Abstract
We investigated the elastically scattered light of single pollen in a large space angle. Scattering pattern of birch, elm, hazel, sunflower, pine, ragweed, chestnut, and willow pollen were recorded in the backward and sideward hemisphere. Characteristic features could be detected, that may help identify the pollen by light scattering. We also show scattering pattern of a willow pollen rotated relative to the laser beam, and the relations between the sideward and backward scattering hemispheres.
INTRODUCTION
Every moment in our life we have an interaction with surrounding aerosols—in our home, in the car on the way to and at the work place, even in the hospital. Only extremely clean rooms are nearly free from airborne microparticles. Dependent on their size (smaller than 10 μ m) aerosols can be inhaled to deposit down in the alveolar region; aerosols bigger in diameter can be filtered in the head airway region, which includes the nose, mouth, pharynx, and larynx (CitationHinds 1999). Some of the aerosols we inhale, like sea salt particles or pharmaceutical sprays, can be healthy; others, like mold spores or some bacteria, can cause serious harm and lifelong damage to our health. Aerosols are an inherent part of our environment.
One relatively new but increasing problem are pollen. A great number of pollen or fragments of pollen dispersed in the air from spring until late summer are present even in the urban air of big cities. More and more people are suffering from allergies caused by pollen or similar particles of biological origin. A link between grass pollen and asthma was observed by CitationTaylor et al. (2002). Especially ragweed (Ambrosia) is identified as dangerous for people suffering from hay fever because of severe allergic shocks caused by the pollen of this flowering plant. Measurement, control, identification, and characterization of bioaerosols is of great practical importance to our health. Therefore, it is highly desirable to have a fast, reliable, and sensitive system to detect and identify in real time and in-situ bioaerosols. The classical method still widely used to identify airborne biological particles such as pollen is the identification by optical or scanning electron microscopy.
Laser-based methods were developed in the last decade to identify bioaerosols fast and reliably. It was shown that it is possible to distinguish different pollen “signatures” in Raman spectra (CitationLaucks et al. 2000) by using a 785 nm or 514 nm laser as excitation source. Similar work was done with Raman microscopy using additionally a He-Ne laser with an excitation wavelength of 632 nm (Ivleva et al. 2004).
Much effort was invested in the use of natural fluorescence as a fast and sensitive method. The spectral distribution of intrinsic fluorescence of biological aerosol particles was investigated by several groups (Pan et al. 1999; CitationHill et al. 1999; CitationHill et al. 2001; CitationPan et al. 2003a, Citation2003b; CitationEversole et al. 2001; CitationSivaprakasam et al. 2004). A fundamental problem with the intrinsic fluorescence is that the various particles contain more or less the same fluorophores. Theoretical investigations (CitationWeigel et al. 2004; CitationSchulte et al. 2004) have shown that the angular distribution of inelastically scattered light caused by homogeneously distributed molecules depends on the morphology of the particle.
Experimental results have shown that the angular distribution of the fluorescence from dyed pollen indeed depends on the shape of the particles and their orientation relative to the incident laser beam (CitationSurbek et al. 2008). The angular distribution of elastically scattered light is more complicated than that of fluorescence and contains more information. Therefore, a combination of these two methods may increase the specificity of light scattering techniques. In this article we investigated the two-dimensional scattering pattern generated by large angle elastic light scattering on a variety of different pollen. The angular distribution of polarized elastically scattered light for various particles was already investigated (CitationPhilips et al. 1972). The scattering pattern of various agglomerates of polystyrene latex spheres or single rod-shaped silicon dioxide fibers were investigated by CitationKaye et al. (1992), CitationHoller et al. (1998), CitationSecker et al. (2000), and CitationMishchenko et al. (1996). CitationHoller et al. (1999) show how elastic light scattering pattern changes when droplets evolve into clusters. A further advancement was the use of two-dimensional optical scattering (TAOS) and later the large-angle-range two-dimensional optical scattering (LA-TAOS) method. With the LA-TAOS technique the elastically scattered light of a particle could be recorded for almost the complete hemisphere (either the backward or the sideward hemisphere). The application of the LA-TAOS technique to the observation of airborne and respirable aerosols is a promising tool for identification and characterization (CitationSindoni et al. 2006; CitationPan et al. 2003; CitationFernandes et al. 2006; CitationChang et al. 2007; CitationAptowicz et al. 2005; CitationAptowicz et al. 2006; CitationAuger et al. 2007).
We applied the large-angle-range two-dimensional optical scattering techniques to get elastic scattering pattern in the sideward and backward hemisphere. We then analyzed these patterns to learn to what extent these patterns may help for the identification of pollen. In the present case, the pollens were attached on the end of a silica fiber. We also investigated the effect of the orientation of a football-shaped salix pollen relative to the direction of the incident laser beam.
CHOICE AND PREPARATION OF THE SAMPLES
The selection of pollen for our investigation was guided by their importance for allergic reactions. Samples of pollen from trees as hazel (Corylus avellana), birch (Betula), elm (Ulmus), willow (Salix alba), pine (Pinus sylvestris), and red chestnut (Aesculus) were collected. Furthermore pollen of common ragweed (Ambrosia) and sunflower (Helianthus) were investigated. These pollen are also well-known sources of allergies.
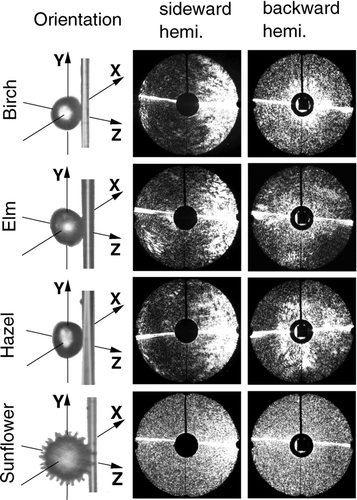
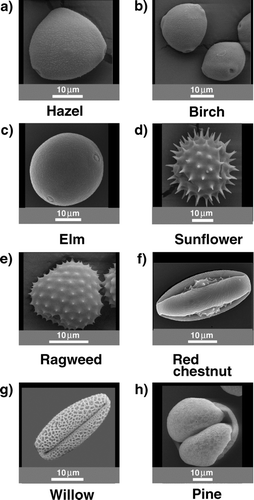
The pollen used for the experiment are shown in the SEM images in . In each image the size of the pollen can be estimated by the length of a 10 μ m measuring bar on the bottom of the photograph. We can divide the pollen into four groups: spherical with a smooth surface, spherical barbed, rod shaped or football shaped, and pollen with a shape not assignable to the other groups.
FIG. 1 SEM images of observed pollen. Divided into four groups: spherical with a smooth surface (hazel, birch, elm), barbed (ragweed, sunflower), rod shaped or football like (red chestnut, willow), and pollen with an unassignable shape (pine) related to the others.
The elastically scattered pattern depends strongly on the surface and the shape of the pollen.
Due to dehydration after harvesting the pollen the shape and the surface of the pollen could slightly be different than in the field under ideal or real conditions. This deformation could be reversed by putting the pollen into ethanol or water shortly before the measurement. As mentioned in the literature (CitationMiguel et al. 2006) we observed that pollen undergo an osmotic shock when immersed into a liquid. Potentially, the cytoplasmic material spilled out of the pollen has affected the scattering pattern. However, if the consistency of the pollen is changed, the scattering pattern might change too and is slightly different than the scattering pattern of natural pollen in the field.
EXPERIMANTAL SETUP
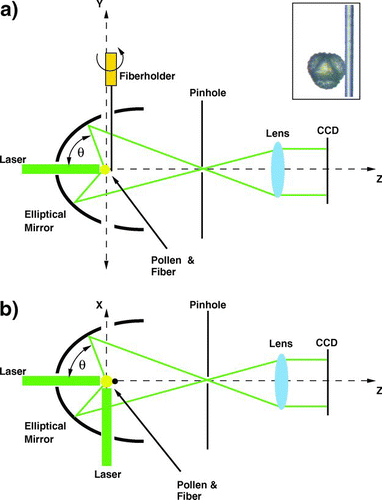
The setup is shown in and b in the side and top view. The pollen is attached on a tapered standard silica fiber with a diameter of approximately 8–10 μ m (shown in the inset of ) and placed in the first focal point of the elliptical mirror. The coating of the fiber was flame removed before tapering. The sample holds on a fiber simply by adhesive forces. A frequency doubled Q-switched Nd-YAG laser (BMI 510 DNS 100, λ = 532 nm, pulse duration 7 ns) was used to produce the elastically scattered light pattern. The laser beam was polarized in the y,z plane and propagated in positive z-direction or in the positive x-direction. The laser beam was focused by a lens to a waist diameter of approximately 100 μ m. The beam waist was located in the first focal point of the mirror. To avoid bleaching of the fluorophores before the measurements, two laser diodes were used to build a reticule, so the pollen could be placed correctly in the scattering volume. The positioning of the pollen was guided by the observation of the scattering pattern produced by the two laser diodes. Forward scattered light was blocked by a pinhole placed at the second focal point of the elliptical mirror.
FIG. 2 (a) Side view of the setup, and a microscope image of a rag weed pollen placed on the fiber. (b) Top view of the setup. (Figure provided in color online.)
A lens was used to transform the divergent beam of scattered light in a nearly parallel beam. The focal length of this lens was appropriately selected to match the beam diameter to the size of the detector chip. A nitrogen cooled CCD camera (Wright Instruments, Model 1, 770 × 1152 pixels) was used as a detector. After the shutter of the camera was opened the laser was triggered to illuminate the pollen with a single laser pulse.
As shown in the and b the scattering angles θ and φ were limited due to the holes in the mirror whereby the laser and the fiber with the pollen could be fed in. In the backward direction the scattering angle θ covered a range from 95° to 165°, and the scattering angle φ ran from 0° to 360°. In the sideward direction the scattering angle θ covered a range from 5° to 175°, and φ covered a range from 90° to 270°. To observe the pollen from different angles relative to the incident laser beam the fiber holder could be rotated around the y-axis.
RESULTS
For better visibility a histogram equalization was applied to all the patterns presented in this article. The scattering of the silica fiber can be seen as a thin bright line in each pattern. As the fiber is not always perfectly straight the scattering pattern of the fiber is also a little bit tilted.
shows the variation of the scattering pattern of a willow pollen as it is rotated in steps of 30° around the y-axis. The long main axis of the particle is tilted by approximately 45° relative to the y-axis. The laser beam propagates as already mentioned in the positive x-direction for scattering in the sideward hemisphere (center column) and in the positive z-direction for scattering in the backward hemisphere (right column). The orientation of the pollen on the fiber was controlled by a microscope before the pollen was placed in the scattering volume. To change the orientation of the pollen the fiber was rotated appropriately. In the first column of the orientation of the particle is shown.
FIG. 3 Sideward and backward scattering pattern of a rotated willow pollen. The rotation is around the y-axis in steps of 30° from a to e. (Figure provided in color online.)
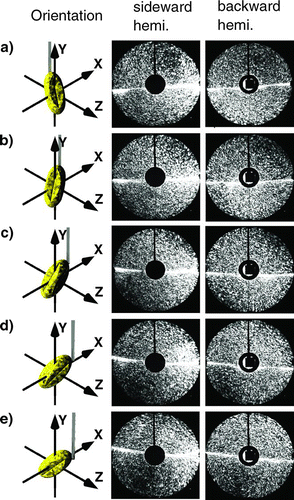
The interpretation of the scattering pattern is as follows: The particle is rotated around the y-axis in steps of 30° from , , , , .
Going down the right column the dark region rotates first clockwise, then in a new dark region appears between 12 and 3 o'clock and finally, the dark region between 6 and 9 o'clock has completely disappeared in . In the case of , the orientation of the particle relative to the laser beam has two symmetry axes perpendicular to each other and tilted to the x-axis by 45°. The scattering pattern displayed in the right column of reflects this symmetry.
As predicted by theory the intensity of the scattered light in the forward direction is much higher than in the backward direction. Therefore, the forward direction can be easily identified in the sideward scattering pattern (left column) as the bright region on the right. The change of orientation of the pollen can also be observed in the sideward scattering pattern.
Relations between the scattering in the sideward and backward hemispheres for pollen with different orientations relative to the laser beam are shown in . To facilitate the assignment of corresponding scattering pattern the scattering range is divided into mathematical quadrants (Qn). For example, the second and third quadrant (Q2S, Q3S) of the sideward scattering pattern has to be compared with the first and fourth quadrant (Q1B, Q4B) of the backward scattering pattern after the pollen is turned by 90° relative to the y-axis. Due to the imaging of the scattering pattern on the camera by the elliptical mirror the scattering pattern are distorted. Scattering in the angle range close to the mirror axis are stretched compared with scattering in the angle range perpendicular to the axis.
FIG. 4 Relations between the sideward and backward scattering hemispheres for a willow pollen with different orientations relative to the laser. (Figure provided in color online.)
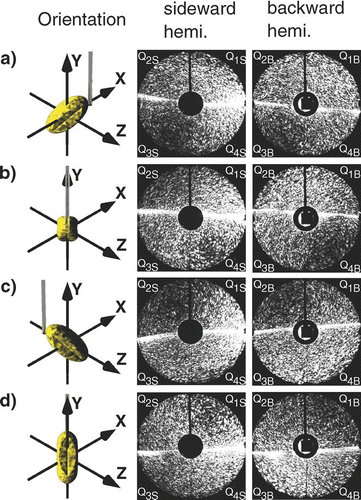
Following the procedure described above correspondence should exist between: Q2S and Q3S of with Q1B and Q4B of ; Q2S and Q3S of with Q1B and Q4B of ; Q2S and Q3S of sideward scattering with Q1B and Q4B of backward scattering, and Q2S and Q3S of sideward scattering with Q1B and Q4B of backward scattering show good agreement with each other.
The effect of the shape of the pollen on the scattering pattern is illustrated in and . The microscope images in the first column show the pollen with their orientation with respect to the incident laser beam. The corresponding SEM images are shown in . In scattering patterns of pollen are shown that have a more or less spherical shape. The birch, elm, and hazel pollen have a relatively smooth surface with small bumps, whereas the sunflower pollen looks more like a morning star. The similarity between the birch and hazel pollen is not surprising, because both belong to the same family (Betulaceae). This similarity between the birch and hazel pollen can also be seen in the SEM photos in . If we start with the interpretation of patterns from scattering in the sideward hemisphere, the large maximum in the forward direction on the left side of the images can be seen. In the original figures bright arcs are also visible, which are characteristic for scattering on spheres. These patterns are quite washed out by the surface needles of the sunflower pollen. The scattering patterns from scattering in the backward hemisphere show the loss of symmetry the larger the deviation from the spherical shape becomes. Again, the needles on the surface of the sunflower pollen cause a smoothing of the angular light distribution.
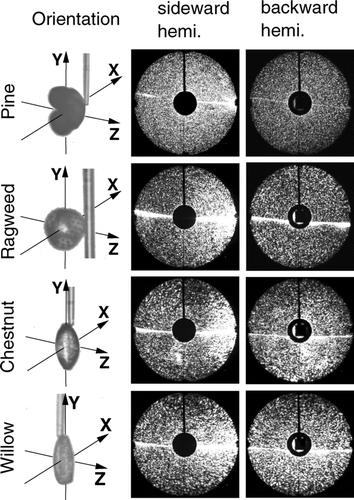
As examples of scattering patterns from pollens with quite different shapes, scattering experiments were carried out on pine, ragweed, chestnut, and willow pollen grains.
The results are shown in . The scattering pattern of the pine and the ragweed pollen apparently do not show significant structures. Nevertheless, the speckles of the scattered light are fine and narrow spaced by the pine pollen with a diameter of 40 μ m, while they are coarser for scattering on a ragweed pollen with a diameter of 20 μ m.
The football-shaped chestnut and the longish willow pollen were placed with the long axis parallel to the fiber. The pattern for both the football shaped pollen and the longish pollen show distinctive larger speckles than the pine and ragweed pollen. The reason possibly is that the shorter axis is distinctively shorter than the size of the pine and ragweed pollen. In addition the intensity gradient in radial direction for backward scattering is higher for the stretched pollen (chestnut and willow) compared to backscattering from ragweed and pine pollen.
CONCLUSION
Our investigation have shown that the large-angle-range two-dimensional optical scattering (LA-TAOS) method can be applied to obtain scattering pattern of pollen placed on a tapered silica fiber either in the sideward or in the backward direction. The scattering patterns were disturbed by the fiber in the equatorial region. Nevertheless, due to the large scattering angle range the influence of the fiber is small. The shape of the pollen investigated may deviate from the shape of freshly collected pollen. Especially pollen distributed by the wind have air bags that can collapse, due to dehydration. Comparing the scattering pattern of all pollen in and significant differences for pollen that differ in shape, size, surface, and orientation are shown. However, these figures show also that the resolution of the images is higher than necessary. Comparison of the scattering pattern of the same sort of pollen has shown that the fine structure of the scattering pattern differ from individual to individual pollen even if the pollen belong to the same plant. The use of a longer wavelength would not reduce the content of useful information but may decrease the signal to noise ratio due to the decrease of the sensitivity of the detector. For a decision as to what the optimum special resolution would be, we intend to apply various image processing techniques to the complete data. As a summary, the results are encouraging for further work on elastic light scattering in a large angle range to assist in a rapid pollen identification. However, the database is still much too small to decide whether or not this is a promising way for fast identification of bioaerosols.
REFERENCES
- Aptowicz , K. B. and Chang , R. K. 2005 . Angular Resolved Elastic Scatter from Single Particles Over a Large Solid Angle and with High Resolution . J. Physics: Conference Series , 6 : 90 – 96 .
- Aptowicz , K. B. , Pinnick , R. G. , Hill , S. C. , Pan , Y. L. and Chang , R. K. 2006 . Optical Scattering Patterns from Single Urban Aerosol Particles at Adelphi, Maryland, USA; A Classification Relating to Particle Morphologies . J. Geophys. Res. , 111 : D12212
- Auger , J. C. , Aptowicz , K. B. , Pinnick , R. G. , Pan , Y. L. and Chang , R. K. 2007 . Angularly Resolved Light Scattering from Aerosolized Spores: Observations and Calculations . Opt. Let. , 32 : 3358 – 3360 .
- Chang , R. K. and Pan , Y.-L. 2007 . Linear and Non-Linear Spectroscopy of Microparticles: Basic Principles, New Techniques and Promising Applications . Far. Discus. , 137 : 9 – 36 .
- Eversole , J. D. , Cary , W. K. Jr. , Scotto , C. S. , Pierson , R. , Spence , M. and Campillo , A. J. 2001 . Continous Bioaerosol Monitoring Using UV Excitation Fluorescence: Outdoor Test Results . Field Anal. Chem. Technol. , 15 : 205 – 212 .
- Fernandes , G. E. , Pan , Y.-L. , Chang , R. K. , Aptowicz , K. B. and Pinnick , R. G. 2006 . Simultaneous Forward- and Backward-Hemisphere Elastic-Light-Scattering Patterns of Respirable-Size Aerosols . Opt. Lett. , 31 : 3034 – 3036 .
- Hill , S. C. , Pinnick , R. G. , Niles , S. , Pan , Y.-L. , Holler , S. , Chang , R. K. , Bottiger , J. R. , Chen , B. T. , Orr , C.-S. and Feather , G. 1999 . Real-Time Measurement of Fluorescence Spectra from Single Airborne Biological Particles . Field Anal. Chem. Technol. , 3 : 221 – 239 .
- Hill , S. C. , Pinnick , R. G. , Niles , S. , Fell , N. F. , Pan , Y.-L. , Bottiger , J. , Bronk , B. V. , Holler , S. and Chang , R. K. 2001 . Fluorescence from Airborne Microparticles: Dependence on Size, Concentration of Fluorophores, and Illumination Intensity . Appl. Opt. , 40 : 3005 – 3013 .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : Wiley-Interscience .
- Holler , S. , Pan , Y.-L. and Chang , R. K. 1998 . Two-Dimensional Angular Optical Scattering for the Characterization of Airborne Microparticles . Opt. Lett. , 23 : 1489 – 1491 .
- Holler , S. , Surbek , M. , Chang , R. K. and Pan , Y.-L. 1999 . Two-Dimensional Angular Optical Scattering Patterns as Droplets Evolve into Clusters . Opt. Lett , 24 : 1185 – 1187 .
- Ivleva , N. P. , Niessner , R. and Panne , U. 2005 . Characterization and Discrimination of Pollen by Raman Microscopy . Anal. Bioanal. Chem. , 381 : 261 – 267 .
- Kaye , P. H. , Hirst , E. , Clark , J. M. and Micheli , F. 1992 . Airborne Particle Shape and Size Classification from Spatial Light Scattering Profiles . J. Aerosol Sci. , 23 : 597 – 611 .
- Laucks , M. L. , Roll , G. , Schweiger , G. and Davis , E. J. 2000 . Physical and Chemical (Raman) Characterization of Bioaerosols-Pollen . J. Aerosol Sci. , 31 : 307 – 319 .
- Miguel , A. G. , Taylor , P. E. , House , J. , Glovsky , M. M. and Flagan , R. C. 2006 . Meteorological Influence on Respirable Fragment Release from Chinese Elm Pollen . Aerosol Sci. Technol. , 40 : 690 – 696 .
- Mishchenko , M. I. , Travis , L. D. and Mackowski , D. W. 1996 . T-matrix Computations of Light Scattering by Nonspherical Particles: A Review . J. Quant. Spectrosc. Rad. , 55 : 535 – 575 .
- Pan , Y.-L. , Aptowitcz , K. B. , Chang , R. K. , Hart , M. and Eversole , J. D. 2003a . Characterizing and Monitoring Respiratory Aerosols by Light Scattering . Opt. Lett. , 28 : 589 – 591 .
- Pan , Y.-L. , Hartings , J. , Pinnick , R. G. , Hill , S. C. , Halverson , J. and Chang , R. K. 2003b . Single-Particle Fluorescence Spectrometer for Ambient Aerosols . Aerosol Sci. Technol. , 37 : 628 – 639 .
- Pan , Y.-L. , Surbek , M. , Aptowicz , K. B. and Chang , R. K. Rapid classification of aerosols by single-laser-shot fluorescence spectra and elastic scattering patterns . CLEO1903 Conference on Lasers and Electro-Optics .
- Philips , D. T. and Wyatt , P. J. 1972 . Single-Particle Light Scattering Measurement: Photochemical Aerosols and Atmospheric Particulates . Appl. Opt. , 11 : 2082 – 2087 .
- Secker , R. , Kaye , P. H. , Greenaway , R. S. , Hirst , E. , Bartley , D. and Videen , G. 2000 . Light Scattering from Deformed Droplets and Droplets with Inclusions. I. Experimental Results . Appl. Opt. , 39 : 5023 – 5030 .
- Sindoni , O. I. , Saija , R. , Iati , M. A. , Borghese , F. , Denti , P. , Fernandes , G. E. , Pan , Y.-L. and Chang , R. K. 2006 . Optical Scattering by Biological Aerosols: Experimental and Computational Results on Spore Simulants . Opt. Express , 14 : 6942 – 6950 .
- Sivaprakasam , V. , Huston , A. L. , Scotto , C. and Eversole , J. D. 2004 . Multiple UV Wavelength Excitation and Fluorescence of Bioaerosols . Opt. Express , 12 : 4457 – 4466 .
- Schulte , J. , Weigel , T. and Schweiger , G. 2004 . Inelastic Scattering at Arbitrary Shaped Particles . J. Aerosol Sci. , 35 : 485 – 486 .
- Surbek , M. , Esen , C. and Schweiger , G. 2008 . Angular Distribution of Inelastic Light Scattering by Dyed Doped Pollen on a Silica Fiber . Aerosol Sci. Technol. , 42 : 742 – 747 .
- Taylor , P. E. , Flagan , R. C. , Valenta , R. and Glovsky , M. M. 2002 . Release of Allergens as Respirable Aerosols: A Link between Grass Pollen and Asthma . Allergy Clin. Immunol , 109 : 51 – 58 .
- Weigel , T. , Schulte , J. and Schweiger , G. 2004 . Determination of the Composition and Shape of a Particle with Help of Inelastic Light Scattering . J. Aerosol Sci. , 35 : 1171 – 1172 .