Abstract
In this study the coagulation, condensation, and sintering of nanoparticles is investigated using a stochastic particle model. Each stochastic particle consists of interacting polydisperse primary particles that are connected to each other. In the model sintering occurs between each individual pair of neighboring primary particles. This is important for particles in which the range of the size of the primary particles varies significantly. The sintering time is obtained from the viscous flow model. The model is solved using a stochastic particle algorithm. The particles are represented in a binary tree that contains the connectivity as well as the degree of sintering information. Particles are forme, coagulate, sinter, and experience condensation according to known rate laws. The particle binary tree, along with it the degree of sintering, is updated after each time step according to the rates of the different processes. The stochastic particle method uses the technique of fictitious jumps and linear process deferment. The theoretical results are fitted against experimental values for the formation of SiO 2 nanoparticles and computer generated TEM pictures are presented and compared to experiments.
1. INTRODUCTION
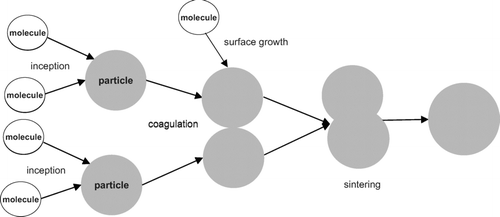
The formation of nanoparticles from gaseous precursors is becoming more and more important in the production of new materials. Inception, coagulation, condensation, and sintering are the important steps to form these particles. The inception of a particle is induced by a collision of two precursors from the gas-phase. These first particles grow due to condensation of molecules from the surrounding gas-phase or further collisions with other particles. Condensation leads to an increase of particle volume and increases the sphericity. Sintering reduces the surface of the particles and makes them rounder whereas the coagulation of particles decreases the sphericity of the particles. The relative timescale of the sintering and the coagulation processes determines the shape and the size of the particles. The faster the sintering is, the rounder the particles become.
CitationKoch and Friedlander (1990) have developed a model explaining particle growth by coagulation and sintering. They have generalized the Smoluchowski equation to incorporate the coalescence rate into a model for aerosol dynamics. CitationXiong and Pratsinis (1993) have established a two-dimensional sectional model to describe the evolution of the particle size and shape. Seto et al. (Citation1995, Citation1997) have studied the sintering of SiO2 and TiO2 particles in a heated pipe and determined the structure and size distribution of the particles. They have also used a two-dimensional sectional model to represent the size distribution and to solve the population balance equation. Their model explains the growth of primary particles and the shrinkage of the particles due to sintering and they concluded that the temperatures at which sintering occurs are 50–100% of the bulk melting points. This work has been extended in a later study by CitationNakaso et al. (2001). The dependence of the melting point on the size of the particles is also studied by CitationKarlsson et al. (2005). The computational performance of different population balance models has been investigated by CitationMühlenweg et al. (2002).
Some researchers have found that the liquidity of the primary particles depends strongly on the size of the primary particles (CitationRogak 1997; CitationTsantilis and Pratsinis 2000; CitationTsantilis et al. 2001). The melting point decreases with the size of the primaries, therefore CitationRogak (1997) introduced a critical primary size Dmelt
. He assumes that the primaries are liquid like below Dmelt
and therefore they coalesce instantly. Rogak also concluded that the primary particle diameter is much less sensitive to temperature than would be expected from simple sintering models. CitationTsantilis and Pratsinis (2000) have developed a simple sectional model describing the evolution of primary and aggregate size distributions. Their model has been applied to aerosol synthesis of TiO2 by TiCl4 oxidation in a hot wall reactor. They also stated that sintering is quite slow for large primary particles compared to small primaries. In a further study CitationTsantilis et al. (2001) have introduced a critical primary particle diameter ![]() in the mathematical expression for the characteristic sintering time to take the change of fluidity of small primaries into account. CitationEhrman (1999)has developed a sintering model assuming an atomistic diffusion mechanism to correct the characteristic sintering time for small primary particles. This model takes the effect of internal pressure within the particles into account by modifying the characteristic sintering time accordingly. CitationKim et al. (2003) and CitationLee et al. (2001) studied the growth of non-spherical silica particles in a counter-flow diffusion fame. They used different models to calculate the characteristic sintering time and concluded that the viscous flow sintering model combined with the atomistic diffusion for small particles yields to the best agreement with experimental data. In most of these studies equal sized primary particles are assumed, however CitationYadha and Helble (2004) investigated the coalescence of unequal-sized amorphous primary particles. Their results indicate that the normalized sintering rate is independent of the size ratio of the two interacting primary particles and that the smaller primary particle determines primarily the sintering rate. CitationHeine and Pratsinis (2007) have developed a two dimensional sectional population balance model that relates the rate of sintering to each individual primary particle size.
in the mathematical expression for the characteristic sintering time to take the change of fluidity of small primaries into account. CitationEhrman (1999)has developed a sintering model assuming an atomistic diffusion mechanism to correct the characteristic sintering time for small primary particles. This model takes the effect of internal pressure within the particles into account by modifying the characteristic sintering time accordingly. CitationKim et al. (2003) and CitationLee et al. (2001) studied the growth of non-spherical silica particles in a counter-flow diffusion fame. They used different models to calculate the characteristic sintering time and concluded that the viscous flow sintering model combined with the atomistic diffusion for small particles yields to the best agreement with experimental data. In most of these studies equal sized primary particles are assumed, however CitationYadha and Helble (2004) investigated the coalescence of unequal-sized amorphous primary particles. Their results indicate that the normalized sintering rate is independent of the size ratio of the two interacting primary particles and that the smaller primary particle determines primarily the sintering rate. CitationHeine and Pratsinis (2007) have developed a two dimensional sectional population balance model that relates the rate of sintering to each individual primary particle size.
Schmid et al. (Citation2004, Citation2006) have presented a three-dimensional model for aggregates undergoing sintering and coagulation where the particles are modeled as overlapping spheres. They concluded that the fractal dimension depends strongly on the concurrent coagulation and sintering process.
CitationAkhtar et al. (1994) have developed a Monte Carlo simulation on a 2-D lattice to describe the coagulation and sintering of nanoclusters. Sintering has been implemented in their model using a random particle walk on the surface of the cluster. They have also investigated the fractal dimension of the particles and concluded that the surface area of the particles is more sensitive to changes in the sintering rate than the fractal dimension. The effect of heat release on the particle formation due to the sintering process has been studied by CitationLehtinen and Zachariah (2002).
In our work a stochastic method is used to solve the population balance equation and the sintering is calculated individually for each pair of neighboring primary particles. The system is simulated using a stochastic ensemble consisting of interacting particles. This technique does not suffer from the numerical diffusion inherent in sectional methods and is able to provide the full multivariate particle population density without additional constraints. Various algorithms have been developed to reduce the computational time required for the solution of the population balance equation (Patterson et al. 2006a). Stochastic particle models have already been used to investigate the particle size distribution of soot (CitationCelnik et al. 2009; CitationMorgan et al. 2007; CitationRaj et al. 2009; CitationSander et al. 2009; CitationSingh et al. 2005, Citation2006). CitationMorgan et al. (2005) have used a stochastic particle model to simulate coagulation, condensation, and sintering, however in contrast to our work the sintering is calculated as an average for the entire particle.
The processes described by the current model are: coagulation, condensation, and sintering. The simulation in this work models the particle formation process starting from the first sticking of two molecules from the gas-phase. The model maintains a list of particles where each particle contains a data structure that stores the primary particles. This data structure also contains the level of sintering between touching primary particles. The coagulation of particles is implemented using the free collision kernel. Sintering, which is driven by the excess surface area of the particle compared to a spherical particle with the same volume (CitationKoch and Friedlander 1990; CitationXiong and Pratsinis 1993), is not calculated as an average for the entire particle, but is implemented for each pair of neighboring primaries individually. This is important if the sintering time depends on the size of the primary particles as reported in (CitationRogak 1997; CitationTsantilis and Pratsinis 2000; CitationTsantilis et al. 2001). The primary particles are not equal-sized and change their size due to sintering and condensation processes. The current sintering model is included in the stochastic population balance solver developed by CitationCelnik et al. (2007).
In the next section the different processes implemented in the model are described in more detail. Afterwards the mathematical implementation is presented. Finally the model is fitted against experiments using data for the formation of SiO2 nanoparticles in a hot wall reactor from CitationSeto et al. (1997). Agglomerates are generated at 900°C and the change of the shape of the particles due to sintering is evaluated by heating the particles to different final temperatures. The primary particle diameter distribution and the average primary particle diameter is evaluated and computer generated TEM style images are created to compare the model with experimental data. The data structure is presented in the supplementary material.
2. PARTICLE STRUCTURE MODEL
2.1. General Description and Reaction Rates
In the current work the system is represented by a stochastic particle ensemble. These particles interact according to the rates for the different processes: sintering, condensation, coagulation and inception. The number of stochastic particles is limited by the maximum number of particles Nmax , which determines the accuracy of the simulation. Eibeck and Wagner (Citation2001, Citation2003) have demonstrated for a time-independent univariate system undergoing coagulation and fragmentation that the system converges to the solution of the model if N →∞. Each particle contains a data structure where the primary particles are stored that belong to the particle. The data structure stores also the connections between the primaries and the sintering level between each pair of touching primaries.
The rates for the processes () included in the current model are:
-
Inception: A new particle is inserted in the particle ensemble each time two molecules from the gas-phase collide. The rate is calculated using the transition kernel (Patterson et al. 2006b) which is the harmonic mean of the slip-flow and free molecular kernels.
-
Coagulation: The coagulation rate R coag of two particles Pi and Pj is proportional to the free molecular collision kernel:
where m is the mass and dc the collision diameter. -
Condensation: A molecule from the gas-phase which collides with a particle sticks on the particle and increases the mass of the particle. This process is implemented in the model assuming that the collision of a molecule from the gas-phase and a particle can be calculated using the free molecular collision kernel for the particle and a single molecule from the gas phase. The rate takes the form
where C, m, and d are the gas-phase concentration, mass, and collision diameter of the condensing species, respectively. dc is the collision diameter of the particle, η the efficiency of the collision. -
Sintering: The sintering rate
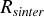 depends mainly on the excess surface of the particle over a spherical particle with the same mass. A particle reduces the free surface to minimize the free energy. This results in the rounding of the particle, finally to a sphere. The asymptotic equation (CitationKoch and Friedlander 1990)
describing the sintering of a particle with the surface a, a spherical surface
depends mainly on the excess surface of the particle over a spherical particle with the same mass. A particle reduces the free surface to minimize the free energy. This results in the rounding of the particle, finally to a sphere. The asymptotic equation (CitationKoch and Friedlander 1990)
describing the sintering of a particle with the surface a, a spherical surface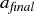 (the surface of a sphere with the same volume as the particle) and a characteristic sintering time τ, developed by CitationFrenkel (1945), has been used to calculate the sintering. The sintering has been calculated by solving Equation (Equation4) individually for each pair of neighboring primary particles. More details about the implementation of sintering will be given in the next section.
(the surface of a sphere with the same volume as the particle) and a characteristic sintering time τ, developed by CitationFrenkel (1945), has been used to calculate the sintering. The sintering has been calculated by solving Equation (Equation4) individually for each pair of neighboring primary particles. More details about the implementation of sintering will be given in the next section.
This model extends the previous two-dimensional surface-volume model (CitationPatterson and Kraft 2007) and the primary particle list model (CitationWest et al. 2007). The surface-volume model tracks the volume and the surface area of each particle as independent variables. The primary particle list model extends the surface-volume model by adding a list of primary particles for each particle. This primary particle list allows the generation of TEM like pictures, however the actual structure of the particle is not stored in these models and the extent of sintering is calculated by solving Equation (Equation4) as an average for the entire particle. This is an issue especially when the characteristic sintering time depends on the size of each primary particle as reported in previous studies (CitationRogak, 1997; CitationTsantilis and Pratsinis 2000; CitationTsantilis et al. 2001) and the primaries have a broad size distribution.
2.2. State Space
In this model it is assumed that each particle
-
[C] ij stores the common surface of primary Pi and Pj . This value is changed due to sintering.
-
[I] ij provides the sum of the surfaces of primary Pi and Pj before they start to sinter.
-
[S] ij contains the surface of a sphere with the same volume than primary Pi and Pj .
The surface of the particle is approximated by the sum of the surface of all the primary particles assuming that the primaries are spherical:
2.3. Jump Processes
The mathematical implementation of the inception, coagulation, condensation, and sintering processes are presented in this section.
2.3.1. Inception
The collision of two molecules in the gas phase is treated as a particle coagulation event and a spherical particle consisting of one primary containing the volume of the two molecules is inserted into the particle ensemble. An inception event increases the number of particles N by 1.
2.3.2. Coagulation
The coagulation of particle pi and particle Pj is implemented as follows in the model:
The matrix C(Pk ) has the dimension l × l and is calculated as:
2.3.3. Condensation
In a condensation process a molecule from the gas phase attaches to a particle Pj . The rate for this process is calculated using the free molecular collision kernel for a collision between the molecule from the gas-phase and the particle (Equation [3]). This is modeled by increasing the volume of a randomly selected primary Pi of particle Pj by the volume of the colliding molecule. It is assumed that the sintering rate is not affected by a condensation process.
2.3.4. Sintering
The sintering process is modeled assuming that the excess agglomerate surface area over that of a spherical particle with the same mass decays exponentially (CitationFrenkel 1945; CitationKoch and Friedlander 1990; CitationXiong and Pratsinis 1993). The model described in these studies, which originally describes the average sintering of the entire particle has been modified to describe the sintering between two neighboring primaries Pi and Pj . The matrix C, that contains the common surface of the neighboring primaries is changed as follows:
In the following we illustrate this using a simple example. The sintering process of a particle consisting of three primaries p 1, p 2, and p 3 is illustrated in . Assuming that the three primaries just collided and have not yet sintered (), the matrices C, I, and S are:
FIG. 2 Evolution of a particle consisting of three primary particles due to sintering. (a) shows the particle assuming the primaries p 1, p 2, and p 3 just collided. The primaries are just touching. The primaries have started to sinter in (b). After some time primary p 1 and p 2 are completely sintered and replaced by a new primary with the same mass than p 1 and p 2 ((c)). The numbering of the primaries is updated. The particle is completely sintered in (d).
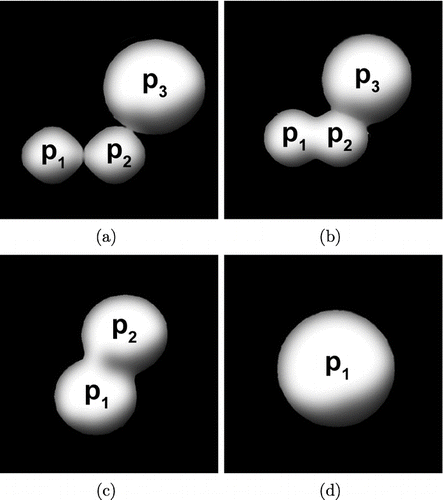
The new primary p 1 has the same volume as the former primaries p 1 and p 2 before the sintering event. The primaries p 1 and p 2 continue to sinter until one spherical primary remains ().
A detailed description of the data structure can be found in the supplementary material.
2.4. Algorithm
The implementation of the algorithm involves the following steps:
-
Set start time t ← t0 and the initial system x ← x0
-
Calculate an exponentially distributed waiting time
-
Increment time variable t←t+dt.
-
If
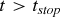 then end.
then end. -
Update the sintering level by integrating Equation (Equation16) for the time dt for all the particles.
-
Choose a process i according to the probability:
-
Perform process.
-
Go to step 2.
3. VALIDATION OF THE MODEL
The model has been fitted against experimental results from CitationSeto et al. (1997). The simulation consists of three consecutive steps:
-
The particles are formed at 900°C starting from a gas mixture of SiO2, O2, and N2 for the time th .
-
After the creation time th the particles are reheated within 0.1 s to different final temperatures.
-
The particles remain at the final temperature Tf .
FIG. 3 Different steps in the simulation: The particles grow due to coagulation and condensation at 900°C. The temperature is increased at t = th to the final temperature Tf in 0.1 s. At the final temperature the particles shrink and the sphericity increases due to sintering.
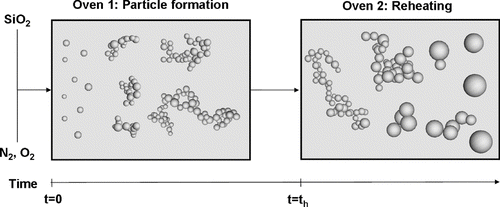
The timescale on the graphs represents the entire simulation time. The simulation starts with a mixture of silica (SiO2), nitrogen, and oxygen at 900°C. Chemical reactions in the gas-phase are not included due to the lack of reaction rates and thermochemistry. The pressure was set to 1 bar during the entire simulation. In the beginning of the particle formation each time two silica molecules collide a particle consisting of one primary with the mass of two silica molecules is created. The collision of a particle with a SiO2 molecule increases the mass of the particle by the mass of a SiO2 molecule. The SiO2 molecules are quickly completely converted to particles.
The material dependant sintering parameters A, E, and ![]() shown in have been determined by fitting the model to the experiments of CitationSeto et al. (1997). The determined values are in the same order as those reported in a study from CitationTsantilis et al. (2001).
shown in have been determined by fitting the model to the experiments of CitationSeto et al. (1997). The determined values are in the same order as those reported in a study from CitationTsantilis et al. (2001).
TABLE 1 Parameters
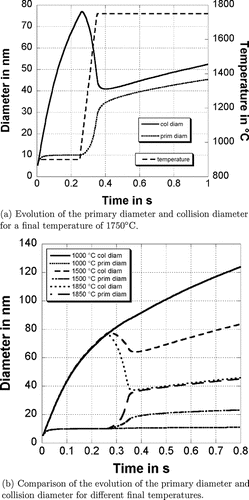
shows the average primary particle diameter, the collision diameter and the temperature profile for a simulation where the temperature has been increases at th =0.25 s from 900°C to 1750°C within 0.1 s. In the beginning the particles remain spherical and consist of only one primary particle because the primaries are quite small and therefore the average sintering time is much shorter than the average time between two collision events. The sintering time increases due to the growth of primaries and when the average primary diameter reaches about 7 nm, the particles become nonspherical (the particles consist of more than one primary). This stage is reached after 0.008 s.
The average primary diameter of the particles grows due to sintering and condensation processes up to 10 nm. At this stage the sintering time is much higher than the average time between two collision events. Therefore further collision events result in the formation of agglomerates consisting of many primary particles and sintering is negligible. The average primary diameter remains constant whereas the collision diameter increases. The temperature is increased after 0.25 s to 1750°C within 0.1 s. The temperature increase reduces the characteristic sintering time and the primary particles sinter together. The average primary diameter increases to 36 nm and the collision diameter decreases to 40 nm shortly after the temperature increase. The particles reach their final shape very quickly after the temperature increase. The primary particle and collision diameter size distribution follows the step function of the temperature instantaneously; however the collision diameter and the primary diameter still increase slightly due to further collisions between the particles and sintering between the primaries after the temperature increase. The collision diameter is close to the average primary diameter because the particles reach a nearly spherical shape and are composed of only a small number of primary particles. presents the evolution of the average primary and collision diameter for different final temperatures. The temperature has again been increased after 0.25 s from 900°C to the final temperature during 0.1 s. The average collision diameter of the particles before reheating depends strongly on the time the particles are in the first reactor. The longer the particles are in the first reactor (where the particles are formed), the bigger is the collision diameter. In contrast the primary particle diameter does not depend so strongly on the time in the first reactor, only on the temperature.
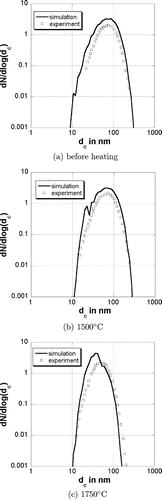
shows the primary particle size distribution of the particles before reheating after 0.25 s at 900°C, after reheating to 1500°C and after reheating to 1750°C. The temperature profile is the same as in the previous simulations. The distribution has been normalized to the total number of primary particles and divided by Δlog(d p ). The dots are experimental values from CitationSeto et al. (1997). The primary particle size distribution before heating is quite narrow and no particle is bigger than 20 nm. The primary particle size increases at higher temperatures and the distribution gets broader. The corresponding collision diameter distributions are presented in . The experimental values from CitationSeto et al. (1997) are the mobility diameter of the particles. In the comparison it is assumed that the mobility diameter is equal to the collision diameter. The collision diameter distribution gets narrower with an increasing temperature due to the sintering of the particles.
FIG. 5 Primary particle size distribution for different final temperatures. The dots are experimental values from CitationSeto et al. (1997).
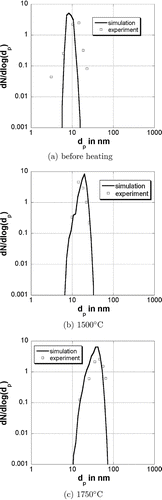
FIG. 6 Collision diameter size distribution for different final temperatures. The dots are experimental values from CitationSeto et al. (1997).
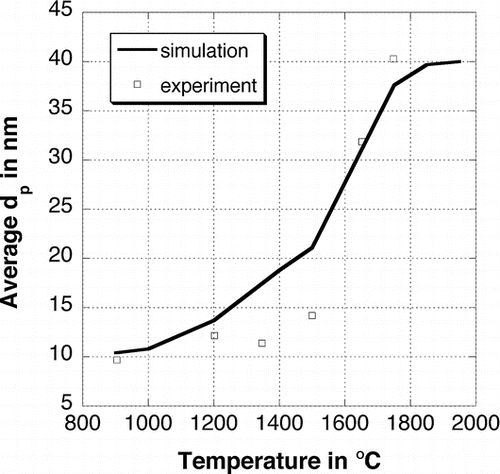
The average primary particle diameter for different final temperatures is shown in . The dots are again experimental values from CitationSeto et al. (1997). The particles do not sinter significantly before reaching 1500°C. After 1500°C the primary particle size increases strongly with increasing temperature. The particles are nearly spherical at 1850°C and most of the particles consist of only one primary particle, therefore further sintering processes are not possible. The primary particle diameter is equal to the collision diameter.
FIG. 7 Average primary particle diameter for different final temperatures. The dots are experimental values from CitationSeto et al. (1997).
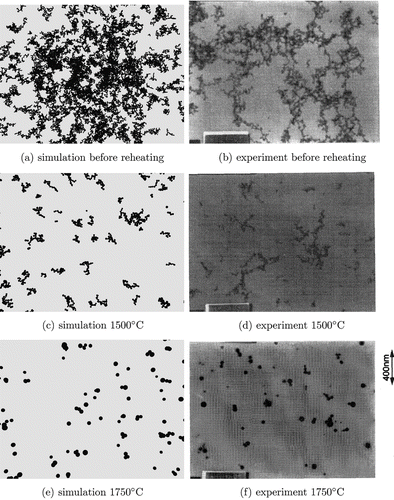
The particle structure does not depend on the collision diameter of the particles before heating. illustrates the average primary particle and collision diameters found for different times at which the temperature is increased (and hence for different collision diameters because particles held for a longer time at 900°C have bigger collision diameters). The particles have a collision diameter of 69 nm and 83 nm for a formation time of 0.2 s and 0.3 s, respectively. It is demonstrated that neither the primary particle diameter nor the collision diameter after the reheating depends on the particle size before reheating. Also, the primary particle size distribution (see ) does not depend on the size of agglomerates before heating. Only the final temperature determines the shape of the particles. This is important if experimental values, where the residence time in the furnace is not clear, are compared to theoretical values. Computer generated TEM style images are compared to experimental values from CitationSeto et al. (1997) in . The theoretical pictures have been created by colliding the primary particles that belong to each particle randomly. These particles have been shot at a plane to simulate the collection of particles using a TEM grid in an experiment. It is demonstrated that the particles shrink with increasing temperature and get rounder. The particles are nearly spherical at 1750°C.
FIG. 8 Average primary particle and collision diameter for different times th where the temperature has been increased from 900°C to 1750°C during 0.1 s.
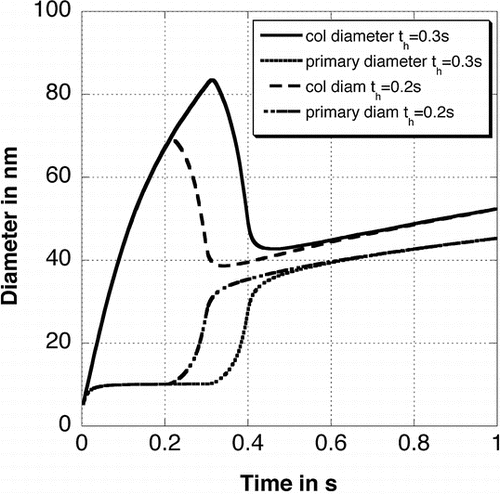
FIG. 9 Primary particle size distribution for different creation times t h where the temperature has been increased from 900°C to 1750°C during 0.1 s.
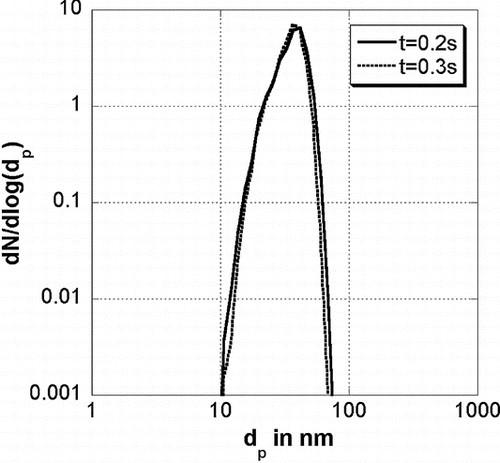
FIG. 10 TEM style images at different final temperatures compared against experimental pictures from CitationSeto et al. (1997).
4. DISCUSSION
The sintering parameters A, E, and d p,min, presented in , depend on the material and are determined by fitting the model to experimental data from Seto et al. The parameters presented by CitationTsantilis et al. (2001) have been used as a starting guess. The difference in the parameters presented here and those used by Tsantilis arises from the different sintering models.
The sintering process starts already at temperatures that are lower than the melting point of the material; however, even at temperatures higher than the melting point the particles are not completely spherical. It is demonstrated that the primary particle diameter after reheating does not depend on the size of the particles before reheating. It should also be noted that the residence time of the particles at the final temperature does not have a significant influence on the shape of the particle. The particles reach their final shape shortly after the temperature has been increased. The dependence of the primary particle size of SiO2 concentration in the gas-phase has also been investigated. The final primary particle size distribution is very insensitive to a change of the concentration of SiO2 molecules in the gas-phase. A higher concentration of SiO2 increases only the growing rate of the diameter of the particles and consequently the number of primary particles in each particle, but the primary particle diameter is not affected.
A sintering level (Equation [8]) has been defined to include the mass transfer between neighboring particles in the calculation of the characteristic sintering time. This is an empirical definition; however it is able to match experimental data. The sintering time without the mass transfer between the primary particles would be unrealistic low. In a further study a more physical expression for the sintering level and the mass transfer between the particles using stochastic Monte Carlo algorithms similar to the method presented by CitationAkhtar et al. (1994) will be developed.
The characteristic sintering time is calculated assuming that it is determined by the smaller primary particle (CitationYadha and Helble 2004), which is especially important when a big primary particle is connected to a small one. This assumption has been fortified by simulating the system using the average primary particle diameter of the two neighboring particles as diameter. In this case the sintering time for small primaries connected to large primaries is very long and the final particles contain much more small primaries which is not consistent with experimental results.
The model enhances the previous primary particle list model (CitationWest et al. 2007) where sintering has been calculated as an average for the entire particle. This is especially an issue when the primary particle size is broad because sintering of small primaries is underestimated. A comparison of the two models has demonstrated that the primary particle list model is not able to match the experimental observations for the formation of SiO2 nanoparticles (the data obtained from the primary particle list model is not presented in this study).
The particle size and the primary particle size distributions are calculated from the first coagulation of two molecules and no assumption is made concerning the size distribution of the first particles. It is only determined by the coagulation of molecules from the gas-phase, whereas most sectional models assume a uniform primary particle size distribution at the beginning of the simulation.
The gas-phase chemistry is not yet included in the current work due to the lack of existing thermochemistry and reaction rates for the formation of SiO2. However the model is able to include a full gas-phase chemistry and in a further study a chemical mechanism for the formation of SiO2 from TEOS will be developed using quantum chemical methods like density functional theory. The computational performance of the model has been increased by using a binary tree data structure to store the particles and the primary particles. A typical simulation with 16384 stochastic particles takes about 3 h of CPU time on one core of a 3 GHz Intel Harpertown cluster.
5. CONCLUSION
A stochastic particle model including unequal-sized primary particles and the structure of the particle has been presented and fitted to experimental data. This model includes coagulation, condensation, inception, and sintering. Each particle is described by the number of primary particles, the sintering level between neighboring primary particles and the volume of each primary particle. No assumption about the initial particle size distribution is made. The sintering is not calculated as an average but individually for each pair of neighboring particles. This is important if the primary particle size distribution is broad because the characteristic sintering time depends strongly on the size of the primaries. The underprediction of the sintering rate of small primary particles is avoided. The particles have been stored in a binary tree data structure to enhance the computational performance. The model is able match experimental data for the formation of SiO2 nanoparticles in a hot wall reactor. The computer generated TEM images are comparable to experimental observations. The primary particle size distribution depends only on the highest temperature in the reactor and is very insensitive to the SiO2 concentration in the gas-phase and the size of the particles before reheating.
Financial support from the EPSRC Grant No. EP/E01724X/1 and EP/C547241/1 is gratefully acknowledged. The authors thank Dr. Murray Height from HeiQ Materials for financial and scientific support.
REFERENCES
- Akhtar , M. K. , Lipscomb , G. G. and Pratsinis , S. E. 1994 . Monte Carlo Simulation of Particle Coagulation and Sintering . Aerosol Sci. Tech. , 21 : 83 – 93 .
- Celnik , M. S. , Patterson , R. I. A. , Kraft , M. and Wagner , W. 2007 . Coupling a Stochastic Soot Population Balance to Gas-Phase Chemistry Using Operator Splitting . Combust. Flame , 148 ( 3 ) : 158 – 176 .
- Celnik , M. S. , Sander , M. , Raj , A. , West , R. H. and Kraft , M. 2009 . Modelling Soot Formation in a Premixed Fame Using an Aromatic-Site Soot Model and an Improved Oxidation Rate . Proc. Combust. Inst. , 32 : 639 – 646 .
- Ehrman , S. H. 1999 . Effect of Particle Size on Rate of Coalescence of Silica Nanoparticles . J. Colloid Interface Sci. , 213 ( 1 ) : 258 – 261 .
- Eibeck , A. and Wagner , W. 2001 . Stochastic Particle Approximation for Smoluchowski's Coagulation Equation . Ann. Appl. Probab. , 11 ( 4 ) : 1137 – 1165 .
- Eibeck , A. and Wagner , W. 2003 . Stochastic Interacting Particle Systems and Nonlinear Kinetic Equations . Ann. Appl. Probab. , 13 ( 3 ) : 845 – 889 .
- Frenkel , J. 1945 . Viscous Flow of Crystalline Bodies Under the Action of Surface Tension . J. Phys. , 9 : 385 – 391 .
- Heine , M. and Pratsinis , S. E. 2007 . Polydispersity of Primary Particles in Agglomerates Made by Coagulation and Sintering . J. Aerosol Sci. , 38 : 17 – 38 .
- Karlsson , M. N. A. , Deppert , K. , Karlsson , L. S. , Magnusson , M. H. , Malm , J.-O. and Srinivasan , N. S. 2005 . Compaction of Agglomerates of Aerosol Nanoparticles: A Compilation of Experimental Data . J. Nanopart. Res. , 7 : 43 – 49 .
- Kim , H. J. , Jeong , J. I. , Park , Y. , Yoon , Y. and Choi , M. 2003 . Modeling of Generation and Growth of Non-Spherical Nanoparticles in a Co-Flow Flame . J. Nanopart. Res. , 5 : 237 – 246 .
- Koch , W. and Friedlander , S. K. 1990 . The Effect of Particle Coalesecence on the Surface Area of Coagulating Aerosol . J. Colloid Interface Sci. , 140 : 419 – 427 .
- Lee , B. W. , Jeong , J. I. , Hwang , J. Y. , Choi , M. and Chung , S. H. 2001 . Analysis of Growth of Non-Spherical Silica Particles in a Counterflow Diffusion Flame Considering Chemical Reactions, Coagulation and Coalescence . J. Aerosol Sci. , 32 : 165 – 185 .
- Lehtinen , K. E. J. and Zachariah , M. R. 2002 . Energy Accumulation in Nanoparticles Collision and Coalescence Processes . J. Aerosol Sci. , 33 : 357 – 368 .
- Morgan , N. , Wells , C. , Kraft , M. and Wagner , W. 2005 . Modelling Nanoparticles Dynamics: Coagulation, Sintering, Particle Inception and Surface Growth . Combust. Theor. Model. , 9 ( 3 ) : 449 – 461 .
- Morgan , N. , Kraft , M. , Balthasar , M. , Wong , D. , Frenklach , M. and Mitchell , P. 2007 . Numerical Simulations of Soot Aggregation in Premixed Laminar Flames . Proc. Combust. Inst. , 31 : 693 – 700 .
- Mühlenweg , H. , Gutsch , A. , Schild , A. and Pratsinis , S. E. 2002 . Process Simulation of Gas-to-Particle-Synthesis Via Population Balances: Investigation of Three Models . Chem. Eng. Sci. , 57 : 2305 – 2322 .
- Nakaso , K. , Fujimoto , T. , Seto , T. , Shimada , M. , Okuyama , K. and Lunden , M. M. 2001 . Size Distribution Change of Titania Nano-Particle Agglomerates Generated by Gas Phase Reaction, Agglomeration, and Sintering . Aerosol Sci. Tech. , 35 : 929 – 947 .
- Patterson , R. I. A. and Kraft , M. 2007 . Models for the Aggregate Structure of Soot Particles . Combust. Flame , 151 : 160 – 172 .
- Patterson , R. I. A. , Singh , J. , Balthasar , M. , Kraft , M. and Norris , J. 2006 . The Linear Process Deferment Algorithm: A New Technique for Solving Population Balance Equations . SIAM J. Sci. Comput. , 28 : 303 – 320 .
- Patterson , R. I. A. , Singh , J. , Balthasar , M. , Kraft , M. and Wagner , W. 2006 . Extending Stochastic Soot Simulation to Higher Pressures . Combust. Flame , 145 ( 3 ) : 638 – 642 .
- Raj , A. , Celnik , M. S. , Shirley , R. S. , Sander , M. , Patterson , R. I. A. , West , R. H. and Kraft , M. 2009 . A Statistical Approach to Develop a Detailed Soot Growth Model Using Pah Characteristics . Combust. Flame , 156 : 896 – 913 .
- Rogak , S. N. 1997 . Modeling Small Cluster Deposition on the Primary Particles of Aerosol Agglomerates . Aerosol Sci. Tech. , 26 : 127 – 140 .
- Sander , M. , Raj , A. , Inderwildi , O. , Kraft , M. , Kureti , S. and Bockhorn , H. 2009 . The Simultaneous Reduction of Nitric Oxide and Soot in Emissions from Diesel Engines . Carbon , 47 : 866 – 875 .
- Schaefer , D. W. and Hurd , A. J. 1990 . Growth and Structure of Combustion Aerosols: Fumed Silica . Aerosol Sci. Tech. , 12 : 876 – 890 .
- Schmid , H. , Al-Zaitone , B. , Artelt , C. and Peukert , W. 2006 . Evolution of the Fractal Dimension for Simultaneous Coagulation and Sintering . Chem. Eng. Sci. , 61 : 293 – 305 .
- Schmid , H. , Tejwani , S. , Artelt , C. and Peukert , W. 2004 . Monte Carlo Simulation of Aggregate Morphology for Simultaneous Coagulation and Sintering . J. Nanopart. Res. , 6 : 613 – 626 .
- Seto , T. , Shimada , M. and Okuyama , K. 1995 . Evaluation of Sintering of Nanometer-Sized Titania Using Aerosol Method . Aerosol Sci. Tech. , 23 : 183 – 200 .
- Seto , T. , Hirota , A. , Fujimoto , T. , Shimada , M. and Okuyama , K. 1997 . Sintering of Polydisperse Nanometer-Sized Agglomerates . Aerosol Sci. Tech. , 27 : 422 – 438 .
- Singh , J. , Balthasar , M. , Kraft , M. and Wagner , W. 2005 . Stochastic Modelling of Soot Particle Size and Age Distribution in Laminar Premixed Flames . Proc. Combust. Inst. , 30 : 1457 – 1465 .
- Singh , J. , Patterson , R. I. A. , Kraft , M. and Wang , H. 2006 . Numerical Simulation and Sensitivity Analysis of Detailed Soot Particle Size Distribution in Laminar Premixed Ethylene Flames . Combust. Flame , 145 : 117 – 127 .
- Tsantilis , S. and Pratsinis , S. E. 2000 . Evolution of Primary and Aggregate Particle-Size Distributions by Coagulation and Sintering . AIChE , 46 : 407 – 415 .
- Tsantilis , S. , Briesen , H. and Pratsinis , S. E. 2001 . Sintering Time for Silica Particle Growth . Aerosol Sci. Tech. , 34 : 237 – 246 .
- West , R. H. , Celnik , M. S. , Inderwildi , O. R. , Kraft , M. , Beran , G. J. O. and Green , W. H. 2007 . Towards a Comprehensive Model of the Synthesis of TiO2 Particles from TiCl4 . Ind. Eng. Chem. Res. , 46 ( 19 ) : 6147 – 6156 .
- Xiong , Y. and Pratsinis , S. E. 1993 . Formation of Agglomerate Particles by Coagulation and Sinterings—Part I. A Two-Dimensional Solution of the Population Balance Equation . J. Aerosol Sci. , 24 : 283 – 300 .
- Yadha , V. and Helble , J. 2004 . Modeling the Coalescence of Heterogenous Amporphous Particles . J. Aerosol Sci. , 35 : 665 – 681 .