , H. , Anttila , T. and Kulmala , M. 2007 . New Parameterization of Sulfuric Acid-Ammonia-Water Ternary Nucleation Rates at Tropospheric Conditions . J. Geophys. Res. , 112 : D15207 doi: 10.1029/2006JD007977
- Modgil , M. S. , Kumar , S. , Tripathi , S. N. and Lovejoy , E. R. 2005 . A Parameterization of Ion-Induced Nucleation of Sulphuric Acid and Water for Atmospheric Conditions . J. Geophys. Res. , 110 : D19205 doi: 10.1029/2004JD005475
- Napari , I. , Noppel , M. , Vehkamaki , H. and Kulmala , M. 2002 . Parametrization of Ternary Nucleation Rates for H2SO4-NH3-H2O Vapors . J. Geophys. Res. , 107 : 4381 doi: 10.1029/2002JD002132
- Pierce , J. R. and Adams , P. J. 2006 . Global Evaluation of CCN Formation by Direct Emission of Sea Salt and Growth of Ultrafine Sea Salt . J. Geophys. Res. , 111 : D06203 doi: 10.1029/2005JD006186
- Pierce , J. R. and Adams , P. J. 2008a . Can Cosmic Rays Affect Clouds by Altering New Particle Formation Rates? . Geophysical Research Letters , submitted
- Pierce , J. R. and Adams , P. J. 2008b . Uncertainty in Global CCN Concentrations from Uncertain Aerosol Nucleation and Primary Emission Rates . Atmos. Chem. Phys. Discuss. , 8 : 16291 – 16333 .
- Pierce , J. R. , Chen , K. and Adams , P. J. 2007 . Contribution of Primary Carbonaceous Aerosol to Cloud Condensation Nuclei: Processes and Uncertainties Evaluated with a Global Aerosol Microphysics Model . Atmos. Chem. Phys. , 7 : 5447 – 5466 .
- Pöschl , U. , Canagaratna , M. , Jayne , J. T. , Molina , L. T. , Worsnop , D. R. , Kolb , C. E. and Molina , M. J. 1998 . Mass Accommodation Coefficient of H2SO4 Vapor on Aqueous Sulfuric Acid Surfaces and Gaseous Diffusion Coefficient of H2SO4 in N2/H2O . J. Phys. Chem. A , 102 : 10082 – 10089 .
- Seinfeld , J. H and Pandis , S. N. 2006 . Atmospheric Chemistry and Physics, , 2nd , New York : John Wiley and Sons .
- Speizer , F. E. 1989 . Studies of Acid Aerosols in 6 Cities and in a New Multi-City Investigation—Design Issues . Environmental Health Perspectives , 79 : 61 – 67 .
- Spracklen , D. V. , Carslaw , K. S. , Kulmala , M. , Kerminen , V. M. , Mann , G. W. and Sihto , S. L. 2006 . The Contribution of Boundary Layer Nucleation Events to Total Particle Concentrations on Regional and Global Scales . Atmos. Chem. Phys. , 6 : 5631 – 5648 .
- Spracklen , D. V. , Pringle , K. J. , Carslaw , K. S. , Chipperfield , M. P. and Mann , G. W. 2005 . A Global Off-Line Model of Size-Resolved Aerosol Microphysics: I. Model Development and Prediction of Aerosol Properties . Atmos. Chem. Phys. , 5 : 2227 – 2252 .
- Stanier , C. O. , Khlystov , A. Y. and Pandis , S. N. 2004 . Nucleation Events during the Pittsburgh Air Quality Study: Description and Relation to Key Meteorological, Gas Phase, and Aerosol Parameters . Aerosol Sci. Technol. , 38 : 253 – 264 .
- Stier , P. , Feichter , J. , Kinne , S. , Kloster , S. , Vignati , E. , Wilson , J. , Ganzeveld , L. , Tegen , I. , Werner , M. , Balkanski , Y. , Schulz , M. , Boucher , O. , Minikin , A. and Petzold , A. 2005 . The Aerosol-Climate Model ECHAM5-HAM . Atmos. Chem. Phys. , 5 : 1125 – 1156 .
- Tang , I. N. and Munkelwitz , H. R. 1994 . Water Activities, Densities, and Refractive Indices of Aqueous Sulfates and Sodium Nitrate Droplets of Atmospheric Importance . J. Geophys. Res. , 99 : 18801 – 18808 .
- Vehkamäki , H. , Kulmala , M. , Napari , I. , Lehtinen , K. E. J. , Timmreck , C. , Noppel , M. and Laaksonen , A. 2002 . An Improved Parameterization for Sulfuric Acid-Water Nucleation Rates for Tropospheric and Stratospheric Conditions . J. Geophys. Res. , 107 : 4622 doi: 10.1029/2002JD002184
- Weber , R. J. , Marti , J. J. , McMurry , P. H. , Eisele , F. L. , Tanner , D. J. and Jefferson , A. 1997 . Measurements of New Particle Formation and Ultrafine Particle Growth Rates at a Clean Continental Site . J. Geophys. Res. , 102 : 4375 – 4385 .
- Yu , F. Q. 2006 . Effect of Ammonia on New Particle Formation: A Kinetic H2SO4-H2O-NH3 Nucleation Model Constrained by Laboratory Measurements . J. Geophys. Res. , 111 : D01204 doi: 10.1029/2005JD005968