Abstract
Evaporation of water changes the response of photoacoustic instruments to light-absorbing particles. Previous calculations of this effect are valid for particles much larger than the mean free path of air. These calculations are extended here to include transition and molecular flow as well as various mass accommodation coefficients for water. For commonly encountered conditions, evaporation can significantly reduce the photoacoustic signal if the mass accommodation coefficient of water on aerosol particles is larger than about 0.01. Unlike the growth of cloud droplets, the photoacoustic signal is very sensitive to changes in the accommodation coefficient between 0.1 and 1. This may provide a way to measure large accommodation coefficients. For a given accommodation coefficient, the change in the photoacoustic signal depends more on absolute than relative humidity. To minimize the effects of evaporation it is better to remove water from the air rather than reduce relative humidity with heating.
NOMENCLATURE
| Values in parentheses are those used for water vapor in air at 295 K, 1000 mbar, and 50% RH. | ||
| c a | = |
specific heat of aerosol particle (4184 J kg−1 K−1) |
| c p,g | = |
specific heat of gas at constant pressure (1006 J kg−1 K−1) |
| D | = |
diffusion coefficient for vapor in air (2.49e-5 m2 s−1) |
| E | = |
energy flux from light absorption |
| f M | = |
ratio of latent to conductive heat transfer in continuum flow |
| I | = |
mass flux |
| K | = |
thermal conductivity of air (0.025 W m−1 K−1) |
| Kn M | = |
Knudsen number for mass transfer |
| Kn T | = |
Knudsen number for heat transfer |
| L | = |
latent heat of evaporation (2.45e6 J kg−1) |
| m a | = |
mass of aerosol particle |
| M g | = |
molecular weight of gas molecules (0.029 kg mol−1) |
| M v | = |
molecular weight of vapor molecules (0.018 kg mol−1) |
| Q | = |
heat transfer flux |
| S | = |
photoacoustic signal |
| S dry | = |
photoacoustic signal with no evaporation |
| r 0 | = |
particle radius |
| R | = |
gas constant (8.31447 J mol−1 K−1) |
| T a | = |
temperature at particle surface |
| T ∞ | = |
temperature far from particle |
| p | = |
total pressure (1.0e5 Pa) |
| p v | = |
partial pressure of the vapor (1311 Pa) |
| α M | = |
mass accommodation coefficient |
| α T | = |
thermal accommodation coefficient (0.97) |
| β M | = |
transition flow correction for mass transfer |
| β T | = |
transition flow correction for heat transfer |
| λ g | = |
mean free path for bulk gas molecules (67 nm) |
| λ v | = |
mean free path for vapor molecules (102 nm) |
| σ g | = |
collision parameter for gas molecules (0.372 nm) |
| σ v | = |
collision parameter for vapor molecules (0.264 nm) |
| τ | = |
thermal time constant |
| ω | = |
applied angular frequency |
INTRODUCTION
Photoacoustic spectroscopy has been developed as a way of measuring the light absorption of aerosol particles (CitationLin and Campillo 1985; CitationArnott et al. 1999; CitationLack et al. 2006). An advantage of photoacoustic spectroscopy is that light absorption is measured when particles are still suspended in air. Methods that depend on the darkening of filters measure extinction through a loaded filter and then must be corrected for scattered light from particles and the filter. Other corrections are necessary, such as for water on the filter and for subtle changes that are correlated with organic content (CitationArnott et al. 2003; CitationVirkkula et al. 2005; CitationSubramanian et al. 2007; CitationCappa et al. 2008; CitationLack et al. 2008).
In photoacoustic instruments, particles absorb light from a laser modulated at an acoustic frequency. Sound waves are produced as this heat is transferred to the surrounding gas. Any evaporation of water changes the heat transfer and acoustic signal. Calculations have previously been made of the change in photoacoustic signal due to water evaporation (CitationBaker 1976; CitationRaspet et al. 2003; and the related CitationArnott et al. 2003). All used heat transfer equations for continuum flow around the particles. Since primary emissions of soot often produces light-absorbing particles smaller than 100 nm, it is important to consider the photoacoustic signal in transition and free molecular flow.
Heat and Mass Transfer Calculations
There are a number of references for the coupled heat and mass transfer to particles that are equivalent to each other except for some small corrections. The equations here are taken from CitationWagner (1982) and CitationWinkler et al. (2006). These include the effect of the different mean free paths of air and water molecules. The main approximations made in the calculations in this article are:
-
The temperature change of the particle is sufficiently small. This enters the calculations in four ways. First and most important, the change in vapor pressure of water with temperature is linearized about the temperature of the system. Second, terms such as the diffusion coefficient are not considered to be a function of distance from the particle. When there is a large difference in temperature between the particle and the surrounding gas the diffusion coefficient and other “constants” have different values near and far away from the particle. Third, large temperature changes imply large departures from equilibrium that can affect accommodation coefficients. Finally, large temperature gradients within about a mean free path of the particle surface induce corrections to the heat and mass fluxes (CitationWagner 1982; CitationZientara et al. 2008) that are not included here. The magnitude of temperature changes is examined in a later section.
-
Water is the only evaporating substance of interest (CitationArnott et al. 2003). Evaporation is proportional to the absolute difference of the partial and vapor pressures of the evaporating substance. The partial and vapor pressures of water are orders of magnitude larger than any other substance likely to be present in atmospheric aerosols. Note that the relevant vapor pressure is that over the solution of interest rather than that of pure water. An aerosol particle near equilibrium with a relative humidity of 10% will have an activity coefficient of 0.1, or equivalently a water vapor pressure 10% of that of pure water. That is why in the results here evaporation can be slower at low relative humidity than at high relative humidity.
-
Water vapor is a trace species so the thermal conductivity and other bulk properties of air do not change as water evaporates.
-
Corrections involving nearest neighbor particles are small. This assumption is examined in CitationRaspet et al. (2003) and for instruments operating near 1 kHz the corrections are only important for particle concentrations well above 105 cm− 3.
-
Temperature gradients inside each particle are small. The heat capacity of a particle is taken into account under the assumption that the particle heats and cools uniformly.
-
The temperature and concentration gradients in the gas surrounding the particle approach a steady state quickly compared to the photoacoustic frequency (CitationRaspet et al. 2003). This along with assumption #5 allows the use of quasi steady state equations.
-
The calculations are for spherical particles. Although many light-absorbing soot particles are highly non-spherical, the results include a ratio of heat to mass transfer that will be less sensitive to shape than either one alone. Even so, it is likely that the interpretation of the mass accommodation coefficient will be complicated for highly irregular particles. Also, the freshest, most non-spherical soot may also be hydrophobic, introducing a different kind of correlation with shape that is beyond the scope of this article.
-
The Kelvin effect is neglected. In the figures below it should be comparable to changes in relative humidity (RH) of at most a few percent. Also, the Kelvin effect changes the amount of water in a particle more than it changes the rates of heat and mass transfer to that particle.
If light absorption causes an aerosol particle to have a temperature T a that is slightly different than the ambient temperature T ∞, the sensible heat flux Q and mass flux I away from the particle are approximately given by
The thermal transition flow correction factor is (CitationWinkler et al. 2006)
Calculations of the change in photoacoustic signal due to evaporation start with the power balance equation
The solution to Equation (6) is straightforward for a sinusoidal applied energy absorption E(t) = E 0 exp(-iωt) where ω is the angular frequency. In a real photoacoustic instrument there is a constant (DC) offset because the laser intensity cannot go negative like a sine wave, but the sinusoidal solution is sufficient for the acoustic response because the offset does not contribute any acoustic signal. After substituting Equations (1) and (2) into (6) and applying the sinusoidal modulation, the temperature change of the particle above and below its constant value is
Physically, τ is the time constant for the thermal inertia of the particle in dry continuum flow and f M is the ratio of heat carried from the particle by latent heat compared to conduction. The case with no evaporation can be obtained by setting f M = 0.
Both heating and evaporation of water induce photoacoustic signals because both change the volume of gas surrounding a particle. The ratio of the volume changes per watt of heat transfer for evaporation and heat conduction is Tc p,g /L, where c p,g is the specific heat of the gas at constant pressure. At 295 K each joule that evaporates water gives about 12% of the volume change compared to the same energy conducted into the gas phase. Finally, expressing the photoacoustic signals in terms of the signal per unit energy gives the ratio of the magnitudes of the wet and dry photoacoustic signals as
Evaporation Effect on Photoacoustic Signals
shows the result of Equation (10) for various values of the mass accommodation coefficient and relative humidities. The effect of evaporation on the photoacoustic signal is very dependent on the accommodation coefficient. At room temperature and moderate relative humidity the change in photoacoustic signal is significant for α M larger than about 0.01.
FIG. 1 Relative photoacoustic signals compared to no evaporation at surface pressure, 295 K, and a photoacoustic frequency of 1 kHz. The upper panel shows various values of the mass accommodation coefficient and the lower curve shows various relative humidities. The heavy curve is repeated between the panels. The case for 274 K and 75% RH has the same absolute humidity as 295 K and 20% RH.
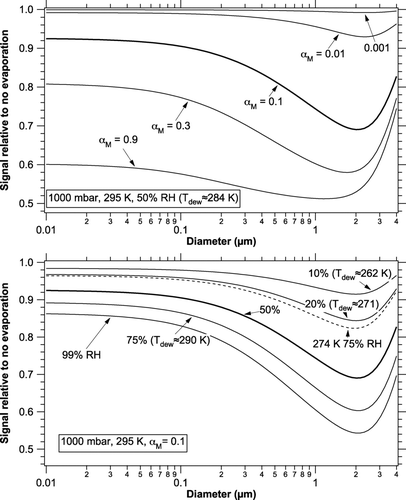
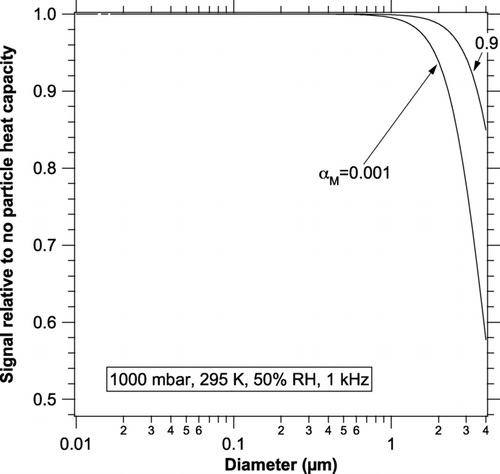
The increase in relative signal for particles larger than about 2 μ m is because the signal for particles that large is dropping because of the internal heat capacity of the particles (CitationRaspet et al. 2003) and the decrease is larger for dry particles than it is for wet particles. shows the effect of heat capacity on particles with the thermal properties of water at a photoacoustic frequency of 1 kHz. For comparison, the NOAA instrument operates at about 983 Hz and versions of the Desert Research Institute instrument at 500 and 1500 Hz (CitationArnott et al. 2003; CitationLack et al. 2006; P. Arnott private communication 2008). Just as in an electrical RC circuit, the time constant induces a phase shift that could be measured to determine the presence of absorption by coarse particles.
FIG. 2 The effect of internal heat capacity on the photoacoustic signal at 1 kHz for particles with the specific heat of water. Internal heat capacity is more important for a small mass accommodation coefficient because then latent heat transfer does not help heat and cool a particle.
It is important to note that particles are not totally dry even at “dry” conditions that are often operationally defined as 20 to 40% RH. The complex mixtures in atmospheric particles can have low deliquescence points and resist efflorescence more than pure substances (CitationMarcolli et al. 2004; CitationKhlystov et al. 2005). Even solid particles will usually have some water adsorbed on their surfaces. When particles are in equilibrium with the surrounding air the equilibrium pressure (including the activity coefficient) of solution or adsorbed water must be equal to the partial pressure of water vapor. For example, the p v term in Equation (1) for adsorbed water on a particle in equilibrium with 20% RH is simply one quarter the p v of a solution droplet at 80% RH.
Calculations show that even relatively dry particles contain sufficient water to allow evaporation to affect the photoacoustic signal. To calculate the evaporation, the energy absorption is first calculated, from Mie theory for uniform spheres or other theory as appropriate, and the temperature change is calculated from Equation (7). Then the mass loss can be calculated from Equation (2) and integrating over half of a sine wave. shows the change in temperature and the amount of water lost from highly absorbing particles (the imaginary refractive index of 0.1 may be compared to about 0.3 for nigrosin dye). The conditions are for the laser intensity in the NOAA photoacoustic instrument (CitationLack et al. 2006). The mass loss is much less than 1% of a particle's mass. For comparison, a monolayer of water on a 100 nm diameter particle is roughly 2% of the mass. A very small amount of sulfate or other ions should retain enough water for evaporation to continue through a laser cycle and even adsorbed water can be sufficient.
FIG. 3 Temperature rise and mass loss of water (dotted curve) from highly absorbing particles during a single photoacoustic cycle for conditions typical of the NOAA photoacoustic instrument. The dashed curve shows the amount of heating for a lower pressure case, as might occur during an aircraft measurement or downstream of a critical orifice.
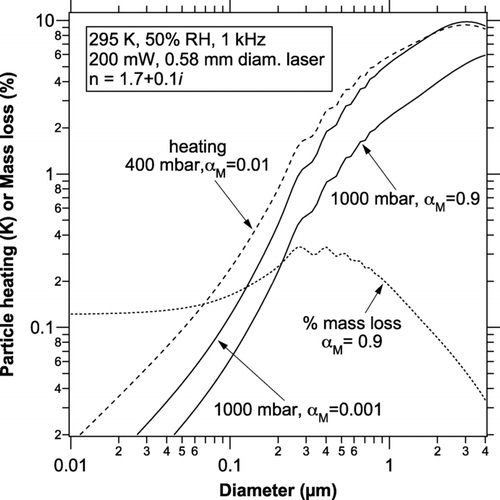
Except for particles larger than 1 μ m, temperature increases are less than a few K so the assumption of small temperature rise in the equations above is justified. For the approximations made here the temperature rise is linear in laser power density. The use of multiwatt lasers or tighter focusing could lead to undesirably large temperature changes in the particles.
DISCUSSION
Implications for Operating Photoacoustic Instruments
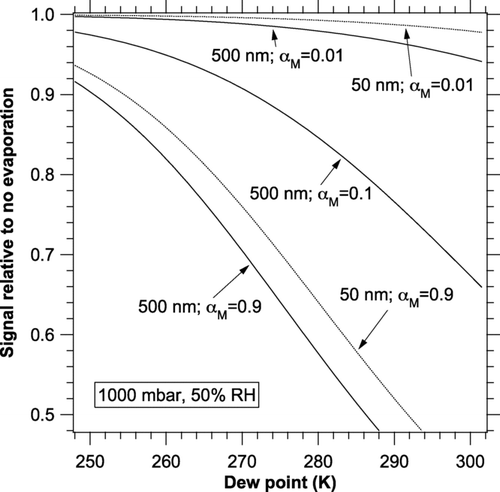
In Equation (2), the mass flux of water is proportional to the partial pressure of water vapor. This means that drying the air by removing water vapor is a better way to reduce the error due to evaporation than reducing the relative humidity by heating. Dew points below about 250 K are preferable if α M is greater than 0.5, or dew points below about 270 K if α M is greater than about 0.03. shows the dew point dependence at a constant 50% RH. The main effect of reducing relative humidity with heating is probably to reduce the accommodation coefficient. At lower RH the reduced water content means that any organic surface layers will become thicker. Both removing water vapor and heating would produce smaller errors from evaporation than either treatment alone.
FIG. 4 Dew point dependence of the change in photoacoustic signal due to evaporation. Calculations were done at 50% RH, 1 kHz, and a range of temperatures (8 to 12 K greater than the dew points). Curves are shown for 50 and 500 nm diameter particles.
In contrast, the same absolute humidity should be maintained if the goal is to measure the change in light absorption as particles take up water. The lower panel on includes a case that represents cooling the 20% RH case by enough to raise the RH to 75%. The curves are very similar, meaning that the effects of evaporation would largely cancel and small changes in light absorption could be measured. The small remaining temperature effect is of much less concern than possible changes in mass accommodation coefficient as the particle surface changes with relative humidity. One practical difficulty is that the resonant frequency of a photoacoustic cell is temperature dependent, making it difficult to use the same laser modulation and possibly introducing different errors in measuring the cavity Qs.
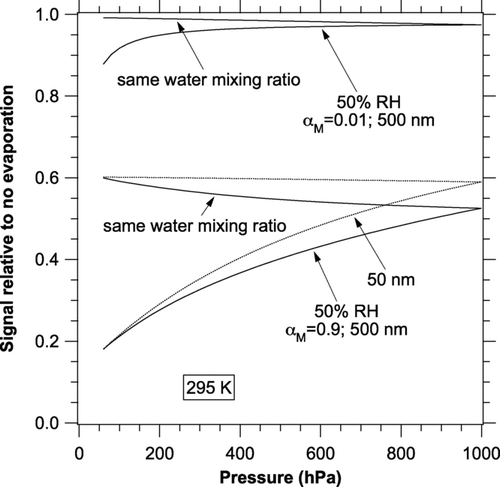
The pressure dependence of the change in signal due to evaporation is shown in . There are two limiting cases. One is constant relative humidity, applicable if samples are taken from environments at different pressures. In this case the evaporation effect is much larger at lower pressures because there is less conductive heat transfer at lower pressure. The other case is constant water mixing ratio, applicable if air at atmospheric pressure is brought through a critical orifice and then returned to the initial temperature. In this case the error is independent of pressure for particles small enough to be in the molecular flow limit because it depends only on the number of molecules of gas and vapor hitting a particle. For larger particles, a pressure drop causes a complicated interplay of reduced heat transfer, reduced relative humidity, and increased mean free path. The net result is a slight reduction in the error due to evaporation.
FIG. 5 Pressure dependence of the change in photoacoustic signal due to evaporation. Calculations were done at 295 K and 1 kHz. Curves are shown for both constant RH and constant water mixing ratio. For α M = 0.01 the values for 50 nm diameter particles (not shown) are larger than those for 500 nm particles.
Accommodation Coefficient
The sensitivity to accommodation coefficient raises the question of what the value of the coefficient is. There has been a wide spread in estimates of the mass accommodation coefficient for pure liquid water, with values from about 0.04 to near 1.0 (CitationMozurkewich 1986). Recently there has been experimental evidence from two independent measurements for values above 0.6 (CitationSmith et al. 2006; CitationWinkler et al. 2006) whereas two other groups have found temperature-dependent values between about 0.12 and 0.3 (CitationDavidovits et al. 2004; CitationZientara et al. 2008).
Ambient aerosols in the atmosphere, however, almost always contain measurable amounts of organics (CitationMurphy et al. 2006) that can reduce water uptake (e.g., CitationXiong et al. 1998). Droplet growth kinetics indicate a range of accommodation coefficients, even in the same air mass (CitationRuehl et al. 2008). In order to impede droplet growth by reducing accommodation coefficients to below about 0.001, organic coatings must be so thick that the organic material is tens of percent of the particle mass (CitationCruz and Pandis 1998; CitationChan and Chan 2007). However, there are few experimental data to determine whether trace organic species reduce the accommodation coefficient by a lesser amount. The accommodation coefficient can depend on relative humidity since as a particle takes up water it grows and any organic films become thinner or broken.
Droplet growth in a Continuous-Flow Streamwise Thermal Gradient CCN Counter implies an accommodation coefficient of water on deliquesced ammonium sulfate of about 0.05 and somewhat lower values on particles coated with secondary organic aerosol (CitationAsa-Awuku et al. 2008). However, this instrument is better suited to determining relative rather than absolute values for the accommodation coefficient.
The mass accommodation coefficient for water adsorbed on flame soot is less than 0.002 with considerable variability depending on the type of flame (CitationAlcala-Jornod and Rossi 2004). Moreover, the number of adsorption sites is only a few percent of a monolayer. The water loss for aged soot in the atmosphere might be larger as the soot acquires hydrophilic coatings.
In contrast to the results here, the growth of aerosol particles into cloud droplets is not sensitive to differences in accommodation coefficients above about 0.1 (CitationChuang 2006; CitationRuehl et al. 2008). One reason is that the particles quickly grow to sizes where further growth is less sensitive to the accommodation coefficient. In photoacoustic spectroscopy, the particles do not significantly change their initial diameter, so the sensitivity to the accommodation coefficient remains large. Growth curves also tend to be very sensitive to the exact starting time of the growth, and the photoacoustic signal has a defined phase relative to the heating. Finally, the change in the photoacoustic signal arises from a competition between heat and mass transfer whereas in droplet growth heat and mass transfer work together to limit growth rates.
Measuring Accommodation Coefficients
The sensitivity of the photoacoustic signal to evaporation could be exploited to measure large accommodation coefficients (or the ratio of the mass to thermal accommodation coefficients). For example, the photoacoustic signals could be measured from dilute deliquesced salt particles at the same relative humidity but different temperatures and hence absolute humidities. Operation at low absolute pressure magnifies the effect of evaporation if needed for measurements at lower temperature or smaller accommodation coefficients. The particles could either be seeded with an absorbing substance or one could use an infrared laser operating on a water absorption line.
The simplified equations used here are intended for evaluating photoacoustic signals. Determination of the accommodation coefficient of water would probably benefit from more accurate mass transfer equations that include terms such as Stefan flow and the thermal jump at the particle surface (e.g., CitationWagner 1982; CitationZientara et al. 2008). The use of Equations (2) and (3) along with experimental values for the diffusion coefficient of water in air, which include non-ideal gas effects, does not necessarily yield the exact free molecular flow limit (see section 8.7 in CitationSeinfeld 1986). In addition, for accommodation coefficients near unity the gas molecules near an extended surface no longer have a Maxwell distribution of velocities. For infinite flat surfaces it is customary to compensate by setting αM = αM′/(1–αM′/2). where αM is an effective accommodation coefficient and αM′ is the true probability of surface uptake (CitationMotz and Wise 1960). Dealing with a non-Maxwellian velocity distribution in transition flow is theoretically difficult.
A possible advantage to using photoacoustic spectroscopy to study accommodation coefficients is that some of these theoretical mass transfer issues could be addressed. The temperature changes () are small and moreover can be varied by using different laser powers. Photoacoustic signals can be obtained for a wide range of particle sizes, including the theoretically simpler free molecular flow regime (Kn ≫ 1).
Previous Data
The sensitivity to accommodation coefficient could affect some comparisons of photoacoustic absorption to other methods. In particular, CitationLack et al. (2008) found systematic changes in the relative response of the photoacoustic and filter-based absorption with organic content of the particles. The difference was more highly correlated with absolute organic loading than the organic mass fraction that should affect accommodation coefficients. Still, it is possible that the mass accommodation coefficient was a function of the organic content. If so, it would mean that the photoacoustic absorption would have been slightly too low for the particles with the least organic content and presumably smallest α M . This would make the slope of the response of the filter-based absorption to organic content worse rather than better. The Lack et al. measurements were made in a humid environment (the Gulf of Mexico) but the absolute humidities were reduced with a Nafion drier to dew points of about 277 K (about 22% RH, D. Lack, personal communication 2008). The laboratory comparison of photoacoustic and filter absorption by CitationCappa et al. (2008) specified 10% RH at room temperature or a dew point of about 262 K.
CONCLUSIONS
Given the uncertainty in the accommodation coefficient, it seems prudent to operate photoacoustic instruments in a regime where corrections are small. The errors can be large if the mass accommodation coefficient is as large as that for pure water. There are some limited laboratory data that would suggest lower accommodation coefficients for laboratory particles, at least below 80% RH (M. Greenslade et al. manuscript in preparation). A special worry is that evaporation could cause the photoacoustic signal to depend on the presence of organic films, leading to incorrect inferences about the effect of organics on light absorption.
Drying particles until they effloresce will reduce the evaporation error in photoacoustic measurements not so much because there is insufficient water but rather because adsorption and thicker organic layers probably slow the kinetics of evaporation compared to dilute solution droplets. Data are needed on mass accommodation coefficient of water for realistic particles. This is especially true at low relative humidities when the evaporation would come from adsorbed water. There is enough water in an adsorbed monolayer to affect the photoacoustic signal, but only if the mass accommodation coefficient is sufficiently large.
REFERENCES
- Alcala-Jornod , C. and Rossi , M. J. 2004 . Chemical Kinetics of the Interaction of H2O Vapor with Soot in the Range 190 K <= T <= 300 K: A Diffusion Tube Study . J. Phys. Chem. A. , 108 : 10667 – 10680 .
- Arnott , W. P. , Mossmüller , H. , Rogers , C. F. , Lin , T. and Bruch , R. 1999 . Photoacoustic Spectrometer for Measuring Light Absorption by Aerosols: Instrument Description . Atmos. Environ. , 33 : 2845 – 2852 .
- Arnott , W. P. , Mossmüller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. , Slaton , W. V. , Hand , J. L. , Kreidenweis , S. M. and Collett , J. L. Jr. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res. , 108 ( D1 ) article no. 4034, doi:10.1029/2002JD002165.
- Asa-Awuku , A. , Engelhart , G. J. , Lee , B. H. , Pandis , S. N. and Nenes , A. 2008 . Relating CCN Activity, Volatility, and Droplet Growth Kinetics of Beta-Caryophyllene Secondary Organic Aerosol . Atmos. Chem. Phys. Discuss. , 8 : 10105 – 10151 .
- Baker , M. B. 1976 . Energy Absorption by Volatile Atmospheric Aerosol Particles . Atmos. Environ. , 10 : 241 – 248 .
- Cappa , C. D. , Lack , D. A. , Burkholder , J. B. and Ravishankara , A. R. 2008 . Bias in Filter-Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Laboratory Measurements . Aerosol Sci. Technol. , 42 : 1022 – 1032 .
- Chan , M. N. and Chan , C. K. 2007 . Mass Transfer Effects on the Hygroscopic Growth of Ammonium Sulfate Particles with a Water-Insoluble Coating . Atmos. Environ. , 41 : 4423 – 4433 .
- Chuang , P. Y. 2006 . Sensitivity of Cloud Condensation Nuclei Activation Processes to Kinetic Parameters . J. Geophys. Res. , 111 ( D9 ) Article no. D09201, doi: 10.1029/2005JD006529.
- Cruz , C. N. and Pandis , S. N. 1998 . The Effect of Organic Coatings on the Cloud Condensation Nuclei Activation of Inorganic Atmospheric Aerosol . J. Geophys. Res. , 103 : 13111 – 13123 .
- Davidovits , P. , Worsnop , D. R. , Jayne , J. T. , Kolb , C. E. , Winkler , P. , Vrtala , A. , Wagner , P. E. , Kulmala , M. , Lehtinen , K. E. J. , Vesala , T. and Mozurkewich , M. 2004 . Mass Accommodation Coefficient of Water Vapor on Liquid Water . Geophys. Res. Lett , 31 Article no. L22111, doi: 10.1029/2004GL020835.
- Fuchs , N. A. 1964 . The Mechanics of Aerosols , 408 Pergamon Press, Reprinted by Dover Publications .
- Khlystov , A. , Stanier , C. O. , Takahama , S. and Pandis , S. N. 2005 . Water Content of Ambient Aerosol During the Pittsburgh Air Quality Study . J. Geophys. Res , 110 ( D7 ) Article no. D07S10, doi: 10.1029/2004JD004651.
- Lack , D. A. , Lovejoy , E. R. , Baynard , T. , Pettersson , A. and Ravishankara , A. R. 2006 . Aerosol Absorption Measurement Using Photoacoustic Spectroscopy: Sensitivity, Calibration, and Uncertainty Developments . Aerosol Sci. Technol. , 40 : 697 – 708 .
- Lack , D. A. , Cappa , C. D. , Covert , D. S. , Baynard , T. , Massoli , P. , Sierau , B. , Bates , T. S , Quinn , P. K. , Lovejoy , E. R. and Ravishankara , A. R. 2008 . Bias in Filter Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loadings: Evidence from Ambient Sampling . Aerosol Sci. Technol. , 42 : 1033 – 1041 .
- Lin , H.-B. and Campillo , A. J. 1985 . Photothermal Aerosol Absorption Spectroscopy . Appl. Optics. , 24 : 422 – 433 .
- Marcolli , C. , Luo , B. P. and Peter , T. 2004 . Mixing of the Organic Aerosol Fractions: Liquids as the Thermodynamically Stable Phases . J. Phys. Chem. A. , 108 : 2216 – 2224 .
- Motz , H. and Wise , H. 1960 . Diffusion and Heterogeneous Reaction III. Atom Recombination at a Catalytic Boundary . J. Chem. Phys. , 32 : 1893 – 1894 .
- Mozurkewich , M. 1986 . Aerosol Growth and the Condensation Coefficient for Water: A Review . Aerosol Sci. Technol. , 5 : 223 – 236 .
- Murphy , D. M. , Cziczo , D. J. , Froyd , K. D. , Hudson , P. K. , Matthew , B. M. , Middlebrook , A. M. , Peltier , R. E. , Sullivan , A. , Thomson , D. S. and Weber , R. L. 2006 . Single-Particle Mass Spectrometry of Tropospheric Aerosol Particles . J. Geophys. Res. , 111 : D23S32 doi:10.1029/2006JD007340.
- Raspet , R. , Slaton , W. V. , Arnott , W. P. and Mossmüler , H. 2003 . Evaporation-Condensation Effects on Resonant Photoacoustics of Volatile Aerosols . J. Atmos. Oceanic Technol. , 20 : 685 – 695 .
- Ruehl , C. R. , Chuang , P. Y. and Nenes , A. 2008 . How Quickly do Cloud Droplets Form on Atmospheric Particles? . Atmos. Chem. Phys , 8 : 1043 – 1055 .
- Seinfeld , J. H. 1986 . Atmospheric Chemistry and Physics of Air Pollution , 738 New York : John Wiley and Sons .
- Smith , J. D. , Cappa , C. D. , Drisdell , W. S. , Cohen , R. C. and Saykally , R. J. 2006 . Raman thermometry Measurements of Free Evaporation from Liquid Water Droplets . J. Amer. Chem. Soc. , 128 : 12892 – 12898 .
- Subramanian , R. , Roden , C. A. , Boparai , P. and Bond , T. C. 2007 . Yellow Beads and Missing Particles: Trouble Ahead for Filter-Based Absorption Measurements . Aerosol Sci. Technol. , 41 : 630 – 637 .
- Virkkula , A. , Ahlquist , N. C. , Covert , D. S. , Arnott , W. P. , Sheridan , P. J. , Quinn , P. K. and Coffman , D. J. 2005 . Modification, Calibration and a Field Test of an Instrument for Measuring Light Absorption by Particles . Aerosol Sci. Technol. , 39 : 68 – 83 .
- Wagner , P. E. 1982 . “ Aerosol Growth by Condensation ” . In Aerosol Microphysics II , Edited by: Marlow , W. J. 189 Berlin : Springer-Verlag .
- Winkler , P. M. , Vrtala , A. , Rudolf , R. , Wagner , P. E. , Riipinen , I. , Vesala , T. , Lehtinen , K. E. J. , Viisanen , Y. and Kulmala , M. 2006 . Condensation of Water Vapor: Experimental Determination of Mass and Thermal Accommodation Coefficients . J. Geophys. Res. , 111 ( D19 ) Article no. D19202, doi: 10.1029/2006JD007194.
- Xiong , J. Q. , Zhong , M. H. , Fang , C. P. , Chen , L. C. and Lippman , M. 1998 . Influence of Organic Films on the Hygroscopicity of Ultrafine Sulfuric Acid Aerosol . Environ. Sci. Technol. , 32 : 3536 – 3541 .
- Zientara , M. , Jakubczyk , D. , Kolwas , K. and Kolwas , M. 2008 . Temperature Dependence of the Evaporation Coefficient of Water in Air and Nitrogen Under Atmospheric Pressure: Study in Water Droplets . J. Phys. Chem. A. , 112 : 5152 – 5158 .