Abstract
Experimentally measured deposition of ultrafine particles, ranging from 13–100 nm in diameter, in nasal airway replicas of ten infants aged 3–18 months is presented. The replicas included the face, nostrils, and nasal airways including the upper trachea. A differential mobility analyzer (DMA) and a condensation particle counter (CPC) were used to quantify the nasal deposition by comparing the number of polydisperse sodium chloride particles, generated by evaporation from a Collison atomizer, at the inlet and outlet of the replicas. Particles were individually classified in size by DMA and subsequently were counted one size bin at a time by CPC upstream and downstream of each replica. Since in vivo data is not available for infants to compare to, we validated our experimental procedure instead by comparing deposition in nasal airway replicas of six adults with in vivo measurements reported in literature. In the infant replicas, tidal inhalation was simulated at two physiologically compatible flow rates and the effect of flow rate on deposition was found to be small. Deposition obtained at constant flow rates is lower than with tidal breathing, indicating the importance of unsteadiness, in contrast to similar data in adults where unsteadiness is known to be unimportant. An empirical equation, containing geometrical features of the nasal airways in the form of related non-dimensional dynamical parameters (Reynolds, Schmidt, and Womersley numbers), was best fitted to the infant data. This equation may be useful for a priori prediction of nasal deposition and intersubject variability during exposure of infants to ultrafine aerosols.
Introduction
Ultrafine particles (UFP, with a diameter of less than 100 nm) are ubiquitous in ambient and indoor air from several natural and anthropogenic sources. Epidemiological studies have raised concerns over adverse health effects associated with exposure to ultrafine particles (CitationKreyling et al. 2006). The cardiovascular and pulmonary systems have been diagnosed as the main targets of this unwanted exposure. However, recent studies have shown stronger affiliations between inhaled particles and respiratory failure compared to cardiovascular outcomes (CitationHalonen et al. 2009). Exposure to radon and its progeny, which attaches to dusts and airborne particles, is associated with an increased risk of lung cancer (NRC 1988). Viral lower respiratory tract infections in infants and young children are also a major public health issue (CitationVan Woensel et al. 2003). On the other hand, effects of UFPs on organs other than the lungs, especially, the brain and central nervous system (CNS) have received special attention recently. Researchers have found that the CNS can be a target of ultrafine aerosols and the most probable mechanism behind this process is from deposits on the olfactory mucosa in the nasopharyngeal region of the respiratory tract and subsequent translocation via the olfactory nerve (CitationOberdorster et al. 2004).
Nanomedicine, including the use of nanoparticles, has potential for the therapeutic treatment of diseases via manipulation of particle characteristics such as size, surface chemistry, surface charge, and surface area (CitationGill et al. 2007). Conceptually, drug delivery directly to the site of infection reduces the systemic side effects of therapeutic agents, which in turn makes pulmonary targeted drug delivery of ultrafines a plausible research area. Moreover, deposition of inhaled pharmaceutical aerosols in the olfactory region may be an efficient method for treatment of central nervous system disorders. However, the dosimetry, which involves regional deposition patterns in the respiratory tract and the biokinetic fate of inhaled ultrafine particles, is not yet fully understood (CitationKreyling et al. 2006).
Human extrathoracic airways filter inhaled pharmaceutical particles and hinder their penetration to targeted regions in the lung. Therefore, knowledge of the filtration efficiency of the upper airways (naso/oropharyngeal regions) and the influence of intersubject differences on this mechanism is essential for evaluating delivered dose to the lungs, as well as assessing the risks of exposure to toxic ultrafine particles in different environments. In an attempt to understand how physical properties of particles and respiratory parameters influence the deposition of aerosols in extrathoracic airways of adults, many experimental measurements (in vivo and in vitro), numerical analyses and computational fluid dynamics (CFD) studies have been performed. For example, in vivo adult studies have been performed by Cheng, Y. S. et al., Cheng, K. H. et al., and CitationSwift and Strong (1996). These in vivo studies have demonstrated that considerable intersubject variability is present in the diffusion deposition regime (CitationCheng 2003). Although in vivo studies on human subjects are desirable, the invasive and hazardous nature of the employed aerosols limits the extent of such studies. Experimental measurements of deposition of ultrafine particles in physical casts of extrathoracic airways (in vitro) have been used as a substitute for in vivo studies (CitationCheng et al. 1988, Citation1990, Citation1993; CitationSwift et al. 1992; CitationYamada et al. 1988; Gradon and Yu 1989; CitationGuilmette et al. 1994; CitationKelly et al. 2004).Computational fluid dynamic (CFD) simulations have been useful for improving our understanding of deposition mechanisms correlated to flow patterns in nasal airways (CitationYu et al. 1998; CitationZamankhan et al. 2006; CitationShi et al. 2006; CitationWang et al. 2009; CitationXi and Longest 2008).
In spite of all the above noted progress on aerosol deposition in adults, our knowledge of aerosol behavior in children and specifically in infants is limited. Practical difficulties with using in vivo imaging in infants and children could be one of the reasons for this limitation. The youngest subject for which in vitro deposition of ultrafine particles in extrathoracic airways has been measured is reported in the study of CitationCheng et al. (1995). They measured the deposition of ultrafine particles and radon progeny within three nasal airways replicas of 1.5-, 2.5-, and 4-year-old children using monodispersed NaCl and Ag aerosols ranging from 0.0046 μm to 0.2 μm in diameter at inspiratory and expiratory flow rates of 3 L/min, 7 L/min, and 16 L/min. In that study, the deposition efficiency was found to decrease with increasing age for a given particle size between 0.001 to 0.2 μm. To our knowledge, however, no studies have measured the deposition of ultrafine particles in the extrathoracic airways of children younger than 1.5 years (i.e., infants).
For micron-sized particles, comparison of the deposition data of CitationSwift (1991), CitationMinocchieri et al. (2008), and the SAINT replica (CitationSchuepp et al. 2005) raised the following question: assuming there are large intersubject variations in extrathoracic airways of infants, as there are in adults, how much of the variability in deposition is due to differences in airway morphology or breathing pattern at a given age and how much is due to age alone (CitationFinlay 2008)? While Storey-Bishoff et al. (2008) address this question for micron-sized particles, ultrafine aerosol deposition in infants has not been explored in this regard.
It has been suggested that infants breathe exclusively through their nose from birth to between 6 weeks and 6 months of age (CitationPolgar and Kong 1965). Moreover, face masks are normally used for pharmaceutical aerosol administration to infants because infants are unable to use the mouthpieces associated with standard inhalers and nebulizers; therefore, predicting deposition of aerosols in nasal airways of infants is important in exposure assessments and determining lung drug dose.
Comparisons of ultrafine particle deposition in models and live subjects confirm in vitro measurement as an acceptable method for the simulation of particle behavior in human nasal airways (CitationMartonen and Zhang 1992). Experiments using nasal replicas are popular because they can be used for systematic studies in laboratories without the ethical limitations of human studies. Given that nasal airway cross sections do not change noticeably during the breathing cycle (CitationArens et al. 2005), collecting images of infant extrathoracic airways is an alternative option to avoid the complexities of subject recruitment and in vivo studies. Moreover, although deposition of ultrafine aerosols in replicas with nasal hair is higher than without nasal hair for adults (CitationCheng et al. 1993), this is not an issue for infants because they do not yet have any nasal hair. Also, surface roughness characteristics of replicas have not been found to be important in deposition of ultrafine particles less than 150 nm in diameter (CitationKelly et al. 2004). For the above reasons, in vitro measurements of deposition in infants nasal airways are expected to be an acceptable alternative to in vivo studies.
In the present study we characterize ultrafine particles deposition in nasal airways of infants, supplementing a recent communication whose focus was on the deposition of micrometer-sized particles in replicas of nasal airways of infants (CitationStorey-Bishoff et al. 2008).
EXPERIMENTAL PROCEDURE
For the infant part of our study, computed tomography (CT) scans of upper airways of 10 infants (3–18 months) were obtained from the medical imaging archive at Stollery Children's Hospital, Edmonton, Canada with the approval of the Alberta Health Research Ethics Board. The replicas included the face, nostrils and nasal airways to the level of the upper trachea. Details of model construction and subject parameters can be found in a recent communication (CitationStorey-Bishoff et al. 2008).
For validation purposes, magnetic resonance imaging (MRI) scans of six adults during nasal breathing were obtained in coronal slices using a Turbo Spin Echo sequence using a Siemens MRI scanner (Sonata, Siemens Healthcare, Erlangen, Germany) with a standard head coil for signal reception under the approval of the University of Alberta Health Research Ethics Board. In-plane spatial resolution in the coronal plane was 0.33 mm with a slice thickness of 1.5 mm. Coronal slice orientations ensured the lower resolution slice-dimension was oriented predominantly along the length of the nasal passages, while the high resolution in-plane dimensions were oriented perpendicular to the nasal wall. Manual dynamic region growing using Mimics (Materialise, Leuven, Belgium) was used for segmenting airways in the MRI data. Replicas included all facial features and smoothed nasal airways proximal to trachea were subtracted from the face. shows smoothed nasal airways of one male (subject 3), one female (subject 5), and one of the infants (subject 2). The replicas were built in an Invision SR 3-D printer (3D Systems, Rock Hill, SC, USA) in three parts using an acrylic-build material and wax support, which was melted and removed later from the replicas by heating them to 60°C. Some geometrical parameters of the adult subjects are given in .
FIG. 1 Nasal airways of one infant subject and two adult subjects (one male and one female) are shown.
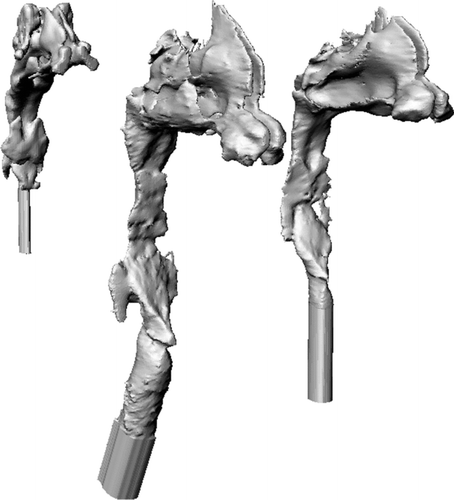
TABLE 1 Adult subject parameters
To facilitate the tube connections, noses of all replicas (adults and infants) were fitted with size 1 PARI BABYTM nebulizer silicone face masks (PARI, Inc. Midlothian, VA, USA). This method was chosen instead of using an exposure chamber because our preliminary experiments using an exposure chamber resulted in excessive fluctuations of concentration at the sampling point and close to the nostrils due to diffusion of particles as well as flow circulation within the chamber.
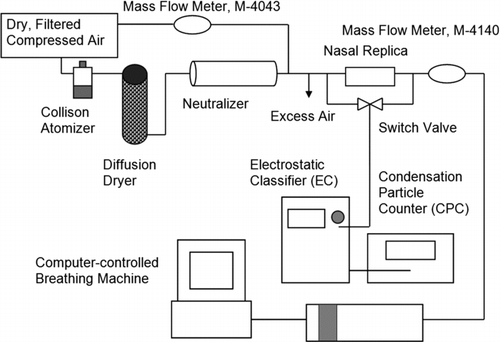
Polydisperse salt particles were generated from saline solution at a solution concentration of 0.24 ± 0.028 mg/ml using a 6-jet Collison atomizer (BGI, Inc., Waltham, MA, USA). Aerosol was passed through a silica gel diffusion dryer and subsequently passed through a Kr-85 neutralizer (Model 3054, TSI, Inc., St. Paul, MN, USA). To reduce coagulation, aerosol was then diluted upstream of the nasal replica with clean dry dilution air at a flow rate of 17 L/min (infant setup) or 31 L/min (adult setup). A mass flow meter (Model 4043, TSI, Inc., St. Paul, MN, USA) was used to measure flow rates of dilution air. Since a tidal breathing pattern was simulated, an outlet was provided to release the excess dilution air out of the system. A Traceable Digital Hygrometer Thermometer Dew point (Fisher Scientific, USA) was used to measure the relative humidity just upstream of the nasal replica; the maximum relative humidity was 31%. Dry salt particles are achieved (complete crystallization) below a relative humidity of 40% (CitationOrr et al. 1958; CitationTang and Murkelwitz 1984). The deposition efficiency for ultrafine particles in the size range of 13–100 nm in diameter in nasal replicas was measured using the setup illustrated in . Different components of the experimental setup were all connected using Teflon tubes (TSI, Inc., St. Paul, MN, USA) and metal fittings.
Two flow rates that were physiologically compatible with natural tidal breathing in infants were programmed and generated with an in-house breathing machine to produce a sinusoidal breathing pattern. For the present subjects, resting tidal volumes of 30–88 mL and breathing rate of 44–34 bpm, respectively, have been considered physiologically realistic (CitationStorey-Bishoff et al. 2008). A mass flow meter (Model 4140, TSI, Inc., St. Paul, MN, USA) was used for recording tidal breathing patterns. Periodic patterns were used since previous researchers have shown differences in total deposition of particles during tidal and steady flow through airway replicas at an equal average velocity (CitationHaussermann et al. 2002; CitationShi et al. 2006). Only the inhalation half of the breathing pattern was used. Actual values of breaths/min and tidal volume for each test were measured by analyzing recorded flow patterns versus time, given that the tidal volume is the area under the curve. The following measured breathing patterns were used: 42.09 ± 0.16 breaths/min (bpm) with average flow rate 3.07 ± 0.03 L/min, and 33.98 ± 0.09 bpm with average flow rate 7.06 ± 0.06 L/min, corresponding to minimum and maximum flow rates. Dividing average flow rate by twice the breaths per minute gives the tidal volume for a sinusoidal wave. To determine the effect of flow rate on deposition for three subjects (2, 6, and 10), a third flow pattern with a middling flow rate (5.34 ± 0.25 L/min, 39.78 ± 0.12 bpm) was also tested. Similarly, a small number of tests were done with two infant subjects (subjects 3 and 8) at two constant flow rates similar to two tested tidal flow patterns (3 and 7 L/min).
For six adult replicas, three constant flow rates of 4, 10, and 20 L/min were used to allow comparison with literature data. An additional set of experiments was performed with three adult subject replicas (subjects 1, 3, and 6) using two physiological tidal flow patterns with the average of approximately 9.7 ± 0.03 L/min (9.6 ± 0.05 bpm and tidal volume Vt= 0.506 ± 0.002 L) and 19.6 ± 0.4 L/min (16.86 ± 0.34 bpm and Vt=0.58 ± 0.02 L) to obtain preliminary data on the difference in deposition between constant versus tidal flow.
A scanning mobility particle sizer (Model 3936, TSI, Inc., Shoreview, MN, USA), consisting of an electrostatic classifier (EC, Model 3080, TSI, Inc., Shoreview, MN, USA) with a nano differential mobility analyzer (DMA, 3085, TSI, Inc., Shoreview, MN, USA) and a condensation particle counter (CPC, Model 3776, TSI, Inc., Shoreview, MN, USA), was used to count the particles. Deposition efficiency (η) was determined based on the number concentration before (C in) and after (C out) each nasal airway replica, as follows:
A typical experiment consisted of two 125-s samples from each side of the replica at ten sizes of particles (13 and 20–100) at 10 nm size interval increments (20, 30 nm, etc.). Preliminary experiments showed that one minute after switching valves, the concentration was steady; therefore, a one-minute time interval occurred between the two aforementioned samples to eliminate errors due to valve switching. Each deposition data point is an average of three experiments. Error bars are not displayed because they were approximately the same size of symbols. The first set of measurements for each replica was excluded after realizing that it was higher than the other measurements due to electrostatic surface charge on the airway surfaces of replicas, which was eliminated after having enough mass deposited on those surfaces. A single CPC was used after realizing that concentration was steady during the time of each test (310 s) for the selected particle sizes. Using a single CPC also eliminated any systematic error in determining the deposition efficiency. Count concentration of smaller particles (<13 nm) was too variable over the time of experiment (coefficient of variation: COV > 5%) due to the low concentration of those particles coming out of the Collison atomizer; therefore, only deposition of particles larger than 13 nm was determined.
Correction was needed for multicharged particles passing through the DMA with the mobility of the singly charged target size. For example, when the electrostatic classifier was set at 30 nm (n= 1), in actuality a fraction of 43 nm (n= 2) and 54 nm (n= 3) particles would also be counted by the CPC. Therefore, C in and C out in Equation (Equation1) for 30 nm particles are in fact given by:
Since pressure drop measurements are a means of indirectly validating the build procedure of the models, the pressure drop across each infant and adult replica was measured with a low range digital manometer (OMEGA HHP-103). Steady inspiratory flow rates in the ranges of 4–75 L/min and 0–16 L/min were used to obtain pressure measurements for the adult and infant replicas, respectively. The pressure drops of the connections were subtracted from the total pressure drop and the presented net values are averages of triplicate measurements.
RESULTS AND DISCUSSION
Method Validation
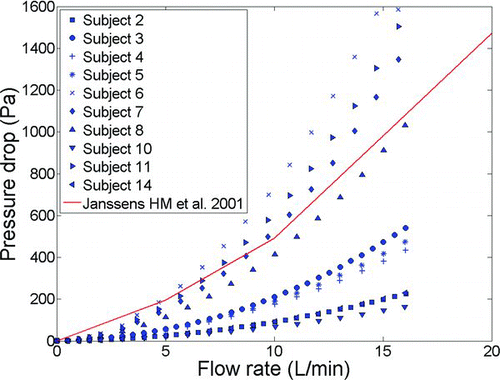
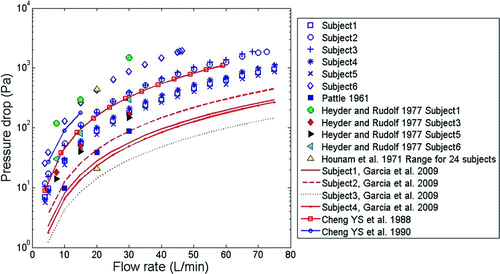
The transnasal pressure drop measurements in our six adult replicas for inspiratory flow rates ranging from 4–75 L/min are compared to literature values in . Comparison of the in vitro data of Cheng et al. (1988, 1990) and CitationGarcia et al. (2009) as well as the in vivo measurements of pressure drop across the nasal passages and nasopharynx on human volunteers performed by CitationPattle (1961), CitationHounam et al. (1971), and CitationHeyder and Rudolf (1977) shows that despite all the obvious intersubject variability, airway resistance across our adult replicas is within the range of existing data in the literature. This gives us confidence that the methodology we have chosen for fabricating replicas is adequate.
FIG. 3 Pressure drop data points across six adult human nasal replicas as a function of inspiratory air flow rate compared with available in vivo and in vitro measurements in the literature.
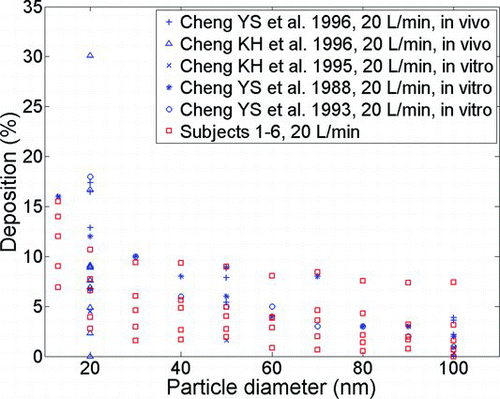
Deposition in our six adult replicas is compared for a flow rate of 20 L/min with existing in vivo and in vitro data in . Good agreement is seen. A paired t-test between mean deposition in six replicas versus the mean of all previous studies at a given flow rate (4 and 10 L/min) and particle size (20, 50, and 100 nm) yielded no significant difference between our in vitro data and that given by these previous studies (p> 0.05).
FIG. 4 Deposition data for adults at a flow rate of 20 L/min compared to available in vivo and in vitro data.
The above pressure and deposition data provides validation of our experimental method, given that no similar in vivo data on infants exists for validation purposes.
The pressure drop data for inspiratory flow in ten infant replicas are shown in . The range of in vivo physiologic data for upper airway resistance (ratio of pressure drop to airflow at a particular time) reported in the literature is 3.7–23.9 cm H2O. L–1.sec (362.85–2343.8 Pa. L–1.sec) for Caucasian infants weighing 1.5–10.2 kg (CitationStocks and Godfrey 1978). The CitationJanssens et al. (2001) Sophia Anatomical Infant Nose-Throat (SAINT) replica, an anatomically correct model of the upper airways of a 9-month-old child, has previously been found to lie in this range. shows that our replicas have similar values. Additional post-built validation of geometrical features of our replicas (volume, airway surface, minimum cross sectional area, and length) is given elsewhere (CitationStorey-Bishoff et al. 2008).
Comparison of Infant Data with Existing Adult Correlations
Having obtained deposition measurements in our ten infant replicas, let us first examine whether this data can be predicted using existing correlations developed for adults. summarizes all the available correlations that we have found in the literature for prediction of deposition of ultrafine particles in nasal airways of adults. They have been fitted to our infant data and their attributed R-squared values are given.
TABLE 2 Summary of equations in the literature for predicting deposition of ultrafine particles in nasal airways of adults and their related R2 values when fitted to our infant data
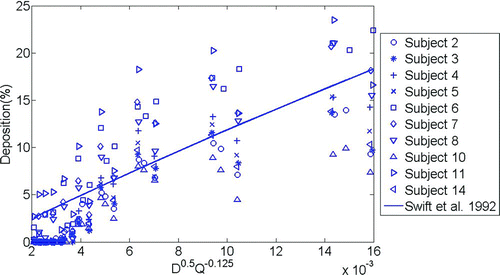
compares deposition values calculated using an equation developed for adults by CitationSwift et al. (1992), which gives the highest R-squared value when compared with our infant experimental data. Similar to , we find that most of the literature equations (given in ) pass through the cloud of our infant data. However, there is large intersubject variability that scatters the data and reduces the ability of any of the above equations to accurately predict deposition in a given individual.
FIG. 6 The correlation of CitationSwift et al. (1992) developed for adults is shown with our infant deposition data. Error bars on our experimental data points are approximately the same size as the symbols and so are not shown.
The respiratory tract is still under development in children and the airway dimensions are a function of age (CitationPhalen et al. 1985), which causes age-associated changes in nasal deposition. The effect of age on nasal deposition has been included in the equation developed by CitationCheng et al. (1995) as given in Equation (Equation10), in which parameter a(t) is a function of age in years as given in Equation (Equation11):
Here, the diffusion coefficient (D) is in cm2/s and Q is in L/min. Comparison of expected deposition in 5 of our infant replicas (subjects 2, 3, 4, 5, and 6) using Equations (Equation10) and (Equation11) is given with our measured experimental data in .
FIG. 7 The correlation of CitationCheng et al. (1995) developed for older children (1.5-, 2.5-, and 4-year-olds) is shown with infant deposition data in this study.
Previous authors provide predictions with much reduced intersubject variability by using dimensionless analysis with subject specific length scales (CitationStorey-Bishoff et al. 2008; CitationGarcia et al. 2009; CitationGrgic et al. 2004). Developed for adults, Equation (9) includes the subject specific dimensionless parameters Sc and Re. However, it does not include a dimensionless parameter governing unsteady effects, which is reasonable for adults (CitationKelly et al. 2004; CitationSwift and Strong 1996; CitationWang et al. 2009), but may not be reasonable in infants for particles governed by diffusion as is seen in , where deposition with constant flow rate is compared to that occurring with sinusoidal tidal breathing. It is seen that deposition is higher for unsteady versus steady flow rates. The breathing frequency in infants is much higher than adults and is apparently responsible for the increased importance of unsteadiness in infants. CitationHaussermann et al. (2002) found unsteadiness to be important at high breathing frequency in adults for micron-sized particles.
FIG. 8 Comparison of deposition in infant replicas vs. particle diameter (dp) using tidal breathing (solid markers) and constant flow rate (empty markers).
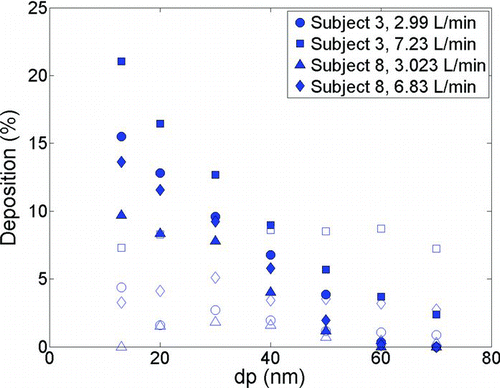
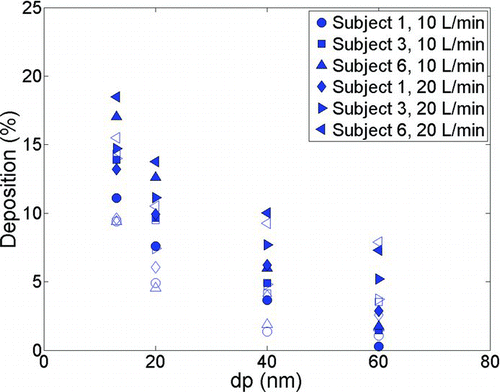
While the difference between steady and tidal flow deposition is noticeable in infants, this is in contrast to what we observe for adults as shown in . Although our data for detailed comparison of inhalation patterns in adults is limited (since adults are not the focus of this study), careful examination of shows that in most cases tidal deposition is slightly higher than constant flow deposition. CitationHeyder et al. (1982) noted enhanced deposition in adults with tidal flow patterns for micron sized particles compared to deposition using a controlled breathing pattern. However, Heyder et al. proposed that most of the intersubject variability was due to morphological parameters and the effect of physiological parameters was not significant in their study.
FIG. 9 Comparison of deposition in adult replicas vs. particle diameter (dp) using tidal breathing (solid markers) and constant flow rate (empty markers).
As will be noted later, prolonged residence in recirculation regions may play a stronger role in causing deposition in infants and may explain these differences between infant and adult deposition of ultrafine particles. In mass transport applications with large Peclet number, similar to this study, it is known that flow-assisted diffusion is present as a result of enhanced cross-stream advection due to recirculation regions and the recirculation zone resembles a well mixed region at steady-state with a resulting reduced distance for diffusive transport (CitationTrevelyan et al. 2002).
Predictive Correlations for Infants
Given the above considerations, unsteadiness appears to be important in the deposition of ultrafine particles in infants and we therefore add the dimensionless Womersley number, Wo (defined in Equation (Equation15)), to the list of governing dimensionless dynamical parameters. Following Finlay and Martin (2008), we then define the parameter X as a rational combination of the governing dimensionless parameters and determine the best fit form of X. However, it cannot be known a priori which characteristic diameter used in the calculation of dimensionless parameters will result in the best predictive fit. Some of the characteristic diameters that have been proposed in literature for reducing intersubject variability either in micron-sized or ultrafine range and tested in this study have been summarized in .
TABLE 3 Characteristic diameters that are suggested in literature and tested in this study for calculation of dimensionless parameters to reduce intersubject variability
Further study showed that the following equation gave the best fit to our deposition data:
Using different combinations of non-dimensional numbers to define X with various choices of characteristic diameters, R-squared values were obtained for each combination and are given in . shows that the characteristic diameter denoted in the third row from the bottom of , which uses the length of the nasal airway and its resistance, gives the best fit compared to other characteristic diameters.
TABLE 4 R-squared values of Equation (Equation12) fitted to infant data using different characteristic diameters
It can be seen that including the Womersley number also gives a better fit with a higher R-squared value. Although including the ratio of ![]() , where
, where ![]() is the average characteristic diameter for all subjects, improves R-squared values as much as including Womersley number does, Womersley number is included in the following further exploration of the parameter X due to our observed effect of unsteady flow. Inclusion of the Womersley number may be expected to improve the fit compared to inclusion of the ratio of characteristic diameters if the number of breaths per minute varies by a few orders of magnitude, however, such range in breathing frequency is not physiologically realistic; therefore it has not been explored.
is the average characteristic diameter for all subjects, improves R-squared values as much as including Womersley number does, Womersley number is included in the following further exploration of the parameter X due to our observed effect of unsteady flow. Inclusion of the Womersley number may be expected to improve the fit compared to inclusion of the ratio of characteristic diameters if the number of breaths per minute varies by a few orders of magnitude, however, such range in breathing frequency is not physiologically realistic; therefore it has not been explored.
The values of the six constants (a−f) of Equations (Equation12) and (13) were obtained using least squares fitting and their values are given in . The exponent b involving Re is much smaller than the exponent d including Wo, indicating that breathing frequency is more important than flow rate in affecting deposition. This supports our earlier supposition that breathing frequency underlies the explanation for the importance of unsteadiness in infants.
TABLE 5 Values of constants in equations (Equation12) and (13) for four diameters with the highest R 2 values for infant data
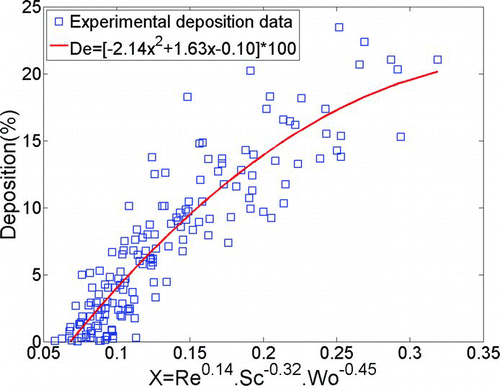
shows the best fit using characteristic diameter defined as dc =(0.0181 L nose/R nose)4/19 from Equations (Equation12) and (13).
FIG. 10 Deposition in infant replicas vs. non-dimensional deposition parameter using characteristic diameter defined as dc =(0.0181L nose/R nose)4/19.
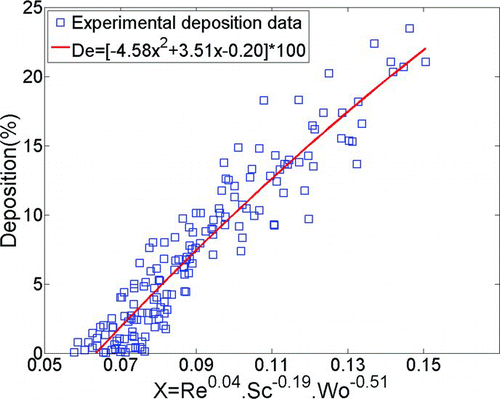
The characteristic diameter dc =(0.0181 L nose/R nose)4/19 was introduced by CitationGarcia et al. (2009) as the best parameter for the estimation of deposition of micron-sized particles in adult nasal airways. To examine the validity of using the correlation for turbulent flow pressure drops in a pipe of diameter d and length L for nasal airways, given by (Blasius H. 1911)
0.06), which is close to the value of 1.75 seen in the turbulent flow pressure drop correlation for pipes (Equation (Equation17)). Therefore, nasal resistance R nose was calculated by fitting the following equation to our measured infant replica pressure drop data:
TABLE 6 Nasal resistance dependent characteristic diameter of infants
Although the diameter that includes nasal resistance (dc
=(0.0181 L
nose/R
nose)4/19) yields the highest R-squared value for our infant data, other diameters (dc
=![]() , and
, and ![]() may be more convenient for use in a priori prediction of a deposition in a given subject. The constants used in defining the fits with those diameters are given in . Among
may be more convenient for use in a priori prediction of a deposition in a given subject. The constants used in defining the fits with those diameters are given in . Among ![]() and
and ![]() , if subject specific predictions are desired, both dc
=V/As
and
, if subject specific predictions are desired, both dc
=V/As
and ![]() require knowledge of the given subjects’ airway dimensions and thus require imaging of the nasal airway. However, to quantify
require knowledge of the given subjects’ airway dimensions and thus require imaging of the nasal airway. However, to quantify ![]() , acoustic rhinometry can be used to measure the volume and length of the nasal airways in a given subject. Average cross section can be calculated as volume divided by length. Thus, while the R-squared value is not as high,
, acoustic rhinometry can be used to measure the volume and length of the nasal airways in a given subject. Average cross section can be calculated as volume divided by length. Thus, while the R-squared value is not as high, ![]() may be more convenient to use than these other diameters. The fit function using
may be more convenient to use than these other diameters. The fit function using ![]() is shown in .
is shown in .
Use of Infant Correlation for Adults
While the correlation shown in provides good prediction of our infant deposition data, it is interesting to explore whether this correlation is suitable for predicting deposition in adults. For this reason, the values of L nose and R nose for three adult subjects that had been tested by us with tidal breathing patterns were quantified similarly and the values of those parameters are given in .
TABLE 7 Nasal resistance dependent characteristic diameter of adults
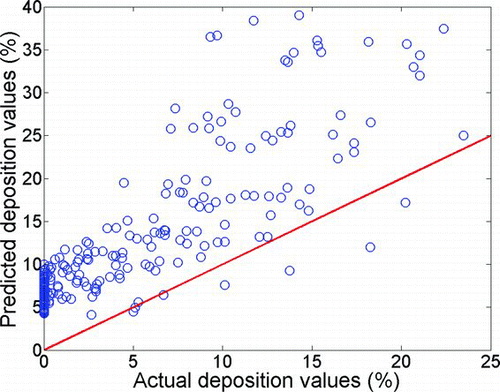
We compared our measured adult deposition data with the values obtained from Equations (Equation12) and (13) with related Reynolds and Womersley numbers. The fit is much worse than that for infants and gives a low R-squared value (R 2= 0.37). The number of adult subjects that were tested for tidal flow rate is not appropriate for an extensive comparison of adult deposition with our curve-fit obtained for infants; however, based on our relatively small dataset, the equation predicts somewhat higher deposition values in infants compared with adults at a fixed X value. This suggests that nasal deposition increases with decreasing age within the range of experiments in this study. CitationCheng et al. (1995) also found higher nasal deposition in younger children (1.5-year-old) compared with 2.5- and 4-year-old children.
The utility of our infant correlation to predict our adult data may also be reduced by the fact that deposition in adults is largely unaffected by unsteadiness, so that the inclusion of the Womersley number is not appropriate in adults. As mentioned earlier, the higher breathing frequency in infants appears to make unsteadiness important and cause this difference between infant and adult deposition for ultrafine particles. Unsteadiness is known to reduce entrainment of jets and affect deposition of micrometer sized particles in the larynx in adults (CitationGrgic et al. 2006). From a fluid mechanics perspective, reduced entrainment in separated shear regions may enhance the size of recirculation regions in our infant replicas with unsteady flow. Increased residence time in such enhanced recirculation regions may result in enhanced diffusional deposition and could explain the importance of unsteady effects in infants that we observe. This explanation is supported by other studies where it is found that the presence of recirculation regions significantly enhances cross-stream diffusional mass transport (CitationTrevelyan et al. 2002).
CONCLUSIONS
Deposition within a subject (intrasubject variability) is found to be a function of Reynolds number, Schmidt number, and Womersley number. Strong dependence of deposition on the steady vs. unsteady nature of the flow was noticed within the range of this study, with deposition using tidal breathing found to be higher than deposition using a constant flow pattern. These observations may be due to the fact that in unsteady flow patterns, a higher proportion of the flow area is part of recirculating flows having lower than average velocity. Enhanced recirculation regions would increase particle residence time and diffusional deposition. For adults, however, deposition for tidal flow was almost identical to deposition using steady flow rates. This may be due to the much lower breathing frequency in adults, and concomitant reduction in differences in recirculating regions between steady and unsteady flow rates.
For our ten infant replicas, the best fit correlation to our data is ![]() where
where ![]() , introduced by CitationGarcia et al. (2009) and obtained by fitting pressure-flow data with Equations (Equation19) and (Equation20), is used as the length scale for calculating Reynolds and Womersley numbers. The average value of d
c=(0.0181 L
nose/R
nose)4/19 for our infants is 2.95 mm. This equation requires knowledge of nasal resistance to predict deposition in a given subject. A correlation that fits nearly as well, but which requires only geometric information is η=−2.14(Re0.14.Sc−0.32.Wo−0.45)2+1.63(Re0.14.Sc−0.32.Wo−0.45)−0.10 where
, introduced by CitationGarcia et al. (2009) and obtained by fitting pressure-flow data with Equations (Equation19) and (Equation20), is used as the length scale for calculating Reynolds and Womersley numbers. The average value of d
c=(0.0181 L
nose/R
nose)4/19 for our infants is 2.95 mm. This equation requires knowledge of nasal resistance to predict deposition in a given subject. A correlation that fits nearly as well, but which requires only geometric information is η=−2.14(Re0.14.Sc−0.32.Wo−0.45)2+1.63(Re0.14.Sc−0.32.Wo−0.45)−0.10 where ![]() , defined as the ratio of the average cross-sectional area to a representative length of the nose from the nostrils to the trachea, is used as the length scale for calculating Reynolds and Womersley numbers. The average value of
, defined as the ratio of the average cross-sectional area to a representative length of the nose from the nostrils to the trachea, is used as the length scale for calculating Reynolds and Womersley numbers. The average value of ![]() for our infants is 1.1 mm. Use of either of these correlations allows fairly accurate subject specific predictions of ultrafine particle deposition in the nasal airways of infants. Such predictive capabilities may be useful in the development of improved health effect models for infants.
for our infants is 1.1 mm. Use of either of these correlations allows fairly accurate subject specific predictions of ultrafine particle deposition in the nasal airways of infants. Such predictive capabilities may be useful in the development of improved health effect models for infants.
The authors gratefully acknowledge the financial support of this work from the following sources: Natural Sciences and Engineering Research Council, Alberta Ingenuity Fund. The technical support of Institute for Reconstructive Sciences in Medicine is also gratefully acknowledged.
REFERENCES
- Arens , R. , Sin , S. , McDonough , J. M. , Palmer , J. M. , Dominguez , T. , Meyer , H. , Wootton , D. M. and Pack , A. I. 2005 . Changes in Upper Airway Size During Tidal Breathing in Children with Obstructive Sleep Apnea Syndrome . Am. J. Respir. Crit. Care Med. , 171 : 1298 – 1304 .
- Blasius , H. 1911 . The Similarity Law in Friction Processes . Phys. Z. , 12 : 1175 – 1177 .
- Cheng , Y. S. , Yamada , Y. , Yeh , H. C. and Swift , D. L. 1988 . Diffusional Deposition of Ultrafine Aerosols in a Human Nasal Cast . J Aerosol Sci. , 19 ( 6 ) : 741 – 751 .
- Cheng , Y. S. , Yamada , Y. , Yeh , H. C. and Swift , D. L. 1990 . Deposition of Ultrafine Aerosols in a Human Oral Cast . Aerosol Sci. Technol. , 12 ( 4 ) : 1075 – 1081 .
- Cheng , Y. S. , Su , Y. F. , Yeh , H. C. and Swift , D. L. 1993 . Deposition of Thoron Progeny in Human Head Airways . Aerosol Sci. Technol. , 18 ( 4 ) : 359 – 375 .
- Cheng , Y. S. , Smith , S. M. , Yeh , H. C. , Kim , D. B. , Cheng , K. H. and Swift , D. L. 1995 . Deposition of Ultrafine Aerosols and Thoron Progeny in Replicas of Nasal Airways of Young Children . Aerosol Sci. Technol. , 23 : 541 – 552 .
- Cheng , Y. S. , Yeh , H. C. , Guilmette , R. A. , Simpson , S. Q. , Cheng , K. H. and Swift , D. L. 1996 . Nasal Deposition of Ultrafine Particles in Human Volunteers and its Relationship to Airway Geometry . Aerosol Sci. Technol. , 25 : 274 – 291 .
- Cheng , K. H. , Cheng , Y. S. , Yeh , H. C. , Guilmette , R. A. , Simpson , S. Q. , Yang , S. Q. and Swift , D. L. 1996 . In vivo Measurements of Nasal Airway Dimensions and Ultrafine Aerosols Depositing in Human Nasal and Oral Airways . J Aerosol Sci. , 27 ( 5 ) : 785 – 801 .
- Cheng , Y. S. 2003 . Aerosol Deposition in the Extrathoracic Region . Aerosol Sci. Technol. , 37 : 659 – 671 .
- Finlay , W. H. 2008 . At The Frontiers of Understanding: Inhaled Aerosols in Neonates . Pediatr. Res. , 64 ( 2 ) : 121 – 122 .
- Finlay , W. H. and Martin , A. R. 2008 . Recent Advances in Predictive Understanding of Respiratory Tract Deposition . J. Aerosol Med. Pulm. Drug Deliv. , 21 ( 2 ) : 1 – 17 .
- Garcia , G. J. M. , Tewksbury , E. W. , Wong , B. A. and Kimbell , J. S. 2009 . Interindividual Variability in Nasal Filtration as a Function of Nasal Cavity Geometry . J. Aerosol Med. Pulm. Drug Deliv. , 22 ( 2 ) : 139 – 155 .
- Gill , S. , Lobenberg , R. , Ku , T. , Azarmi , S. , Roa , W. and Prenner , E. J. 2007 . Nanoparticles: Characteristics, Mechanisms of Action and Toxicity in Pulmonary Drug Delivery—A Review . J. Biomed Nanotech. , 3 : 107 – 119 .
- Grgic , B. , Finlay , W. H. , Burnell , P. K. P. and Heenan , A. F. 2004 . In Vitro Intersubject and Intrasubject Deposition Measurements in Realistic Mouth-Throat Geometries . J. Aerosol Sci. , 35 : 1025 – 1040 .
- Grgic , B. , Martin , A. R. and Finlay , W. H. 2006 . The Effect of Unsteady Flow Rate Increase on in vitro Mouth–Throat Deposition of Inhaled Boluses . J Aerosol Sci. , 37 : 1222 – 1233 .
- Guilmette , R. A. , Cheng , Y. S. , Yeh , H. C. and Swift , D. L. 1994 . Deposition of 0.005-12 μ m Monodisperse Particles in a Computer-Milled, MRI based Nasal Airway Replica . Inhal. Toxicol. , 6 ( s1 ) : 395 – 399 .
- Halonen , J. I. , Lanki , T. , Yli–Tuomi , T. , Tiittanen , P. , Kulmala , M. and Pekkanen , J. 2009 . Particulate air Pollution and Acute Cardiorespiratory Hospital Admissions and Mortality Among the Elderly . Epidemiol. , 20 ( 1 ) : 143 – 153 .
- Haussermann , S. , Bailey , A. G. , Bailey , M. R. , Etherington , G. and Youngman , M. 2002 . The Influence of Breathing Patterns on Particle Deposition in a Nasal Replicate Cast . J. Aerosol Sci. , 33 : 923 – 933 .
- Heyder , J. and Rudolf , G. 1977 . “ Deposition of Aerosol Particles in the Human Nose ” . In Inhaled Particles IV , Edited by: Walton , W. H. 107 – 126 . Oxford, , UK : Pergamon Press .
- Heyder , J. , Gebhart , J. , Stahlhofen , W. and Stuck , B. 1982 . Biological Variability of Particle Deposition in the Human Respiratory Tract During Controlled and Spontaneous Mouth-Breathing . Ann. Occupat. Hyg. , 26 ( 1–4 ) : 137 – 147 .
- Hounam , R. F. , Black , A. and Walsh , M. 1971 . The Deposition of Aerosol Particles in the Nasopharyngeal Region of the Human Respiratory Tract . J. Aerosol Sci. , 2 : 47 – 61 .
- Janssens , H. M. , De Jongste , J. C. , Fokkens , W. J. , Robben , S. G. F. , Wouters , K. and Tiddens , H. A. W. M. 2001 . The Sophia Anatomical Infant Nose–Throat (Saint). Model: A Valuable Tool to Study Aerosol Deposition in Infants . J Aerosol Med. , 14 ( 4 ) : 433 – 441 .
- Kelly , J. T. , Asgharian , B. , Kimbell , J. S. and Wong , B. 2004 . Particle Deposition in Human Nasal Airway Replicas Manufactured by Different Methods. Part II: Ultrafine Particles . Aerosol Sci. Technol. , 38 : 1072 – 1079 .
- Kreyling , W. G. , Semmler-Behnke , M. and Moller , W. 2006 . Ultrafine Particle–Lung Interactions: Does Size Matter? . J. Aerosol Med. , 19 : 74 – 83 .
- Martonen , T. B. and Zhang , Z. 1992 . Comments on Recent Data for Particle Deposition in Human Nasal Passages . J Aerosol Sci. , 23 ( 6 ) : 667 – 674 .
- Minocchieri , S. , Burren , J. M. , Bachmann , M. A. , Stern , G. , Wildhaber , J. , Buob , S. , Schindel , R. , Kraemer , R. , Frey , U. P. and Nelle , M. 2008 . Development of the Premature Neonate for the Study of Aerosol Delivery . Pediatr. Res. , 64 ( 2 ) : 141 – 146 .
- National Research Council (NRC) . 1988 . Health Risks of Radon and Other Internally Deposited Alpha Emitters , Washington, DC : Committee on the Biological Effects of Ionizing Radiations, National Academy Press .
- Oberdorster , G. , Sharp , Z. , Atudorei , V. , Elder , A. , Gelein , R. , Kreyling , W. and Cox , C. 2004 . Translocation of Inhaled Ultrafine Particles to the Brain . Inhal. Toxicol. , 16 : 437 – 445 .
- Orr , C. , Hurd , F. K. and Corbett , W. J. 1958 . Aerosol Size and Relative Humidity . J. Colloid Sci. , 13 : 472 – 482 .
- Park , S. H. , Kim , H. O. , Han , Y. T. , Kwon , S. B. and Lee , K. W. 2001 . Wall Loss Rate of Polydispersed Aerosols . Aerosol Sci. Technol. , 35 : 710 – 717 .
- Pattle , R. E. 1961 . “ The Retention of Gases and Particles in the Human Nose ” . In Inhaled Particles and Vapors , Edited by: Davies , C. N. 302 – 309 . New York : Pergamon Press .
- Phalen , R. F. , Oldham , M. J. , Beaucage , C. B. , Crocker , T. T. and Mortensen , J. D. 1985 . Postnatal Enlargement of Human Tracheobronchial Airways and Implications for Particle Deposition . Anat. Rec. , 212 : 368 – 380 .
- Polgar , G. and Kong , G. P. 1965 . The Nasal Resistance of Newborn Infants . J. Pediatr. , 67 : 557 – 567 .
- Schlichting , H. 1968 . Boundary Layer Theory , Edited by: Kestin , J. Stanford : McGraw-Hill .
- Schuepp , K. G. , Jauernig , J. , Janssens , H. M. , Tiddens , H. A. , Straub , D. A. , Stangl , R. , Keller , M. and Wildhaber , J. H. 2005 . In Vitro Determination of the Optimal Particle Size for Nebulized Aerosol Delivery to Infants . J. Aerosol Med. , 18 : 225 – 235 .
- Shi , H. , Kleinstreuer , C. and Zhang , Z. 2006 . Laminar Airflow and Nanoparticle or Vapor Deposition in a Human Nasal Cavity Model . J. Biomech. Eng. , 128 : 697 – 706 .
- Stocks , J. and Godfrey , S. 1978 . Nasal Resistance During Infancy . Respir. Physiol. , 34 : 233 – 246 .
- Storey-Bishoff , J. , Noga , M. and Finlay , W. H. 2008 . Deposition of Micrometer-Sized Aerosol Particles in Infant Nasal Airway Replicas . J. Aerosol Sci. , 39 : 1055 – 1065 .
- Swift , D. L. , Cheng , Y. S. , Su , Y. F. and Yeh , H. C. In Indoor Radon and Lung Cancer: Reality and Myth? . 29th Hanford Symposium on Health and the Environment . Edited by: Cross , F. pp. 213 Washington : Richland .
- Swift , D. L. 1991 . Inspiratory Inertial Deposition of Aerosols in Human Nasal Airway Replicate Casts: Implications for the Proposed NCRP Lung Model . Radiat. Prot. Dosimetry. , 38 : 29 – 34 .
- Swift , D. L. , Montassier , N. , Hopke , P. K. , Kim , K. H. , Cheng , Y. S. , Su , Y. F. , Yeh , H. C. and Strong , J. C. 1992 . Inspiratory Deposition of Ultrafine Particles in Human Nasal Replicate Cast . J. Aerosol Sci. , 23 ( 1 ) : 65 – 72 .
- Swift , D. L. and Strong , J. C. 1996 . Nasal Deposition of ulTrafine 218Po Aerosols in Human Subjects . J. Aerosol Sci. , 27 ( 7 ) : 1125 – 1132 .
- Tang , I. N. and Murkelwitz , H. R. 1984 . An Investigation of Solute Nucleation in Levitated Solution Droplets . J. Colloid Interface Sci. , 98 ( 2 ) : 430 – 438 .
- Trevelyan , P. M. J. , Kalliadasis , S. , Merkin , J. H. and Scott , S. K. 2002 . Mass-Transport Enhancement in Regions Bounded by Rigid Walls . J. Eng Math. , 42 : 45 – 64 .
- Van Woensel , J. B. M. , Van Aalderen , W. M. C. and Kimpen , J. L. L. 2003 . Viral Lower Respiratory Tract Infection in Infants and Young Children . Br. Med. J. , 327 : 36 – 40 .
- Vincent , J. H. 2005 . Health–Related Aerosol Measurement: A Review of Existing Sampling Criteria and Proposals for New Ones . J. Environ. Monit. , 7 : 1037 – 1053 .
- Wang , S. C. and Flagan , R. C. 1990 . Scanning Electrical Mobility Spectrometer . Aerosol Sci. Technol. , 13 ( 2 ) : 230 – 240 .
- Wang , S. M. , Inthavong , K. , Wen , J. , Tu , J. Y. and Xue , C. L. 2009 . Comparison of Micron- and Nanoparticle Deposition Patterns in a Realistic Human Nasal Cavity . Respir. Physiol. Neurobiol. , 166 : 142 – 151 .
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range . J. Aerosol Sci. , 19 ( 3 ) : 387 – 389 .
- Xi , J. and Longest , P. W. 2008 . Effects of Oral Airway Geometry Characteristics on the Diffusional Deposition of Inhaled Nanoparticles . J. Biomech. Eng. , 130 ( 011008 ) : 1 – 16 .
- Xi , J. and Longest , P. W. 2008 . Numerical Predictions of Submicrometer Aerosol Deposition in the Nasal Cavity Using a Novel Drift Flux Approach . Int. J. Heat Mass Transfer. , 51 : 5562 – 5577 .
- Yamada , Y. , Cheng , Y. S. , Yeh , H. C. and Swift , D. L. 1988 . Inspiratory and Expiratory Deposition of Ultrafine Particles in a Human Nasal Cast . Inhal. Toxicol. , 1 : 1 – 11 .
- Yu , G. , Zhang , Z. and Lessman , R. 1998 . Fluid Flow and Particle Deposition in the Human Upper Respiratory System . Aerosol Sci. Technol. , 28 : 146 – 158 .
- Zamankhan , P. , Ahmadi , G. , Wang , Z. , Hopke , P. H. , Cheng , Y. S. , Su , W. C. and Leonard , D. 2006 . Airflow and Deposition of Nano-Particles in a Human Nasal Cavity . Aerosol Sci. Technol. , 40 : 463 – 476 .