Abstract
Particulate matter (PM) emitted from a dual fuel engine is characterized using thermogravimetry, X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy. Thermogravimetric analysis (TGA) provides the mass fractions of elemental carbon and volatile materials in PM; XPS provides the possible chemical compositions in the topmost layer of PM surface and Raman analysis provides the possible structure of the carbon presented in PM. Dual fuel engine uses both liquid (diesel) and gaseous fuels simultaneously to produce mechanical power and can be switched to only diesel fueling under load. The dual fuel engine is operated with natural gas and simulated biogases (laboratory prepared) and results are compared between the dual fueling and diesel fueling under the same engine operating conditions. Significantly higher volatile fractions in PM are obtained for dual fueling compared to diesel fueling complementing the gravimetric results. The maximum contribution of the graphitic carbon or aliphatic carbon such as hydrocarbons and paraffins (C═C or C─C) are found in the topmost atomic layers of both the diesel and dual fuel PM samples. The other chemical states are found to be the carbon-oxygen functional groups indicating significant oxidation behavior in the PM surface. Lesser aromatic content is noticed in the case of dual fuel PM than diesel PM. The carbon in dual fuel PM is found to be more amorphous compared to diesel PM. These characterizations provide us new information how the PM from a diesel engine can be different from that from a dual fuel engine.
1. INTRODUCTION
Diesel engines are inherently more efficient than gasoline engines and can be operated at higher compression ratios, which permits to use alternative low energy content fuel gases. They are widely used in both stationary and mobile applications, especially where high power output is needed. While often resulting in smaller amounts of carbon monoxide, and hydrocarbons emissions in comparison with gasoline engines, diesel engines emit significantly higher PM. Particulate emissions can be classified as potential occupational carcinogen and can have a number of other negative health impacts associated with exposure (CitationZhu et al. 2005). It is generally agreed that diesel engines used in transport systems represent an important source of ambient particulate matter (CitationVouitsis et al. 2003). Diesel engines emit fine and ultrafine (particles having diameter of less than 100 nm) particles that can easily penetrate deep into the respiratory system (CitationKennedy et al. 2009). There might be a causal relationship between exposure to diesel emissions from mobile sources and the incidence of cancer, respiratory symptoms, and respiratory diseases (CitationEl-Zein et al. 2007). Diesel PM have therefore potential environmental impacts, including health effects, climate change, ecological effects, and visibility. Current regulations concern PM concentrations only, i.e., the mass of all particulates that can be collected from the exhaust. Therefore, the regulation seems to be insufficient with respect to the present health and environmental concerns. Modern engines are meeting the PM mass concentration targets but there is increasing concern about the emission of fine particles in the exhaust (CitationBurtscher 2005). Hence, in addition to the concentration measurements, PM characterization is also important to provide better understanding to address the issues.
Among different approaches to the challenge of minimizing the emissions, one can be considered as the use of gaseous fuels in diesel engines. Gaseous fuels in diesel engines operate in dual fuel mode where the main energy comes from the gaseous fuel and the minimum amount of diesel fuel acts as the ignition source. Fuel gas, which is referred to as the primary fuel, is normally inducted into the diesel engine along with air and compressed as usual. A minimum amount of diesel fuel spray, termed as pilot fuel (minimum quantity equivalent to 5–10% of the total full load fuel flow), is injected through the conventional fuel injection system near the end of compression stroke to initiate combustion. The pilot diesel fuel first self ignites and then acts as a reliable source of ignition for the surrounding gas-air mixture. Since the engines can be operated at high compression ratios, they are suitable for the use of low energy content gaseous fuels. Dual fueling therefore offers opportunities to use alternative (natural gas) and renewable (biogas) fuels in engines.
Biogas can be produced from many different organic wastes by anaerobic fermentation. Developing countries produce enormous amount of fermentable biomass such as agricultural wastes and hence have huge potential to produce and use biogas in engines. The use of stationary small capacity diesel engines in power generation and in agriculture and construction works is quite common in these developing countries. Biogas can provide a good alternative source for those engines and thus can contribute to the national economy and to the environment. The main component of biogas is methane (CH4), which is treated as a very harmful green house gas. In many cases this CH4 can just be escaped to the atmosphere and thus may have harmful effects to the environment. Therefore, the use of biogas has twofold benefits: provides an alternative and renewable energy source and protects the environment.
Significant research gaps are noted in the case of biogas operated dual fuel engines in terms of both the gaseous and PM emissions. Although numerous works have been published over the last decades on the regulated gaseous emissions from natural gas (NG) operated dual fuel engines (CitationBoisvert et al. 1988; CitationWong et al. 1991; CitationWeaver and Turner 1994; CitationAbd Alla et al. 2000; CitationGalal et al. 2002; CitationPapagiannakis and Hountalas 2004; CitationPatterson et al. 2006), only a very few of them are found to investigate either the smoke or PM emissions for the NG-diesel dual fuel engines (e.g., CitationBoisvert et al. 1988; CitationWong et al. 1991; CitationPapagiannakis and Hountalas 2004). On the other hand, a very limited number of published research works are found for biogas-diesel dual fuel engines especially from an emissions perspective such as CitationKarim and Amoozegar (1982), CitationKarim and Weirzba (1992), and CitationHenam and Makkar (1998). Virtually no published research work is found during this research project that deals with the PM emissions from a biogas-diesel dual fuel engine.
Although the use of gaseous fuels in diesel engines may help to reduce PM emissions, the basics are still not clearly understood. It is therefore important to carry on detailed experimental investigations in terms of PM measurements and characterization for the dual fuel applications in order to achieve increased understanding.
In this study, thermogravimetric analysis (TGA) is employed to quantify the mass fractions of the elemental carbon and volatile materials in the sampled PM. The surface characterization technique, X-ray photoelectron spectroscopy (XPS), is used to characterize the PM samples collected on filters. The internal microstructure of the carbon in PM samples is investigated and quantitatively analyzed using Raman spectroscopy. This leads to a better understanding of how the diesel PM changes in terms of chemical composition with different engine operating conditions and fuel qualities, especially the comparative picture between the diesel PM and dual fuel PM under the same operating conditions.
2. EXPERIMENTAL DESCRIPTIONS
2.1. Test Engine Facility and Method
A Lister Petter, single cylinder, direct injection diesel engine is used for the present study. The engine is modified to run in both diesel and dual fuel modes. The original injection system of the engine is maintained for the dual fuel operations. Further detail of the engine and PM sampling system are in CitationMustafi and Raine (2008).
New Zealand low sulfur diesel fuel, limiting sulfur to a maximum of 50 ppm, is used for the experiments. Natural gas (NG) is obtained from the pipeline supply and simulated biogas is prepared in the laboratory by mixing NG with CO2 with desired proportions to obtain different biogas mixtures: biogas-1 (80% CH4 and 20% CO2); biogas-2 (67% CH4 and 33% CO2); biogas-3A (59% CH4, 41% CO2 and about 820 ppm H2S); and biogas-3B (58% CH4 and 42% CO2).
Tests are performed at a constant engine speed of 1750 rpm. Two modes of steady state operation are chosen for diesel fueling: low load (∼ 3 Nm) and high load (∼ 28 Nm), which are approximately 8% and 75% of the rated output of the engine respectively for the mentioned speed. Under dual fuel operation, the amount of pilot diesel is always kept constant and the output torque of the engine is raised to 28 Nm by increasing the flow rate of gaseous fuel. About 62 percent diesel fuel by volume is replaced during the dual fuel operations.
2.2. Exhaust Dilution and PM Sampling Systems
A single stage partial flow dilution system (PFDS) is used in this study to dilute the representative exhaust gas sample drawn from the engine. The details of the system are described in CitationMustafi and Raine (2008). The dilution ratio (DR) is maintained at approximately 10 to 1 for the whole experimental program. The typical values of the flow rates used in the experiment were: exhaust sample flow rate ∼ 14 L/min; dilution airflow rate ∼ 126 L/min; and the total flow rate ∼ 140 L/min. The sample transfer tube (about 1 m of total length and about 8 mm in diameter) between the engine exhaust pipe and the dilution tunnel is insulated and heated, maintaining a wall temperature of 190°C to minimize thermophoretic deposition. Though the PFDS does not reflect the true atmospheric dilution because of the limitations of using higher DR, its use in PM measurements is certified by the standards and is treated as a substitute for full flow constant volume sampling system at steady-state condition (CitationKhalek et al. 2002). It has been established that DR has effects on volatile materials of the measured PM but has little effect on solid carbonaceous materials (CitationLapuerta et al. 1999).
2.3. Thermogravimetric Analysis (TGA)
PM samples are collected on glass fiber filters without Teflon coating (Whatman GF/C 1822/047), as they are suitable for TGA (CitationPrice et al. 2007; CitationLapuerta et al. 2007). After collection, the loaded filters are kept in sealed Petri dishes and are conditioned in an environmental chamber before TGA.
A strip of the loaded filter, about 5 mm wide, is cut and then cut again into small squares which are carefully stacked into the sample pan of the thermogravimetric analyzer. The PM mass collected on the whole filter used for TGA is in the range of 2.6 to 3 mg (as suggested by CitationLapuerta et al. 2007). The mass of a whole clean filter is typically 25 mg. The initial weight of the selected portion of the loaded filter that is put into the sample pan ranged from 2.5 to 3.8 mg. The initial mass gain found in TGA plots (online Supplemental Information, Figures S1–S3) was not investigated during the experiments. However, this symptom was observed for all the samples analyzed and therefore had consistency in the results obtained.
2.3.1. TGA Parameters
A Shimadzu TGA-50 thermogravimetric analyzer facility has been used for the present study. All the PM samples are heated in the TGA according to the heating program listed in which has been adapted from those recommended by CitationLapuerta et al. (2007) and CitationPrice et al. (2007).
TABLE 1 TGA heating program
TGA has limitations that some of the organic material can be pyrolysed during the first part of the test when the environment is inert. This would appear as non-volatile matters and thus lead to that material being classified as elemental carbon. The appropriate term may therefore be solid or non-volatile fraction. However, like other researchers (CitationPrice et al. 2007), elemental carbon (EC) term has been used in this article, as the major part of the non-volatile fraction would be carbonaceous materials.
2.4. XPS Analysis
PM samples are collected onto glass fiber filters without Teflon coating (the same as for TGA). The collection time is usually longer than for gravimetric analysis in order to collect enough sample material on the filter.
XPS examination of the collected PM samples is performed on a Kratos Axis Ultra DLD, using monochromatic Al Kα X-rays (1486.69 eV) having a spectral resolution of 0.1–0.2 eV. The X-ray power used is 150 W (10 mA, 15 kV). Two types of XPS spectra are recorded; survey (or wide) and narrow scans. Survey scans are performed with 160 eV pass energy, while the narrow scans are performed with pass energy of 20 eV. The XPS system included a charge neutralization system, which is used for non-conducting samples. Calibration of the spectrometer is periodically performed using the Au 4f and Cu 2p lines at 84 and 932.67 eV, respectively. The identification of the elements present in a sample specimen is performed by recording a survey scan over the range of 1000-0 eV binding energy (BE), as suggested by CitationMoulder et al. (1992). The carbon (C 1s) peak with the binding energy of 285 eV is commonly used as the reference for calibration of the spectra as suggested by CitationBeamson and Briggs (1992).
Narrow scans are performed for carbon and oxygen elements to provide information regarding the chemical states of the element under examination. Each elemental peak in the spectrum is usually fitted with a few other component peaks. Each of these assigned peaks provide information regarding the corresponding chemical state or environment of the element based on their BE positions. All the spectra of the narrow scans are also calibrated against C 1s (BE: 285 eV) position. A mixed line shape GL(50) (ratio of Gaussian to Lorentzian) is used to fit the individual component peaks and the backgrounds of the spectra are subtracted using a Shirley background.
2.5. Raman Analyses
The microstructures of the sampled PM are quantitatively analyzed by using a Raman spectroscopy system (Renishaw, System 1000). The particulate filters and the dilution and sampling system used here are similar to those used for the XPS studies. The Raman system is coupled with a Lecia optical microscope has two available excitation lasers. The excitation laser used is a Renishaw NIR solid-state diode laser emitting a line at ∼ 785 nm (source power 26 mW). The spectral resolution is about 2 cm−1. The spectrometer is controlled by the Renishaw WiRE (Windows-based Raman Environment) software package.
Raman spectra are collected over the range of 900 to 2000 cm–1. The Raman spectrometer is operated in continuous scanning mode. Laser power, spot diameter and beam exposure time are optimized for the PM samples as 25% of the maximum laser power, fully defocused laser beam and exposure time of 20 s. GRAMS/32 software (Graphic Relational Array Management System) is used to perform the curve fitting of the spectra and to determine the spectral parameters. The spectra are fitted to convergence with four bands without fixing or restricting any spectral parameter in the iteration process. The goodness of the fits is indicated by the reduced values of χ2 where values between 1 and 3 imply the convergence of the curve-fit towards the observed spectrum. In this study the value of χ2 varied from 1.36 to 1.67.
The in-plane crystalline dimension, L a , is determined using the Equation (Equation1) (CitationZhu et al. 2005):
3. RESULTS AND DISCUSSION
3.1. TGA
TGA is used to distinguish between the volatile fraction (VF) and solid fraction of the collected PM samples. Typical TGA plots for diesel low load, diesel high load and diesel-NG PM samples are presented in the online Supplemental Information (Figures S1–S3). The total weight loss is considered to determine the mass fractions of the elemental carbon and volatile fraction of the PM. The volatile fraction is further divided into high and low volatility fractions (CitationPrice et al. 2007). The fractions are defined based on the temperature ranges:
-
High Volatility: Mass/weight loss up to temperature, T ≤ 200°C (nitrogen environment)
-
Low Volatility: Mass/weight loss when 200 < T ≤ 450°C (nitrogen environment)
-
Elemental Carbon: Mass/weight loss when 450 < T ≤ 500°C (air environment)
In addition to these fractions, a non-combustible residual fraction, ash, may remain on the filter after the completion of TGA. However, the ash fraction is not resolved in the present study.
TGA has been performed at least twice for every sample collected and the results are presented in . The sample for biogas2 fueling was not ready during the TGA experiment and is not included in this study. Two filters were found to be stacked together during the sample collection for biogas1 fueling from the engine, which was noticed only at the time of TGA and therefore, the results for this particular sample might have some artifacts due to a proportionally smaller amount of collected mass. In the TGA plots (Figures S1–S3) there are two curves: one for the mass loss and the other for the temperature increase. The third one is the manual selection in mass loss curve for which range the mass loss is counted.
FIG. 1 Elemental carbon and volatile PM mass fractions as determined by TGA for different engine fueling (diesel low: 3 Nm; diesel high and dual fuel: 28 Nm; 1750 rpm; 28°bTDC; 0.6 kg/h pilot for dual fueling). (Figure provided in color online.)
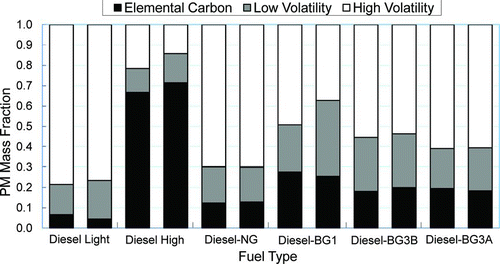
3.1.1. Volatile Fraction (VF)
shows that the mass fraction in the range of high volatility varies between 19 and 78% and that for low volatility varies from 16 to 27% for different engine fueling. The highest value of VF is obtained in the case of diesel (low) fueling and the lowest is for diesel (high) fueling. Higher volatile organic fractions (VOFs) and soluble organic fractions (SOFs) for diesel fueling under low to medium loads compared to high loads are already reported (CitationKweon et al. 2002, Citation2003; CitationNing et al. 2004; CitationLapuerta et al. 2007). The TGA results obtained here for diesel PM therefore agree well with these previous studies.
When we compare between diesel and dual fuel conditions at high load (), a significant difference in the values of VF is measured. VF is always much higher in the case of dual fueling, even though the engine operating condition is the same. VF is between 78 and 87% for dual fueling compared with 35% for diesel fueling. The high volatility fraction varied between 51 and 70% for different dual fueling compared with only 19% for diesel fueling. This indicates that the fuel composition has a great influence on the volatile/EC composition of the emitted PM. The difference is also noticed in filter appearances, as filters for dual fueling appeared to be less black than that for diesel fueling. Higher SOF or ratio of organic carbon to elemental carbon (OC/EC) for NG fueling has been reported in the literature (such as CitationBarbour et al. 1986; CitationWong et al. 1991; CitationLev-On et al. 2002). CitationBarbour et al. (1986) observed about 46% SOF (volatile aldehydes especially formaldehyde) for NG fueling compared to about 20% for diesel fueling for 75% engine load condition. This result is qualitatively similar to ours (87% and 35%, respectively) as VF possibly includes the water fraction and is known to give higher readings than SOF (CitationLapuerta et al. 2007).
According to Kweon et al. (Citation2002, Citation2003) the premixed combustion is a primary controlling factor for the particle-phase organic compounds in diesel PM. As the stoichiometric A/F ratio decreases with the introduction of gaseous fuels in the engine, theoretically richer mixture are in existence in dual fueling condition than diesel (high load) condition. Experimentally, the CO emissions for dual fueling are found to be slightly higher than for diesel fueling, indicating rich mixture combustion in the first case. The higher the proportion of premixed combustion, the higher the tendency to produce organic compounds. Since ignition delay is increased with the addition of gaseous fuels, this causes a higher proportion of premixed combustion and therefore an increase in particle-phase organic compounds.
3.1.2. Elemental Carbon (EC) Fraction
According to , measured elemental carbon mass fraction varies from about 5 (diesel low load) to 65% (diesel high load) indicating that the elemental carbon mass fraction is a function of the amount of diesel injection. Although the amount of diesel injection is the same, higher mass fractions of EC are obtained for the dual fuel conditions compared to diesel low load condition (). This indicates that there is a significant contribution of gaseous fuels on the total elemental carbon mass fractions during dual fueling under the engine operating conditions. However, the net result is far below the level of emissions of diesel (high) load, as gaseous fuels tend to produce much less amount of PM (CitationMustafi and Raine 2008). presents the actual mass of the samples lost during TGA for different engine fueling conditions.
TABLE 2 The actual mass-loss of the samples during TGA for different engine fueling conditions
3.2. XPS Analysis
3.2.1. Survey Scans
The XPS survey scans provided the identification of the elements present in the surface of the PM samples. A survey scan is also taken for the blank filter to know the elements present in the filter material. As there is no easy way to subtract the filter elements to get the net quantification for the detected elements in a loaded filter, only a proportionate picture of the quantity of the elements present in PM is obtained. However, the major elements to consider are carbon and oxygen. When comparing between a blank filter and a loaded (with diesel (high) PM) filter, the blank filter has significantly lower amount of carbon compared to the loaded filter. Since the diesel (high) sample is mainly composed of carbon it can be assumed that the majority of carbon appearing in the XPS spectra has originated from the collected diesel particulate matter on the filter. These carbons appearing on the sampling filters may not reflect the true amount of carbons that originated from the combustion processes in engine. Some of the exhaust gas phase organic compounds may adsorb onto the filter during sampling (CitationLipsky and Robinson 2006). Nearly all of the PM found in the tailpipe before dilution is present as solid carbonaceous agglomerates including a small amount of metallic ash. However there also may be a significant quantity of volatile organic and sulfur compounds in the gas phase at exhaust temperatures that are transformed to diesel particulate matter by nucleation, adsorption, and condensation as the exhaust dilutes and cools (CitationKittelson and Abdul-Khalek 1999).
lists all the detected elements for PM samples and for the blank filter. Since zinc and calcium are still detectable in diesel (high) sample, it seems likely that these two elements are present in PM and may originate from lubrication oil. The highest amount of PM was collected on the filter for the diesel high load condition among all the samples due to the nature of PM emission for this case. Thus a thicker layer of PM covered the filter surface compared to the other cases. It was expected that the elements of the blank filters would not be detected as XPS has limitations in detectable depth of the layer under focus. With this thicker layer, XPS could only detect the elements present in PM and could not reach unto the filter surface. For this reason, the other elements of the blank filter were not detected for this case and lower elemental signals were observed in the XPS spectra than those obtained for the blank filter. These elements have previously been observed in XPS analysis on vehicle exhaust PM samples (CitationHutton and Williams 2000). A sample was also collected for diesel (high) PM on a silver membrane metallic filter to avoid the interference of filter elements such as carbon with the PM elements in XPS analysis. In this case, only three elements were detected: C1s (97.48%), O1s (2.49%), and Zn 2p (0.04%), showing good agreement with the quartz filter samples. In TGA we denote elemental carbon (EC) mass fraction for different PM samples under investigation. In XPS, we also obtain carbon as one of the major elements present in PM surface. Thus, in diesel high load case, we obtained the highest amount of EC by TGA and the highest amount of carbon by XPS. Carbon content on PM surfaces by XPS thus complement the results obtained by TGA.
TABLE 3 Elemental composition of different PM samples detected by XPS survey scans
3.2.2. Narrow Scans
For all the PM samples, carbon and oxygen are found to be the main components and therefore the narrow scans have been done for these two elements. These elements are mainly observed in diesel engine/vehicle exhaust PM and have been analyzed by various researchers (CitationAlbers et al. 2000; CitationHutton and Williams 2000; CitationCollura et al. 2005; CitationMüller et al. 2006). Curve fits are performed for individual elements for each PM sample using Gaussian/Lorentzian (G/L ratio = 0.5) line shapes as mentioned earlier. The computer program, Computer Aided Surface Analysis for XPS (CasaXPS), was used in this study to analyze the XPS spectra. Spectra collected in the standard format were selected, viewed, and processed using CasaXPS. The peaks were selected basically looking at the general practices that have been observed in the literature for the similar applications as cited here (such as CitationAlbers et al. 2000; CitationMüller et al. 2006). The assignment gave a perfect fit between the original spectrum and the resultant curve.
3.2.2.1. Carbon (C1s) spectra. The elemental spectra are resolved into several component peaks, each corresponding to a different surface functionality. Literature has been reviewed to assign appropriate carbon functional groups for these peaks and these are summarized in . Carbon (C1s) spectra of different PM samples are presented in the online Supplemental Information (Figures S4–S8). The C1s spectra have been resolved into five individual component peaks for all PM (Figures S5–S8) except the diesel (low) PM sample where only three individual peaks are fitted (Figure S4). The peaks at higher binding energies refer to oxygenated carbon compounds. This indicates that as the load increases from low to high, different carbon-oxygenated groups are detected implying significant oxidation in the case of diesel (high) and dual fuel PM.
TABLE 4 Assignment of various functional groups for carbon element according to the binding energy
The highest peak of the C1s signals for all the PM samples ranged between 284.9 and 285.0 eV, indicating the presence of graphitic carbon or aliphatic contributions such as hydrocarbons and paraffins (C═C or C─C) in the topmost atomic layers of the PM. Similar observations have been noted previously for diesel exhaust particulates or urban aerosols, and for activated carbon (CitationBiniak et al. 1997; CitationAlbers et al. 2000; CitationHutton and Williams 2000; CitationCollura et al. 2005; CitationMüller et al. 2006). When comparing between diesel (high) and dual fuel PM samples, the peak is broadened in the latter case (FWHM = 1.0–1.2 compared to 0.78) indicating a less developed graphitic structure in dual fuel PM as suggested by CitationMüller et al. (2006). The next dominant peak at BE = 285.47–285.79 eV, is obtained for all PM except the diesel (low) PM sample. According to the literature, this peak refers to the n-paraffin functional group and might be a function of combustion temperature.
The next peaks are for higher binding energies indicating the presence of different carbon-oxygenated functional groups in PM. According to the results, all the PM samples have a peak for carbon with alcohol or ether groups except for diesel (high) PM. Quantitatively it is observed that this particular type of carbon structure is in higher proportion (6.3–16.2%) for the dual fuel PM samples in comparison to diesel PM samples (0–3.9%). The C1s peak at 287.34 and 288.15 eV for diesel (high) and (low) PM, respectively, refers to carbonyl functional group (─C═O), which is not seen in dual fuel PM.
The next carbon-oxygen functional group, which refers to the carboxyl, or ester functional group (─C─OOH), is a common oxidized state of carbon for all PM samples. It is observed that this particular carbon structure is approximately constant for all the cases indicating a basic oxygenated carbon structure of diesel PM independent of fuel quality and engine operation.
The final peak which ranges from 291 to 291.6 eV BE may be due to enhanced plasmon-loss features (graphite) or π −π * shake-up satellite signals (aromatics) indicating the presence of uncovered polyaromatic or graphite-like basic structural units in the surface regions of the PM samples (according to CitationBiniak et al. 1997; CitationAlbers et al. 2000). This feature of carbon structure is present in less proportion for dual fuel PM compared to diesel (high) sample indicating less aromatic content in dual fuel PM structures. This is to be expected since the amount of diesel fuel, which can be regarded as the source of aromatics, is minimized in the case of dual fueling conditions.
3.2.2.2. Oxygen (O1s) spectra. In all cases the narrow scan of O1s spectra have been resolved into two individual component peaks except diesel (high) PM sample where three individual peaks are fitted. The maxima of the O1s signals for all the PM samples ranged from 533.3 to 533.8 eV, suggesting the dominant contributions of aliphatic carbon-oxygen functional group, O-C (CitationBeamson and Briggs 1992) in PM surfaces. However, the maximum of the O1s signal for diesel (low) PM appears at 532.75 eV suggesting the dominant contribution of carbon-oxygen functional group, aliphatic C─O─C or C─OH or aromatic O═C (CitationBeamson and Briggs 1992). It is found that the amount of this aliphatic carbon-oxygen functional group O-C is higher for dual fuel PM compared to diesel (high) PM. As the load increased from low to high the peaks of the O1s spectra shifted to higher binding energies (see O1s spectra in the online Supplemental Information; Figures S9–S11).
The other peak in the O1s signals ranged from 532.0 to 532.45 eV, indicating the presence of aliphatic O═C carbon-oxygen functional group (CitationBeamson and Briggs 1992) in the topmost layers of the PM samples. Quantitatively the amount of this carbon-oxygen functional group in dual fuel PM structure is found to be lower than that of diesel (high) PM that is in agreement with the assigned carbon-oxygen functional groups for C1s spectra. The presence of similar carbon-oxygen functional groups in diesel exhaust particles and for urban aerosols and activated carbon particulates is reported (CitationBiniak et al. 1997; CitationAlbers et al. 2000; CitationHutton and Williams 2000; CitationMüller et al. 2006). The component peaks for O1s thus complement the findings of similar carbon-oxygen functional groups that have been observed in the carbon spectra.
In addition to the two major peaks in the O1s spectra, there is a small shoulder at about 535.9 eV for diesel (high) PM sample that has not been observed for the other samples. This peak may appear due to the presence of chemisorbed oxygen and/or water in the diesel (high) PM surface as suggested by CitationBiniak et al. (1997).
3.3. Raman Analyses
The well-known G (or “Graphite”) and D (or “Defect”) bands of graphite are the main features observed, with varying degrees of intensity and width (FWHM) near 1330 cm–1 and 1600 cm–1, respectively, for all PM samples. Typical spectra for diesel (high) and diesel-NG PM are shown in the online Supplemental Information (Figures S12 and S13). Raman signal depends on the size of the particle under focus. A large coagulated particle was easily available on the filter to focus for diesel (high) PM sample. On the other hand, for diesel-NG PM sample, it was difficult to find such a large coagulated particle on the filter to focus, as PM emission in this case was lower. Hence, a poorer signal was obtained for the latter case. Three to five band combinations exist in the literature to fit the visible Raman spectra of amorphous (combustion/diesel PM) carbons (CitationMustafi 2008). However, in the present work, four band combinations (G, D1, D3, and D4) show better fit to the Raman spectra compared to five band combinations (G, D1, D2, D3, and D4).
In the present study, the G band included the D2 band and the other bands were separately considered. The results presented in show that the intensity ratio (I D1/I G ) is similar in the case of diesel (high) and diesel (low) load PM, but the ratio is significantly lower in the case of dual fuel PM. Uncertainties provided in are the variations observed from different runs for a particular PM sample under investigation. This may provide an indication of increasing degree of graphitization of carbonaceous materials in dual fuel PM. The crystallite size increased and FWHM decreased in the case of diesel (high) PM compared to diesel (low) PM. This implies that the disorder of graphite structures decreases with increasing engine load (according to CitationZhu et al. 2005). Although the crystallite size, L a , determined from Equation (Equation1), increases for dual fuel PM compared to diesel PM ().
TABLE 5 Peak intensity ratios and in-plane crystalline size, L a for different PM samples
Previous studies (CitationCuesta et al. 1994; CitationJawhari et al. 1995) have reported an increase of I D3/I G with the proportion of amorphous carbon in PM. In the present study this intensity ratio varied from 0.5 for diesel (light) to 1.3 for dual fuel PM samples () indicating the more amorphous nature of the carbon in dual fuel PM compared to diesel (high) samples.
CitationIvleva et al. (2007) correlated the intensity ratio, I D3/I G , to the ratio of elemental carbon (EC) to total carbon (TC) present in PM. According to the authors the decrease in the intensity ratio with increasing EC/TC ratio implied a decrease in amorphous organic carbon fraction. In the present work, it has been observed that the EC/TC ratio (from TGA analysis) is the highest in the case of diesel (high) PM compared to others and also it has the lowest I D3/I G ratio, which implies that carbon in diesel (high) PM is less amorphous compared to the others.
CitationFerrari and Robertson (2000) proposed that the visible Raman spectra in many experiments can be interpreted by the three-stage model (graphite → nanocrystalline graphite → a−C, amorphous carbon → ta-C) explaining the amorphization trajectory ranging from graphite to ta-C (tetrahedral amorphous or diamond). According to the authors the main effects in the evolution of the Raman spectrum in:
-
Stage 1: From graphite to nanocrystalline graphite
-
the G band moves from 1581 to ∼ 1600 cm–1
-
the D band appears and I D /I G increases
-
no dispersion of the G band
-
-
Stage 2: From nanocrystalline graphite to a−C
-
the G band decreases from 1600 to ∼ 1510 cm–1
-
I D /I G approaches to zero
-
increasing dispersion of the G band
-
Based on this model, it can be assumed that the carbon structure of the diesel PM samples in this study falls in the first stage where the main structural change is passing from graphite to nanocrystalline graphite with virtually no sp 3 sites as the G band is near 1600 cm–1 and the I D /I G increases. The dual fuel PM on the other hand can be assumed to fall in stage-2, i.e., from nanocrystalline graphite to a−C with small sp 3 sites as the G band decreases from 1600 cm–1 and the I D /I G ratio decreases and the FWHM of G band increases.
4. CONCLUSIONS
Characterizations have been done of the sampled PM emitted from a dual fuel engine operated on natural gas and biogas. Based on the TGA, XPS, and Raman analysis the following conclusions can be made:
-
Engine load and the quality of gaseous fuel are found to have significant effects on volatile mass fractions of the measured PM. Significantly higher volatile mass fractions but lower elemental carbon mass fractions are obtained for dual fuel PM compared to diesel (high) PM. This implies that the elemental carbon mass fraction in PM is approximately proportional to the quantity of diesel fuel injected.
-
Carbon and oxygen are the main elements found in the PM structure by XPS studies. Quantitatively the highest carbon fraction is found in diesel (high) PM that has reflected in TGA results. The maximum contribution of the graphitic carbon or aliphatic carbon such as hydrocarbons and paraffins (C═C or C─C) are obtained in the topmost atomic layers of the PM. The other chemical states are mainly the different oxidized states of carbon such as carbon with alcohol (C-OH), carbonyl (-C═O) or ether functional group, carboxyl or ester functional group (-O-C═O or HO-C═O). It can be concluded that the chemical states of carbon and oxygen in dual fuel PM structure are similar to those observed for diesel (high) PM. However, a relatively less amount of aromatic carbon compounds is observed in dual fuel PM surface.
-
Raman spectra for different PM samples are mainly composed of “G” (“graphite”) and “D” (“defects”) bands of graphite carbon. There might be an indication from the band intensity ratio (I D1/I G ) that the carbon in dual fuel PM structure has more graphitization compared to diesel (high) PM. The disorder of graphite structures in PM improves with increasing engine load for diesel fueling. The crystalline size L a , increases for dual fuel PM as compared to diesel (high) PM. There might be an indication that the carbon presented in dual fuel PM is of more amorphous nature compared to diesel (high) PM. It might be possible that the main structural change is from graphite to nanocrystalline graphite with virtually no sp 3 sites in the carbon structure of diesel PM. The dual fuel PM, on the other hand, can be assumed to have a path from nanocrystalline graphite to a−C (amorphous carbon) with a small sp 3 site in the structure.
-
These results show that PM from dual fueling are very different from diesel fueling in terms of volatile matters but similar in terms of PM topmost surface characteristics. The carbon structure is also found to be different for dual fuel PM compared to diesel PM.
uast_a_503668_sup_14862478.zip
Download Zip (585.5 KB)[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Abd Alla , G. H. , Soliman , H. A. , Badr , O. A. and Abd Rabbo , M. F. 2000 . Effect of Pilot Fuel Quantity on the Performance of a Dual Fuel Engine . Energy Conversion and Management , 41 : 559 – 572 .
- Albers , P. W. , Klein , H. , Lox , E. S. , Seibold , K. , Prescher , G. and Parker , S. F. 2000 . INS-, SIMS- and XPS-Investigations of Diesel Engine Exhaust Particles . Physical Chemistry Chemical Physics. , 2 ( 5 ) : 1051 – 1058 .
- Barbour , T. R. , Crouse , M. E. and Lestz , S. S. 1986 . Gaseous Fuel Utilization in a Light-Duty Diesel Engine SAE Paper 860070
- Beamson , G. and Briggs , D. 1992 . High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database , Chichester, England : John Wiley .
- Biniak , S. , Szyman'ski , G. , Siedlewski , J. and S'wiatkoski , A. 1997 . The Characterization of Activated Carbons with Oxygen and Nitrogen Surface Groups . Carbon. , 35 ( 12 ) : 1799 – 1810 .
- Boisvert , J. , Gettel , L. E. and Perry , G. C. 1988 . Particulate Emissions for a Dual-Fuel Caterpillar 3208 Engine 277 – 285 . ASME Paper 88-ICE-18
- Burtscher , H. 2005 . Physical Characterization of Particulate Emissions From Diesel Engines: A Review . J. Aerosol Sci , 36 : 896 – 932 .
- Collura , S. , Chaoui , N. , Azambre , B. , Finqueneisel , G. , Heintz , O. , Krzton , A. , Koch , A. and Weber , J. V. 2005 . Influence of the Soluble Organic Fraction on the Thermal Behavior, Texture and Surface Chemistry of Diesel Exhaust Soot . Carbon. , 43 ( 3 ) : 605 – 613 .
- Cuesta , A. , Dhamelincourt , P. , Laureyns , J. , Martinez-Alonso , A. and Tascon , J. M. D. 1994 . Raman Microprobe Studies on Carbon Materials . Carbon. , 32 ( 8 ) : 1523 – 1532 .
- El-Zein , A. , Nuwayhid , I. , El-Fadel , M. and Mroueh , S. 2007 . Did a Ban on Diesel-Fuel Reduce Emergency Respiratory Admissions for Children? . Sci. Total Environ. , 384 ( 1–3 ) : 134 – 140 .
- Ferrari , A. C. and Robertson , J. 2000 . Interpretation of Raman Spectra of Disordered and Amorphous Carbon . Physical Review , B61 : 14095 – 14107 .
- Galal , M. G. , Abdel Aal , M. M. and El Kady , M. A. 2002 . A Comparative Study between Diesel and Dual-Fuel Engines: Performance and Emissions . Combustion Sci. Technol. , 174 : 241 – 256 .
- Henam , A. and Makkar , M. K. 1998 . Combustion of Simulated Biogas in a Dual-Fuel Diesel Engine . Energy Conversion and Management , 39 : 2001 – 2009 .
- Hutton , B. M. and Williams , D. E. 2000 . Assessment of X-ray Photoelectron Spectroscopy for Analysis of Particulate Pollutants in Urban Air . Analyst , 125 ( 10 ) : 1703 – 1706 .
- Ivleva , N. P. , McKeon , U. , Niessner , R. and Poächl , U. 2007 . Raman Microspectroscopic Analysis of Size-Resolved Atmospheric Aerosol Particle Samples Collected with an ELPI: Soot, Humic-Like Substances, and Inorganic Compounds . Aerosol Sci. Technol. , 41 : 655 – 671 .
- Jawhari , T. , Roid , A. and Casado , J. 1995 . Raman Scattering and the Characterization of Some Commercially Available Carbon Black Materials . Carbon , 33 : 1561 – 1565 .
- Karim , G. A. and Wierzba , I. 1992 . Methane-Carbon Dioxide Mixtures as a Fuel SAE Paper 921557
- Karim , G. A. and Amoozegar , N. 1982 . Examination of the Performance of a Dual Fuel Diesel Engine with Particular Reference to the Presence of Some Inert Diluents in the Engine Intake Charge SAE Paper 821222
- Kennedy , I. M. , Wilson , D. and Barakat , A. I. 2009 . Uptake and Inflammatory Effects of Nanoparticles in a Human Vascular Endothelial Cell Line , Health Effects Institute, USA . Research Report 136
- Khalek , I. A. , Ullman , T. L. , Shimpi , S. A. , Jackson , C. C. , Dharmawardhana , B. , Silvis , W. M. , Kreft , N. , Harvey , R. N. , Munday , D. , Yamagishi , Y. , Graze , R. , Smitherman , J. and Adkins , J. 2002 . Performance of Partial Flow Sampling Systems Relative to Full Flow CVS for Determination of Particulate Emissions under Steady-State and Transient Diesel Engine Operation SAE Paper 2002-01-1718.
- Kittelson , D. and Abdul-Khalek , I. 1999 . Formation of Nanoparticles during Exhaust Dilution University of Minnesota, Department of Mechanical Engineering, EFI Members Conference: “Fuels, Lubricants Engines, and Emissions,” January 18–20
- Kweon , C. -B. , Foster , D. E. , Schauer , J. J. and Okada , S. 2002 . Detailed Chemical Composition and Particle Size Assessment of Diesel Engine Exhaust SAE Paper 2002-01-2670
- Kweon , C-B. , Okada , S. , Foster , D. E. , Bae , M.-S. and Schauer , J. J. 2003 . Effect of Engine Operating Conditions on Particle-Phase Organic Compounds In Engine Exhaust of a Heavy-Duty, Direct- Injection (D. I.) Diesel Engine SAE Paper 2003-01-0. 342
- Lapuerta , M. , Armas , O. , Ballesteros , R. and Durán , A. 1999 . Influence of Mini-Tunnel Operating Parameters and Ambient Conditions on Diesel Particulate Measurement and Analysis SAE Paper 1999-01-3531
- Lapuerta , M. , Ballesteros , R. and Rodri'guez-Fernández , J. 2007 . Thermogravimetric Analysis of Diesel Particulate Matter . Measurement Sci. Technol. , 18 ( 3 ) : 650 – 658 .
- Lev-On , M. , LeTavec , C. , Uihlein , J. , Alleman , T. L. , Lawson , D. R. , Vertin , K. , Thompson , G. J. , Wayne , W. S. , Clark , N. , Okamoto , R. , Rieger , P. , Yee , G. , Zielinska , B. , Sagebiel , J. , Chatterjee , S. and Hallstrom , K. 2002 . Chemical Speciation Of Exhaust Emissions From Trucks and Buses Fueled on Ultra-Low-Sulfur Diesel and CNG SAE Paper 2002-01-0432
- Lipsky , E. M. and Robinson , A. L. 2006 . Effects of Dilution on Fine Particle Mass and Partitioning of Semivolatile Organics in Diesel Exhaust and Wood Smoke . Environ. Sci. Technol , 40 : 155 – 162 .
- Matthews , M. J. , Pimenta , M. A. , Dresselhaus , G. , Dresselhaus , M. S. and Endo , M. 1999 . Origin of Dispersive Effects of the Raman D Band in Carbon Materials . Physical Review B. , 59 : R6585 – R6588 .
- Moulder , J. F. , Stickle , W. F. , Sobol , P. E. and Bombon , D. E. 1992 . Handbook of X-Ray Photoelectron Spectroscopy , Edited by: Chastain , J. Eden Prairie, Minnesota, USA : Perkin Elmer Corporation .
- Müller , J.-O. , Su , D. S. , Jentoft , R. E. , Wild , U. and Schlögl , R. 2006 . Diesel Engine Exhaust Emission: Oxidative Behavior and Microstructure of Black Smoke Soot Particulate . Environ. Sci. Technol. , 40 ( 4 ) : 1231 – 1236 .
- Mustafi , N. N. and Raine , R. R. 2008 . A Study of the Emissions of a Dual Fuel Engine Operating with Alternative Gaseous Fuels SAE Paper 2008-01-1394
- Mustafi , N. N. 2008 . Particulate Emissions from a Dual Fuel Engine , Doctoral thesis Auckland, New Zealand : Department of Mechanical Engineering, University of Auckland .
- Ning , Z. , Cheung , C. S. and Liu , S. X. 2004 . Experimental Investigation of the Effect of Exhaust Gas Cooling on Diesel Particulate . J. Aerosol Sci. , 35 ( 3 ) : 333 – 345 .
- Papagiannakis , R. G. and Hountalas , D. T. 2004 . Combustion and Exhaust Emission Characteristics of a Dual Fuel Compression Ignition Engine Operated with Pilot Diesel Fuel and Natural Gas . Energy Conversion and Management , 45 : 2971 – 2987 .
- Patterson , J. , Clarke , A. and Chen , R. 2006 . Experimental Study of the Performance and Emissions Characteristics of a Small Diesel Genset Operating in Dual-Fuel Mode with Three Different Primary Fuels SAE PAPER 2006-01-0050
- Price , P. , Stone , R. , OudeNijeweme , D. and Chen , X. 2007 . Cold Start Particulate Emissions from a Second Generation DI Gasoline Engine SAE Paper 2007-01-1931 (JSAE 20077213)
- Vouitsis , E. , Ntziachristos , L. and Samaras , Z. 2003 . Particulate Matter Mass Measurements For Low Emitting Diesel Powered Vehicles: What's Next? . Progress in Energy and Combustion Sci. , 29 ( 6 ) : 635 – 672 .
- Weaver , C. S. and Turner , S. H. 1994 . Dual Fuel Natural Gas/Diesel Engines: Technology, Performance, and Emissions SAE Paper 940548
- Wong , W. Y. , Midkiff , K. C. and Bell , S. R. 1991 . Performance and Emissions of a Natural Gas Dual-Fueled, Indirect Injected Diesel Engine SAE Paper 911766
- Zhu , J. , Lee , K. O. , Yozgatligil , A. and Choi , M. Y. 2005 . Effects of Engine Operating Conditions on Morphology, Microstructure, and Fractal Geometry of Light-Duty Diesel Engine Particulates . Proc. Combustion Institute , 30 : 2781 – 2789 .