Abstract
Equipment consisting of annular denuders, a filter, and a polyurethane foam adsorbent was used for sampling 15 PAHs from the diluted emission from a heat-storing masonry heater. The denuder method was compared to the ISO 11338 method which was used for the sampling from hot and undiluted exhaust gas. The denuder method used with the exhaust dilution gave a realistic gas–particle distribution of PAHs in more atmospheric-like conditions compared to the sampling from undiluted exhaust gas where PAHs were almost totally in the gas phase. The results gained with the denuder method from the diluted exhaust are more relevant, e.g., from exposure and atmospheric processes point of view. The emissions from smoldering combustion conditions (SC) were compared with the emissions from normal combustion conditions (NC). The emission of each PAH was 7 to 14 times higher from SC than from NC, and the gas–particle distribution was shifted towards the particle phase due to increased condensation of PAHs. The PAHs could be divided into three groups based on their phase distributions. In the first group, PAHs existed mostly in the gas phase in both combustion cases; the vapor pressures of PAHs were lower than the saturation vapor pressures. In the second group, the gas phase was saturated and the concentration was almost the same in both combustion cases, whereas the particle phase concentration was higher in SC. In the third group, PAHs were mostly in the particle phase where the concentration was higher in SC.
1. INTRODUCTION
With global warming being of concern to governments and the public, there is pressure to substitute fossil fuels with renewable fuels like wood. Nowadays, biomass accounts for about 13–14% of the primary energy consumption in the world and about 20% in Finland. In Finland, about 20% of the energy used for space heating is provided by wood combustion, and about 15% of the wood used for energy production is combusted in small-scale combustion devices like fireplaces, stoves and boilers (CitationMattila et al. 2003).
Polycyclic aromatic hydrocarbons, PAHs, are formed in the oxygen-deficit area of a flame where polymerization rather than oxidation occurs (CitationFlagan and Seinfeld 1988). PAHs are of great interest because of their adverse health effects. Exposure to PAHs increases the risk of cancer in humans. In animal experiments, PAHs have also given raise to immunologic and reproductive effects (CitationBoström et al. 2002).
In many countries, residential wood combustion, RWC, has been found to cause a great proportion of emissions of PAHs. Yet it must be added that the comparison of emission estimates is rather difficult due to the different sets of compounds included and the uncertainties existing in the evaluations. In Finland, residential combustion, of which wood combustion has a very large share, was estimated to cause 64% (11 tons/year) of the total emission of four PAHs (benzo(a)pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene and indeno(1,2,3–cd)pyrene) in 2003 (CitationKoskinen et al. 2005). In Sweden it was estimated that RWC produces about 60% of the PAH-emissions (about 100 tons/year with a minor contribution from oil heating) whereas the share of transport and working machinery was about 30% (CitationBoström et al. 2002). In the United States, RWC has been evaluated to produce 33% (8860 tons/year) (CitationUS Environmental Protection Agency 1998) of the total emission of 16 US-EPA PAHs (CitationUS Environmental Protection Agency 1984). In the UK, RWC emission represents 1% (31 tons/year) of the total emission of 16 US-EPA PAHs, whereas the residential combustion of coal and wood together represent 17% of the total national emission (CitationLee et al. 2005).
Based on radiocarbon analysis of atmospheric PAHs, biomass combustion contributed about 50% of total PAHs in the atmosphere at a Swedish background site. In two southern European background sites, the contribution was lower, about 10% (CitationMandalakis et al. 2005).
In urban air, more than 100 PAH compounds have been identified. Their properties, like saturation vapor pressures, vary greatly. At room temperature in equilibrium, bicyclic species like naphthalene are present in the gas phase, compounds like coronene, formed of seven or more benzene rings, in the particle phase and intermediate PAHs like pyrene and anthracene in both phases (CitationSeinfeld and Pandis 1998). Partitioning depends also on, e.g., temperature, concentrations in the gas phase, and the chemical composition of the particles (CitationTurpin et al. 1999; CitationTsapakis and Stephanou 2005).
To be able to assess the fate of PAHs in the atmosphere, it is important to study the distribution of PAHs between the gas and the particle phase. This enables an evaluation of human exposure and health effects of PAHs, and choosing correct control strategies to prevent emissions of PAHs into the air (CitationGundel et al. 1995). Knowing the gas–particle distribution of PAHs is useful also in order to couple the emission measurement results with the PAH content of ambient air particles in source apportionment.
In the traditional PAH sampling methods, a filter is usually followed by an adsorbent in the sampling line. The particle phase organic species are collected on the filter, and the gas-phase PAHs on the adsorbent. The problem is that during the sampling, semi-volatile organics can desorb from the particle mass collected on the filter which leads to underestimation of the concentration of the particle-phase PAHs. This is called negative particle phase artefact. On the other hand, the gas-phase organics can adsorb on the filter material and/or on particle mass on the filter, causing correspondingly overestimation of the concentration which is called positive particle phase artefact (CitationGundel et al. 1995).
Another aspect to be considered in the PAH emission measurement is the dilution of the exhaust gas. Partitioning of semivolatiles depends on, e.g., the saturation vapor pressure (which depends on temperature) and the partial vapor pressure of the species. In hot and undiluted exhaust gas, semivolatiles are mostly in gaseous form, and they are left out of the particle sample drawn from these conditions. In the dilution, where clean dilution air at ambient temperature is mixed with the exhaust gas sample, the temperature of the sample is decreased lowering the saturation vapor pressure. On the other hand, dilution decreases the partial vapor pressure of the gaseous species. The decrease of the temperature affects more strongly the equilibrium between gas and particle phase, and the net effect is the increased condensation of vapors on particle surface. Further increase of the dilution of the exhaust gas at ambient temperature changes the equilibrium to evaporation phase. CitationLipsky and Robinson (2006) found out that changing the dilution ratio, DR, from 20:1 to 120:1 decreased PM2.5 mass emission from a wood stove by over 60% because the organic semivolatiles started to shift to the gas phase as the dilution was increased. This was verified with the measured emissions of artefact-corrected, particulate organic carbon which showed similar trend to that of PM2.5 (CitationLipsky and Robinson 2006).
In this study, we used the measurement methodology which utilizes annular denuders covered with an adsorbent in PAH sampling. The denuders are placed prior to the filter in the sampling line; thus gaseous species are collected before particles. In addition, polyurethane foam adsorbent, PUF, was used for the filter as a back-up sampler which collects the semivolatiles that possibly have evaporated from particles on the filter during the sample collection. This way the measurement results are not biased by the artefacts present in the traditional PAH-sampling methods. From the samples, 15 PAHs were analyzed: acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fle), phenantrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3–cd)pyrene (I123-cdP), dibenzo(a,h)anthracene (DahA), and benzo(g,h,i)perylene (BghiP). The compounds belong to 16 US-EPA PAHs (CitationUS Environmental Protection Agency 1984); naphthalene was excluded because its analysis is often semi-quantitative due to the higher volatility of the compound. Many of the compounds in the selection are carcinogenic and/or abundant in combustion emission. The measurements were done from diluted exhaust gas in order to have the heavier PAHs associated in the particles in the same way as they appear also in the atmosphere. To the authors' knowledge the emission data gained from RWC with this kind of method has not been widely published. Related to RWC, CitationSchauer et al. (2001) used denuder method for studying gas–particle partitioning theory, and CitationFan et al. (1996) used the method in a chamber study. Besides the characterization and the quantification of the PAH emissions from a Finnish heat storing masonry heater, our main goal was to study the gas–particle distribution of PAHs in the emissions of small scale wood combustion and compare two sampling methods: the method in which the sampling is done with two denuders, a filter and a PUF from exhaust gas diluted with a porous tube diluter, and a sampling method according to ISO 11338 (2003) for hot, undiluted exhaust gas.
2. EXPERIMENTAL METHODS
2.1. Combustion Appliance, Fuel, and Operation
The PAH measurement was one part of a complete set of emission measurements performed in a test cell for batch combustion appliances at the University of Kuopio in spring 2006. The facilities are described by CitationSippula et al. (2007) in greater detail. The emissions of CO, CO2, NOx, and several VOCs were measured and a wide range of fine particle measurements and analyses, including, for instance, particle mass, number, size distribution, morphology, OC/EC, ions, and ash were conducted. Results of these measurements have been published by CitationTissari et al. (2008) and CitationFrey et al. (2008).
One aim of the study was to investigate the influence of the combustion appliance operation on the emissions. Two combustion cases, normal combustion (NC) and smoldering combustion (SC) were studied. In the case of NC, the masonry heater was operated as well as possible, whereas in the case of SC, the combustion air of the same masonry heater was intentionally restricted. After the ignition batch of about 2 kg, which was similar in NC and SC, two or three batches of wood followed.
The firewood was dry split logs of seasoned birch. The total wood fuel load was almost the same (9 kg) in both combustion cases. There were differences in the log size, batch size, and in the piling of the logs between the two combustion cases: in NC, big logs were arranged compactly in the firebox, whereas in SC, smaller logs were piled crosswise. In SC, the restricted combustion air decreased the gasification rate of wood, but on the other hand the use of small logs and large batch (i.e., total area of wood logs) increased it. As a consequence, the combustion rates in NC and SC were almost similar, on average 7.8 kg wood/h in NC, and 7.1 kg wood/h in SC. However, pyrolysis gases were combusted clearly more incompletely in SC than in NC which could be observed as higher particle and gaseous emissions.
The combustion appliance used was a traditional Finnish masonry heater. It was made of soap stone, weighed about 800 kg and was equipped with a grate. This kind of a massive masonry heater stores the heat and releases it still after many hours of the dying of the fire. Operational practices and the combustion appliance are described in greater detail in CitationTissari et al. (2008).
2.2. Sampling Methods
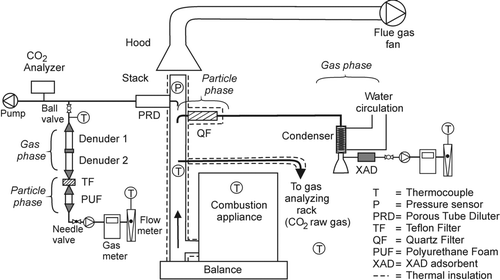
The emissions of 15 PAHs were investigated. Sampling was done with a denuder method, and in the NC test, in order to validate the method, sampling was done simultaneously with a standard method ().
In the NC tests, the sampling time was 80–85 minutes and it covered the whole test. In the SC tests, the sampling time was 35 minutes and it represented the first half of the combustion test. If the whole test would have been covered in SC, there would have been a risk of overloading the samplers. Likely the PAH concentration is in general higher in the beginning part of the combustion test because of the cold start; the organic content has been seen to be higher in the particles sampled in the beginning of the combustion tests (CitationTissari et al. 2007) and gaseous organics typically peak in the beginning of a batch, especially the first one. This may have caused some extra increase in the emissions from SC compared to NC.
In the denuder method, two 8-channel annular denuders made of glass (URG Corp.) and coated according to CitationGundel et al. (1995)with grounded Amberlite® XAD–4 (Supelco) were used in series for the collection of the gas phase compounds. The particle phase compounds were collected on a teflon filter (Pall, 47 mm). A PUF-plug was used to adsorb possible compounds which would volatilize from the particles on the filter (). The samples were drawn from the exhaust gas diluted with a porous tube diluter (CitationLyyränen et al. 2004) with a pump followed by a gas meter. The dilution ratios and the sample temperatures are presented in . In the NC tests, another sampling method, heated filter/condenser/adsorber method for stationary sources described in ISO 11338 (2003), was also used (). To describe the method briefly, the used sampling device was made of glass and consisted of a probe and a heated filter holder with a cup-shaped quartz filter (Munktell Filter Ltd.) inside, a condenser and a flask for the condensate collection, Amberlite® XAD-2 (Supelco) adsorbent and a pump, a gas meter and a rotameter. In this method, the sampling was done from the raw, undiluted gas.
TABLE 1 Average temperatures and dilution ratios in normal combustion (NC) and smoldering combustion (SC) tests
The adsorbents and the filters were pre-cleaned with solvent extraction before the sampling. The purity was assured by extracting some cleaned adsorbents and filters as samples.
2.3. Handling of the Samples and Analysis
PAHs were analyzed separately from each adsorbent and filter. The internal standard of deuterated PAHs (product Z–014J, AccuStandard Inc.) was used to define response factors and to prevent the effect of possible losses in the analysis results. Because the mass of a deuterated PAH in the spiked sample should be close to the mass of a native compound to be analyzed, different concentrations and amounts of an internal standard solution was used for spiking; for the ISO 11338 method samples, an aliquot of 1 ml of the internal standard solution of 5000 ng/ml was used, for the denuder method samples, 1 ml of solution of 1000 ng/ml and for blanks, 0.5 ml of solution of 1000 ng/ml.
For the denuder method samples, the extraction of the denuders was done with a 100 ml mixture of toluene and dichloromethane (1:1). The first denuder in line was extracted 3 times, the second twice. Each extraction was analyzed separately. The teflon filters of the denuder method sampling system were extracted with 10 ml of methanol:toluene (6:1) in ultrasonic bath for an hour (CitationJonker and Koelmans 2002). The PUF-adsorbents were extracted in the Soxhlet-apparatus with an aliquot of 200 ml of toluene:hexane (1:1) for 16 h. For ISO 11338 method samples, the filter and the XAD were both extracted in Soxhlet apparatus for 16 h, the filter with 200 ml of methanol:toluene (6:1) and the XAD with 200 ml of dichloromethane.
After the extraction, the denuder and the PUF extracts were evaporated to a volume of some milliliters with a rotary evaporator. The extracts of the filter samples were evaporated in the flux of nitrogen and with warming. After the first evaporation, solid matter was separated from the extracts of the filters and the denuders with activated alumina columns. The samples were eluted from the columns with 10 ml of dichloromethane. Finally, all the extracts were evaporated with nitrogen flux to the volume of approximately 2 ml and transferred to vials.
Concerning the particle phase samples sampled according to ISO 11338 method, also the nozzle and sampling line before the filter were rinsed with toluene and combined with filter extract. The solvent of the mixture was exchanged with hexane. The extract was concentrated with a rotary evaporator and fractionated in a silica gel column to pentane, dichloromethane:pentane and methanol fractions. The dichloromethane:pentane fraction was used for the PAH analysis. This fraction was concentrated with the rotary evaporator and the nitrogen evaporation after which it was transferred to a vial.
The sampling line after the filter was rinsed with toluene and combined with the condensate. Water and toluene were separated in a separation funnel, after which the toluene was dried with Na2SO4, filtered, and combined with adsorbent extract. The solvent of the mixture was exchanged with hexane. The water fraction separated by the funnel was liquid extracted with hexane in a separation funnel three times, after which the water fraction was discarded. The hexane fraction was dried with Na2SO4, filtered and combined to adsorbent extract in hexane. As the particle phase samples, also the gas phase samples were concentrated and fractionated. More information about the procedure is provided in ISO 11338-2 (2003).
All samples were analyzed with a GC–MS made by Agilent (6890N GC/5973inert MSD). For the separation of the compounds in GC, HP–5–MS (length 30 m, i.d. 0.250 mm, film thickness 0.25 μm) 5%-phenyl-methylpolysiloxane column was used. Usually splitless injection was used, and the injection volume was 2.0 μl. The injector temperature was 275°C. The GC oven temperature program was from 50°C (hold 1 min) to 150°C at 25°C/min, from 150°C to 280°C at 5°C/min (hold 9 min). The carrier gas was helium.
For the analysis, selective ion monitoring (SIM) mode was used. An external standard (product M–610A, AccuStandard Inc.) was used in every analysis. The external standard mixture included also the compounds in the internal standard. Possible sample losses occurred in the evaporation or in the other phases of the sample handling were managed by using the relation of the response factors of a native compound and a deuterated compound analyzed in the external standard mixture in the quantitative analysis (ISO 11338-2, 2003).
The detection limit of the GC–MS was determined to be 3 times and quantitation limit 10 times the standard deviation of the mass of analyte at a mass level not higher than 10 times the estimated detection limit (). The standard deviation was determined from the results of 8 analyses. For the compounds Fla, Pyr, Chr, BbF, BkF, BaP, I123-cdP, DahA, and BghiP, dilution of 1:100 of the standard was used for the determination, for the rest the dilution of 1:1000, respectively.
TABLE 2 The detection and quantitation limits for GC–MS
2.4. Quality Assurance
Blank values were used in assessing the reliability of analyzed results. If a corresponding blank value exceeded 20% of the analyzed mass, the result was discarded. The analysis result of previous extraction of the adsorbent in question, or extraction of unused filter or adsorbent was used as a blank value.
After the test period, a blank test was done for the porous tube diluter. Sampling was done in the same way as in the actual tests, only the ingoing sample was pure nitrogen instead of stack gas. The results of the test were used similarly as other blanks for denuder method, except that the proportion was calculated from the concentrations of the gas and particle phase.
3. RESULTS AND DISCUSSION
3.1. Collection Efficiency of the Denuders
The collection efficiency of the denuders is a fundamental aspect in the determination of the phase distribution. Break through of the gas phase compounds could skew the phase distribution since these gas phase compounds could be adsorbed on the particle material collected on the filter or on the PUF adsorbent.
The break through has been studied with the help of masses gained in the subsequent denuders (e.g., CitationGundel et al. 1995). The proportions of the masses of gaseous PAHs collected on the first denuder are presented in . The compounds compete for the adsorption sites. According to Fick's law of diffusion, the diffusion flux depends on the concentration gradient and the diffusion coefficient of a substance. Since the lighter PAHs like Acy and Ace are more abundant in the gas phase, they are probably adsorbed readily on the resin surface of the first denuder, and the adsorption sites farther from the entering point are left to less abundant compounds. Of the studied compounds that were mainly in the gas phase, almost the entire gaseous fraction (on average, 95% of mass) was adsorbed on the first of the two denuders. Of the compounds for which 10–50% of the mass was in the gas phase, the corresponding fraction was 72%. The diffusion coefficients of the PAHs which exist rather in the particle phase than in the gas phase are usually lower than those of lighter PAHs, although some exceptions exist (CitationUS Environmental Protection Agency 1996).
TABLE 3 The proportion of the gas phase emission collected on the first denuder in the three repeats of normal combustion (NC) and smoldering combustion (SC) tests [%]. The results which were rejected due to break through are marked with *. If a cell is blank, the analysis result has been below the detection limit
Because of these differences in the properties and the behavior of the studied PAHs another approach for studying the break through was made. Firstly, an equal fraction of the mass of a compound was estimated to break through both the first and the second denuder. Secondly, the mass broken through the second denuder was compared to the mass of the compound found in particle phase. If more than 10% of the particle phase mass of the compound could be estimated to be caused by the break through, the result was not included in the calculations of phase distributions or average emissions. In NC tests, some compounds were estimated to be affected by the break through ().
Dilution seemed to affect to some extent the distribution of the compounds between the two denuders; for example in the third repeat of SC, the proportions of PAHs of higher mole masses adsorbed on the first denuder were lower than in the previous two repeats. The dilution ratio was clearly higher, 176:1, in the third SC test as compared to the previous two (86:1 and 69:1) and the concentration of PAHs was lower after the dilution of the exhaust gas. Thus the increased penetration through the first denuder to the second due to occupancy of adsorption sites is not very probable.
3.2. The Effectiveness of the Denuder Extractions
The effectiveness of the extractions was studied by comparing the PAH masses analyzed from the successive extractions. The proportion of the mass eluted in the first extraction was 96% on average and at least 87% for all the other compounds but DahA which was hardly at all adsorbed on the denuders. Several extractions were needed, however, not so much to improve the intake but to clean the denuders for the following sampling.
3.3. Particle Losses on Denuders
Diffusion of particles on the surface of the denuders would cause error to the determination of the gas–particle distribution. The denuders have been designed so that in the case of laminar flow, there should be no intake of the particles on the surfaces; compared to gas molecules, particles have diffusion coefficients several orders of magnitude lower (CitationFebo, Perrino, and Allegrini 1999).
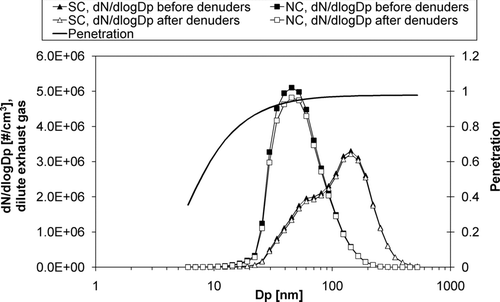
The effect of Brownian diffusion on particle transmission was studied with modelling, the principles of which have been presented by CitationTemime-Roussel et al. (2004). For modelling, the particle number distribution measured with a Fast Mobility Particle Sizer, FMPS (TSI Inc.) from diluted exhaust was used. The loss of total particle number was 3–6%, being more significant for the small particles. The proportion of the particles penetrating through the denuders was more than 0.94 for mode sizes both in NC and SC (). Based on the modelling results, particle losses were low and thus acceptable. CitationTemime-Roussel et al. (2004) studied similar denuders and found out that the transmission efficiencies were over 90% in number (for the particles from 0.04 nm to 8.27 μm in aerodynamic diameter) and over 80% in mass (no pre-cut) for sample flows of 17 and 34 l/min.
FIG. 2 Particle number distributions in diluted exhaust gas of normal combustion (NC) and smoldering combustion (SC) before and after the denuders, and size-segregated penetration of particles through the denuders. As distributions before the denuders, the actual particle number distributions measured from the diluted exhaust gas with the FMPS were used; the distributions after denuders have been modelled.
3.4. The Proportions of the Particle-Phase Emission Adsorbed on PUFs
Of the compounds existing in the particle phase, only the lightest compounds were adsorbed on PUF; it was the most important for the collection of Phe, Ant, Fla, and Pyr. Of the particle phase emission of Phe and Ant, usually about 90% was adsorbed on the PUF. It was the same case for Ant and Fla in NC, but in SC their proportion on PUF were only 6–26%.
The back-up adsorbent plays an important role in the determination of particle phase emission. Without PUF, the analyzed particle phase PAH emission (15 PAHs) would have been, on average, 80% of the true value.
3.5. PAH Emission Factors
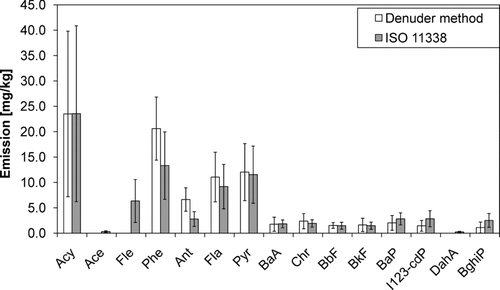
The average emissions of 15 PAHs measured with the denuder method are presented in . As expected, PAH emissions were higher from SC than from NC; the emissions of every measured PAH were 7–14 times higher. The emissions of Acy and I123-cdP increased the most.The gas phase dominates the total PAH emission from both NC and SC; the main components were Acy, Fle, Phe, Ant, Fla, and Pyr. In the emission from NC, the gas phase PAH emission was more evenly distributed between the compounds, whereas in SC, Acy clearly dominated.
TABLE 4 The emission factors of 15 PAHs in the gas phase and in the particle phase at 24–25°C [mg/kg]
The particle phase emission from SC was dominated by Fla and Pyr, while in the NC emission the main component was Pyr followed by many compounds with even proportions. Compared to NC, in SC the particle phase emission of lighter PAHs was emphasized.
Acy, Phe, Fla, and Pyr have been found to dominate PAH emission from RWC in several studies; the profile of PAH emission from RWC in this study is fairly similar to the results in studies by, e.g., CitationBoman et al. (2005b), CitationSchauer et al. (2001), and CitationMcDonald et al. (2000).
The emission of 11 particle phase PAHs reported for NC (see ) contributed, on average, 0.7% of particle mass emission from NC and 2.1% of particle mass emission from SC. The particle mass samples were for PM1 whereas there was no pre-cut for PAH sampling. This has probably no major effect on the result since PAHs are mostly associated with particles which have aerodynamic diameter smaller than 1 μm; according to results of CitationHays et al. (2003), the modes of size distributions of selected PAHs measured in the emissions from different combinations of combustion appliances and wood fuels were between 100–400 nm.
CitationHays et al. (2003) mostly measured considerably lower particle phase PAH emissions from residential fireplace and non-catalytic woodstove than those presented in . This may be partly due to their relatively high dilution ratios, which, as previously discussed, induce shifting of the semivolatiles to gaseous phase. Instead, emissions of some PAHs measured by CitationHedberg et al. (2002) were higher than the emissions from SC in the present study. Emission values published by CitationMcDonald et al. (2000) and CitationSchauer et al. (2001) are somewhat lower compared to the emissions from NC, as are also the emissions from different batch combustion appliances excluding sauna stove measured in the field by CitationTissari et al. (2007). Considering the methods used in other studies, CitationHays et al. (2003) analyzed PAHs from the stages of electrical low pressure impactor, ELPI (Dekati Ltd.), whereas CitationHedberg et al. (2002), CitationMcDonald et al. (2000), CitationSchauer et al. (2001), and CitationTissari et al. (2007) all sampled with a filter followed by a PUF adsorbent or PUF/XAD/PUF adsorbent. In all of these studies, sampling was done from the diluted exhaust.
Nevertheless, one must consider that comparison of the emission factors is not straightforward since the emissions are influenced by, for example, the combustion appliance, fuel and operational practices. Also the sampling equipment affects the measurements results. In addition, the variation of PAH emissions even in the tests conducted with same procedure can be relatively high due to the nature of batch combustion process.
The denuder method was compared to ISO 11338 sampling method in the NC tests. The total emissions of each PAH determined with the two methods were well comparable ().
3.6. Gas-Particle Distribution
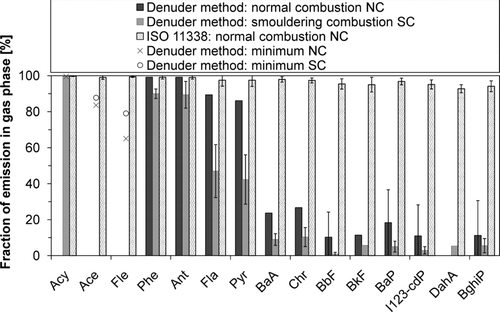
The proportion of the gas phase emission of each PAH was lower in SC as compared to NC (, bars with “denuder method” in title). The gas–particle distribution of total PAH emission was, on average, 88%/12% in NC and 77%/23% in SC. The difference between the two combustion cases is particularly great for Fla and Pyr of which in NC more than 80% but in SC less than 50% was in the gas phase. Since these compounds are major components, they have a great influence on the gas–particle distribution of total PAH emission. CitationBoman et al. (2005a) found that the phase distribution of 12 PAHs was influenced by the sampling conditions (mainly temperature, which ranged from 40 to 80°C); among these compounds were Fle, Phe, Ant, Fla, and Pyr. In our study, these compounds also showed great variability in their phase distribution between the two combustion cases. On the other hand, many of the PAHs which we found in both phases were found only in the particle phase by CitationBoman et al. (2005a). This could be due to low dilution; CitationBoman et al. (2005a) used dilution ratios from 3:1 to 7:1.
FIG. 4 The average gas particle distribution of PAHs determined with the denuder method in NC and SC and with ISO 11338 in NC. The standard deviation (error bar) is represented if n = 3; if not enough accurate data was available the minimum fraction in the gas phase has been estimated on the basis of the contaminated samples.
Numerous chemical reaction pathways and physical processes are present in the formation of PAHs in combustion (reviewed by CitationRichter and Howard, 2000) and in their conversion in the cooling and the dilution of the exhaust gas (e.g., CitationHays et al. 2003; CitationLipsky and Robinson 2006). Organic compounds may, for example, be driven to particle phase by adsorption, absorption, nucleation, chemical reaction or condensation.
The gas-particle distribution of different species in the exhaust gas is dynamic and adapts to the prevailing circumstances. Equations for gas/particle partitioning have been presented for example by CitationPankow (1999). Partitioning dominated by adsorption is dependent on, e.g., surface concentration of adsorption sites, surface concentration of particles, temperature, and properties of compounds like vapor pressure (CitationPankow 1999) whereas condensation, for one, occurs when the partial vapor pressure exceeds the saturation vapor pressure of the species (CitationHinds 1999).
Condensation probably explains most of the differences in phase distributions between the combustion cases. The saturation vapor pressures and partial vapor pressures of studied PAHs in the temperature of the diluted exhaust gas are presented in . Saturation vapor pressures at 25°C given in ISO 11338 (2003), and estimated with a UNIFAC-based group contribution method (CitationAsher et al. 2002; CitationAsher and Pankow 2006) were used. At 25°C, UNIFAC-estimations seem to be lower than the saturation vapor pressures given in ISO 11338. Nevertheless, the determination or estimation of very low saturation pressures is not straightforward in general.
FIG. 5 Vapor pressures of PAHs in diluted exhaust gas in the temperature of 25°C in normal combustion (NC) and smoldering combustion (SC).
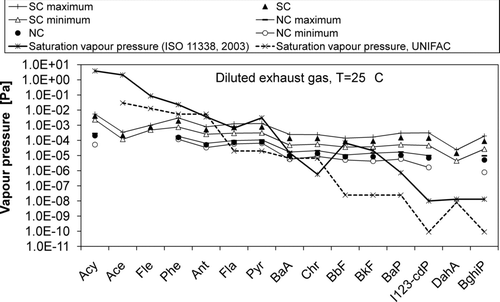
In the solution consisting of several chemical components, the saturation vapor pressure of the species is dependent on the mole fraction of the species in the solution (Raoult's law). Because of the unknown properties of the condensed phase of combustion aerosols (e.g., composition and activities) we used the pure compounds assumption in our calculations.
For compounds from Fla to BkF, the vapor pressures both in SC and NC were close to the saturation vapor pressures (ISO 11338-2, 2003) or even exceeded them, whereas the vapor pressures of the lighter compounds stayed below the saturation pressures. For heavier compounds, the saturation vapor pressures were exceeded clearly. For every compound, the vapor pressure was higher in SC than in NC; condensation was evidently more present in SC driving PAHs to the particle phase. This explains well the findings concerning gas–particle distributions ().
Besides condensation, adsorption also drives PAHs to the particle phase since there was low concentration of PAHs in the particle phase even if saturation had not been achieved. Nevertheless, if adsorption would have been the reason for the shift of the phase distribution towards the particle phase in SC, the particle surface area could be one thing to consider. The average surface area of particles in the size range 5.6–560 nm measured with FMPS in wood smoke ranged between 3.34 × 106–5.23 × 106μm2/cm3 in NC and between 9.08 × 106–1.16 × 107μm2/cm3 in SC. However, surface area against particle mass (PM1) was higher for NC. On the other hand, the surface area has been calculated as if the particles were perfect spheres, whereas the particles were here agglomerates, and the form of the particles probably differed remarkably in NC and SC due to increased condensation in SC. Thus it is rather difficult to compare the particle surface areas.
The gas-particle distributions determined with the denuder method resembled the phase distribution of PAHs determined in the ambient air (e.g., CitationTsapakis and Stephanou 2005; CitationPossanzini et al. 2004). By sampling with the denuder method from the diluted exhaust gas, more relevant data for, e.g., the evaluation of human exposure and health effects of PAHs can be produced.
In order to promote the possible further comparison with the ambient air phase distributions, the partitioning coefficients for PAHs from two SC tests were calculated as described in CitationYamasaki, Kuwata and Miyamoto (1982):
FIG. 6 Partitioning coefficient values Kp in diluted exhaust gas at 25°C in two smoldering combustion (SC) tests (PM1 concentration in diluted exhaust gas in SC2 20.1 mg/m3 and in SC3 2.1 mg/m3, dilution ratios 69:1 and 176:1).
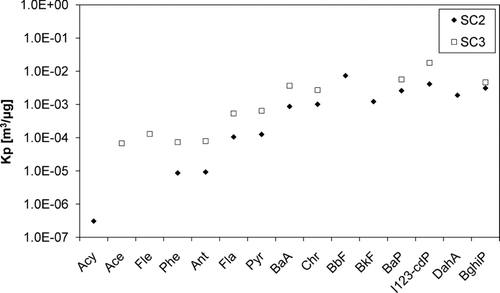
The gas–particle distribution was completely different in the samples taken with the denuder method and ISO 11338 method (). The reason for this is the dilution and the condensation of the semivolatiles within. In the dilution, as the temperature decreases the required vapor pressure for the saturation decreases more steeply than the actual partial pressure of the species. Thus, at some dilution ratio, the saturation pressure of the semivolatile species is reached. This leads to the condensation of the semivolatiles and thus the gas–particle distribution of PAHs shifts towards the particle phase in the diluted exhaust.
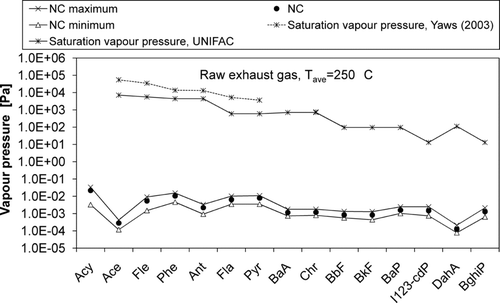
Vapor pressures of PAHs in raw gas in NC were clearly below the saturation pressures of pure compounds (), which explains the fact that over 90% of the emission of each PAH was in the gas phase when measured with the ISO 11338 method (). The saturation vapor pressures of the PAHs presented in were either taken from the literature (CitationYaws, 2003) or estimated with a UNIFAC–based method. In case of the UNIFAC-estimations, however, it should be borne in mind that the method has been developed and tested mostly for temperatures below 250°C (CitationAsher et al. 2002). This naturally results in uncertainties in the predicted values. The reasonable agreement between the UNIFAC-predictions and the values reported by CitationYaws (2003) (), however, gives some confidence on the predictions.
FIG. 7 Vapor pressures of PAHs in raw exhaust gas in the temperature of 250°C in normal combustion (NC).
By their phase distribution, the PAHs could be roughly divided into three groups (). The groups are defined by the physical properties of the compounds in (after CitationLide, 2005). In the first group, the light PAHs are not at all saturated and thus these compounds are almost totally in the gas phase (for example Acy), and the concentration is considerably higher in the emission from SC due to more incomplete combustion. In the second group there are the greatest differences in the gas–particle distribution between the NC and the SC; anyhow, the compounds are either saturated or close to the saturation, and that is why the concentration in the gas phase is fairly similar in the both combustion cases. In the SC, however, the higher concentration can be seen in the particle phase (for example Chr). In the third group, which consists of the heaviest PAHs, compounds are almost totally in the particle phase in both combustion cases, but the concentration is clearly higher in the SC once again (for example I123-cdP). The reason for the differences in gas phase concentrations between the combustion cases can be the low diffusion constants of these heavy compounds; thus longer time is needed for them to reach the equilibrium.
FIG. 8 Gas phase concentration and total concentration of example compounds of three groups of PAHs in the NC and in the SC conditions (bars) and the saturation vapor pressures of Acy, Ace, Fle … at 25°C (stars).
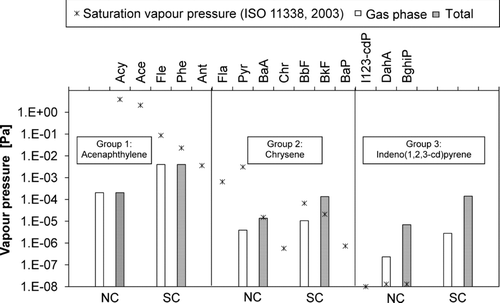
TABLE 5 Physical properties of grouped PAHs (CitationLide, 2005)
4. CONCLUSIONS
The denuder method proved to be suitable for PAH emission measurements from residential wood combustion. For successful use of the method, it is important not to overload the samplers, and to consider the possible contamination of the samples.
More relevant data on the gas–particle distribution of PAHs can be achieved with denuder method than with traditional methods. The denuder method can be used for sampling from the diluted exhaust gas where the phase distribution is more similar to the one reached in the atmosphere as the exhaust gas cools down. Also possible artefacts present in the sampling methods where adsorbent is placed after the filter can be avoided.
Emissions of each PAH from intentionally created smoldering combustion condition (SC) were 7–14 times higher compared to the emissions from normal combustion condition (NC). Additionally, the gas–particle distribution was shifted to particle phase in SC, caused by the increased condensation due to higher amount of PAH vapors.
The gas–particle distribution of PAHs can be qualitatively explained by dividing the compounds into three groups. The first group contains the lighter PAHs (C10–C14) which are far from saturation concentration of pure compounds in both combustion cases and thus almost totally in the gas phase. The second group contains the PAHs (C16–C20) which have very similar concentrations in the gas phase in both combustion cases because they are saturated or near the saturation concentration. However, the concentration in the particle phase is higher in SC. The third group includes the heavy PAHs (C22) which are mostly in the particle phase in both combustion cases, but in SC in higher concentration. The gas phase species can be a consequence from the slow diffusion of the heavy PAH molecules.
In the future, the denuder method can be a useful tool in studying the gas–particle distribution in dilution with different dilution ratios, or in studying the change in the phase distribution as the aerosol ages in the chamber. The emission factors and the gas-particle distributions gained with this method can be used in emission inventories and in source apportionment studies.
Acknowledgments
The funding from Graduate School in Physics, Chemistry, Biology and Meteorology of Atmospheric Composition and Climate Change, Tekes, The Finnish Funding Agency for Technology and Innovation, and Ministry of the Environment is gratefully acknowledged. We thank the staff of Fine Particle and Aerosol Technology Laboratory and Juhani Tarhanen from Department of Environmental Science, University of Kuopio for all the help.
Notes
a : n = 2.
b : there was not enough valid data to calculate averages.
REFERENCES
- Asher , W. E. , Pankow , J. F. , Erdakos , G. B. and Seinfeld , J. H. 2002 . Estimating the Vapor Pressures of Multi-Functional Oxygen-Containing Organic Compounds Using Group Contribution Methods . Atmos. Environ. , 36 : 1483 – 1498 .
- Asher , W. E. and Pankow , J. F. 2006 . Vapor Pressure Prediction for Alkenoic and Aromatic Organic Compounds by a UNIFAC-Based Group Contribution Method . Atmos. Environ. , 40 : 3588 – 3600 .
- Boman , C. , Nordin , A. , Westerholm , R. and Pettersson , E. 2005a . Evaluation of a Constant Volume Sampling Setup for Residential Biomass Fired Appliances—Influence of Dilution Conditions on Particulate and PAH Emissions . Biomass Bioenergy , 29 : 258 – 268 .
- Boman , C. , Pettersson , E. , Nordin , A. , Westerholm , R. and Boström , D. 2005b . “ Gaseous and Particulate Emissions from Residential Wood Log and Pellet Stoves—Experimental Characterization and Quantification ” . In Particulate and Gaseous Emissions from Residential Biomass Combustion , Umeå : C. Boman, Umeå University .
- Boström , C.-E. , Gerde , P. , Hanberg , A. , Jernström , B. , Johansson , C. , Kyrklund , T. , Rannug , A. , Törnqvist , M. , Victorin , K. and Westerholm , R. 2002 . Cancer Risk Assessment, Indicators, and Guidelines for Polycyclic Aromatic Hydrocarbons in the Ambient Air . Environ. Health Perspect. , 110 : 451 – 488 .
- Fan , Z. , Kamens , R. M. , Hu , J. , Zhang , J. and McDow , S. 1996 . Photostability of Nitro-Polycyclic Aromatic Hydrocarbons on Combustion Soot Particles in Sunlight . Environ. Sci. Technol. , 30 : 1358 – 1364 .
- Febo , A. , Perrino , C. and Allegrini , I. 1999 . “ Selective Gas and Particle Sampling: Diffusion Denuders ” . In Gas and Particle Phase Measurements of Atmospheric Organic Compounds , Edited by: Lane , D. A. 127 – 175 . Amsterdam : Gordon and Breach Science Publishers .
- Flagan , R. C. and Seinfeld , J. H. 1988 . Fundamentals of Air Pollution Engineering. 215 Prentice Hall , NJ
- Frey , A. K. , Tissari , J. , Saarnio , K. M. , Timonen , H. J. , Tolonen-Kivimäki , O. , Aurela , M. A. , Saarikoski , S. K. , Makkonen , U. , Hytönen , K. , Jokiniemi , J. , Salonen , R. O. and Hillamo , R. E. J. 2008 . Chemical Composition and Mass Size Distribution of Fine Particulate Matter Emitted by a Small Masonry Heater . Accepted to Boreal Environ. Res. ,
- Gundel , L. A. , Lee , V. C. , Mahanama , K. R. R. , Stevens , R. K. and Daisey , J. M. 1995 . Direct Determination of the Phase Distributions of Semi-Volatile Polycyclic Aromatic Hydrocarbons Using Annular Denuders . Atmos. Environ. , 94 : 1719 – 1733 .
- Hays , M. D. , Smith , N. D. , Kinsey , J. , Dong , Y. and Kariher , P. 2003 . Polycyclic Aromatic Hydrocarbon Size Distribution in Aerosols from Appliances of Residential Wood Combustion as Determines by Direct Thermal Desorption-GC/MS . J. Aerosol Sci. , 34 : 1061 – 1084 .
- Hedberg , E. , Kristensson , A. , Ohlsson , M. , Johansson , C. , Johansson , P.-Å. , Swietlicki , E. , Vesely , V. , Wideqvist , U. and Westerholm , R. 2002 . Chemical and Physical Characterization of Emissions from Birch Wood Combustion in a Wood Stove . Atmos. Environ. , 36 : 4823 – 4837 .
- Hinds , W. C. 1999 . Aerosol Technology. Properties, Behaviour, and Measurement of Airborne Particles. , 278 New York : John Wiley & Sons .
- ISO 11338–1:2003(E) . Stationary Source Emissions—Determination of Gas and Particle—Phase Polycyclic Aromatic Hydrocarbons—Sampling , Switzerland
- ISO 11338–2:2003(E) . Stationary Source Emissions—Determination of Gas and Particle—Phase Polycyclic Aromatic Hydrocarbons—Sample Preparation, Clean-Up and Determination , Switzerland
- Jonker , M. T. O. and Koelmans , A. A. 2002 . Extraction of Polycyclic Aromatic Hydrocarbons from Soot and Sediment: Solvent Evaluation and Implications for Sorption Mechanism . Environ. Sci. Technol. , 36 : 4107 – 4113 .
- Koskinen , P. , Silvo , K. , Mehtonen , J. , Ruoppa , M. , Hyytiä , H. , Silander , S. and Sokka , L. 2005 . Preliminary Assessment of Releases of Certain Hazardous Substances in Finland. , The Finnish Environment 801, Finnish Environment Institute. In Finnish, English abstract. Edita Prima Ltd., Helsinki
- Lee , R. G. M. , Coleman , P. , Jones , J. L. , Jones , K. C. and Lohmann , R. 2005 . Emission Factors and Importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the Domestic Burning of Coal and Wood in the U.K. . Environ. Sci. Technol. , 39 : 1436 – 1447 .
- Lide , D. R. , ed. 2005 . CRC Handbook of Chemistry and Physics , 3-4 – 3-448 . London : Taylor & Francis Group .
- Lipsky , E. M. and Robinson , A. L. 2006 . Effects of Dilution on Fine Particle Mass and Partitioning of Semivolatile Organics in Diesel Exhaust and Wood Smoke . Environ. Sci. Technol. , 40 : 155 – 162 .
- Lyyränen , J. , Jokiniemi , J. , Kauppinen , E. I. , Backman , U. and Vesala , H. 2004 . Comparison of Different Dilution Methods for Measuring Diesel Particle Emissions . Aerosol Sci. Technol. , 38 : 2 – 23 .
- Mattila , L. , Saastamoinen , J. , Helynen , S. , Hämäläinen , J. , Mäkinen , T. , Lohiniva , E. , McKeough , P. , Wolff , J. , Nordman , H. , Tuunanen , J. , Sipilä , K. and Tuhkanen , S. 2003 . “ Energy Production Technologies ” . In Energy Vision 2030 for Finland , Edited by: Kara , M. , Hirvonen , R. , Mattila , L. , Viinikainen , S. , Tuhkanen , S. and Lind , I. 67 – 124 . Helsinki : VTT Energy, Edita Prima Ltd. .
- Mandalakis , M. , Gustafsson , Ö. , Alsberg , T. , Egebäck , A.-L. , Reddy , C. M. , Xu , L. , Klanova , J. , Holoubek , I. and Stephanou , E. G. 2005 . Contribution of Biomass Burning to Atmospheric Polycyclic Aromatic Hydrocarbons at Three European Background Sites . Environ. Sci. Technol. , 39 : 2976 – 2982 .
- McDonald , J. , Zielinska , B. , Fujita , E. , Sagebiel , J. , Chow , J. and Watson , J. 2000 . Fine Particle and Gaseous Emission Rates from Residential Wood Combustion . Environ. Sci. Technol. , 34 : 2080 – 2091 .
- Pankow , J. F. 1999 . “ Fundamentals and Mechanisms of Gas/Particle Partitioning in the Atmosphere ” . In Gas and Particle Phase Measurements of Atmospheric Organic Compounds , Edited by: Lane , D. A. 25 – 37 . Amsterdam : Gordon and Breach Science Publishers .
- Possanzini , M. , Di , Palo, V. , Gigliucci , P. , Tomasi Scianò , M. C. and Cecinato , A. 2004 . Determination of Phase-Distributed PAH in Rome Ambient Air by Denuder/GC-MS Method . Atmos. Environ. , 38 : 1727 – 1734 .
- Richter , H. and Howard , J. B. 2000 . Formation of Polycyclic Aromatic Hydrocarbons and Their Growth to Soot—A Review of Chemical Reaction Pathways . Prog. Energy Combust. Sci. , 26 : 565 – 608 .
- Schauer , J. , Kleeman , M. , Cass , G. and Simoneit , B. 2001 . Measurement of Emissions from Air Pollution Sources. 3. C1–C29 Organic Compounds from Fireplace Combustion of Wood . Environ. Sci. Technol. , 35 : 1716 – 1728 .
- Seinfeld , J. H. and Pandis , S. P. 1998 . Atmospheric Chemistry and Physics. , 744 New York : John Wiley & Sons, Inc. .
- Sippula , O. , Hytönen , K. , Tissari , J. , Raunemaa , T. and Jokiniemi , J. 2007 . Effect of Wood Fuel on the Emissions from a Top-Feed Pellet Stove . Energy Fuels , 21 : 1151 – 1160 .
- Temime-Roussel , B. , Monod , A. , Massiani , C. and Wortham , H. 2004 . Evaluation of an Annular Denuder for Atmospheric PAH Partitioning Studies—2: Evaluation of Mass and Number Particle Losses . Atmos. Environ. , 38 : 1925 – 1932 .
- Tissari , J. , Hytönen , K. , Lyyränen , J. and Jokiniemi , J. 2007 . A Novel Field Measurement Method for Determining of Fine Particle and Gas Emissions from Residential Wood Combustion . Atmos. Environ. , 41 : 8330 – 8344 .
- Tissari , J. , Lyyränen , J. , Hytönen , K. , Sippula , O. , Tapper , U. , Frey , A. , Saarnio , K. , Pennanen , A. S. , Hillamo , R. , Salonen , R. O. , Hirvonen , M.-R. and Jokiniemi , J. 2008 . Fine Particle and Gaseous Emissions from Normal and Smouldering Wood Combustion in a Conventional Masonry Heater . Atmos. Environ. , 42 : 7862 – 7873 .
- Tsapakis , M. and Stephanou , E. G. 2005 . Occurence of Gaseous and Particulate Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: Study of Sources and Ambient Temperature Effect on the Gas/Particle Concentration and Distribution . Environ. Pollut. , 133 : 147 – 156 .
- Turpin , B. J. , Liu , S.-P. , McMurry , P. H. and Eisenreich , S. J. 1999 . “ Definitive Measurement of Semivolatile PAHs with a Diffusion Separator: Design and Investigation of Sampling Artifacts in Filter-Adsorbent Samplers ” . In Gas and Particle Phase Measurements of Atmospheric Organic Compounds , Edited by: Lane , D. A. 369 – 392 . Amsterdam : Gordon and Breach Science Publishers .
- US Environmental Protection Agency . 1984 . Guidelines Establishing Test Procedures for the Analysis of Pollutants Under the Clean Water Act: Method 610. Polynuclear Aromatic Hydrocarbons . Fed Reg , 49 ( 209 ) : 43344 – 43352 . (40 CFR Part 136)
- US Environmental Protection Agency . 1996 . Soil Screening Guidance Technical Background Document , : 137 – 139 . EPA/540/R–95/128
- US Environmental Protection Agency . 1998 . 1990 Emission Inventory of Section 112(c)(6) Pollutants: POM, 2,3,7,8–TCDD/2,3,7,8–TCDF, PCBs, Hexachlorobenzene, Mercury, and Alkylated Lead. Final Report , US Environmental Protection Agency, Emission Factor and Inventory Group (MD–14) and Visibility and Ecosystem Protection group (MD–15). http://www.epa.gov/ttn/atw/112c6/final2.pdf (cited 21.2.2006)
- Yamasaki , H. , Kuwata , K. and Miyamoto , H. 1982 . Effects of Ambient Temperature on Aspects of Airborne Polycyclic Aromatic Hydrocarbons . Environ. Sci. Technol. , 16 : 189 – 194 .
- Yaws , C. L. 2003 . Yaws' Handbook of Thermodynamic and Physical Properties of Chemical Compounds , Knovel .