Abstract
Particles containing potassium salts are common in biomass burning plumes and include compounds such as KCl, KNO 3 , and K 2 SO 4 . We determined the deliquescence and efflorescence relative humidities of single- and multi-component potassium salts using an environmental transmission electron microscope and an environmental scanning electron microscope. KCl particles deliquesced at a relative humidity (RH) of 85% and effloresced at 56%. K 2 SO 4 particles deliquesced at 96% and effloresced at 60%. KNO 3 particles grew continuously with increasing humidity. If KNO 3 particles were heat-treated at 104°C overnight, they deliquesced at an RH above 90%. Atomized solutions of multi-component potassium compounds (KCl & KNO 3 and KCl & K 2 SO 4 ) formed particles that contained distinct grains of each compound upon drying. Particles formed from the KCl & KNO 3 mixtures grew continuously with increasing humidity until deliquescence occurred at an RH of 82% and efflorescence occurred at 58%. Particles from the KCl & K 2 SO 4 mixture began to take up water at an RH of 84%. They deliquesced at >90% and effloresced at 52%. This work provides new data for mixed aqueous electrolytes that are important for atmospheric science. Efflorescence values for K 2 SO 4 , KNO 3 , and internally mixed particles of K 2 SO 4 and KCl are reported here for the first time.
INTRODUCTION
Biomass burning occurs on a global scale and results in the emission of large amounts of soot, organic, and inorganic material into the atmosphere. Such aerosol particles affect the climate in part because of their capacity to absorb or scatter solar radiation and to act as cloud-condensation nuclei (CitationHobbs et al. 1997; CitationAndreae et al. 2004; CitationIPCC 2007).
Large numbers of organic and inorganic particles occur in biomass plumes. The inorganic particles include compounds such as KCl, KNO3, and K2SO4 (CitationPosfai et al. 2003; CitationRissler et al. 2006). These potassium compounds are commonly used as markers for biomass burning (CitationCachier et al. 1995; CitationRissler et al. 2005). They have also been detected in dust samples, and their importance has been demonstrated in aerosol models (CitationKim and Seinfeld 1995; CitationFountoukis and Nenes 2007).
Several studies examined smoke particles from different biomass fires (CitationCachier et al. 1995; CitationGaudichet et al. 1995; CitationLi et al. 2003; CitationPosfai et al. 2003). Flaming and young smoke includes large numbers of particles containing crystals of KCl. Aged and reacted biomass smoke contains greater amounts of K2SO4 and KNO3 than KCl as well as high concentrations of particles containing C, N, and S (CitationLi et al. 2003). This change with age suggests that KCl particles are converted to K2SO4 and KNO3 through gas-particle reactions with S- and N-bearing species, presumably from the biomass plume. These potassium compounds are emitted as KCl and then partially or fully react with HNO3 and H2SO4 during transport in the atmosphere. Partially reacted particles become internal mixtures of either KCl & KNO3 or KCl & K2SO4, whereas fully reacted particles consist of only KNO3 or K2SO4, with HCl gas presumably liberated. Similar aging of potassium salts was reported for dust samples (CitationRo et al. 2005), where K2CO3 particles became internally mixed with sulfates and, less commonly, nitrates.
The transformation of KCl to K2SO4 and KNO3 with aging leads to concomitant changes in the hygroscopic properties of smoke particles. Whether particles are solid or aqueous has important implications for their environmental effects. Aqueous particles are larger and scatter solar radiation back to space more efficiently than solid particles. They can also modify atmospheric chemistry through reactions such as N2O5 hydrolysis (CitationJacob 2000; CitationKane et al. 2001) and secondary organic aerosol production (CitationLim et al. 2005).
Limited work has been done on the compositions of mixed aqueous electrolytes even though many hygroscopic atmospheric particles consist of several salts (CitationMartin 2000). The goals of this study were to determine the deliquescence and efflorescence relative humidity of single- and multi-component potassium salts. We used scanning and transmission electron microscopes (SEM and TEM, respectively), both fitted with environmental cells. With these instruments we have the ability to detect small changes on the surfaces of individual particles as they are exposed to RH conditions that they encounter in the atmosphere.
EXPERIMENTAL
Aerosols containing particles of KCl, KNO3, K2SO4, and their mixtures were generated from 1 M solutions prepared from analytical reagent-grade chemicals (97% purity, Aldrich). Solutions of K2SO4 were saturated at this concentration. The aerosols were dried using a silicon diffusion drier (TSI Model 3062) to an RH of ∼20% (CitationTSI, 2003). Single particles were deposited by diffusion, in air, onto TEM grids (Ted Pella inc #1820; lacey carbon Type-A, 300 mesh copper). This was achieved by holding a TEM grid directly in front of the output from the silica gel dryer for approximately 2 min.
Water-uptake experiments were carried out using a 200-kV FEI Tecnai F20 TEM fitted with a differentially pumped environmental cell (ETEM) (CitationWise et al. 2005; CitationSemeniuk et al. 2007; CitationWise et al. 2008). The cell was kept at constant temperature through a balance of liquid nitrogen cooling and resistive heating. Liquid nitrogen was contained in a dewar outside the microscope column. The dewar was connected via a conducting rod to the specimen-holder tip. The rod, which was insulated with a vacuum jacket from the environment inside the microscope, contained an electrical heater to control and a silicon diode to measure the sample temperature. The holder was designed so that its tip was kept at a constant temperature. The sample temperature was maintained at 15 ± 0.2°C so that an RH of 90 ± 1% was attained if 15 ± 0.05 torr of water vapor was allowed into the environmental cell.
Water vapor was introduced into the environmental cell of the TEM from a glass bulb containing room-temperature distilled water. No carrier gas was used. The vapor pressure in the environmental cell was regulated using a mechanical leak valve and measured using a capacitance manometer located outside the ETEM. More detailed information on the operation of the ETEM and the calculation of the RH is discussed by CitationWise et al. (2005).
Prior to each water-uptake experiment; the accuracy of the RH measurements was verified by measuring the deliquescence relative humidity (DRH) of laboratory-generated NaCl particles. Their DRH was consistently 75 ± 1%, in agreement with the known DRH of NaCl (DRH = 75.3%) (CitationWise et al. 2005; CitationBiskos et al. 2006). The error was calculated from standard deviations of at least ten measurements. There was no obvious difference in the DRH or ERH values if the substrate was made from silicon, a discontinuous carbon layer, or a Formar®/carbon backing (CitationWise et al. 2005). During these experiments, sequences of images at various increasing and decreasing RH values were recorded for each particle. At an RH between 90% and 95%, water condenses on the TEM stage, making water-vapor pressure difficult to read and control. Therefore, RH readings in the ETEM above 90% no longer agreed with the literature DRH for the potassium compounds. Any DRH or phase changes that occurred at an RH above 90% are labelled “>90%.”
An XL30 scanning electron microscope (SEM FEI, Inc.) fitted with a field-emission gun and an environmental chamber (ESEM) to introduce water vapor (CitationHoffman et al. 2004) was also used for comparison to results from the ETEM. ESEM experiments were performed at a temperature of 5°C. An accelerating voltage of 20 kV, beam current of 148 pA, and slow scanning (line time 16.7 ms and 484 lines per frame) were used.
Detailed chemical and morphological characterization of all particles was performed in vacuum using a Philips CM200 TEM. Semi-quantitative energy-dispersive X-ray spectrometry (EDS) was used for chemical characterization.
RESULTS AND DISCUSSION
Deliquescence and Efflorescence of Particles Atomized from Simple Solutions
A series of ETEM measurements in which the RH was increased from 0% to >90% were carried out for KCl, K2SO4, and KNO3 aerosol particles (, , and respectively). The measurements are summarized in .
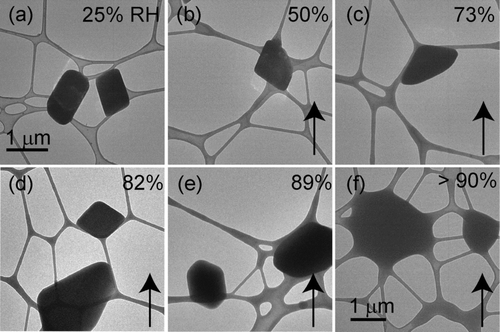
FIG. 1 KCl particles deposited onto a lacey-carbon TEM grid. All images are at the same magnification. The RH was increased from (a) 0% to (d) 85% and then decreased to 56%. Closed black arrows indicate whether the image was obtained as the RH was being increased (↑) or decreased (↓). The open arrows in (c) indicate water uptake prior to deliquescence. Deliquescence occurred at an RH of (e) 85% and efflorescence occurred at an RH of (f) 56%.
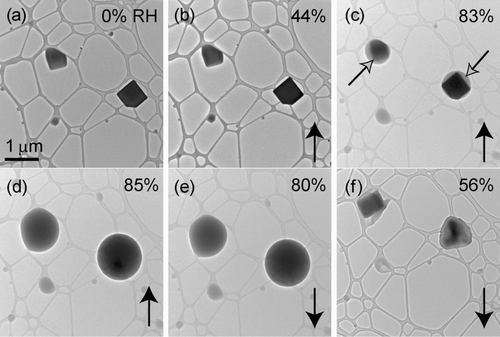
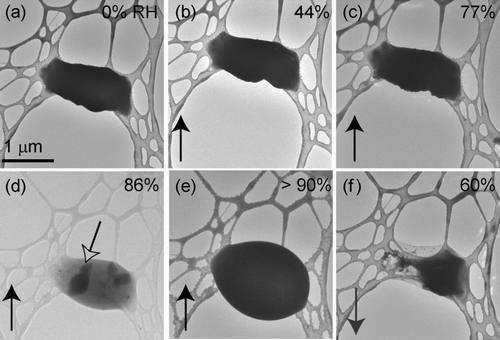
FIG. 2 K2SO4 particles deposited onto a lacey-carbon TEM grid, (a), (b), and (c) are of a different field of view than (d), (e), and (f). Relative humidity was increased from (a) 0% to (d) >90% and then decreased back to 60%. The open black arrow in (c) indicates water uptake prior to deliquescence. Deliquescence occurred at an RH (d) >90% and efflorescence occurred at an RH of (f) 60%. ∗Measurements above 90% are not accurate.
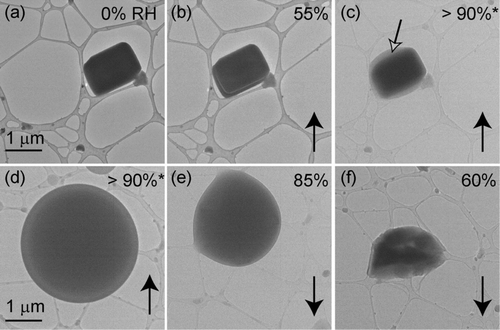
FIG. 3 KNO3 particles deposited onto a lacey-carbon TEM grid. All images are at the same magnification. No abrupt deliquescent phase transition was recorded as the RH increased from (a) 0% to (f) >90%. Each image is of a different field of view.
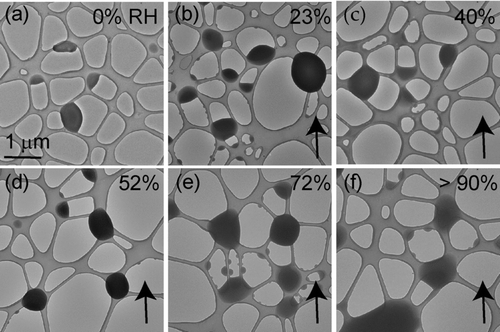
TABLE 1 Summary of deliquescence and efflorescence measurements made using both the ETEM and ESEM together with corresponding literature values
Atomized KCl particles were deposited onto a TEM grid and exposed to an increasing RH in the ETEM (). KCl particles deliquesced at an RH of 85 ± 1%, consistent with the literature value of 84% (CitationCohen et al. 1987a; CitationHamza et al. 2004; CitationSemeniuk et al. 2007). shows no visible morphological changes until the RH was increased to 83%, at which point each particle took up an observable quantity of water before deliquescing at 85%. This water uptake prior to deliquescence was reversible, meaning that when the RH was dropped by 1 or 2% the water disappeared. A similar effect was reported and discussed for NaCl particles by CitationWise et al. (2008). When the RH was decreased to 56 ± 1%, the KCl particles recrystallized (), consistent with efflorescence RH (ERH) measurements of CitationCohen et al. (1987a) and CitationHamza et al. (2004).
For K2SO4 particles, deliquescence did not occur in the ETEM until the RH was above 90% (). Similar to the other salts studied, reversible water uptake prior to deliquescence occurred (). When the RH was decreased, K2SO4 particles effloresced at an RH of 60 ± 2% (). To our knowledge, this is the first report of the ERH of K2SO4.
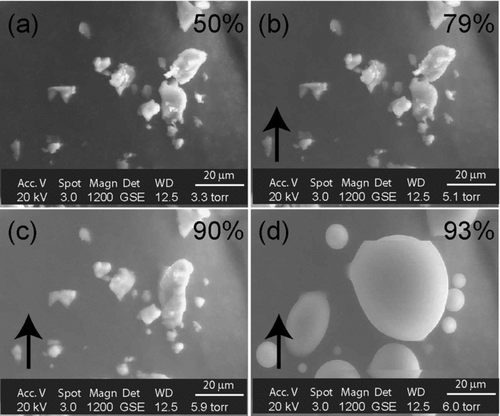
The DRH of K2SO4 was verified using an ESEM, where K2SO4 particles deliquesced at an RH of 96%, consistent with measurements of CitationAdams and Merz (1929) and CitationGoldberg (1981). Repeated experiments on KCl and K2SO4 showed that the electron beam of the TEM did not affect their DRH or ERH values.
The hygroscopic behavior of KNO3 is more complex than the compounds discussed above. It is more sensitive to electron radiation than the other alkali salts studied, and therefore consecutive exposures of the same area of the TEM grid caused damage to the particles, making them difficult to study using electron microscopy. The sensitivity of KNO3 and other alkali metal nitrates to radiation damage is discussed by CitationKulkarni and Garg (1991). In order to examine these particles with minimal damage, each area was exposed to the electron beam for approximately 5 s. The electron beam was then blanked, and the grid position was changed so that each photograph was from a new area of the grid.
Atomized KNO3 particles were exposed to an RH that was increased from 0% to above 90% in the ETEM (). Since the particles lacked well-developed crystal faces, it was difficult to detect morphological changes as the humidity increased. However, the particles spread out on the TEM grid at higher RH values, suggesting that they had absorbed water (). In the ESEM, gradual growth was observed as the humidity was increased. When the RH was decreased to 0%, these particles gradually decreased in size but did not readily crystallize. A range of studies using other approaches suggests that particulate nitrates such as NaNO3, NH4NO3, and Ca(NO3)2 occurred as amorphous solids when the RH was decreased to below 10% after having been above the DRH (CitationTang and Munkelwitz 1994; CitationMcInnes et al. 1996; CitationLee and Hsu 2000; CitationGysel et al. 2002; CitationHoffman et al. 2004; CitationLaskin et al. 2005; CitationSchlenker and Martin 2005; CitationGibson et al. 2006; CitationLiu et al. 2008).
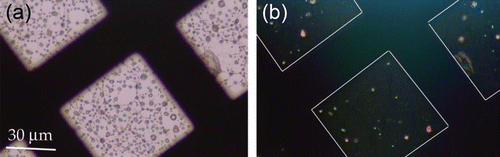
An optical microscope with polarized light illumination was used to view TEM grids containing atomized KNO3 particles. Interference colors () indicate that most such atomized KNO3 particles are crystalline. This result differs from IR and X-ray measurements that indicated that nitrate particles deposited onto a surface occur as amorphous solids (CitationHoffman et al. 2004; CitationLiu et al. 2008).
FIG. 4 Polarized-light optical microscopy images of KNO3 particles deposited onto a lacey-carbon TEM grid. Image (a) is viewed in plane-polarized transmitted-light. Image (b) shows the same area viewed in cross-polarized transmitted light. Only crystalline particles give interference patterns when viewed with cross-polarized light.
In a second set of experiments, the atomized KNO3 particles were heated overnight at 104°C to remove surface and structurally absorbed water prior to being exposed to an RH increasing from 0% to above 90% (). This temperature was not sufficient for the thermal decomposition of KNO3, which occurs at 400°C. Morphological changes occurred only above 90%, when particles became rounded, suggesting that they deliquesced (). In the ESEM, a similar result (DRH = 93%) was obtained when larger, mechanically formed KNO3 particles were studied (). These measurements are compatible with literature DRH values of 92.3% (CitationAdams and Merz 1929). The difference in hygroscopic properties between atomized and oven-treated KNO3 (and mechanically produced KNO3) possibly arises from residual water that remains in atomized particles but was removed upon heating.
Deliquescence and Efflorescence of Particles Atomized from Complex Solutions
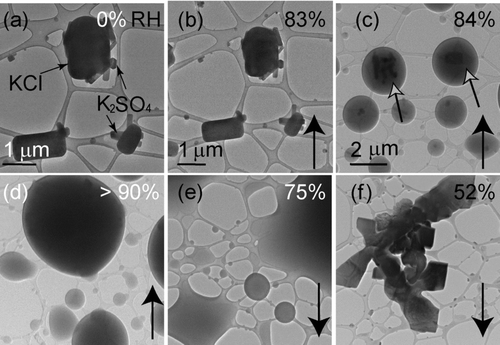
As a model for atmospherically processed KCl particles, we studied the hydration behavior of particles atomized from 1 M solutions of KCl & KNO3 (1:1), KCl & K2SO4(1:1), KCl & HNO3(1:1), and KCl & H2SO4(1:1). Particles atomized from 1M solutions of KCl & KNO3 or KCl & K2SO4 contained distinct grains of both compounds ( and ). Using the Philips CMZOD TEM for imaging and EDS, we identified the shapes and compositions of single grains deposited onto the TEM grids before transferring the grids to the ETEM. As KCl grains are generally more euhedral than KNO3 grains, it was possible to visually distinguish between them in mixed particles. For particles containing both KCl and K2SO4, KCl particles consisted of single grains, whereas the K2SO4 particles consisted of multiple grains of smaller elongated particles. The coexistence of these compounds as separate phases has been reported for ambient particles (CitationPosfai et al. 1994, Citation1995), but differs from reports by CitationGe et al. (1996) and CitationHoffman et al. (2004).
FIG. 7 Particles from an equimolar solution of KCl & KNO3 deposited onto a lacey-carbon TEM grid. Images (b) through (f) are at the same magnification. Particle identities are indicated with the black arrows in (a). Relative humidity was increased from (a) 0% to (d) 82% and then decreased to (f) 58%. Images (a), (d), (e), and (f) are of a different field of view. Solid phases, indicated by the open black arrows, remain visible at an RH of (c) 78%.
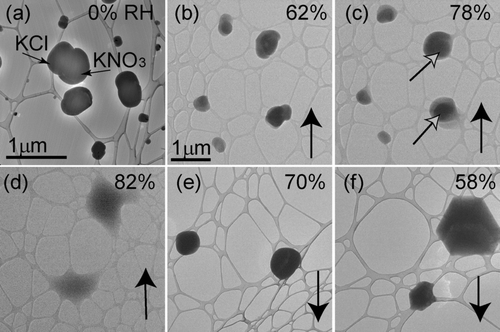
FIG. 8 Particles from an equimolar solution of KCl & K2SO4 deposited onto a lacey-carbon TEM grid. Images (c) through (f) are at the same magnification. Particle compositions are indicated with the black arrows in (a). Relative humidity was increased from (a) 0% to (d) >90% and then decreased to (f) 52%. Solid phases, indicated by the open black arrows, remain visible from an RH of (c) 84% to (d) >90% and are surrounded by a layer of water.
Hygroscopic measurements of particles atomized from the KCl & KNO3 solution showed that the presumed KNO3 fraction of these particles gradually took up water without a distinct DRH. It is difficult to assess the eutonic effect for this mixture since KNO3 does not exhibit a distinct DRH. Solid particles, presumably KCl, were visible inside droplets up to an RH of 78% but were no longer present at 82% ( and ). Tilting of the sample within the ETEM allowed us to see whether solid grains were on the interior or exterior of the droplets. Solid grains inside droplets were viewed by changing the height of the focal plane. The compositions of those solids were assumed to be those of the least soluble species of the internally mixed particle. CitationAdams and Merz (1929) measured the eutonic RH of a solution containing KCl & KNO3 to be 78.6% at 30°C. This value is compatible with ours of 82%, within experimental uncertainty. When the humidity was decreased, particles effloresced at an RH of 58%.
Particles atomized from a 1 M solution of KCl & HNO3 displayed gradual hygroscopic growth. Similar to atomized KNO3 particles, heating overnight at 104°C removed adsorbed water, and deliquescence did not occur until the RH reached values >90%. EDS spectra showed that the particles consisted of K, O, and N, indicating that KNO3 particles formed from the mixture and HCl gas was presumably liberated.
For particles atomized from solutions of KCl & K2SO4, the presumed KCl fraction of the particles began to take up water at an RH of 84% (). This value is only 1% lower than the measured DRH for pure KCl (85%), which is within experimental uncertainty. The eutonic effect can vary depending on the composition of a mixed particle. For example, CitationTang and Munkelwitz (1994) observed that particles containing both NaCl and Na2SO4 took up water initially at 74.2%, an RH close to the RH for NaCl (75.3%). However, particles containing NaCl and NaNO3 took up water at an RH of 68%.
Solid cores, presumably K2SO4, were visible within single droplets from RH values of 84% to >90% (). Full deliquescence occurred at an RH >90% (). The RH at which a mixture of KCl & K2SO4 fully deliquesced was reported to be 83.2% at 30°C (CitationAdams and Merz 1929). The difference between our results and those of CitationAdams and Merz (1929) could result from the temperature differences between these studies. Our experiments were performed at 15°C.
Particles from the solution of KCl & K2SO4 effloresced at an RH of 52%. This value is lower than that of either KCl (ERH 56%) or K2SO4(ERH 60%). To our knowledge this is the first report of the mutual ERH for particles from a KCl & K2SO4 solution. In a similar study (CitationCohen et al. 1987b), solutions of KCl & NaCl (1:1) or NaCl & (NH4)2SO4 (1:1) also effloresced at values lower than the ERH of either of the single salts. However, if soluble inorganic salts are mixed with insoluble dust particles, the ERH of the soluble component increases because of heterogeneous nucleation (CitationHan and Martin 1999).
Particles dried from a solution of KCl & H2SO4 took up water at an RH of 86% (). These particles did not deliquescence until the RH reached >90% (). When the RH was decreased, the particles effloresced at 60% (). These values for deliquescence and efflorescence match those of K2SO4. However, water uptake at an RH of 86% is also compatible with the DRH of KHSO4 (CitationPilinis et al. 1989 Footnote 1 ). At 86%, solid cores remained visible within droplets. If particles did not deliquesce and the RH was decreased below the DRH from 86% to 75%, the particles recrystallized.
FIG. 9 Particles from an equimolar solution of KCl & H2SO4 deposited onto a lacey-carbon TEM grid. All images are at the same magnification. Relative humidity was increased from (a) 0% to (e) >90% and then decreased to (f) 60%. Solid phases remain visible from an RH of (d) 86% to (e) >90% and are surrounded by a layer of water indicated by the open black arrows.
The difference in efflorescence that results from decreasing the RH from >90% to 60% or from 86% to 75% was caused by the solid cores that remained in the latter case. A similar result was observed by CitationLightstone et al. (2000) for mixed ammonium nitrate and succinic acid particles. Our results indicate that the solubility of slightly soluble components and the RH history of a particle are essential in order to successfully model mixed composition aerosol particles in the atmosphere. Our work corroborates recent results by CitationWang et al. (2008), who reported that the ERH can shift depending on the internal mixture of single particles.
SUMMARY AND DISCUSSION
We describe the deliquescence and efflorescence properties of KCl, K2SO4, and KNO3 compounds that are common in atmospheric aerosols. ERH values for KNO3, K2SO4, and mixtures of potassium compounds (e.g., KCl & K2SO4 and KCl & KNO3) are reported here for the first time.
Atomized KNO3 particles deposited onto a TEM grid show optical interference patterns when viewed with cross-polarized light, indicating that they were crystalline. This result differs from measurements where nitrate particles occurred as amorphous solids after they were atomized and deposited onto a surface (CitationHoffman et al. 2004; CitationLiu et al. 2008). Efflorescence measurements provide information on the hygroscopic properties that could enhance gas-particle atmospheric equilibrium models, some of which assume that the water content for KNO3 and K2SO4 below their deliquescence point is zero (CitationKim et al. 1993a, b).
Organic particles, soot, and tar balls dominate the aerosol produced through biomass burning and appear to be hydrophobic (CitationSemeniuk et al. 2007). The hygroscopic properties of biomass aerosol largely depend on the fraction of inorganic particles. CitationSemeniuk et al. (2007) showed that attached or included inorganic grains (e.g., KCl) in organic particles greatly enhance the water uptake ability of carbonaceous particles. Understanding the hygroscopic properties of the inorganic component of biomass-burning particles and their mixtures will allow a better estimate of the behavior of these particles under ambient RH conditions.
This material is based upon work supported by the National Science Foundation under Grant No. 0304213 from the Division of Atmospheric Chemistry. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We gratefully acknowledge the use of the facilities at the John M. Cowley Center for High Resolution Electron Microscopy within the Center for Solid State Science at Arizona State University. We also thank Karl Weiss, Weijun Li, and Dr. Kouji Adachi for useful discussions.
Notes
1Atomized without heating.
2Atomized with heating.
3The RH at which the mixed particles began to take up water.
4The RH at which all components of the mixed particle were in the liquid phase.
5Calculated from data measured by CitationMullin (1961).
∗ETEM only.
1Calculated from data measured by CitationMullin (1961).
REFERENCES
- Adams , J. R. and Merz , A. R. 1929 . Hygroscopicity of Fertilizer Materials and Mixtures . Ind. Eng. Chem. , 21 : 305 – 307 .
- Andreae , M. O. , Rosenfeld , D. , Artaxo , P. , Costa , A. A. , Frank , G. P. , Longo , K. M. and Silva-Dias , M. A. F. 2004 . Smoking Rain Clouds over the Amazon . Science , 303 : 1337 – 1342 .
- Biskos , G. , Paulsen , D. , Russell , L. M. , Buseck , P. R. and Martin , S. T. 2006 . Prompt Deliquescence and Efflorescence of Aerosol Nanoparticles . Atmos. Chem. Phys , 6 : 4633 – 4642 .
- Cachier , H. , Liousse , C. , Buatmenard , P. and Gaudichet , A. 1995 . Particulate Content of Savanna Fire Emissions . J. Atmos. Chem. , 22 : 123 – 148 .
- Cohen , M. D. , Flagan , R. C. and Seinfeld , J. H. 1987a . Studies of Concentrated Electrolyte-Solutions Using the Electrodynamic Balance. 1. Water Activities for Single-Electrolyte Solutions . J. Phys. Chem. , 91 : 4563 – 4574 .
- Cohen , M. D. , Flagan , R. C. and Seinfeld , J. H. 1987b . Studies of Concentrated Electrolyte-Solutions Using the Electrodynamic Balance. 2. Water Activities for Mixed-Electrolyte Solutions . J. Phys. Chem. , 91 : 4575 – 4582 .
- Fountoukis , C. and Nenes , A. 2007 . Isorropia II: A Computationally Efficient Thermodynamic Equilibrium Model for K+-Ca2+-Mg2+-NH+ 4-Na+-SO2 − − 4-NO− − 3-Cl− −-H2O Aerosols . Atmos. Chem. Phys. , 7 : 4639 – 4659 .
- Gaudichet , A. , Echalar , F. , Chatenet , B. , Quisefit , J. P. , Malingre , G. , Cachier , H. , Buatmenard , P. , Artaxo , P. and Maenhaut , W. 1995 . Trace-Elements in Tropical African Savanna Biomass Burning Aerosols . J. Atmos. Chem. , 22 : 19 – 39 .
- Ge , Z. Z. , Wexler , A. S. and Johnston , M. V. 1996 . Multicomponent Aerosol Crystallization . J. Colloid Interf. Sci. , 183 : 68 – 77 .
- Gibson , E. R. , Hudson , P. K. and Grassian , V. H. 2006 . Physicochemical Properties of Nitrate Aerosols: Implications for the Atmosphere . J. Phys. Chem. A , 110 : 11785 – 11799 .
- Goldberg , R. N. 1981 . Evaluated Activity and Osmotic Coefficients for Aqueous Solutions -36 Uni-Bivalent Electrolytes . J. Phys. Chem. Ch. Ref. , 10 : 671 – 764 .
- Gysel , M. , Weingartner , E. and Baltensperger , U. 2002 . Hygroscopicity of Aerosol Particles at Low Temperatures. 2. Theoretical and Experimental Hygroscopic Properties of Laboratory Generated Aerosols . Environ. Sci. Technol. , 36 : 63 – 68 .
- Hamza , M. A. , Berge , B. , Mikosch , W. and Ruhl , E. 2004 . Homogeneous Nucleation of Supersaturated KCl-Solutions from Single Levitated Microdroplets . Phys. Chem. Ch. Ph , 6 : 3484 – 3489 .
- Han , J. H. and Martin , S. T. 1999 . Heterogeneous Nucleation of the Efflorescence of (NH4)2SO4Particles Internally Mixed with Al2O3, TiO2, and ZrO2 . J. Geophys. Res. Atmos , 104 : 3543 – 3553 .
- Hobbs , P. V. , Reid , J. S. , Kotchenruther , R. A. , Ferek , R. J. and Weiss , R. 1997 . Direct Radiative Forcing by Smoke from Biomass Burning . Science , 275 : 1776 – 1778 .
- Hoffman , R. C. , Laskin , A. and Finlayson-Pitts , B. J. 2004 . Sodium Nitrate Particles: Physical and Chemical Properties During Hydration and Dehydration, and Implications for Aged Sea Salt Aerosols . J. Aerosol Sci. , 35 : 869 – 887 .
- IPCC . 2007 . “ Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change ” . 996 Cambridge United Kingdom and New York, NY, USA : Cambridge University Press .
- Jacob , D. J. 2000 . Heterogeneous Chemistry and Tropospheric Ozone . Atmos. Environ. , 34 : 2131 – 2159 .
- Kane , S. M. , Caloz , F. and Leu , M. T. 2001 . Heterogeneous Uptake of Gaseous N2O5 by (NH4)2SO4, NH4HSO4, and H2SO4Aerosols . J. Phys. Chem. A , 105 : 6465 – 6470 .
- Kim , Y. P. and Seinfeld , J. H. 1995 . Atmospheric Gas-Aerosol Equilibrium. 3. Thermodynamics of Crustal Elements Ca2+, K+, and Mg2+ . Aerosol Sci. Technol , 22 : 93 – 110 .
- Kim , Y. P. , Seinfeld , J. H. and Saxena , P. 1993a . Atmospheric Gas-Aerosol Equilibrium. 2. Analysis of Common Approximations and Activity-Coefficient Calculation Methods . Aerosol Sci. Technol , 19 : 182 – 198 .
- Kim , Y. P. , Seinfeld , J. H. and Saxena , P. 1993b . Atmospheric Gas Aerosol Equilibrium. 1. Thermodynamic Model . Aerosol Sci. Technol , 19 : 157 – 181 .
- Kulkarni , S. P. and Garg , A. N. 1991 . An Evaluation of Rate Constants for Radiation Induced Decomposition of Alkali-Metal Nitrates . J. Radioanal. Nucl. Chem. Lett. , 153 : 211 – 219 .
- Laskin , A. , Wietsma , T. W. , Krueger , B. J. and Grassian , V. H. 2005 . Heterogeneous Chemistry of Individual Mineral Dust Particles with Nitric Acid: A Combined CCSEM/EDX, ESEM and ICP-MS Study . J. Geophys. Res. Atmos. , 110 : D10208
- Lee , C. T. and Hsu , W. C. 2000 . The Measurement of Liquid Water Mass Associated with Collected Hygroscopic Particles . J. Aerosol Sci. , 31 : 189 – 197 .
- Li , J. , Posfai , M. , Hobbs , P. V. and Buseck , P. R. 2003 . Individual Aerosol Particles from Biomass Burning in Southern Africa: 2. Compositions and Aging of Inorganic Particles . J. Geophys. Res. Atmos , 108 : 8484 – 8495 .
- Lightstone , J. M. , Onasch , T. B. , Imre , D. and Oatis , S. 2000 . Deliquescence, Efflorescence, and Water Activity in Ammonium Nitrate and Mixed Ammonium Nitrate/Succinic Acid Microparticles . J. Phys. Chem. A , 104 : 9337 – 9346 .
- Lim , H. J. , Carlton , A. G. and Turpin , B. J. 2005 . Isoprene Forms Secondary Organic Aerosol through Cloud Processing: Model Simulations . Environ. Sci. Technol , 39 : 4441 – 4446 .
- Liu , Y. , Yang , Z. , Desyaterik , Y. , Gassman , P. L. , Wang , H. and Laskin , A. 2008 . Hygroscopic Behavior of Substrate-Deposited Particles Studied by Micro-FT IR Spectroscopy and Complementary Methods of Particle Analysis . Anal. Chem. , 80 : 633 – 642 .
- Martin , S. T. 2000 . Phase Transitions of Aqueous Atmospheric Particles . Chem. Rev. , 100 : 3403 – 3453 .
- McInnes , L. M. , Quinn , P. K. , Covert , D. S. and Anderson , T. L. 1996 . Gravimetric Analysis, Ionic Composition, and Associated Water Mass of the Marine Aerosol . Atmos. Environ. , 30 : 869 – 884 .
- Mullin , J. W. 1961 . Crystallization , 246 – 248 . London : Butterworth .
- Pilinis , C. , Seinfeld , J. H. and Grosjean , D. 1989 . Water-content of Atmospheric Aerosols . Atmos. Environ. , 23 : 1601 – 1606 .
- Posfai , M. , Anderson , J. R. , Buseck , P. R. , Shattuck , T. W. and Tindale , N. W. 1994 . Constituents of Remote Pacific Marine Aerosol—A TEM Study . Atmos. Environ. , 28 : 1747 – 1756 .
- Posfai , M. , Anderson , J. R. , Buseck , P. R. and Sievering , H. 1995 . Compositional Variations of Sea-Salt-Mode Aerosol Particles from the North Atlantic . J. Geophys. Res. Atmos. , 100 : 23063 – 23074 .
- Posfai , M. , Simonics , R. , Li , J. , Hobbs , P. V. and Buseck , P. R. 2003 . Individual Aerosol Particles from Biomass Burning in Southern Africa: 1. Compositions and Size Distributions of Carbonaceous Particles . J. Geophys. Res. , 108 : 8482 – 8493 .
- Rissler , J. , Pagels , J. , Swietlicki , E. , Wierzbicka , A. , Strand , M. , Lillieblad , L. , Sanati , M. and Bohgard , M. 2005 . Hygroscopic Behavior of Aerosol Particles Emitted from Biomass Fired Grate Boilers . Aerosol Sci. Technol. , 39 : 919 – 930 .
- Rissler , J. , Vestin , A. , Swietlicki , E. , Fisch , G. , Zhou , J. , Artaxo , P. and Andreae , M. O. 2006 . Size Distribution and Hygroscopic Properties of Aerosol Particles from Dry-Season Biomass Burning in Amazonia . Atmos. Chem. Phys. , 6 : 471 – 491 .
- Ro , C. U. , Hwang , H. , Chun , Y. and Van Grieken , R. 2005 . Single-Particle Characterization of Four “Asian Dust” Samples Collected in Korea, Using Low-Z Particle Electron Probe X-Ray Microanalysis . Environ. Sci. Technol. , 39 : 1409 – 1419 .
- Schlenker , J. C. and Martin , S. T. 2005 . Crystallization Pathways of Sulfate-Nitrate-Ammonium Aerosol Particles . J. Phys. Chem. A , 109 : 9980 – 9985 .
- Semeniuk , T. A. , Wise , M. E. , Martin , S. T. , Russell , L. M. and Buseck , P. R. 2007 . Hygroscopic Behavior of Aerosol Particles from Biomass Fires Using Environmental Transmission Electron Microscopy . J. Atmos. Chem. , 56 : 259 – 273 .
- Tang , I. N. and Munkelwitz , H. R. 1994 . Aerosol Phase-Transformation and Growth in the Atmosphere . J. Appl. Meteorol. , 33 : 791 – 796 .
- TSI . 2003 . Model 3062 Diffusion Dryer Instruction Manual .
- Wang , J. , Hoffmann , A. A. , Park , R. J. , Jacob , D. J. and Martin , S. T. 2008 . Global Distribution of Solid and Aqueous Sulfate Aerosols: Effect of the Hysteresis of Particle Phase Transitions . J. Geophys. Res. Atmos. , 113 : D11206
- Wise , M. E. , Biskos , G. , Martin , S. T. , Russell , L. M. and Buseck , P. R. 2005 . Phase Transitions of Single Salt Particles Studied Using a Transmission Electron Microscope with an Environmental Cell . Aerosol Sci. Technol. , 39 : 849 – 856 .
- Wise , M. E. , Martin , S. T. , Russell , L. M. and Buseck , P. R. 2008 . Water Uptake by NaCl Particles Prior to Deliquescence and the Phase Rule . Aerosol Sci. Technol , 42 : 281 – 294 .