[Supplementary materials are available for this article. Go to the publisher’s online edition of Aerosol Science and Technology to view the free supplementary files.]
1. INTRODUCTION
Smoke emitted by prescribed and wild fires can make a substantial contribution to ambient aerosol (CitationMcMeeking et al. 2006; CitationPark et al. 2007; CitationSpracklen et al. 2007). Approaches to investigate these contributions have used a variety of different chemical smoke markers, including levoglucosan, produced by thermal degradation of cellulose, and water-soluble potassium (CitationAndreae 1983; CitationEngling et al. 2006; CitationHays et al. 2002; CitationSimoneit 2002; CitationWard et al. 2006). Filter sampling is commonly employed to measure smoke markers in ambient and source samples; however, these time-integrated measurements limit knowledge about variability of smoke marker emissions, especially between flaming and smoldering fire phases.
Recently developed instruments, such as the Aerodyne High-Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-AMS, AMS hereinafter for short), allow aerosol composition measurements with time resolution of seconds (CitationCanagaratna et al. 2007; CitationDeCarlo et al. 2006). Fragments with mass-to-charge (m/z) ratios of 60.021 (C2H4O+ 2) and 73.029 (C3H5O+ 2) have been suggested as AMS biomass burning markers (CitationAlfarra et al. 2007; CitationMohr et al. 2009; CitationSchneider et al. 2006). CitationWeimer et al. (2008) reported quadrupole AMS observations of primary smoke particles from flaming and smoldering phases of domestic wood burning. In this article, AMS data are used for the first time to examine key smoke markers emitted during the flaming and smoldering phases from open burning of numerous wildland fuel types. Key AMS smoke marker data presented include m/z 38.964 and m/z 60.021 (referred to as K+ and C2H4O+ 2 hereafter). We also compare C2H4O+ 2 measured by the AMS to concentrations of anhydrosugars (levoglucosan, mannosan, and galactosan) obtained by analyzing filter samples (CitationSullivan et al. 2008). Such observations are key to understanding what ambient AMS marker measurements reveal about primary particle contributions from biomass burning.
2. INSTRUMENTATION
During the second Fire Lab at Missoula Experiment (FLAME II), small quantities (∼250 g) of more than 20 different biomass fuel types were combusted in an “open burning” configuration. More information on the combustion facility, fuels used, types of burns conducted, and chemical analysis of collected filter samples can be found in CitationSullivan et al. (2008) and CitationMcMeeking et al. (2009). The composition of emitted smoke particles was continuously monitored across evolving flaming and smoldering combustion conditions using the AMS, which has been described in detail elsewhere (CitationCanagaratna et al. 2007; CitationDeCarlo et al. 2006). The fuel was burned under a 1.5 m diameter exhaust stack and the smoke forced up the stack to ∼16 m above ground where emissions cool to near ambient conditions. A high-volume air pump pulled diluted smoke emissions from the top of the stack to the AMS through a 3.2 cm diameter, 20 m long stainless steel tube. A typical time from biomass combustion to smoke particle analysis by the AMS was ∼30 s. The “W” ion path was used to provide higher spectral resolution of measured ion fragments. Collected data (∼30 s resolution) were analyzed using AMS data analysis software (SQUIRREL and PIKA) (CitationDeCarlo et al. 2006; CitationSueper 2009). Details about identification and quantification of smoke marker species measured are provided in the online supplemental information.
3. RESULTS AND DISCUSSION
Biomass burning emissions of gases and particles depend strongly on the combustion conditions, which can be broadly classified as flaming or smoldering. The combustion conditions can be monitored using the modified combustion efficiency (MCE), a ratio of emitted CO2 to the sum of emitted CO2 and CO (CitationChen et al. 2007; CitationMcMeeking et al. 2009; CitationYokelson et al. 1997). In this study MCE values larger than 0.9 and less than 0.8 were used to determine a predominance of flaming or smoldering combustion, respectively. An example timeline of MCE and key particle species emissions is provided in the online supplemental information.
3.1. Emission of Primary Smoke Markers
Previous studies have observed that potassium emission is higher in flaming-dominated combustion (e.g., CitationEchalar et al. 1995) and that levoglucosan emissions are higher during lower temperature (smoldering) burns (CitationShafizadeh 1982). In order to be most useful as a marker for emissions of primary particle mass (or organic aerosol (OA), which often dominates particle mass), an ideal marker would be emitted at a constant ratio to total particle mass (or OA) across combustion phases and for different biomasses.
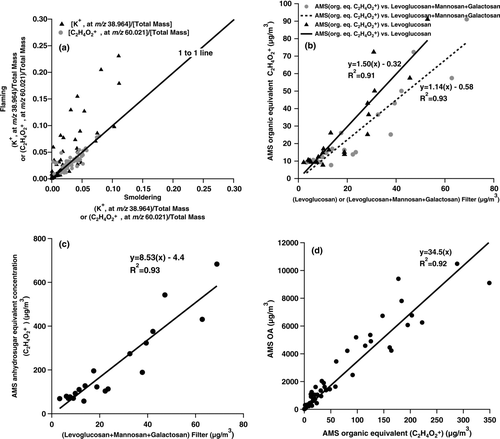
Ratios of K+ and C2H4O+ 2 to particle mass measured by the AMS in the flaming and smoldering phases of individual burns are shown in . K+ was typically emitted at a higher mass fraction in flaming phase combustion; C2H4O+ 2 was emitted at similar ratios to particle mass in flaming and smoldering phases of most burns. These observations are consistent with the poor relationship between potassium and levoglucosan concentrations reported for FLAME filter samples (CitationSullivan et al. 2008). Further, CitationSullivan et al. (2008) reported only modest dependence of the ratio of levoglucosan to aerosol organic carbon on fuel type. Together, these observations suggest that levoglucosan and related products are more universal primary smoke markers than potassium, since they do not depend as strongly on fuel type or combustion conditions.
FIG. 1 Observations of smoke particles during FLAME II open biomass burning experiments: (a) comparisons of the ratios of K+ (at m/z 38.964) and C2H4O+ 2 (at m/z 60.021) to total AMS particle mass for flaming and smoldering fire phases of individual FLAME II burns; (b) relationship of organic equivalent concentrations of C2H4O+ 2 from the AMS to the concentration of levoglucosan or the concentration sum of levoglucosan, mannosan, and galactosan measured from simultaneously collected filter samples (CitationSullivan et al. 2008); (c) a hypothetical computed AMS anhydrosugar-equivalent concentration (determined as 7.5 times AMS C2H4O+ 2) to levoglucosan + mannosan + galactosan concentrations measured in simultaneously collected filter samples; (d) AMS organic aerosol concentration vs. AMS C2H4O+ 2 organic equivalent concentration for individual FLAME II burns. The plotted points in each panel represent the average values measured, typically over several minutes of sampling. The orthogonal distance regression approach was used to compute best fit lines in panels (b)–(d).
3.2. Relationships between Anhydrosugars, AMS C2H4O+ 2, and AMS OA
Levoglucosan is the most abundant thermal breakdown product of cellulose identified in smoke particles. The thermal degradation of cellulose and hemicellulose also produces other anhydrosugars, including mannosan, galactosan, arabinosan, and xylosan. Mass fragment m/z 60 is commonly used as a marker for biomass burning in the AMS, where typically one major ion (C2H4O+ 2) contributes to the observed signal as also observed in this dataset (see the online supplemental information, Figure S1).
presents the correlation between levoglucosan, or levoglucosan + mannosan + galactosan, as determined from filters (CitationSullivan et al. 2008), with organic-equivalent (CitationZhang et al. 2005) C2H4O+ 2 measured simultaneously by the AMS. Strong correlations are observed, with correlation coefficients (R 2) of 0.91 and 0.93, for the relationship with levoglucosan alone or the sum of three anhydrosugar concentrations, respectively. These results, together with the absence of substantial C2H4O+ 2 in most other non-biomass burning aerosol types, confirm that C2H4O+ 2 is a suitable AMS marker for primary particle emissions from biomass burning. Multiple field studies have shown that C2H4O+ 2 is present at a level of ∼0.3 % of total OA during periods without biomass burning influence (e.g., CitationAiken et al. 2009; CitationDocherty et al. 2008). CitationMohr et al. (2009) found C2H4O+ 2 in AMS analysis of meat cooking aerosols and laboratory studies also indicate it can form in AMS analysis of longer chain alkanoic acids. CitationTakegawa et al. (2007) used an AMS to study oxalic acid, several other larger diacids, and glyoxylic acid and found only minor amounts of m/z 60. As CitationTakegawa et al. (2007) discuss, fragmentation patterns in the AMS can differ from standard 70 eV EI spectra due to the higher vaporizer temperature used in the AMS. We hypothesize that the source of the ∼0.3% background C2H4O+ 2 in ambient OA is dominated by acid groups from SOA with a minor contribution from other sources such as meat cooking. The general representativeness of this background value should be evaluated in additional studies.
AMS analyses of levoglucosan, mannosan, and galactosan standards in our laboratory reveal that C2H4O+ 2 comprises 14.1, 13.8, and 12.1% of the total ion fragment pattern, respectively (CitationAiken et al. (2009), CitationSchneider et al. (2006), and CitationAlfarra et al. (2007) previously reported values of approximately 13%, 12%, and 8% for levoglucosan, respectively). Using this information, and assuming for the moment that no other molecules in the smoke particles generate C2H4O+ 2, we can calculate an AMS “anhydrosugar-equivalent mass concentration” (anhyd.-eq. conc.), defined as the sum of the levoglucosan, mannosan, and galactosan concentrations required to produce the observed C2H4O+ 2 signal intensity. We calculate the anhyd.-eq. conc. by multiplying the observed C2H4O+ 2 mass concentration by 7.5 to account for the full anhydrosugar fragment pattern. The results of these calculations are shown in , where the anhyd.-eq. conc. is plotted vs. the sum of the three anhydrosugar concentrations from simultaneous filter samples. If levoglucosan, mannosan, and galactosan were the only molecules in the smoke particles to yield C2H4O+ 2, we would expect the anhyd.-eq. conc. to equal the concentration sum of these three species. Clearly this is not the case. The slope of a best fit line for this dataset is 8.5 with a small intercept, indicating that the AMS C2H4O+ 2
signal is much larger than can be explained by the three measured anhydrosugars. This is not surprising. Other structurally similar molecules, which are likely abundant in smoke, also produce C2H4O+ 2 upon electron impact fragmentation. AMS laboratory tests reveal, for example, that glucose yields an important fragment at C2H4O+ 2, comprising ∼10% of the total ion fragment pattern.
We can also directly investigate the utility of AMS C2H4O+ 2 itself as a smoke marker by examining the correlation between OA and C2H4O+ 2 across all FLAME II burns. A correlation of R2= 0.92 with a slope of 34.5 between biomass burning OA and C2H4O+ 2 was observed (), similar to the relationship determined by CitationAiken et al. (2009) for mostly unprocessed smoke reaching downtown Mexico City from nearby forest fires. CitationAlfarra et al. (2007)previously proposed a ratio of 36 for domestic wood burning in Zurich and indicate a ratio of 30 was estimated for summertime biomass (presumably open) burning in the region. Weimer et al. (2007) report a much more variable range of this ratio for their residential wood burning experiments and a tendency for lower OA to m/z 60 ratios during flaming conditions.
The correlation between OA and C2H4O+ 2 observed here is stronger than the correlation between FLAME filter-based measurements of levoglucosan and OC (R2= 0.68) (CitationSullivan et al. 2008). One possible explanation for the improved relationship is that C2H4O+ 2 appears to reflect a broader range of biomass burning molecules than the three anhydrosugars discussed here. The sum of all compounds yielding C2H4O+ 2 may define a more constant fraction of emitted fine particle OA than does levoglucosan. As CitationSullivan et al. (2008) point out, the levoglucosan/OC yield appears to vary across fuel components (e.g., branches, needles/leaves, grasses, etc.) in accordance with changes in the fuel mass fraction of cellulose. The less variable ratio of OA to C2H4O+ 2 observed here suggests that increases (decreases) in levoglucosan yield in biomass burning smoke may be offset to some extent by corresponding decreases (increases) in other molecules that also yield C2H4O+ 2 in the AMS.
The strength of the OA vs. C2H4O+ 2 relationship in fresh smoke across fuel types and combustion conditions offers promise that AMS measurements may be useful for directly estimating contributions of biomass burning primary emissions to ambient fine particle organic carbon. Additional research is necessary, however, to assess this relationship as a function of other biomass burning source types (e.g., residential wood burning) and various atmospheric processes, including smoke dilution (which may affect organic gas-particle partitioning) and smoke plume aging.
uast_a_499884_sup_14501007.zip
Download Zip (57.9 KB)This work was funded by NPS (CA2380-99001 TO0356), JFSP (05-3-1-06), NSF (ATM-0521643, ATM-0449815), and USEPA (R833747).
REFERENCES
- Aiken , A. , Salcedo , D. , Cubison , M. J. , Huffman , J. A. , DeCarlo , P. F. , Ulbrich , I. M. , Docherty , K. S. , Sueper , D. , Kimmel , J. R. , Worsnop , D. R. , Trimborn , A. , Northway , M. , Stone , E. A. , Schauer , J. J. , Volkamer , R. M. , Fortner , E. , de Foy , B. , Wang , J. , Laskin , A. , Shutthanandan , V. , Zheng , J. , Zhang , R. , Gaffney , J. , Marley , N. A. , Paredes-Miranda , G. , Arnott , W. P. , Molina , L. T. , Sosa , G. and Jimenez , J. L. 2009 . Mexico City Aerosol Analysis During MILAGRO Using High Resolution Aerosol Mass Spectrometry at the Urban Supersite (T0)—Part 1: Fine Particle Composition and Organic Source Apportionment . Atmos. Chem. Phys. , 9 : 6633 – 6653 .
- Alfarra , M. R. , Prevot , A. S. H. , Szidat , S. , Sandradewi , J. , Weimer , S. , Lanz , V. A. , Schreiber , D. , Mohr , M. and Baltensperger , U. 2007 . Identification of the Mass Spectral Signature of Organic Aerosols from Wood Burning Emissions . Environ. Sci. Technol. , 41 : 5770 – 5777 .
- Andreae , M. O. 1983 . Soot Carbon and Excess Fine Potassium: Long-Range Transport of Combustion-Derived Aerosols . Science , 220 : 1148 – 1151 .
- Canagaratna , M. R. , Jayne , J. T. , Jimenez , J. L. , Allan , J. D. , Alfarra , M. R. , Zhang , Q. , Onasch , T. B. , Drewnick , F. , Coe , H. , Middlebrook , A. , Delia , A. , Williams , L. R. , Trimborn , A. M. , Northway , M. J. , DeCarlo , P. F. , Kolb , C. E. , Davidovits , P. and Worsnop , D. R. 2007 . Chemical and Microphysical Characterization of Ambient Aerosols with the Aerodyne Aerosol Mass Spectrometer . Mass Spectrom. Rev. , 26 : 185 – 222 .
- Chen , L. W. A. , Moosmuller , H. , Arnott , W. P. , Chow , J. C. , Watson , J. G. , Susott , R. A. , Babbitt , R. E. , Wold , C. E. , Lincoln , E. N. and Hao , W. M. 2007 . Emissions from Laboratory Combustion of Wildland Fuels: Emission Factors and Source Profiles . Environ. Sci. Technol. , 41 : 4317 – 4325 .
- DeCarlo , P. F. , Kimmel , J. R. , Trimborn , A. , Northway , M. J. , Jayne , J. T. , Aiken , A. C. , Gonin , M. , Fuhrer , K. , Horvath , T. , Docherty , K. S. , Worsnop , D. R. and Jimenez , J. L. 2006 . Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer . Anal. Chem. , 78 : 8281 – 8289 .
- Docherty , K. S. , Stone , E. A. , Ulbrich , I. M. , DeCarlo , P. F. , Snyder , D. C. , Schauer , J. J. , Peltier , R. E. , Weber , R. J. , Murphy , S. M. , Seinfeld , J. H. , Grover , B. D. , Eatough , D. J. and Jimenez , J. L. 2008 . Apportionment of Primary and Secondary Organic Aerosols in Southern California During the 2005 Study of Organic Aerosols in Riverside (SOAR-1) . Environ. Sci. Technol. , 42 : 7655 – 7662 .
- Echalar , F. , Gaudichet , A. and Cachier , H. 1995 . Aerosol Emissions by Tropical Forest and Savanna Biomass Burning: Characteristic Trace Elements and Fluxes . Geophys Res. Lett. , 22 : 3039 – 3042 .
- Engling , G. , Herckes , P. , Kreidenweis , S. M. , Malm , W. C. and Collett , J. L. Jr. 2006 . Composition of the Fine Organic Aerosol in Yosemite National Park During the 2002 Yosemite Aerosol Characterization Study . Atmos. Environ. , 40 : 2959 – 2972 .
- Hays , M. D. , Geron , C. D. , Linna , K. J. , Smith , N. D. and Schauer , J. J. 2002 . Speciation of Gas-Phase and Fine Particle Emissions from Burning of Foliar Fuels . Environ. Sci. Technol. , 36 : 2281 – 2295 .
- McMeeking , G. R. , Kreidenweis , S. M. , Baker , S. , Carrico , C. M. , Chow , J. C. , Collett , J. L. , Hao , W. M. , Holden , A. S. , Kirchstetter , T. W. , Malm , W. C. , Moosmuller , H. , Sullivan , A. P. and Wold , C. E. 2009 . Emissions of Trace Gases and Aerosols During the Open Combustion of Biomass in the Laboratory . J. Geophys. Res. , 114 : D19210
- McMeeking , G. R. , Kreidenweis , S. M. , Lunden , M. , Carrillo , J. , Carrico , C. M. , Lee , T. , Herckes , P. , Engling , G. , Day , D. E. , Hand , J. , Brown , N. , Malm , W. C. and Collett , J. L. Jr. 2006 . Smoke Impacted Regional Haze in California During the Summer of 2002 . Agr. Forest Meteorol. , 137 : 25 – 42 .
- Mohr , C. , Huffman , J. A. , Cubison , M. J. , Aiken , A. C. , Docherty , K. S. , Kimmel , J. R. , Ulbricht , I. M. , Hannigan , M. and Jimenez , J. L. 2009 . Characterization of Primary Organic Aerosol Emissions from Meat Cooking, Trash Burning, and Motor Vehicles with High-Resolution Aerosol Mass Spectrometry and Comparison with Ambient and Chamber Observations . Environ. Sci. Technol. , 43 : 2443 – 2449 .
- Park , R. J. , Jacob , D. J. and Logan , J. A. 2007 . Fire and Biofuel Contributions to Annual Mean Aerosol Mass Concentrations in the United States . Atmos. Environ. , 41 : 7389 – 7400 .
- Schneider , J. , Weimer , S. , Drewnick , F. , Borrmann , S. , Helas , G. , Gwaze , P. , Schmid , O. , Andreae , M. O. and Kirchner , U. 2006 . Mass Spectrometric Analysis and Aerodynamic Properties of Various Types of Combustion-Related Aerosol Particles . Int. J. Mass Spectrom. , 258 : 37 – 49 .
- Shafizadeh , F. 1982 . Intorduction to Pyrolysis of Biomass . J. Anal. Appl. Pyrol. , 3 : 283 – 305 .
- Simoneit , B. R. T. 2002 . Biomass Burning—A Review of Organic Tracers for Smoke from Incomplete Combustion . Appl. Geochem. , 17 : 129 – 162 .
- Spracklen , D. V. , Logan , J. A. , Mickley , L. J. , Park , R. J. , Yevich , R. , Westerling , A. L. and Jaffe , D. A. 2007 . Wildfires Drive Interannual Variability of Organic Carbon Aerosol in the Western US in Summer . Geophys. Res. Lett. , 34 : L16816
- Sueper , D. 2009 . ToF-AMS High Resolution Analysis Software . Online available at http://cires.colorado.edu/jimenez-group/ToFAMSResources/ToFSoftware/index.html.
- Sullivan , A. P. , Holden , A. S. , Patterson , L. A. , McMeeking , G. R. , Kreidenweis , S. M. , Malm , W. C. , Hao , W. M. , Wold , C. E. and Collett , J. L. 2008 . A Method for Smoke Marker Measurements and its Potential Application for Determining the Contribution of Biomass Burning from Wildfires and Prescribed Fires to Ambient PM2.5 Organic Carbon . J. Geophys. Res. , 113 : D22302
- Takegawa , N. , Miyakawa , T. , Kawamura , K. and Kondo , Y. 2007 . Contribution of Selected Dicarboxylic and W-Oxocarboxylic Acids in Ambient Aerosol to the m/z 44 Signal of an Aerodyne Aerosol Mass Spectrometer . Aerosol Sci. Technol. , 41 : 418 – 437 .
- Ward , T. J. , Hamilton , R. F. , Dixon , R. W. , Paulsen , M. and Simpson , C. D. 2006 . Characterization and Evaluation of Smoke Tracers in PM: Results from the 2003 Montana Wildfire Season . Atmos. Environ. , 40 : 7005 – 7017 .
- Weimer , S. , Alfarra , M. R. , Schreider , D. , Mohr , M. , Prevot , A. S. H. and Baltensperger , U. 2008 . Organic Aerosol Mass Spectral Signatures from Wood-Burning Emissions: Influence of Burning Conditions and Wood Type . J. Geophys. Res. , 113 : D10304
- Yokelson , R. J. , Susott , R. , Ward , D. E. , Reardon , J. and Griffith , D. W. T. 1997 . Emissions from Smoldering Combustion of Biomass Measured by oPen-Path Fourier Transform Infrared Spectroscopy . J. Geophys. Res. , 102 : 18865 – 18877 .
- Zhang , Q. , Alfarra , M. R. , Worsnop , D. R. , Allan , J. D. , Coe , H. , Canagaratna , M. R. and Jimenez , J. L. 2005 . Deconvolution and Quantification of Hydrocarbon-Like and Oxygenated Organic Aerosols Based on Aerosol Mass Spectrometry . Environ. Sci. Technol. , 39 : 4938 – 4952 .