Abstract
A model is presented to describe the collection of ultrafine particles by the UNC passive aerosol sampler. In this model, particle deposition velocity is calculated as a function of particle size, shape and other properties, as well as a function of sampler geometry. To validate the model, deposition velocities were measured for ultrafine particles between 15 and 90 nm in diameter. Passive aerosol samplers were placed in a 1 m 3 test chamber and exposed to an ultrafine aerosol of ammonium fluorescein. SEM images of particles collected by the samplers were taken at 125 kX magnification. Experimental values of deposition velocity were then determined using data from these images and from concurrent measurements of particle concentration and size distribution taken with an SMPS. Deposition velocities from the model and from the experiments were compared and found to agree well. These results suggest that the deposition velocity model presented here can be used to extend the use of the UNC passive aerosol sampler into the ultrafine particle size region.
INTRODUCTION
The rapid development and growth of industries that employ nanotechnology have raised concerns about potential health risks from the inhalation of ultrafine particles (dp ⩽ 100 nm). Inhalation of these particles has been linked to the increased production of reactive oxygen species in the lung (CitationOberdorster et al. 2005; CitationDonaldson et al. 2002), which leads to oxidative stress and results in inflammation and interstitial fibrosis (CitationDonaldson et al. 2002). Ultrafine particles also cause protein denaturation, membrane damage, DNA damage, immune reactivity, perturbation of phagocytic function, and foreign body granulomas (CitationNel et al. 2006). As a result, the assessment of exposures to ultrafine particles has increased in importance.
Equipment to monitor ultrafine particles is often large, requires electrical power, and expensive; characteristics that make these instruments undesirable for some exposure studies. A need exists for an unobtrusive and economical way to characterize exposure to ultrafines. The UNC passive aerosol sampler appears to meet this need, and to have potential as a method for the collection and analysis of ultrafine particles (CitationWagner and Leith 2001a, Citation2001b).
This sampler consists of an aluminum scanning electron microscope (SEM) stub with a collection substrate, and a stainless steel cap. The cap has a round 6 mm hole in its center and is covered with metal mesh that prevents inadvertent damage to the substrate and intrusion of debris into the sampler. Particles pass through the mesh and into the cavity between the mesh and substrate by gravitational settling and diffusion, then deposit on the substrate. After exposure, the substrate is examined using an optical or electron microscope to determine the number, size, and properties of the particles collected. These data are then used to determine the average concentration and size distribution of the particles to which the sampler was exposed using methods described by Wagner and Leith (2001a, 2001b), CitationLeith et al. (2007) and CitationWhitehead and Leith (2008). The passive aerosol sampler can be placed in a stationary location or can be clipped to a worker's lapel. When worn as a personal sampler, its unobtrusive size allows freedom of movement, important because altered behavior resulting from carrying large or heavy personal monitoring equipment can lead to an unrepresentative sample (CitationWiener and Rodes 2001).
To date, the performance of the UNC passive aerosol sampler has been characterized only for particles larger than about 100 nm. The objective of this study was to extend the range of this sampler into the ultrafine size region; that is, to determine its ability to characterize the concentration and size of particles between 10 and 100 nm in diameter.
DEPOSITION VELOCITY MODEL
A model to describe particle deposition velocity onto the passive sampler substrate is necessary to relate particles collected on the substrate to airborne particle concentration. diagrams the parameters relevant to such a model.
FIG. 1 Schematic of a subsection of the passive aerosol sampler around a single, representative hole in the mesh cap (not to scale).
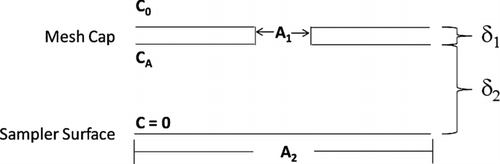
In , δ1 represents the thickness of the mesh cap; δ2 represents the distance between the bottom of the mesh cap and the substrate surface; A 1 represents the area for each of the many round holes in the mesh cap; and A 2 represents the proportional area of the substrate below each hole. Three concentrations are also depicted. C 0 is the aerosol concentration in the air above the sampler, CA is the concentration immediately beneath the hole, and the concentration at the substrate surface is zero.
The model below rests on an assumption that particle collection occurs at steady state. Both gravity and diffusion contribute to the flux, J, of particles to the substrate surface. These processes are assumed to operate independently. The flux due to gravity, JG , is
Fick's first law gives the flux due to diffusion, JD , as
Substitution of Equation (Equation4) into Equation (Equation5) and rearrangement yields
The flux to the substrate due to both gravity and diffusion becomes
By combining Equations (Equation7) and (Equation8), V is represented by
The model presented above is based on the assumption that the deposition processes are at steady state. For particles from 10 to 100 nm and for the geometry of the UNC passive aerosol sampler, this velocity is from 10–5 to 10–6 m/s according to Equation (Equation9). Because the diffusion distance in the passive sampler, δ1+δ2, is about 1 mm, the deposition time is on the order of 100 to 1000 s. Thus, if the aerosol concentration above the sampler is stable for 15 min or more the steady state assumption should be met for particles up to 100 nm in size. For 10 nm particles, the aerosol needs to be stable for only a few minutes for deposition to be steady state.
This model does not account for particle collection by diffusion on the interior walls of the sampler. To the extent that particles diffuse to these surfaces, Equation (Equation9) will overestimate deposition velocity. The cylindrical passage between the mesh and the substrate is 6 mm in diameter and 1 mm high, so that relatively few particles that originate near its centerline will reach its walls by diffusion. If the mesh cap is always horizontal as intended, gravity will pull particles away from the back side of the mesh cap and help reduce diffusional deposition there. This effect will be less important as particle size decreases.
Ordinarily, passive aerosol samplers would be used to determine a size frequency distribution. In this case, concentration would be determined from measured values of particle flux to the substrate, C 0=J/V, a simple rearrangement of Equation (Equation8). This expression can be rewritten as
EXPERIMENTS
Properties of the Mesh Cap
Equation (Equation9) shows that particle deposition velocity depends, in part, on the fractional open area of the metal mesh that covers the sampler cap, A 1/A 2. Photographs of this mesh were taken using an optical microscope and analyzed using ImageJ (CitationNational Institutes of Health 2010) to determine the fractional open area of the mesh surface.
Sampler Substrate
Ultrafine analysis with an electron microscope requires a very smooth collection substrate. Because an SEM stub has many grooves and imperfections on its surface, a 200 mesh copper TEM grid (Product No. 01810, Ted Pella Inc., Redding, CA) was fixed to the stub using two drops of carbon black (Ted Pella Inc., Redding, CA) on opposite edges of the grid. The TEM grid has a formvar film that is coated with a thin layer of carbon, providing a smooth substrate surface that is electrically conductive and stable under electron microscopy conditions. These features provide an ideal background to image ultrafine particles with high beam stability and without interfering structures or surface defects. Once the TEM grid was fixed on the SEM stub to provide a collection substrate, a mesh cap was immediately placed on the sampler and covered to prevent contamination. The sampler was then ready for use.
Experimental Setup
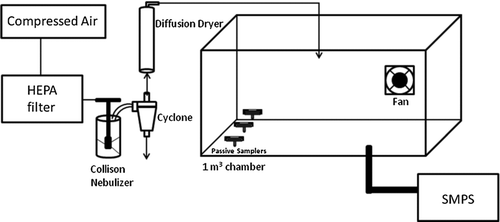
The experimental setup shown in and used here is similar to that used by CitationSheehan et al. (2009). HEPA filtered compressed air passed through a three-jet Collison nebulizer (BGI Inc., Waltham, MA) containing 0.03% (by volume) ammonium fluorescein in water. The resultant aerosol then flowed through a cyclone (BGI Inc., Waltham, MA) and diffusion dryer. The cyclone removed larger droplets that would have dried to form larger particles, and the diffusion dryer ensured that all water droplets evaporated fully to leave behind solid ammonium fluorescein particles (ρ p = 1.35 g/cm3) (CitationVanderpool and Rubow 1988) with a number concentration of about 106/cm3. These ultrafine particles flowed into the 1 m3 chamber where a fan ensured that the aerosol was well mixed. Excess air flowed out of the chamber through exit ports. Three passive samplers (RJLee Group, Monroeville, PA) were placed in the chamber. Simultaneously, a scanning mobility particle sizer (SMPS, Model 3080, TSI Inc. Shoreview, MN) measured and recorded the particle concentration and size distribution in the chamber every 3 min throughout the 39.5 h exposure period. At the conclusion of this period, the passive samplers were removed from the chamber and placed in a capped container for later analysis.
Imaging the Sampler Substrate
Analysis of the passive sampler substrates was performed using a Zeiss Supra 25 field emission scanning electron microscope (FESEM) (Zeiss Inc., Thornwood, NY) with a resolution of 2 nm. After removal of the mesh cap, the sampler substrate was placed in the SEM and imaged with a 1.5 keV electron beam and a 10 μm aperture. Images were obtained by collecting secondary electrons with an in-lens detector at a magnification of 125 k×. Image fields were chosen randomly at 120×; the magnification was then increased to 125 k× and an image was taken. Additional fields were chosen until approximately 150 particles had been imaged.
Image Analysis: Particle Sizing and Binning
SEM images of the substrate surface were analyzed using ImageJ. Brightness and contrast thresholds were adjusted to ensure that all imaged particles were detected. Image J then numbered and calculated the projected area, Feret diameter and Feret angle of each particle. Particle size bins were constructed to match those used by the SMPS.
The SMPS categorizes particles according to volume equivalent diameter, d ev , for spherical particles. Projected area diameters, dpa , determined with Image J for particles on the substrate, were converted to dev according to dev =dpa /Sv , where Sv is the volume shape factor. In this work, Sv was assumed to be 1.6, the value previously used by CitationWagner and Leith (2001b).
Only particles wholly contained within the image field were counted by the ImageJ software. Because particles that are large relative to the image size are less likely to be completely contained within the image and less likely to be counted, the “count” for each particle was adjusted according to
RESULTS
The fractional open area of the passive sampler mesh, A 1/A 2, was measured to be 0.292. The hole diameters were consistent and 287 μm. The thickness of the mesh cap, δ1, was 0.132 mm and the distance between the bottom of the mesh and the top of the substrate, δ2, was 0.982 mm.
shows an example of a photograph of particles collected by the passive aerosol sampler in these tests. Particle shape was roughly circular. Particle loading, L (count/area) on the substrate surface was calculated as a function of d ev by dividing the total of all particle counts from Equation (Equation11) in each size bin by the total area of all SEM images for each sampler. Particle loading divided by the total sampling time gave the experimentally determined particle flux to the collection surface, J exp. Experimentally determined deposition velocities, V exp, were then calculated for particles in each bin size using
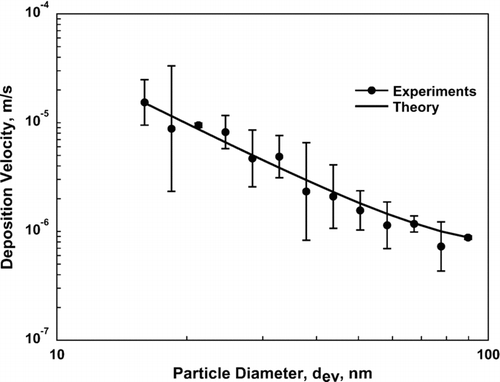
compares deposition velocities determined from the experiments with those from the theory presented in Equation (Equation9). Error bars for the experimental data are based on logarithms of the measurements. The agreement between theory and experimental data is good, with a correlation coefficient of 0.994.
FIG. 4 A comparison of deposition velocities determined via experiment (the average of V exp determined for each of the three samplers independently) and theory given by Equation (Equation9) as a function of particle diameter, d ev. Error bars for the experimental values represent one standard deviation.
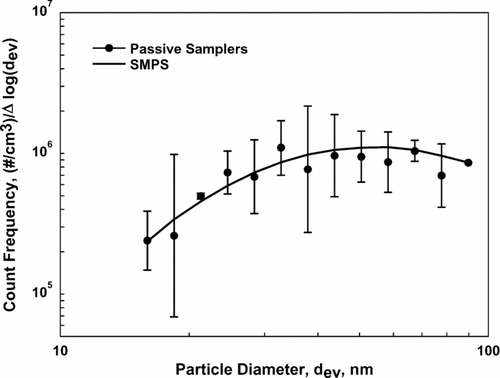
The data in are re-plotted as a size frequency distribution by count in . Here the concentration values from the SMPS are plotted against concentrations measured using the passive sampler as determined from Equation (Equation10) where deposition velocity, V, was found using Equation (Equation9). This plot is representative of the kind that would ordinarily be produced using measurements from passive aerosol samplers. This figure, like , shows that passive samplers analyzed using deposition velocities from Equation (Equation9) produce measured concentrations in good agreement with those measured using an SMPS.
FIG. 5 Size distribution of ammonium fluorescein measured with an SMPS and with the UNC passive aerosol samplers analyzed using deposition velocity from Equation (Equation9). Error bars represent one standard deviation.
DISCUSSION
This work presents a new theory to estimate the number concentration of ultrafine particles collected passively. The integral part of this theory is a model that describes particle deposition velocity as a function of particle size. The deposition velocity model depends upon both particle and sampler characteristics.
Although Equation (Equation9) for deposition velocity contains no empirical constants, the experimental data presented in Figures and depend on the conversion of projected area diameter, dpa , to equivalent volume diameter, dev , using a volume shape factor, Sv , where dev =dpa /Sv . An Sv value cannot be determined from two-dimensional photographs of particles such as those used here, so it must be assumed. The S v value of 1.6 used here was reported by CitationLin et al. (1994) for outdoor aerosol in Chicago, and has been used in previous work with the passive aerosol sampler. To investigate the sensitivity of results to assumptions about Sv , a series of log-log plots was made for measured deposition velocity using alternative values of Sv versus deposition velocity calculated from the theory, Equation (Equation9). For Sv values from 1.0 to 2.0, the best-fit lines for these plots all had slopes from 0.98 to 1.02, suggesting that the choice for Sv has little effect on the results.
shows that the size distribution measured by the passive aerosol samplers followed the SMPS data very well. Concentrations measured by the passive samplers showed little bias, as they differed from those measured by the SMPS by an average of about 6%. Additionally, all passive sampler data lie within one standard deviation of the SMPS measurements with the exception of one point (dev = 21nm). For the smallest particles used in this study, accuracy might be improved by examining the sampler substrate at a higher magnification; however, depending on particle composition, the higher beam energy required could lead to the partial destruction of particles or to changes in their morphology (CitationTumolva et al. 2010).
Relative standard deviations for the measured values of deposition velocity in range from 3.5% to 89.7% with an average of 43%; relative standard deviations in range from 4.9% to 90% with an average of 49%. These levels of precision are worse than the 32% that Wagner and Leith (2001) reported for PM10 measurements with the passive sampler. Precision can be improved by imaging more of the substrate area to count more particles, but at the cost of additional time on the SEM. The data found with the passive aerosol sampler in these experiments match well with data from the SMPS so that the effort to count additional particles beyond the 150 or so used here seems unwarranted.
The number concentration of ammonium fluorescein particles generated in this study was about 106/cm3 and particles were collected for 39.5 h. These conditions yielded a particle loading on the passive sampler that required approximately an hour of SEM time to image 150 particles. If concentration or sampling time had been higher, less SEM time would have been required. To achieve this particle loading on a sampler deployed in a typical occupational setting such as an engineered nanoparticle synthesis reactor where the particle concentration is orders of magnitude lower, 104–105 particles/cm3 (CitationSahu and Biswas 2010), would require sampling for weeks to months. Workers could wear the passive sampler and, like radiation badges, they could be sent for analysis on a quarterly basis. Sampling for a month is appropriate for the ultrafine particle concentrations found in urban environments, whereas rural environments might require season-long sampling (CitationHerring et al. 2007). These sampling periods could be shortened if a larger substrate area were examined, or lengthened by examining a smaller substrate area.
The particles generated in this study were roughly spherical and unagglomerated. A substantial increase in particle agglomeration would have an important effect on deposition velocity. Further study is necessary to determine how well the deposition velocity model presented here describes collection for these less-ideal aerosols.
CONCLUSIONS AND SUMMARY
This work shows that the UNC passive aerosol sampler can be used to measure the concentration and size distribution of ultrafine particles from 10 to 100 nm in diameter. Key to this development is a new model for particle deposition velocity in the sampler. The model is based on a theory for particle flux to the sampler substrate due to diffusion and gravitational settling. Agreement between particle concentrations measured using an SMPS and concentrations measured using passive samplers analyzed with the new model for particle deposition velocity was good; the passive sampler data showed little bias and reasonably good precision.
he authors gratefully acknowledge NIEHS grant T33 ES7018 and funds from the Environment Agency Abu Dhabi for supporting this work. They also thank Dr. Robert Bagnell, director of the UNC Microscopy Services Laboratory, for his help with the SEM work.
REFERENCES
- Donaldson , K. and Tran , C. L. 2002 . Inflammation Caused by Particles and Fibers . Inhal. Toxicol. Intl. Forum for Resp. Research , 14 ( 1 ) : 5 – 27 .
- Herring , S. V. , Kreisberg , N. M. , Stolzenburg , M. R. and Lewis , G. S. 2007 . Comparison of Particle Size Distributions at Urban and Agricultural Sites in California's San Joaquin Valley. . Aerosol Sci. Technol. , 41 : 86 – 96 .
- Lin , J. , Noll , K. E. and Holsen , T. M. 1994 . Dry Deposition Velocities as a Function of Particle Size in the Ambient Atmosphere . Aerosol Sci. Technol. , 20 : 239 – 252 .
- Leith , D. , Sommerlatt , D. and Boundy , M. G. 2007 . Passive Sampler for PM10−2.5 Aerosol . J. Air Waste Manage. Assoc. , 57 ( 3 ) : 332 – 336 .
- National Institutes of Health . 2010 . ImageJ: Image Processing and Analysis in Java. http://rsb.info.nih.gov/ij/index.html (accessed 5/10/2010).
- Nel , A. , Xia , T. , Madler , L. and Li , N. 2006 . Toxic Potential of Materials at the Nanolevel . Science , 311 : 622 – 627 .
- Oberdorster , G. , Oberdorster , E. and Oberdorster , J. 2005 . Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles . Env. Health Persp. , 113 ( 7 ) : 823 – 839 .
- Reist , P. 1993 . Aerosol Science and Technology, , 2nd ed. , 132 New York : McGraw Hill .
- Russ , J. C. 1995 . The Image Processing Handbook, , 2nd ed. , Boca Raton, FL : CRC Press .
- Sahu , M. and Biswas , P. 2010 . Size Distributions of Aerosols in an Indoor Environment with Engineered Nanoparticle Synthesis Reactors Operating under Different Scenarios . J. Nanopart. Res. , 12 : 1055 – 1064 .
- Sheehan , M. J. , Peters , T. M. , Cena , L. , O'Shaughnessy , P. T. and Gussman , R. A. 2009 . Generation of Nanoparticles with a Nebulizer-Cyclone System . Aerosol Sci. Technol. , 43 : 1091 – 1098 .
- Tumolva , L. , Park , J. Y. , Jae-suk , K. , Miller , A. L. , Chow , J. C. , Watson , J. G. and Park , K. 2010 . Morphological and Elemental Classification of Freshly Emitted Soot Particles and Atmospheric Ultrafine Particles using the TEM/EDS . Aerosol Sci. Technol. , 44 : 202 – 215 .
- Vanderpool , R. W. and Rubow , K. L. 1988 . Generation of Large, Solid, Monodisperse Calibration Aerosols . Aerosol Sci. Technol. , 9 ( 1 ) : 65 – 69 .
- Wagner , J. and Leith , D. 2001a . Passive Aerosol Sampler. Part I: Principle of Operation . Aerosol Sci. Technol. , 34 : 186 – 192 .
- Wagner , J. and Leith , D. 2001b . Passive Aerosol Sampler. Part II: Wind Tunnel Experiments . Aerosol Sci. Technol. , 34 : 193 – 201 .
- Whitehead , T. and Leith , D. 2008 . Passive Aerosol Sampler for Particle Concentrations and Size Distributions . J. Environ. Monitor. , 10 : 331 – 335 .
- Wiener , R. and Rodes , C. E. 2001 . “ Indoor Aerosols and Exposure Assessment ” . In Aerosol Measurement , Edited by: Willeke , K. and Baron , P. A. 859 – 886 . New York : Wiley & Sons, Inc. .