Abstract
A photo-acoustic absorption spectrometer (PAS) and a cavity ring down aerosol extinction spectrometer (CRD-AES) were used, in conjunction with Mie Theory, to measure the refractive index (RI) of absorbing polystyrene spheres (APSS). The PAS and CRD-AES were also used to measure the absorption and extinction enhancement after these APSS were coated in oleic acid. The experimental enhancements were then compared to predictions from coated-sphere Mie Theory. The measured absorption and extinction enhancements both agreed with modeled enhancements to within an average of 5%. A filter-based absorption technique (particle soot absorption photometer, PSAP) was also used to measure the absorption by the APSS and showed a significant size-dependent bias, as evidenced by the filter-based method measuring significantly lower absorption for both uncoated and coated APSS compared to the PAS. These results suggest the validity of applying photo-acoustics to measure the absorption enhancement created by semi-volatile atmospheric species coating absorbing particles.
INTRODUCTION
More certain in-situ measurements of aerosol absorption are required to improve the understanding of the direct radiative impact of atmospheric aerosols. While aerosol single scattering albedos (SSAs) of 0.7 to 0.9 are common for mixed aerosol types in many air masses (CitationBergstrom et al. 2007), black carbon (BC), the strongest aerosol radiative absorber in the atmosphere, can absorb up to 90% of the radiation encountered (SSA of 0.1 at 532 nm) (CitationZhang et al. 2008). Absorbing aerosol act to heat the atmospheric layer containing them and, in fact, aerosol layers with SSA values as high as 0.95 can have a positive top-of-atmosphere forcing depending on the underlying surface albedo (CitationRussell et al. 2002). Even though BC constitutes a small fraction of the global total aerosol mass burden, BC may contribute the equivalent of 1/4 of the warming caused by CO2 (IPCC 2007). Given this significant impact, it is necessary to obtain in-situ measurements of aerosol absorption to calculate the localized direct radiative forcing of these absorbing layers (CitationBates et al. 2006), to compare and calibrate remotely sensed data, such as sun photometers and satellites (CitationRussell et al. 2002; CitationSchmid et al. 2000; CitationSchmid et al. 2006), and to compare to and validate models (e.g., CitationMallet et al. 2006). Some in-situ measurements of absorption are also used to estimate mass loadings of BC (CitationBaltensperger 2003).
A complication in measuring aerosol absorption is the potential for absorbing and non-absorbing coatings on highly absorbing cores. Such coatings may enhance absorption through lensing of the incident light (CitationBond et al. 2006). The absorption enhancement effect has been studied through modeling studies (CitationBond et al. 2006; CitationFuller et al. 1999; CitationJacobson 2001) and some laboratory studies that used indirect techniques to measure absorption (e.g., the difference between extinction and scattering, CitationSchnaiter et al. 2005; CitationZhang et al. 2008). Recently, it has been shown that biases exist when measuring scattering by nephelometry for highly absorbing particles (CitationBond et al. 2009; CitationMassoli et al. 2009). Realistic absorption enhancement factors of 1 to 3 are predicted depending on the coating thickness and underlying core diameter (CitationBond et al. 2006) and enhancement factors up to 2 have been measured in the laboratory (CitationSchnaiter et al. 2005). CitationSchwarz et al. (2008) estimated absorption enhancement factors of 1.3 to 1.5 for internally mixed BC in the tropical troposphere. However, indirect techniques of measuring absorption are not likely to be accurate or sensitive enough to measure the enhancement effect under most ambient conditions. Direct measurement of the enhancement effect in ambient aerosol ensembles has so far not been achieved. Such direct field measurements require instrumentation capable of measuring the real atmospheric radiative effects of the coatings.
Traditional in-situ filter-based instruments that measure aerosol absorption are shown to have moderate uncertainties under ideal conditions (CitationArnott et al. 2005; CitationBond et al. 1999; CitationVirkkula et al. 2005) and large uncertainties when sampling complicated mixed aerosol ensembles (CitationLack et al. 2008; CitationSchmid et al. 2006). The deposition of the aerosol onto the filter, particularly liquid-like organic aerosol, potentially alters the physical state of the particles (CitationSubramanian et al. 2007) and introduces uncertainty to the absorption measurement. Therefore, it is necessary to explore the most appropriate methods of directly measuring absorption enhancement effects to ensure the magnitudes of absorption measured in the field and laboratory settings are accurate and can be accounted for in climate models.
The photo-acoustic spectrometer (PAS) is one method capable of directly measuring aerosol absorption with higher certainty than traditional filter-based methods (CitationArnott et al. 2003; CitationLack et al. 2006). This technique, however, has yet to be reliably validated with respect to absorption enhancement measurements. CitationCappa et al. (2008a) demonstrated the potential for the PAS to measure the enhancement effect by coating calibrated absorbing polystyrene spheres (CitationLack et al. 2006) with oleic acid and measuring the resulting absorption enhancement using a PAS. CitationSlowik et al. (2007) also used a PAS system to measure the absorption cross section of denuded and non-denuded soot from a fuel-rich ethylene flame, indicating an enhancement factor of ∼1.6 for the non-denuded compared to denuded soot.
A potential bias in the PAS technique arises whereby semi-volatile material within the aerosol may be evaporated by the incident laser energy. Although most of the energy absorbed by the aerosol is converted to acoustic energy, some can be converted to latent energy needed to evaporate semi-volatile material. This may lead to an under representation of the total absorption. CitationRaspet et al. (2003) investigated this effect theoretically (in the continuum flow regime) using conditions of a highly absorbing aerosol having the volatility of water and found that significant depression (in excess of 50%) of the PAS signal could result if the relative humidity of the aerosol was above 65%. In extending the CitationRaspet et al. (2003) calculations to the transition and molecular flow regime, CitationMurphy (2009) showed that the loss in signal is related to the absolute humidity and the accommodation coefficient of water and that acoustic energy loss would be minimized at low absolute humidities and small (⩽200 nm diameter) particle sizes. In fact, the signal depression is proportional to the vapor pressure of the semi-volatile material (CitationMurphy 2009). Given that most semi-volatile organics that partition significantly to the aerosol phase have vapor pressures several orders of magnitude lower than water (e.g., CitationCappa et al. 2008b), it is unlikely that semi-volatile organic material will depress the PAS signal. The PAS technique therefore is expected to provide a robust measure of the absorption enhancement effect created by organic coatings on absorbing cores. Here we present a study of the PAS response to organic coated and uncoated absorbing polystyrene spheres that provides experimental confirmation of this expectation.
Measurements
The PAS used in this study is similar to that described in CitationLack et al. (2006) and measures light absorption (b abs in Mm−1) at 532 nm. Aerosol extinction (b ext in Mm−1) was measured at 532 nm using a cavity ring down–aerosol extinction spectrometer (CRD-AES, CitationBaynard et al. 2007). Uncertainties in b abs and b ext are 5 and 1%, respectively, with detection limits of 0.5 Mm−1 for 5 s data acquisition interval. Aerosol number concentrations (CNAerosol) were measured using a condensation particle counter (CPC, TSI 3022AFootnote 1 ). The 3022A instrument measures all particles larger than about 7 nm. Reported measurement uncertainties are ±5% for all CN measurements. Multiple sizes of mono-disperse absorbing polystyrene spheres (APSS) (Duke Scientific Corp.1, Palo Alto, CA) were atomized using a constant output atomizer (TSI 30761), dried with a diffusion drier, and size selected using a differential mobility analyzer (DMA, TSI 30711). A Radiance Research1 3-wavelength Particle Soot Absorption Photometer (PSAP) also measured aerosol absorption. The PSAP data were corrected using the correction described in CitationLack et al. (2008). This correction includes the filter transmission, flow and basic sample area corrections from CitationBond et al. (1999), an additional filter sample area correction (CitationSheridan et al. 2005) and the scattering corrections of CitationVirkkula et al. (2005). Absorption at 530 nm is presented in this study.
The dried, size-selected APSS were distributed directly to the PAS, PSAP, CRD-AES, and CPC instruments or were coated with oleic acid (Aldrich Chemical Co.1, refractive index (RI) at 532 nm = 1.46, density 0.895 g cm−3), size selected for a second time (DMA, TSI 3080), and then directed to the instruments. The coatings were produced by passing the aerosol flow over a heated reservoir of oleic acid. The reservoir consisted of a “V” shaped Pyrex tube (1.2 cm diameter, 30 cm long) with ∼2 ml of oleic acid in the bottom of the tube and the bend in the tube was wrapped in heating tape. Increasing the reservoir temperature generated thicker coatings on the APSS particles via gas-to-particle condensation. The thickness of oleic acid coatings was determined from the difference in the DMA-measured diameters of the coated and uncoated APSS.
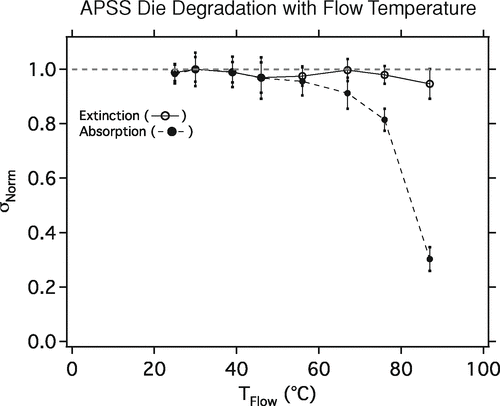
It was found that elevated flow temperatures degraded the APSS dye (see , in Appendix). Therefore, the heated section length was minimized to include only 2 inches of heating tape on either side of the liquid reservoir. This configuration allowed the aerosol flow temperatures to be maintained between 20°C and 40°C where degradation was not a problem.
Modeling
The CitationBohren and Huffman (1983) implementation of Mie Theory was first used to determine the effective refractive index (RI) of the uncoated APSS particles. As the manufacturer's dying process was done after the spheres were formed, the resulting APSS particles may not have a homogeneous distribution of dye throughout. Thus, any derived refractive index may represent an effective refractive index for the potentially inhomogeneous material. To model the expected absorption enhancement of APSS due to organic coatings, the coated systems were modeled using Mie Theory adapted for the coated sphere model (CitationBohren and Huffman 1983) having an absorbing core and a non-absorbing organic coating. Further details are provided in the following section.
RESULTS
Uncoated APSS
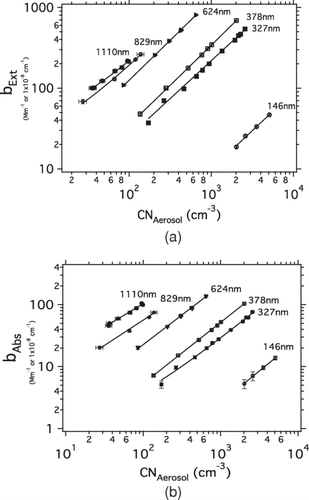
Regression of the CRD-AES measured extinction versus particle number concentration provides the extinction cross-sections (σExt) for each APSS size (). Normalizing this cross-section to the geometric cross-section of the APSS particles provides the per-particle extinction efficiency, or QExt. The corresponding absorption versus aerosol number concentration () provides the absorption cross-section (σAbs) from which the absorption efficiency (QAbs) was determined.
FIG. 1 (a) bExt versus CNAerosol for all APSS Sizes, (b) bAbs versus CNAerosol for all APSS Sizes. Slopes of these data represent the optical cross-sections (σ) for each APSS size. The quality of each fit (hidden by the log/log nature of the plot) is provided in as a 2 – sigma precision in each fit.
The RI of the spherical APSS was determined by using Mie theory to fit the real and imaginary RI to measured size dependent QExt and QAbs (). Note that we simultaneously fit QExt and QAbs. Using Mie theory and Chi-square minimization, we were able to determine a single RI for the APSS, indicating that the manufacturers dye penetrated through every APSS size for the single batch of APSS particles studied here. Previous batches indicated incomplete dyeing. A RI of 1.60(±0.03) + 0.045(±0.004)i was determined for the APSS, with uncertainties calculated from the Chi Square minimization results as outlined in Press et al. (1986, p. 533). It must be noted that the derived real RI compares well to the reported real RI for non-dyed polystyrene spheres (Real RI = 1.6, CitationPettersson et al. 2004). The RI derived from provides the RI for the absorbing cores in the Coated APSS section. provides the details of the APSS studied, including measured size, σExt, σAbs, QExt, QAbs, and SSA.
FIG. 2 Experimental QExt and QAbs (█ and ⊗) versus particle diameter. Lines are the modeled QExt (- – -) and QAbs (____) from fitting the real and imaginary refractive index to the experimental data using Mie Theory.
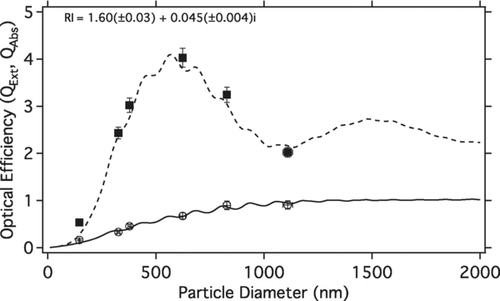
TABLE 1 Measured APSS diameters, extinction and absorption cross-sections, extinction and absorption efficiency factors, and SSA
Coated APSS
The σExt and σAbs for APSS coated in non-absorbing material were determined in the same manner as for uncoated APSS, shown in and . Up to 7 coating thicknesses were applied to each of the 327, 378, and 624 nm APSS. For each coating thickness at least 3 levels of CNAerosol were measured to obtain the regressions, similar to those shown in .
Experimental extinction enhancement factors (EExt-Exp) were calculated as the ratio of coated APSS σExt to uncoated APSS σExt (from ). The ratio of coated APSS σAbs to uncoated APSS σAbs (from ) provides the experimental absorption enhancement factor, EAbs-Exp. shows EExt-Exp (symbols) and EAbs-Exp (symbols) for the 327, 378, and 624 nm diameter APSS sizes coated with varying thicknesses of oleic acid.
FIG. 3 (a) Measured extinction enhancement factors (EExt-Exp) for all coated APSS sizes. (b) Measured absorption enhancement factors (EAbs-Exp) for all coated APSS sizes. The 327 nm data (gray) is placed on an offset axis (right axis) for clarity. Uncertainty in EExp is that propagated through both uncoated and coated optical cross sections. The extinction and absorption enhancement predicted by 2-Layer Mie theory (EExt-Mod, EAbs-Mod) is shown as dashed lines. Uncertainty in modeled EExt-Mod and EAbs-Mod shown as solid lines. All uncertainties shown are 1 standard deviation.
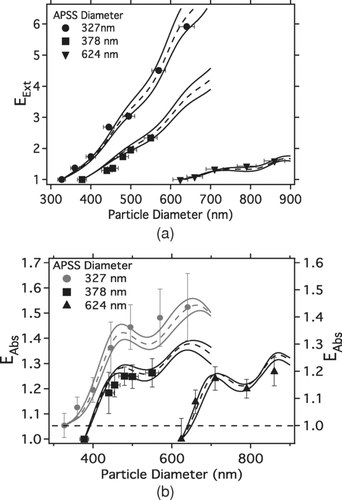
Coated sphere Mie theory was used to model the expected enhancements using the absorbing APSS core RI (from ), the non-absorbing oleic acid coating RI (RI = 1.46 + 0.0i), and the oleic acid coating thickness (from the DMA diameter measurements of coated and uncoated APSS). The theoretical σAbs for the coated APSS was then compared to the theoretical σAbs for the corresponding uncoated APSS size (determined from coated sphere Mie theory). The ratio of the two provided the modeled absorption enhancement factor (EAbs-Mod), which is also shown in as the dashed lines. The modeled extinction enhancement (EExt-Mod) was also calculated in this way. The solid lines within show the 1-standard deviation for the modeled enhancements, calculated using the uncertainties in the derived APSS RI.
DISCUSSION
Measured extinction and absorption enhancements due to the oleic acid coating () compare very well with coated sphere Mie theory. The EExt-Exp and EAbs-Exp are, on average, within 5% of their expected, modeled ranges. These results suggest that the complex RI determined for the uncoated APSS by the combination of the PAS and CRD-AES results () is an appropriate representation for the uncoated APSS system and the coated sphere Mie model appropriately predicts the measured optical properties of the particles.
Previous validation of the coated sphere Mie theory was not to be found in the literature, despite its wide use. If we assume that the coated sphere Mie theory provides accurate results for 2-layer systems, then the differences between EAbs-Exp and EAbs-Mod must indicate how well the PAS can measure the absorption enhancements. The comparison between the measured and modeled absorption enhancements for all coated APPS sizes imply an accuracy of 5% for the PAS measurements.
Potential sources for inaccuracies in the PAS measurements may arise through three mechanisms. First, the PAS cannot physically measure EAbs to better than 5% due to fundamental instrument limitations (CitationLack et al. 2006), indicating that a 5% accuracy in measured EAbs is a reasonable result. The second possible mechanism is a low bias in the PAS measurement due to evaporation of volatile material, as described in the introduction. If such a bias were significant, then the observed absorption would be too low due to suppression of the acoustic signal from evaporation of oleic acid. The absence of a systematically low bias in EAbs-Exp experimentally demonstrates that evaporation is not significantly influencing the PAS signal. This is consistent with oleic acid having a vapor pressure that is very low compared to water. The third possible bias mechanism involves the possibility that the energy relaxation kinetics of large particles might be slow relative to the acoustic period within the PAS (CitationRaspet et al. 2003). This mechanism could plausibly affect the coated systems for the largest APSS diameters studied here; however, a systematically low bias relative to Mie theory was not observed at the larger sizes (). The calculated magnitude of this kinetic effect is negligible for these experiments (see Appendix).
In summary, within the boundaries of the experimental system, the PAS measured the expected absorption enhancement to within 5% of that expected by Mie theory. Therefore, we expect that under conditions reasonably representative of the ambient atmosphere, absorption measured by the PAS will include accurate representation of the absorption enhancement of absorbing cores coated by a semi-volatile material (excluding, under some conditions, water).
Comparison of Absorption Methods
A filter-based absorption technique (PSAP) was also operated during the APSS experiments. Here we compare the PSAP and PAS measurements in an effort to assess the utility of the filter-based techniques for the study presented. The ratio of PSAP-measured and PAS-measured absorption at ∼532 nm (RAbs= bAbs,PSAP/bAbs,PAS) for un-coated APSS is shown in (solid circles). Overall the PSAP shows a systematic size dependent bias, compared to the PAS absorptions. For smaller sizes of APSS (up to ∼350 nm diameter) R abs was 1.05–1.10. This difference between the PSAP and the PAS is within the combined uncertainties of the techniques and is consistent with other PSAP and PAS comparisons from ambient sampling (CitationLack et al. 2009; CitationVirkkula et al. 2005). For larger particle diameters, however, RAbs decreased to ∼0.5. Similar behavior is observed for the 327 nm APSS coated in oleic acid (open circles in ) and for the larger coated APSS sizes (not shown).
FIG. 4 Ratio of PSAP and PAS measured absorption for all uncoated APSS (solid circles) and the 327 nm APSS coated with Oleic acid (open circles).
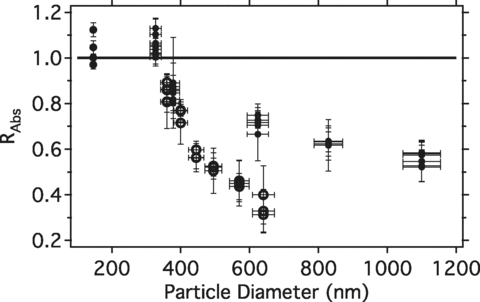
FIG. A1 Response of extinction and absorption optical cross-sections (σExt and σAbs) of APSS to sample flow temperature. Above approximately 50°C the APSS dye appears to degrade.
It is likely that this low bias in the PSAP measurement is linked to the enhanced forward scattering from the larger particles increasing the light transmission through the filter leading to reduced absorption readings. An increase in light transmission through the filter may also provide more opportunity for multiple scattering within the filter, which is known to lead to erroneously large absorption measurements. A full evaluation of this size dependent bias is beyond the scope of this study. Note that in these mono-disperse experiments the standard correction for the PSAP scattering bias (CitationBond et al. 1999; CitationVirkkula et al. 2005), which was developed for poly-disperse aerosols, does not apply. CitationSheridan et al. (2005) also observed a breakdown in the standard correction for aerosol with low single scattering albedos (<0.8). Fundamentally, any filter-based technique will have potential errors when studying enhancement due to coatings because the morphology of the coatings can change when the particles are deposited on the filter.
It is difficult to discuss the current size dependent bias without considering a previously identified absorption enhancement bias from organic aerosol (CitationCappa et al. 2008a; CitationLack et al. 2008), which has recently been indicated within the study of CitationKondo et al. (2009). The organic bias may be contributing to the data presented here, however the exact mechanisms of that bias are unknown and here the size dependent biases are obviously outweighing any enhancement effect by the coated APSS in the PSAP (either the expected enhancement or PSAP bias enhancement due to organic coated particles). These results should be considered carefully for any experimental attempt to separate and quantify the recently identified biases. Combining the size dependent uncertainties with the other recently identified uncertainties for the filter-based methods (i.e., CitationCappa et al. 2008a; CitationLack et al. 2008) suggests caution in the interpretation of aerosol absorption measurements (based on filter transmission) containing mono-disperse or large (>500 nm diameter) particles, air masses with variable size distributions, or air masses containing organic aerosol.
CONCLUSION
Measurements using a CRD-AES and PAS demonstrate that the techniques are capable of measuring complex refractive indices for absorbing spheres and the enhancements in extinction and absorption, respectively, caused by organic coatings on absorbing spherical particles to within ∼5% of the values expected from Mie theory and coated sphere Mie theory. Two potential biases in the PAS instrument (volatile compound evaporation and slow energy dissipation for large particles) were shown to be insignificant for the coated particle system studied. This instrument validation allows for future evaluation of absorption enhancements in the atmosphere using the PAS technique. A filter-based absorption technique (PSAP) was shown to have significant particle size dependent biases under the conditions of this study. This may have implications for the accuracy of absorption measured by these filter-based techniques.
APPENDIX
Calculation of Loss in PAS Signal Due to Heat Transfer in Large Particles
The time constant for particle heating in dry continuum flow (t) is given by Equation (Equation1) (CitationMurphy 2009);
This work was funded, in part, by the NOAA Climate Change Program, the Office of Science (BER), Department of Energy (Atmospheric Science Program) grant No. DE-FG02–05ER63995, the Atmospheric Chemistry Program of the National Science Foundation, grant No. ATM-0525355. ESC was funded by the NASA Earth System Science Fellowship program.
Notes
1Certain commercial equipment, instruments, or materials are identified in this article in order to adequately specify the experimental procedure. Such identification does not imply recognition or endorsement by the National Oceanic and Atmospheric Administration, nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
REFERENCES
- Arnott , W. P. , Hamasha , K. , Moosmuller , H. , Sheridan , P. J. and Ogren , J. A. 2005 . Towards Aerosol Light-Absorption Measurements with a 7-Wavelength Aethelometer: Evaluation with a photoacoustic Instrument and 3-Wavelength Nephelometer . Aerosol Sci. Technol. , 39 : 17 – 29 .
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. , Slaton , W. , Hand , J. L. , Kreidenweis , S. M. and Collet , J. L. Jr. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res. , 106 ( AAC ) : 15 – 11 .
- Baltensperger , U. 2003 . The Global Atmospheric Watch Aerosol Programme . Geophys. Res. Abstracts , 5 : 12284
- Bates , T. S. , Anderson , T. L. , Baynard , T. , Bond , T. , Boucher , O. , Carmichael , G. , Clarke , A. , Erlick , C. , Guo , H. , Horowitz , L. , Howell , S. , Kulkarni , S. , Maring , H. , McComiskey , A. , Middlebrook , A. , Noone , K. , O'Dowd , C. D. , Ogren , J. , Penner , J. , Quinn , P. K. , Ravishankara , A. R. , Savoie , D. L. , Schwartz , S. E. , Shinozuka , Y. , Tang , Y. , Weber , R. J. and Wu , Y. 2006 . Aerosol Direct Radiative Effects over the Northwest Atlantic, Northwest Pacific, and North Indian Oceans: Estimates Based on In-Situ Chemical and Optical Measurements and Chemical Transport Modeling . Atmos. Chem. Phys. , 6 : 1657 – 1732 .
- Baynard , T. , Pettersson , A. , Lovejoy , E. , Brown , S. , Lack , D. , Massoli , P. , Osthoff , H. , Ciciora , S. , Dube , B. and Ravishankara , A. R. 2007 . Design and Application of a Pulsed Cavity Ring-Down Aerosol Extinction Spectrometer for Field Measurements . Aerosol Sci. Technol. , 41 : 447 – 462 .
- Bergstrom , R. W. , Pilewskie , P. , Russell , P. B. , Redemann , J. , Bond , T. , Quinn , P. K. and Sierau , B. 2007 . Spectral Absorption Properties of Atmospheric Aerosols . Atmos. Chem. Phys. , 7 : 5937 – 5943 .
- Bohren , C. F. and Huffman , D. R. 1983 . Absorption and Scattering of Light by Small Particles , John Wiley & Sons Inc. .
- Bond , T. , Covert , D. and Müller , T. 2009 . Truncation and Angular-scattering Corrections for Absorbing Aerosol in the TSI 3563 Nephelometer . Aerosol Sci. Technol. , 43 : 866 – 871 .
- Bond , T. , Habib , G. and Bergstrom , R. W. 2006 . Limitations in the Enhancement of Visible Light Absorption Due to Mixing State . J. Geophys. Res. , 111
- Bond , T. C. , Anderson , T. L. and Campbell , D. 1999 . Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols . Aerosol Sci. Technol. , 30 : 582
- Cappa , C. , Lack , D. , Burkholder , J. and Ravishankara , A. 2008a . Bias in Filter Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Laboratory Measurements . Aerosol Sci. Technol. , 42 : 1022 – 1032 .
- Cappa , C. D. , Lovejoy , E. R. and Ravishankara , A. R. 2008b . Evaporation Rates and Vapor Pressures of the Even-Numbered C8-C18 Monocarboxylic Acids . J. Phys. Chem. A , 112 : 3959 – 3964 .
- Fuller , K. A. , Malm , W. C. and Kreidenweis , S. M. 1999 . Effects of Mixing on Extinction by Carbonaceous Particles . J. Geophys. Res , 104 ( 15 ) : 954 – 915,954 .
- Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M. and Miller , H. L. 2007 . “ Climate Change 2007: The Physical Science Basis. Contribution of Working Group 1 to the Fourth Assessment Report. Report of the Intergovernmental Panel on Climate Change ” . Cambridge and New York : Cambridge University Press .
- Jacobson , M. Z. 2001 . Strong Radiative Heating due to the Mixing State of Black Carbon in Atmospheric Aerosols . Nature , 409 : 695 – 697 .
- Kondo , Y. , Sahu , L. , Kuwata , M. , Miyazaki , Y. , Takegawa , N. , Moteki Imaru , J. , Han , N. S. , Nakayama , T. , Kim-Oanh , N. T. , Hu , M. , Kim , Y. J. and Kita , K. 2009 . Stabilization of the Mass Absorption Cross Section of Black Carbon for Filter-Based Absorption Photometry by the Use of a Heated Inlet . Aerosol Sci. Technol. , 43:DOI: 10.1080/02786820902889879
- Lack , D. , Lovejoy , E. , Baynard , T. , Pettersson , A. and Ravishankara , A. 2006 . Aerosol Absorption Measurement using Photoacoustic Spectroscopy: Sensitivity, Calibration, and Uncertainty Developments . Aerosol Sci. Technol. , 40 : 697 – 708 .
- Lack , D. A. , Cappa , C. D. , Covert , D. S. , Baynard , T. , Massoli , P. , Sierau , B. , Bates , T. S. , Quinn , P. K. , Lovejoy , E. R. and Ravishankara , A. R. 2008 . Bias in Filter Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Ambient Measurements . Aerosol Sci. Technol. , 42 : 1033 – 1041 .
- Lack , D. A. , Corbett , J. , Lerner , B. , Massoli , P. , Quin , P. K. , Coffman , D. , Bates , T. S. , Covert , D. S. , Sierau , B. , Herndon , S. , Onasch , T. , Baynard , T. , Lovejoy , E. R. , Ravishankara , A. R. and Williams , E. 2009 . Particulate Emissions from Commercial Shipping. Optical, Physical and Chemical Properties . J. Geophys. Res. , 114:doi:10.1029/2008JD011300
- Mallet , M. , Pont , V. , Liousse , C. , Roger , J. C. and Dubuisson , P. 2006 . Simulation of Aerosol Radiative Properties with the ORISAM-RAD Model During a Pollution Event (ESCOMPTE 2001) . Atmos. Environ. , 40 : 7696 – 7705 .
- Massoli , P. , Murphy , D. M. , Lack , D. A. , Baynard , T. , Brock , C. A. and Lovejoy , E. R. 2009 . Uncertainty in Light Scattering Measurements by TSI Nephelometer: Results from Laboratory Studies and Implications for Ambient Measurements . Aerosol Sci. Technol. , in press
- Murphy , D. M. 2009 . The Effect of Water Evaporation on Photoacoustic Signals in Transition and Molecular Flow . Aerosol Sci. Technol. , 43 : 356 – 363 .
- Pettersson , A. , Lovejoy , E. R. , Brock , C. A. , Brown , S. and Ravishankara , A. R. 2004 . Measurement of Aerosol Optical Extinction at 532 nm with Pulsed Cavity Ring Down Spectroscopy . J. Aerosol Sci. , 35 : 995 – 1011 .
- Press , W. H. , Flannery , B. P. , Teukolsky , S. A. and Vetterling , W. T. 1986 . Numerical Recipes. The Art of Scientific Computing , New York : Cambridge University Press .
- Raspet , R. , Slaton , W. , Arnott , W. P. and Moosmuller , H. 2003 . Evaporation–Condensation Effects on Resonant Photoacoustics of Volatile Aerosols . J. Atmos. Oceanic Technol. , 20 : 685
- Russell , P. B. , Redemann , J. , Schmid , B. , Bergstrom , R. W. , Livingston , J. M. , McIntosh , D. M. , Ramirez , S. A. , Hartley , S. , Hobbs , P. V. , Quinn , P. K. , Carrico , C. M. , Rood , M. J. , Ostrom , E. , Noone , K. J. , von Hoyningen-Huene , W. and Remer , L. 2002 . Comparison of Aerosol Single Scattering Albedos Derived by Diverse Techniques in Two North Atlantic Experiments . J. Atmos. Sci. , 59 : 609 – 619 .
- Schmid , B. , Livingston , J. M. , Russell , P. B. , Durkee , P. A. , Jonsson , H. H. , Collins , D. R. , Flagan , R. C. , Seinfeld , J. H. , Gassó , S. , Hegg , D. A. , Öström , E. , Noone , K. J. , Welton , E. J. , Voss , K. J. , Gordon , H. R. , Formenti , P. and Andreae , M. O. 2000 . Clear Sky Closure Studies of Lower Tropospheric Aerosol and Water Vapor during ACE 2 using Airborne Sunphotometer, Airborne In-Situ, Space-Borne, and Ground-Based Measurements . Tellus , B52 : 568 – 593 .
- Schmid , O. , Artaxo , P. , Arnott , W. P. , Chand , D. , Gatti , D. , Frank , G. P. , Hoffer , A. , Schnaiter , M. and Andreae , M. O. 2006 . Spectral Light Absorption by Ambient Aerosols Influenced by Biomass Burning in the Amazon Basin. I: Comparison and Field Calibration of Absorption Measurement Techniques . Atmos. Chem. Phys. , 6 : 3443 – 3462 .
- Schnaiter , M. , Linke , M. , Möhler , O. , Naumann , K.-H. , Saathoff , H. , Wagner , R. , Schurath , U. and Wehner , B. 2005 . Absorption Amplification of Black Carbon Internally Mixed with Secondary Organic Aerosol . J. Geophys. Res. , 110:doi:10.1029/2005JD006046
- Schwarz , J. P. , Spackman , J. R. , Fahey , D. W. , Gao , R. S. , Lohmann , U. , Stier , P. , Watts , L. A. , Thomson , D. S. , Lack , D. A. , Pfister , L. , Mahoney , M. J. , Baumgardner , D. , Wilson , J. C. and Reeves , J. M. 2008 . Coatings and Their Enhancement of Black Carbon Light Absorption in the Tropical Atmosphere . J. Geophys. Res. , 113:doi:10.1029/2007JD009042
- Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. , Andrews , E. , Atkinson , D. , Covert , D. , Moosmuller , H. , Petzold , A. , Schmid , B. , Strawa , A. , Varma , R. and Virkkula , A. 2005 . The Reno Aerosol Optics Study: An Evaluation of Aerosol Absorption Measurement Methods . Aerosol Sci. Technol. , 39 : 1 – 16 .
- Slowik , J. G. , Cross , E. , Han , J.-H. , Davidovits , P. , Onasch , T. B. , Jayne , J. T. , Williams , L. R. , Canagaratna , M. R. , Worsnop , D. R. , Chakrabarty , R. K. , Moosmüller , H. , Arnott , W. P. , Schwarz , J. P. , Gao , R.-S. , Fahey , D. W. , Kok , G. L. and Petzold , A. 2007 . An Inter-Comparison of Instruments Measuring Black Carbon Content of Soot Particles . Aerosol Sci. Technol. , 41 : 295 – 314 .
- Subramanian , R. , Roden , C. A. , Boparai , P. and Bond , T. C. 2007 . Yellow Beads and Missing Particles: Trouble Ahead for Filter-Based Absorption Measurements . Aerosol Sci. Technol. , 41 : 630 – 637 .
- Virkkula , A. , Ahlquist , N. C. , Covert , D. S. , Arnott , W. P. , Sheridan , P. J. , Quinn , P. K. and Coffman , D. J. 2005 . Modification, Calibration and a Field Test of an Instrument for Measuring Light Absorption by Particles . Aerosol Sci. Technol. , 39 : 68
- Zhang , R. , Khalizov , A. F. , Pagels , J. , Zhang , D. , Xue , H. and McMurry , P. H. 2008 . Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols During Atmospheric Processing . Proceedings of the National Academy of Sci. , 105 : 10291 – 10296 .