Abstract
In this study, iron silicon oxide particles were generated in a one-step flame assisted spray pyrolysis (FASP) process using H2/air or H2/O2 diffusion flames. A colloidal precursor solution was used, which contained dissolved iron nitrate and stably suspended silica nanoparticles. H2/air flames resulted in magnetic FexOy/silica core-shell particles. There was a correlation between particle size and particle structure; particles larger than 500 nm had the core-shell structure, but smaller particles had non-core-shell structures. H2/O2 flames only resulted in nanoparticles that had non-core-shell structures. The core-shell particles had a iron oxide core that was hermetically enclosed in a uniform silica shell with a typical thickness of approximately 100 nm; they were superparamagnetic with a room-temperature saturation magnetization greater than 24 emu/g. Temperature history of the particles may be used to explain the correlation between flame type and particle structure. The correlation between particle size and structure may be due to size-dependent thermodynamic stability of the structures, or kinetics of heat and mass transfer. The results from this study suggest that micrometer sized iron oxide silica core-shell magnetic particles could be generated from a one-step flame aerosol process, but FexOy/silica nanoparticles (< 100 nm) with the core-shell structure cannot be generated in a one-step flame aerosol process.
INTRODUCTION
Magnetic FexOy/silica (Fe x O y = Fe3O4 or γ -Fe2O3) core-shell particles have attracted much research interest due to the many potential applications for such particles, for example, immobilization and separation of biomolecules, magnetically controlled drug delivery, and MRI imaging enhancement (CitationGupta and Hung 1989; CitationWeissleder et al. 1990). There have been numerous publications on the synthesis of core-shell magnetic particles with an iron oxide core and a silica shell. Most of the reported synthesis methods for iron-oxide/silica core-shell magnetic particles were “wet” methods (CitationPhilipse et al. 1994), involving multi-step, batch-mode processes in a solution. An alternative to the “wet” methods is aerosol synthesis. Aerosol methods can offer continuous production of particle materials, and they are flexible in scale. Therefore, aerosol processes capable of generating core-shell magnetic FexOy/silica particles may offer potential advantage over the “wet” methods. In the past, aerosol methods based on flame and furnace have been used for the synthesis of iron oxide silica composite particles. For example, McMillin et al. and Ehrman et al. used Bunsen burner type premixed flame processes (CitationEhrman et al. 1999; CitationMcMillin et al. 1996), CitationLi et al. (2006) used a flame spray pyrolysis approach to synthesize iron silicon oxide particles. The products from these processes were nanoparticles that had non-core-shell structures. That is, these nanoparticles were composed of segregated iron oxide and silica regions that did not have the FexOy-core/silica-shell structure.
Recently, a furnace-based aerosol process was reported to be capable of generating magnetic core-shell iron oxide silica nanoparticles (CitationBasak et al. 2007). Most recently, a two-step approach based on a flame reactor was reported for the synthesis of Fe2O3/SiO2 core-shell nanoparticles (CitationTeleki et al. 2009). Teleki et al. used a flame spray pyrolysis (FSP) reactor to generate Fe2O3 nanoparticles; by introducing hexamethyldisiloxane vapor in the post-flame region, they were able to encapsulate the Fe2O3 nanoparticles with a very thin silica coating.
The motivation of this study was to answer the question: Is it possible at all to use a one-step flame process to generate FexOy/silica core-shell particles? Here a one-step flame aerosol process is defined as a process in which all the ingredients of the product particles are introduced into the flame reactor at a single inlet simultaneously with the fuel gas. Due to its relative simplicity, a one-step flame process apparently can offer some advantages over the furnace-based processes or multi-step flame processes, provided each process is capable of the same function.
As reviewed above, previous studies have shown that one-step flame processes in general do not produce FexOy/silica core-shell particles. However, this study was inspired by the hypothesis that a novel combination of flame aerosol methods might be capable of producing magnetic FexOy/silica core-shell particles. Flame assisted spray pyrolysis (FASP) using a colloidal solution was identified as a novel approach for synthesizing FexOy/silica particles. A colloidal solution is a solution that contains dissolved metal salt as well as stably suspended colloidal particles. Colloidal solutions had been previously used as starting materials in furnace and flame based aerosol synthesis processes, as shown in . To the best of our knowledge, prior to this work FASP with a colloid solution precursor had not been used for the synthesis of FexOy/silica particles.
TABLE 1 Previous studies using colloidal solutions in aerosol synthesis
In this study, either air or O2 was used as the oxidant stream to result in either a H2/air flame or a H2/O2 flame for the FASP. Two different precursor concentrations were used while maintaining the Fe/Si ratio constant. Thermophoretic sampling was used to help understand the particle evolution in the flame. In the following sections descriptions of the experimental methods are given. The results are described and discussed, followed by the conclusions.
Experimental
The experimental methods are described below. The experimental conditions of the major tests are also summarized at the beginning of the Results section in .
TABLE 2 Experimental conditions, characterization methods, and major results (Fe/Si molar ratio = 1; MS: Mössbauer Spectroscopy; MM: Magnetization Measurement; TS: Thermophoretic Sampling)
Synthesis Conditions
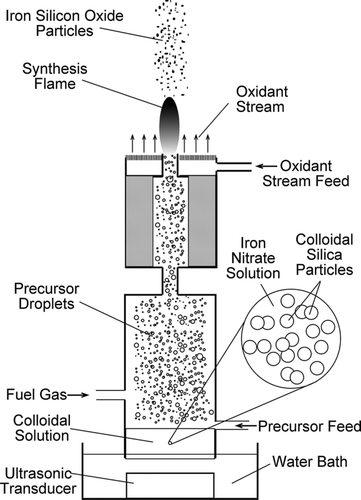
The FASP apparatus used in this study was similar to that used in previous studies (CitationGuo and Luo 2008; CitationGuo et al. 2009a). A schematic illustration of the process is shown in . The colloidal solution was prepared by dissolving Fe(NO3)3· 9H2O (Alfa Aesar, Ward Hill, MA) in a silica colloid (Silicon(IV) oxide, particle size 10 nm, 30% in H2O, colloidal dispersion, CAS# 7631-86-9, Stock # 43111, Lot # K11Q038, Ward Hill, MA). The Fe(NO3)3· 9H2O was first dissolved in deionized water; then the aqueous solution was mixed with the silica colloid. The concentration of the iron nitrate solution and the mixing ratio between the iron nitrate solution and the silica colloid were controlled so that the Fe/Si molar ratio was maintained constant at 1. The colloidal precursor solution was fed into an atomization vessel at 5 mL/h and atomized continuously by a 1.7 MHz ultrasonic transducer (CitationGuo and Luo 2008). The H2 fuel gas, at a flow rate of 1 standard liter per minute (SLM), carried the droplets of the colloidal precursor solution from the atomization vessel through a tubular burner. At the burner mouth, the H2 gas formed a stable, self-sustained coflow diffusion flame supported by air or O2 at 10 SLM. Particles were formed in the flame after complex physical changes and chemical reactions. A vacuum pump drew the post-flame aerosol through a filter holder (not shown in schematic) and product particles were collected on a filter (CitationGuo and Luo 2008). The particles collected on filter were characterized by the various methods described below. Particles were also sampled from the interior of the flame by thermophoretic sampling, and then analyzed by electron microscopy, as described below.
Particle Characterization
To determine their microstructure and size, the particles were analyzed by transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS) on a JEOL 2010 microscope (JEOL Ltd., Tokyo, Japan). For TEM sample preparation, the particles were carefully scraped off the filter and suspended in isopropanol. The suspension was dropped on a copper TEM grid with carbon film support. The particles were left on the grid after evaporation of isopropanol. Using representative TEM images of a sample, particle size distribution was obtained. A total of more than 500 particles were measured to obtain the size distribution for a sample.
To obtain chemical structure information of the product particle, X-ray diffraction (XRD) was carried out on a Bruker-AXS D8 Powder X-ray diffractometer (Bruker, Madison, WI) by placing the filter samples directly on the sample stage. Also, Mössbauer spectroscopy was carried out to obtain information of iron speciation and crystallite size. Mössbauer spectra of the particles were collected on a Wissel spectrometer (Wissenschaftliche Elektronik GmbH, Starnberg, Germany) at room temperature using 57Co source in Rh matrix. Measurements were calibrated with alpha-Fe standard absorber sample. Spectra were fitted by Lorentzian shape components providing values of isomer shift, quadrupole splitting, absorption line area, and line width for phase, iron valence state, and relative abundance analysis.
To determine the magnetic properties of the particles, magnetization measurements were carried out in a MPMS® SQUID VSM dc magnetometer (Quantum Design, San Diego, CA). The room-temperature M(H) measurement was carried out by setting temperature at 300K and scanning 7T → 0T → −7T → 0T → 7T at a step size of 50 Oe/s (10,000 Oe = 1T). For the low temperature measurement, the temperature was set at 5K (sample field cooled in 7T) and the scan was run 7T → 0T → −7T → 0T → 7T at a step size of 50 Oe/s (10,000 Oe = 1T).
Thermophoretic Sampling
To help understand the particle formation mechanism, particle samples were taken thermophoretically from the flame interior in two of the main tests. The thermophoretic sampling was carried out by quickly inserting a TEM grid into the flame center at various heights above the burner. The TEM grid typically remained in the flame for a few milliseconds to tens of milliseconds before being quickly retracted. Particles in the vicinity of the TEM grid were driven by thermophoresis towards the TEM grid due to the large temperature gradient between the flame gases and the cold TEM grid. The particles were thus deposited on the TEM grid. Particle growth was “frozen” at the moment the particles were deposited on the cold TEM grid. These particles “frozen” in a certain growth stage could be later viewed in a microscope. Therefore the particle characteristics at various growth stages could be learned. Thermophoretic sampling has been used repeatedly in understanding particle evolution in flames (CitationDobbins and Megaridis 1987; CitationGuo and Kennedy 2004, Citation2007; CitationGuo et al. 2009b; CitationMueller et al. 2004).
RESULTS
A strong correlation was found between the flame type and particle structure/size. There was also a correlation between particle structure and particle size. The experimental conditions, characterization methods and main particle characteristics of the four major tests are summarized in .
H2/Air Flames Resulted in Core-Shell Particles
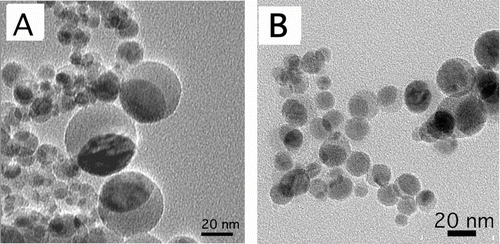
H2/air flames resulted in core-shell particles, as shown in . With 0.65 M precursor (Test 1), the mean particle diameter was approximately 1 μ m, and the majority of the particles had the core-shell structure. The core-shell particles had a silica shell with low electron density relative to the core, which generated clear contrast in the TEM image for identification of the core-shell structure. The silica shell thickness was uniform for each core-shell particle. From particle to particle the shell thickness varied from 80 nm to 120 nm.
FIG. 2 Representative TEM images of particles from H2/air flames: (A) as-synthesized core-shell particles from Test 1 (some appear “hairy” due to presence of nanoparticle aggregates); (B) a higher magnification image showing nanoparticle aggregates; (C) particles from Test 1 after washing in 0.75 M sulfuric acid for 24 h to remove the nanoparticle aggregates (white arrow points to a sub-300 nm particle with non-core-shell structure), and (D) as-synthesized particles from Test 2 (black arrows point to particles with non-core-shell structures).
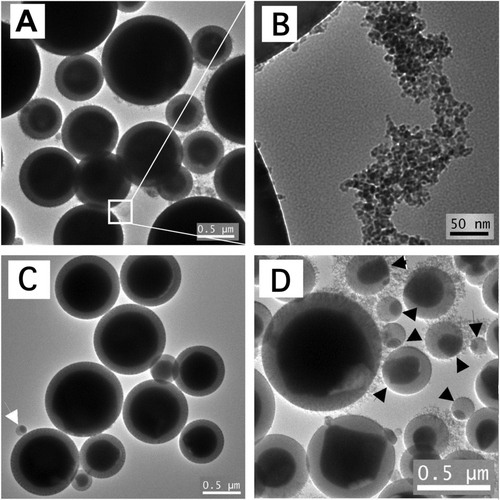
Nanoparticle aggregates were found among the core-shell micrometer-sized core-shell particles. In lower magnification images, the core-shell particles appeared “hairy” due to the presence of these nanoparticle aggregates. The primary particle size of these aggregates was about 10 nm, as shown in . These aggregated nanoparticles did not have the core-shell structure. EDS analysis suggested that these nanoparticles were mostly iron oxide. To verify the chemical nature of these nanoparticles, some of the sample from Test 1 was placed in 0.75 M sulfuric acid at room temperature for 24 h. After the acid treatment, the particles were washed with deionized water and their TEM images were taken, as shown in . Aggregated nanoparticles were no longer visible in the acid-treated sample. The fact that these nanoparticles dissolved in acid further suggested that they were iron oxide (CitationLide 2001). Similar nanoparticle aggregates were found among larger particles in a previous study on formation of Fe2O3 particles in a flame aerosol process (CitationGuo and Kennedy 2007).
There was a correlation between size and structure of particles from the H2/air flames. Larger particles (> 500 nm) had the core-shell structure. However, particles smaller than about 300 nm all had non-core-shell structures. This was true with either precursor concentration level. With 0.65 M precursor (Test 1), most of the particles were larger than 500 nm and had the core-shell structure, but the very few sub-300 nm particles had non-core-shell structures, an example of which is seen in . With 0.03 M precursor concentration (Test 2) the mean particle diameter was approximately 0.5 μ m; many particles were smaller than 300 nm and had non-core-shell structures, as seen in .
H2/O2 Flames Resulted in Non-Core-Shell Nanoparticles
H2/O2 flames with both precursor concentration levels (Test 3 and Test 4) resulted in nanometer sized non-core-shell particles. As shown in , in TEM images the particles were nearly spherical, with a high electron density (darker) region that was distinct from a low electron density region. The particles were all smaller than 50 nm in diameter. The particle size with the 0.03-M precursor was smaller than that with the 0.65-M precursor.
Thermophoretic Sampling Results
Particles were taken from the flame interior by thermophoretic sampling and viewed by TEM, in order to obtain “snapshots” of particles at various stages of growth. This was done in for Tests 1 and 3. The most notable finding was that micrometer-sized core-shell particles formed at intermediate stages of both the H2/air flame and the H2/O2 flame. However, in the H2/O2 flame (Test 3) the core-shell particle formed in early stages of the flame, and then disappeared in the maximum temperature region. In contrast, in the H2/air flame the core-shell particles formed near the maximum temperature region and remained through the flame. The TEM images of particles thermophoretically sampled from the flame interior are shown in .
FIG. 4 Representative TEM images of thermophoretic particle samples and flame photos of (A) an H2/air flame in Test 1 and (B) an H2/O2 flame in Test 3. (Figure provided in color online.)
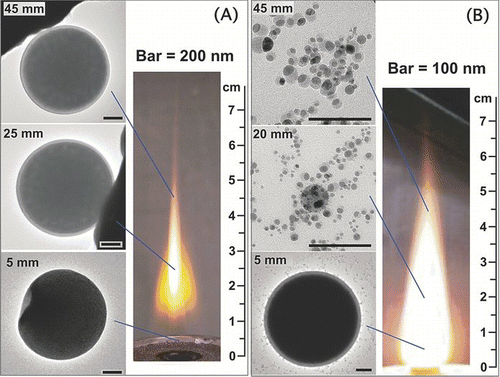
At 5 ∼ 10 mm above the burner exit (ABE) of the H2/air flame, micrometer sized spherical homogeneous particles were found; at this height of the H2/O2 flame, micrometer sized core-shell particles were found, along with some homogeneous particles. At 15 ∼ 25 mm ABE of the H2/air flame, micrometer-sized core-shell particles were found; at the same height of the H2/O2 flame, only nanoparticles (mostly < 20 nm) were found, and there were no micrometer-sized particles any more. At 35 ∼ 45 mm ABE of the H2/air flame, micrometer-sized core-shell particles were found; at the same height of the H2/O2 flame, only nanoparticles were found. All the nanoparticles from the thermophoretic samples had non-core-shell structures. Major findings of the thermophoretic samples are summarized in . The significance of the findings is discussed in the Discussion section.
TABLE 3 Summarized results of the thermophoretic samples
Combining the experimental findings presented above, an insight may be gained into the correlation between the flame type and the particle structure. This is described in detailed in the Discussion section.
Characterization of Iron Species
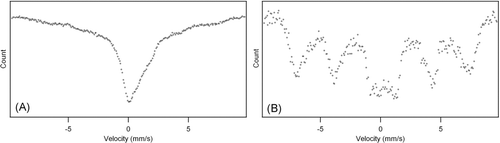
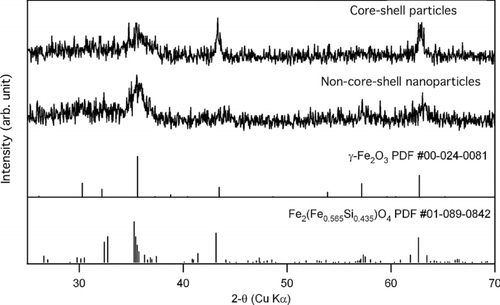
XRD and Mössbauer spectroscopy were used to characterize the iron species in the particles. Representative XRD patterns of the core-shell particles from H2/air flame (Test 1) and the non-core-shell particles from H2/O2 flame (Test 3) are shown in . Maghemite (γ -Fe2O3) and Fe2(Fe0.565Si0.435)O4were found to be two most probable matches, the standard patterns of which are also shown in . It is possible that the core-shell particles and the non-core-shell particles both contained γ -Fe2O3 and Fe2(Fe0.565Si0.435)O4. However, the peak at 2-θ of approximately 43° was more prominent with the core-shell particles. Both phases have a peak near this angle, but that with the iron silicon oxide Fe2(Fe0.565Si0.435)O4 has higher relative intensity (see the standard XRD patterns). Therefore it appears that the core-shell particles had a higher fraction of Fe2(Fe0.565Si0.435)O4 than did the non-core-shell particles.
FIG. 5 Representative XRD patterns of the core-shell and the non-core-shell particles; vertical lines show the peak location and intensity of the standard XRD diffraction patterns of γ -Fe2O3 and Fe2(Fe0.565Si0.435)O4.
The Mössbauer results () suggested that the crystallite size, and possibly the iron valence state, were different in the core-shell particles (Test 1, H2/air flame) than in the non-core-shell particles (Test 3, H2/O2 flame). Both spectra exhibited relaxation of the magnetic order, which resulted in the collapse of sextet components into a doublet in the middle of the spectrum. However, this effect was especially pronounced in the spectrum of the core-shell particles, as seen in . The non-core-shell spectrum showed magnetically split sextet, as seen in , indicating the presence of maghemite (γ -Fe2O3) (CitationLin et al. 1996). The relaxation of the magnetic order could be attributed to very small crystallite size (CitationLin et al. 1996). The core-shell spectrum showed no sextet pattern, suggesting that the crystallite size was smaller in the core-shell particles than in the non-core-shell particles. Also, the core-shell spectrum showed substantial bulge on the right side shoulder of the central absorption peak. This suggested that the core-shell particles contained Fe2 + (CitationMurad and Cashion 2004). Thus the Mössbauer results suggested that, in the core-shell particles, a fraction of the iron could be Fe2 +; while in the non-core-shell particles, apparently the iron was mostly Fe3 +.
Magnetization Properties of Core-Shell Particles and Non-Core-Shell Nanoparticles
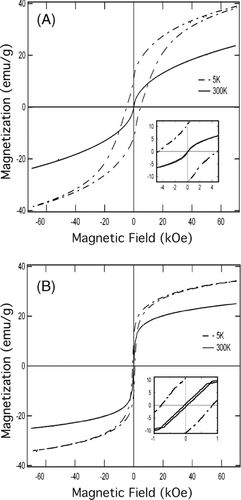
Magnetization measurements were carried out for core-shell particles from H2/air flame (Test 1) and non-core-shell nanoparticles from H2/O2 flame (Test 3). The hysteresis loops are shown in and . Both the core-shell particles and the non-core-shell nanoparticle exhibited superparamagnetic behavior at room temperature (300 K). At 5 K, the particles became blocked, resulting in significant increases in coercivity and remanence. The core-shell particles and the non-core-shell particles apparently had different magnetic domain sizes, and hence different temperature-dependence of the magnetic properties (CitationTartaj et al. 2002). The main magnetic properties of the core-shell particles and the non-core-shell nanoparticles are shown in , in comparison with magnetic properties of iron oxide silica core-shell nanoparticles generated in other aerosol processes (CitationLi et al. 2006; CitationTeleki et al. 2009). The core-shell particles in this study exhibited very soft room-temperature magnetization behaviors (low coercivity and remanence) in comparison to particles from the other studies. This difference in magnetization properties may be attributed to the difference in crystallinity and domain size. The difference in iron speciation (e.g., γ -Fe2O3 and Fe2(Fe0.565Si0.435)O4) may also be responsible for the difference in magnetization properties.
TABLE 4 Comparison of particle magnetic properties with other studies
DISCUSSION
The findings of this study suggest that the FASP method using colloidal solution precursor is capable of generating micrometer-sized iron oxide particles that are hermetically encapsulated with a silica shell. However, this capability is dependent on the type of flame and affected by the correlation between particle structure and particle size. Herein we give an explanation for the effect of flame type. In addition, particle size-structure correlation and the relevance of the micrometer-sized magnetic core-shell particles are also discussed.
Correlation of Flame Type and Particle Structure
The different results from the two different flame types (H2/air and H2/O2) may be explained with the temperature history of the particles. Based on the experimental observations, a phenomenological particle formation mechanism is proposed and shown schematically in . The figure illustrates the events that take place from when the precursor droplet enters the flame aerosol reactor, to when the product particles leave the flame aerosol reactor. Along the axis of the burner and in the flow direction, the gas temperature first rises to reach a maximum due to the combustion reaction, then decreases as the coflowing room-temperature gas (air or O2) cools the combustion products. In general, from precursor droplets entering the flame to product particle leaving the flame, the possible physical and chemical events are: solvent evaporation, metal salt decomposition and metal oxide formation, melting and gasification of metal oxides, re-solidification of molten metal oxide and/or condensation of gaseous species. This depiction of particle growth does not include particle coagulation and coalescence. Nevertheless, this approach has been found useful for understanding metal oxide particle formation mechanisms in co-flow diffusion flames (CitationGuo and Kennedy 2007; CitationGuo et al. 2009b).
FIG. 8 Schematic illustrations of the different temperature history in a H2/air flame and in a H2/O2 flame. (Figure provided in color online.)
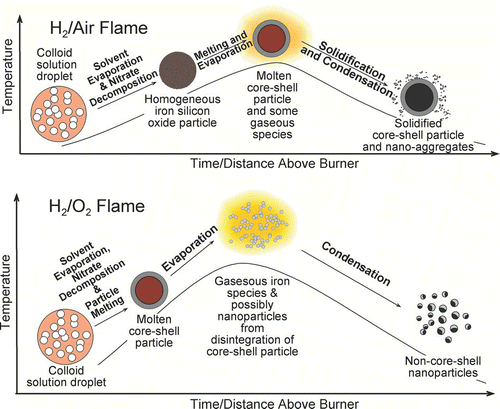
The magnitude of the above-mentioned events and their locality within the flame are dependent on the temperature history of the particles. In the H2/air flame, the highest temperature is about 2200 K; in the H2/O2 flame, the highest temperature can reach approximately 2700 K (CitationGuo and Kennedy 2004; CitationGuo and Luo 2008). The maximum temperature of the flames used in this study was approximately 20 ∼ 25 mm above the burner exit.
In the beginning, solvent evaporation and nitrate decomposition take place. The gas temperature at the burner exit (base of flame) is similar to 600 K, which reaches over 2200 K within 20 mm above the burner exit (CitationGuo and Kennedy 2004; CitationGuo and Luo 2008). With a typical precursor droplet diameter of approximately 4 μ m in diameter (CitationGuo et al. 2009a), the solvent evaporation may complete in less than a millisecond after the droplet enters the flame, during which time the droplet would travel less than a millimeter (See Appendix). As the solvent evaporates, the iron nitrate starts to nucleate and precipitate on the surface of the silica colloidal nanoparticles (CitationWang et al. 2007). Then at a temperature below 400°C the iron nitrate decomposes to form iron oxide (CitationWieczorek-Ciurowa and Kozak 1999). Gases that are generated from nitrate decomposition escape; iron oxide, the solid product of the nitrate decomposition, coats the surface of the silica nanoparticles. The iron-oxide-coated silica nanoparticles form a close-packed spherical structure. The particles that are formed immediately after nitrate decomposition do not yet have the desired core-shell structure. The thermophoretic sampling results from 5 ∼ 10 mm above burner support this description of events. The presence of core-shell particles at this height of the H2/O2 flame suggests that particle melting occurs in this region of the H2/O2 flame, as discussed below.
As the particle approaches the maximum temperature region, melting occurs. At temperatures about 1950 K the particles would melt; the molten iron oxide and silicon oxide would form two immiscible liquid phases (CitationEhrman et al. 1999). The two immiscible liquid phases form a core-shell structure, with the silica phase being the shell. In the H2/air flame, this takes place near the maximum temperature region. In the H2/O2 flame, this takes place at a location closer to the burner because of the higher heating rate; the particle becomes molten well before reaching the maximum temperature region. The experimental results support this description: core-shell particles were found at 15 ∼ 25 mm of the H2/air, and 5 ∼ 10 mm of the H2/O2 flame.
In the maximum temperature zone, vaporization of iron oxide takes place. Chemical equilibrium calculations have shown that Fe and FeO vapors, rather than solid iron oxide, are thermodynamically in the maximum temperature region (CitationGuo and Kennedy 2007). In the H2/air flame, the flame temperature is relatively low, and the vaporization does not significantly change the size or structure of the core-shell particles. However, in the H2/O2 flame, the extremely high temperature (∼ 2700 K) leads to rapid vaporization of iron oxide, so that the core-shell particle disintegrates to form gaseous iron species and silica nanoparticles. The thermophoretic sampling results support this description: in the H2/air flame the core-shell particles maintained their size and structure; while no micrometer-sized particles were found in and above the maximum temperature region of the H2/O2 flame. Additional tests were done with 0.65 M silica colloid (no iron nitrate dissolved therein) in a H2/O2 flame. The post-flame filter samples revealed silica particles with a mean diameter of approximately 1 μ m. This result suggests that the vaporization of silica is not significant in comparison to that of iron oxide.
In the cooling zone of the flame, the gaseous iron species nucleate and condense to form nano-aggregates in the H2/air flame, which are found around the dominant, micrometer-sized core-shell particles. In the H2/O2 flame, the gaseous iron species would condense onto the silica nanoparticles and form nanoparticles with the non-core-shell structure.
Correlation of Particle Size and Particle Structure
There are two possible explanations for the observed correlation between particle size and particle structure. It is possible that the core-shell structure is thermodynamically stable in larger particles, but unstable in smaller particles. A possible analogous phenomenon may be the correlation between particle size and crystal structure that had been found in flame-synthesized particles (CitationGuo and Luo 2008). However, the correlation between particle size and particle structure may also be a kinetic effect. In this study, the particle diameter ranged from tens of nanomaterials to over one micrometer. The heat transfer time constant and the solid phase diffusion time constant could vary by several orders of magnitude. Therefore the correlation between particle size and particle structure might be kinetic in nature. It is not yet possible to give a definitive answer for the size-structure correlation with this study. Nevertheless, the results suggest that it would be impossible to generate FexOy/silica core-shell nanoparticles in a one-step flame process, “one-step” as being defined earlier in the Introduction. This is consistent with the findings from previous studies (CitationEhrman et al. 1999; CitationLi et al. 2006; CitationMcMillin et al. 1996).
Relevance of Micrometer-Sized Magnetic Core-Shell Particles
In comparison to the large number of studies on core-shell nanoparticles, there are relatively few studies on the synthesis of micrometer-sized magnetic core-shell particles. However, micrometer-sized magnetic core-shell particles are useful for separation of biological molecules (CitationBoom et al. 1990), and even in vivo magnetic resonance imaging (CitationHamoudeh and Fessi 2006; CitationMcAteer et al. 2007). Therefore, it appears that micrometer-sized magnetic core-shell particles are relevant to existing and potential applications in their own right.
In particular, the magnetic core-shell particles generated in this study had relatively thick and dense silica shells, which afforded the iron oxide core good protection against corrosive environment. Some of the core-shell particles from this study remained intact (based on TEM observation) after being placed in 0.75 M sulfuric acid at room temperature for over a month (data not shown). This suggests that these core-shell particles generated with this method could be used in corrosive (non-hydrofluoric) environment where magnetic particles are useful. The chemical robustness of these core-shell particles also means that they can withstand harsh preparation and cleaning processes, which makes it possible for them to be reusable.
CONCLUSION
Iron silicon oxide particles were generated by modified FASP in H2/air and H2/O2 diffusion flames, using a precursor solution that contained dissolved iron nitrate and colloidal silica nanoparticles at two different concentration levels. Magnetic particles with a full, uniform silica shells were obtained from the H2/air flames. There was a correlation between particles size and structure; particles larger than 500 nm had the core-shell structure, but those smaller had non-core-shell structures. H2/O2 flames only resulted in nanoparticles smaller than 50 nm, which all had non-core-shell structures. Formation of nanoparticles in the H2/O2 flames was attributed to the very high maximum temperature in these flames. The correlation between size and structure might be a result of size-dependent stability of the structures, or the size-dependent time constants of mass and heat transfer. Combining the results from this study and other studies, it may be concluded that it is possible to form micrometer-sized, but not nanometer-sized (< 100 nm), FexOy/silica core-shell particles, in a one-step flame process as defined in the Introduction of this article.
APPENDIX: CALCULATION OF PRECURSOR DROPLET DRYING TIME
The drying time of a pure water droplet was calculated to approximate the drying time of an actual precursor droplet. The gas temperature at the exit of the burner was estimated to be 600 K according to previous measurements (CitationGuo and Kennedy 2004). The objective of the calculation was to determine the drying time of a 4 μ m water droplet suspended in air with an ambient temperature of 600 K and ambient water vapor partial pressure of 5000 Pa. The drying time thus calculated was considered a conservative (over) estimate of the actual drying time, because in the flame the gas temperature would increase rapidly above 600 K. The assumed ambient water vapor pressure of 5000 Pa corresponded to 50% of the water vapor that would result from 5 mL/h of liquid water evaporating into a gas flow of 1 standard liter per minute (SLM).
The drying time was calculated with the following equation:
The temperature of the droplet was found by solving the following equation through iteration:
Financial support for this work was provided by Texas A&M University and Texas Engineering Experiment Station. Technical assistance from Dr. Ibrahim Karaman, Mr. Steven Eli Rios, and Mr. Wonjoong Hwang in magnetization measurement is gratefully acknowledged.
REFERENCES
- Basak , S. , Tiwari , V. , Sethi , V. and Biswas , P. One Step Aerosol Route Synthesis of Silica Coated γ -Fe2O3 Nanoparticles for Biomedical Applications . Proc. 5th Asian Aerosol Conference . August 26–29 2007 , Kaohsiung, Taiwan.
- Boom , R. , Sol , C. J. A. , Salimans , M. M. M. , Jansen , C. L. , Wertheimvandillen , P. M. E. and Vandernoordaa , J. 1990 . Rapid and Simple Method for Purification of Nucleic-Acids . J. Clin. Microbiol. , 28 : 495 – 503 .
- Dobbins , R. A. and Megaridis , C. M. 1987 . Morphology of Flame-Generated Soot as Determined by Thermophoretic Sampling . Langmuir , 3 : 254 – 259 .
- Ehrman , S. H. , Friedlander , S. K. and Zachariah , M. R. 1999 . Phase Segregation in Binary SiO2/TiO2 and SiO2/Fe2O3 Nanoparticle Aerosols Formed in a Premixed Flame . J. Materials Res. , 14 : 4551 – 4561 .
- Guo , B. and Kennedy , I. M. 2004 . The Speciation and Morphology of Chromium Oxide Nanoparticles in a Diffusion Flame . Aerosol Sci. Technol. , 38 : 424 – 436 .
- Guo , B. and Kennedy , I. M. 2007 . Gas-Phase Flame Synthesis and Characterization of Iron Oxide Nanoparticles for Use in a Health Effects Study . Aerosol Sci. Technol. , 41 : 944 – 951 .
- Guo , B. and Luo , Z. P. 2008 . Particle Size Effect on the Crystal Structure of Y2O3 Particles Formed in a Flame Aerosol Process . J. Amer. Ceramic Society , 91 : 1653 – 1658 .
- Guo , B. , Mukundan , M. and Yim , H. 2009a . Flame Aerosol Synthesis of Phase–Pure Monoclinic Y2O3 Particles via Particle Size Control . Powder Technol. , 191 : 231 – 234 .
- Guo , B. , Yim , H. and Luo , Z.-P. 2009b . Formation of Alumina Nanofibers in Carbon-Containing Coflow Laminar Diffusion Flames . J. Aerosol Sci. , 40 : 379 – 384 .
- Gupta , P. K. and Hung , C. T. 1989 . Magnetically Controlled Targeted Micro-Carrier Systems . Life Sci. , 44 : 175 – 186 .
- Hamoudeh , M. and Fessi , H. 2006 . Preparation, Characterization and Surface Study of Poly-Epsilon Caprolactone Magnetic Microparticles . J. Colloid and Interface Sci. , 300 : 584 – 590 .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : Wiley .
- Li , D. , Teoh , W. Y. , Selomulya , C. , Woodward , R. C. , Amal , R. and Rosche , B. 2006 . Flame-Sprayed Superparamagnetic Bare and Silica-Coated Maghemite Nanoparticles: Synthesis, Characterization, and Protein Adsorption-Desorption . Chem. of Mat. , 18 : 6403 – 6413 .
- Lide , D. R. 2001 . CRC Handbook of Chemistry and Physics , Boca Raton : CRC Press .
- Lin , S. Y. , Ferg , J. , Biswas , P. , Enzweiler , R. and Boolchand , P. 1996 . Characterization of Maghemite Ferric Oxide Crystals Processed by an Aerosol Technique . J. Magn. Magn. Mater. , 159 : 147 – 158 .
- McAteer , M. A. , Sibson , N. R. , von zur Muhlen , C. , Schneider , J. E. , Lowe , A. S. , Warrick , N. , Channon , K. M. , Anthony , D. C. and Choudhury , R. P. 2007 . In Vivo Magnetic Resonance Imaging of Acute Brain Inflammation Using Microparticles of Iron Oxide . Nat. Med. , 13 : 1253 – 1258 .
- McMillin , B. K. , Biswas , P. and Zachariah , M. R. 1996 . In Situ Characterization of Vapor Phase Growth of Iron Oxide–Silica Nanocomposites. 1. 2-D Planar Laser-Induced Fluorescence and Mie Imaging . J. Materials Res. , 11 : 1552 – 1561 .
- Meyra , A. G. , Kuz , V. A. and Zarragoicoechea , G. J. 2004 . Universal Behavior of the Enthalpy of Vaporization: An Empirical Equation . Fluid Phase Equilibria , 218 : 205 – 207 .
- Mueller , R. , Jossen , R. , Kammler , H. K. and Pratsinis , S. E. 2004 . Growth of Zirconia Particles Made by Flame Spray Pyrolysis . Aiche J , 50 : 3085 – 3094 .
- Murad , E. and Cashion , J. 2004 . Mössbauer Spectroscopy of Environmental Materials and Their Industrial Utilization , Boston : Kluwer Academic Publishers .
- Philipse , A. P. , Vanbruggen , M. P. B. and Pathmamanoharan , C. 1994 . Magnetic Silica Dispersions—Preparation and Stability of Surface-Modified Silica Particles with a Magnetic Core . Langmuir , 10 : 92 – 99 .
- Tartaj , P. , Gonzalez-Carreno , T. and Serna , C. J. 2002 . Magnetic Behavior of γ -Fe2O3 Nanocrystals Dispersed in Colloidal Silica Particles . J. Phys. Chem. B , 107 : 20 – 24 .
- Teleki , A. , Suter , M. , Kidambi , P. R. , Ergeneman , O. , Krumeich , F. , Nelson , B. J. and Pratsinis , S. E. 2009 . Hermetically Coated Superparamagnetic Fe2O3 Particles with SiO2 Nanofilms . Chem. of Mat. , 21 : 2094 – 2100 .
- Turns , S. R. 2006 . Thermal-Fluid Sciences , New York : Cambridge University Press .
- Wang , W. N. , Widiyastuti , W. , Lenggoro , I. W. , Kim , T. O. and Okuyama , K. 2007 . Photoluminescence Optimization of Luminescent Nanocomposites Fabricated by Spray Pyrolysis of a Colloid-Solution Precursor . J. Electrochem. Society , 154 : J121 – J128 .
- Weissleder , R. , Elizondo , G. , Wittenberg , J. , Rabito , C. A. , Bengele , H. H. and Josephson , L. 1990 . Ultrasmall Superparamagnetic Iron-Oxide—Characterization of a New Class of Contrast Agents for Mr Imaging . Radiology , 175 : 489 – 493 .
- Wieczorek-Ciurowa , K. and Kozak , A. J. 1999 . The Thermal Decomposition of Fe(NO3)39H2O . J. Therm. Anal. , 58 : 647 – 651 .