Abstract
The present article pertains to a theoretical analysis for thermophoresis of a micro-particle in rarefied gas environment. Different from the conventional analyses, thermal stress effect in velocity slip boundary condition is taken into account. This interfacial thermal characteristic is always ignored in previous theoretical studies. The present theory with consideration of the thermal stress slip provides an appropriate modification and thus agrees well with the measured data of thermophoretic mobility of various particles. Order-of-magnitude analysis also qualitatively demonstrates enhancement of the thermal stress slip with reducing particle size or increasing characteristic temperature gradient. Finally, a parametric analysis shows that the thermal stress slip effect becomes more pronounced at conditions of high particle thermal conductivity and high Knudsen number as well.
1. INTRODUCTION
It has been well known that a micro-particle suspended in a gaseous medium with an existing temperature gradient experiences a thermophoretic force, which drives the particle in motion. This phenomenon is called thermophoresis. Extensive review and historical development of thermophoresis can be found in literature, e.g., CitationZheng (2002) and CitationPiazza and Parola (2008). Thermophoresis is of considerable importance in a variety of practical applications, such as air cleaning, aerosol sampling, and particle contaminant control in the semiconductor industry (CitationChein and Liao 2005). It has been extensively investigated over the last few decades.
The physical interpretation of thermophoresis mechanism is based on the gas kinetic theory. The particle in a gaseous medium of non-uniform temperature field has hot and cold sides. The gas molecules in the region near the hotter side collide with the particle at higher momentum than the molecules in the cold region. It thus leads to a resultant force and in turn migration of the particle in the direction opposite to the temperature gradient (CitationSenchenko and Keh 2007). The interaction between the gas molecules and the particle depends on a parameter termed Knudsen number (Kn), which is defined as the ratio of mean free path l of the gas to a characteristics length scale, e.g., particle diameter in dealing with phoretic motion. A number of theoretical studies on particle thermophoresis have been performed in slip-flow regime with Kn up to O(10−1), for spherical particles (CitationEpstein 1929; CitationBrock 1962; CitationTalbot et al. 1980; CitationKeh and Chen 2003; CitationLu and Lee 2003; CitationKeh and Chang 2006) and non-spherical particles (CitationWilliams 1986; CitationKeh and Tu 2001; CitationKeh and Ou 2004). In the previous theoretical studies of thermophoresis, the boundary condition employed in velocity and temperature solutions is simple slip condition.
In an earlier work of CitationSone (1972), it was demonstrated that a tangential velocity slip now named thermal stress slip can be stemmed from the variation in the normal gradient (∂T/∂n) of the gas temperature in the tangential direction along the gas-solid interface, i.e., ∂(∂T/∂n)/∂s, (n and s stand for normal and tangential directions, respectively). Recently, this thermal stress effect was also employed to develop a generalized second-order slip boundary condition (CitationLockerby et al. 2004) as a modification of the very earlier version of velocity slip condition proposed by CitationMaxwell (1879). The slip condition with thermal stress has been employed in solutions of natural convection of rarefied gas between two non-coaxial cylinders (CitationAoki et al. 1998; CitationLockerby et al. 2004), theoretical analysis of particle photophoresis (Soong et al. 2009), etc. But it has not ever been considered in previous studies of particle thermophoresis.
For thermophoretic motion of spherical particle, with variation of the normal (radial) temperature gradient along the curved particle surface, the thermal stress slip effect should be taken into account, especially in the case of small particle. The present work is to revisit the problem of spherical particle thermophoresis with considering the thermal slip effect in gaseous slip-flows. Besides, the thermophoretic mobility with the modified slip-velocity boundary conditions is to be theoretically evaluated.
2. THEORETICAL ANALYSIS
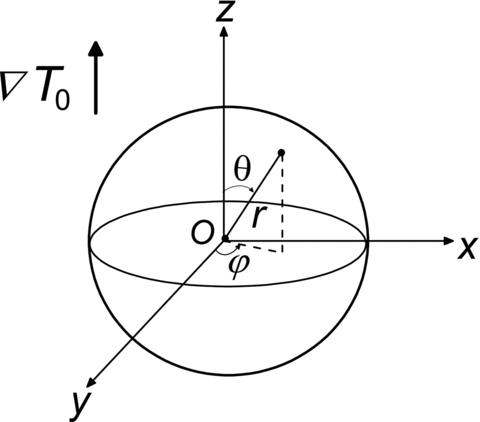
shows a schematic diagram of an isotropic homogenous particle suspended in a gas medium of a prescribed temperature gradient ∇T 0 with T 0 denoting temperature field. A spherical coordinate system O(r, θ, ϕ) is fixed on the center of the particle. Since the thermophoretic motion of the particle is slow, the relative velocity of the gas around the particle and the forced convection is negligible, the energy transfer between the gas and particle is assumed to be dominated by heat conduction. The gas temperature distribution Tg and the temperature field inside the particle Tp are governed by the Laplace equation,
On the particle surface, r=R, the normal heat flux is continuous and a temperature jump is considered, while in the far-field, the gas temperature gradient approaches the prescribed free-stream temperature gradient ∇T 0. Considering the axi-symmetry nature, the temperature fields Tg and Tp in rθ-plane are solved by invoking the following boundary conditions,
The fluid temperature distribution in spherical coordinates satisfying the above boundary conditions is
The Stokes flow around the spherical particle with fluid viscosity μ, the velocity vector v, and pressure field p can be described by the incompressible creeping flow equation.
In solution of the gas velocity field, the following second-order slip boundary condition based on the Maxwell relation with Burnett stress flux and heat flux (CitationLockerby et al. 2004) is considered, viz.,
The thermophoretic motion of the particle is slow; the Reynolds number Re=ρV 0 R/μ is thus very low. In a typical example with the parameters Kn ∼O(10−1), Re ∼O(10−3), ΔTc /Tr ∼O(10−1), the above ratio has the order-of-magnitude of 10−4, which means that the terms involving second-order derivative of velocity are quite smaller than the thermal stress slip. Thus, in the present analysis we adopt slip boundary condition with the second-order terms neglected but only thermal stress slip retained. Using the present nomenclature and coordinates, the boundary conditions can be depicted as
Using the governing equations and boundary conditions above, the force exerted on the particle by the ambient gas can be determined by
The first term on the right hand side is the Stokes drag force with rarefaction correction, and the second term is the thermophoretic force (Fth ). This formula with thermal stress slip effect included is different from that proposed previously by CitationBrock (1962) or CitationTalbot et al. (1980). The values of coefficients cm , ctc , and ctj were developed with kinetic theory for complete energy and momentum accommodations of Maxwellian/hard-sphere molecules (CitationKeh and Chang 2009) but the thermal creep coefficient has been modified to be 1.17 by experimental and theoretical predictions (CitationTalbot et al. 1980). Then, the coefficients, cm =1.14, ctc =1.17, and ctj =2.18, which were extensively used in the previous studies of particle thermophoresis (CitationTalbot et al. 1980; CitationLu and Lee 2003; CitationKeh and Ou 2004; CitationChein and Liao 2005; CitationDavis 2006; CitationKeh and Chang 2009) are adopted in the present analysis. The thermal stress slip coefficient cts =1 was determined on the basis of the linearized Maxwell-Burnett boundary condition (CitationLockerby et al. 2004). For comparison, cts = 0 is taken for the cases with thermal stress slip ignored. Here, the thermal stress coefficient cts (1 or 0) is regarded simply as a switching index for consideration of the thermal stress slip effect. CitationPoddoskin et al. (2001) demonstrated that the slip coefficients for monoatomic and polyatomic gases differ only slightly from one another. The theoretical analysis of CitationLatyshev et al. (2005) also showed that slip coefficients depend on Prandtl number (Pr) considerably. For the gases of Pr around 2/3 (= 0.667), the slip velocity and the rate of thermophoresis in polyatomic gases differ from those in monoatomic gases insignificantly. Therefore, we adopted the coefficients based on monatomic gases for air (Pr ≃ 0.7) and applied the commonly accepted values in the analysis on particle thermophoresis.
For thermophoretic motion at steady state, the resultant force acting on the particle is zero and the particle thermophoretic velocity, Vth , is of opposite direction of the fluid velocity V 0 for transformation of the reference frame, i.e., Vth =−V 0, and one has
Consider the viscosity given by ![]() (CitationKennard 1938), where
(CitationKennard 1938), where ![]() is the mean molecular speed, then μ=4Mkg
/15kB
with kB
denoting the Boltzmann's constant and M the gas molecular mass. We obtain the thermophoretic force and velocity equations in alternative forms,
is the mean molecular speed, then μ=4Mkg
/15kB
with kB
denoting the Boltzmann's constant and M the gas molecular mass. We obtain the thermophoretic force and velocity equations in alternative forms,
3. RESULTS AND DISCUSSION
3.1. Analysis of Order-of-Magnitudes
To check relative importance of the thermal stress slip, an order-of-magnitude analysis is performed. In Equation (Equation8a), the first two terms, r∂(v
θ/r)/∂r+(∂vr
/∂θ)/r, in the square bracket on right hand side (RHS) are concerned with momentum exchange, the third one in the square bracket is the thermal stress slip term, ![]() , and the last term on RHS
, and the last term on RHS ![]() is thermal creep term. The parameter ℜ1 is defined as the ratio of the thermal stress term to the momentum exchange term, and ℜ2 as the ratio of the thermal stress term to the thermal creep, viz.,
is thermal creep term. The parameter ℜ1 is defined as the ratio of the thermal stress term to the momentum exchange term, and ℜ2 as the ratio of the thermal stress term to the thermal creep, viz.,
3.2. Comparison with Previous Predictions and Experimental Data
In slip-flow regime, Borck (1962) and CitationTalbot et al. (1980) developed a theory for particle thermophoresis, in which the thermophoretic force is
With consideration of thermal stress slip effect, we develop a modified expression of thermophoretic force, i.e.,
To demonstrate the validity of the present theory, we try to compare our results with the previous predictions and experimental data. However, for lack of previous experimental data on thermophoresis in slip flow regime, the present theoretical predictions are compared with measurements of thermophoretic force in the range of 0.06⩽Kn⩽1. Figures – show comparisons of our predictions of dimensionless thermophoretic force with previous theory and measurements for three different kinds of particles. For dioctyl phthalate (DOP) particles of thermal conductivity kp = 0.165 W/mK in gaseous medium (air) of kg = 0.0262 W/mK, the present predictions agree well with the experimental data of CitationLi and Davis (1995) in the range of Kn < 0.5. The theory with consideration of thermal stress slip is better than Equation (Equation16) proposed by CitationDavis (2006), which has noticeable deviation from the measurements and the deviations are more pronounced at high Kn. As Knudsen number approaches to the continuum limit, the theories with and without thermal stress slip coincide the measurements due to vanishing thermal stress slip effect. In , for polystyrene latex (PSL) with thermal conductivity kp = 0.195 W/mK in air, it can be observed that our predictions at Kn < 0.6 are closer to the experimental data (CitationLi and Davis 1995; CitationSantachiara et al. 2002) than the theoretical results of CitationDavis (2006) without thermal stress slip. In Figures and , data from CitationLi and Davis (1995) are of a little abrupt change with increase in Kn and become closer to Davis's predictions (2006). So far we do not have any speculation about this tendency of the measurements.
FIG. 2 Comparison of the present theory with measured thermophoretic velocity of DOP (dioctyl phthalate) particle in air with k*=6.298.
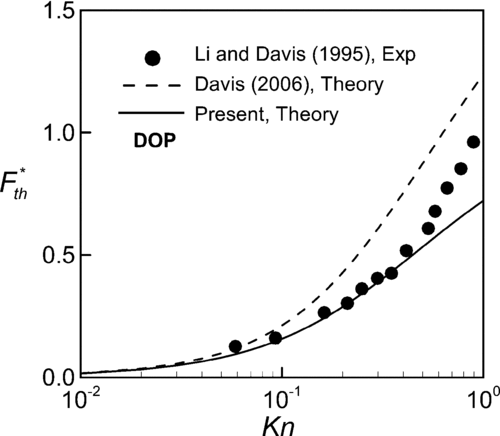
FIG. 3 Comparison of the present theory with measured thermophoretic velocity of PSL (polystyrene latex) particle in air with k*= 7.443.
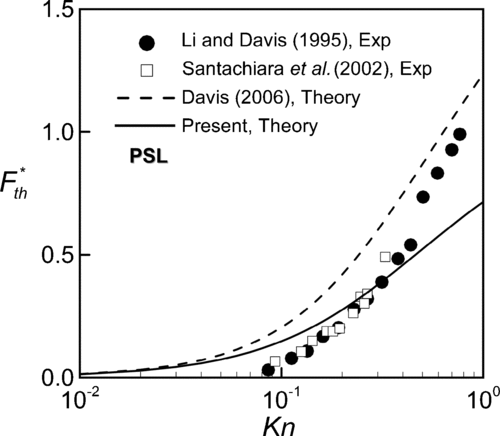
FIG. 4 Comparison of the present theory with measured thermophoretic velocity of NaCl particle in air with k*= 244.3.
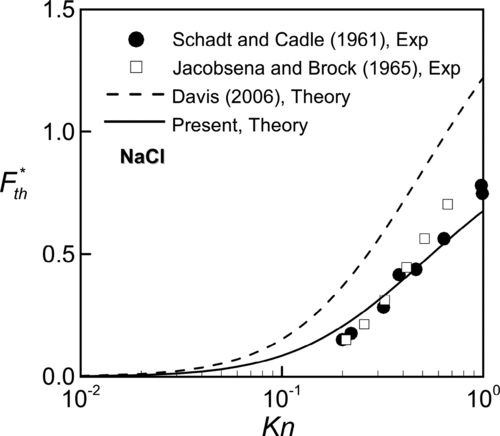
For a NaCl particle of thermal conductivity kp = 6.4 W/mK in air, presents the predictions of dimensionless thermophoretic force and compared with the previous experimental data (CitationSchadt and Cadle 1961; CitationJacobsen and Brock 1965) and theoretical predictions (CitationDavis 2006). The present predictions with thermal stress slip effect are more comparable with experimental data and the qualitative features about the thermal stress slip presented in Figures and are similar. The deviation between the theories with (present) and without (CitationDavis 2006) thermal stress slip effect is enlarged as Kn increases or, equivalently, the particle size reduces.
3.3. Parametric Analysis of Thermophoretic Mobility
With Vth
normalized as ![]() , it can be expressed in a dimensionless form, viz.
, it can be expressed in a dimensionless form, viz.
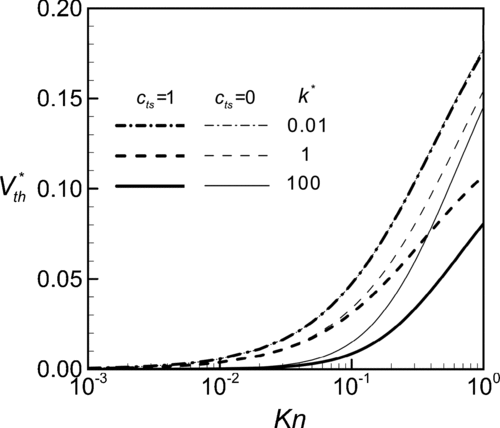
To further understand the thermal stress slip effect on thermophoretic mobility, we perform a detailed parametric analysis. In Equation (Equation18), the dimensionless thermophoretic velocity V* th is a function of two major parameters. The Knudsen number, Kn≡l/R, characterizes the rarefaction of the gas and, by its definition, it is also an indication for the influence of the particle size. The other is the dimensionless thermal conductivity of the particle, k*≡kp /kg . presents the influences of the thermal conductivity ratio k* on the thermophoretic velocity V* th at various conditions of Kn with and without considering thermal stress slip. It can be observed that V* th monotonically decreases with k* for all cases. It agrees with the conclusions of previous works (CitationOhwada and Sone 1992; CitationKeh and Tu 2001). Physically, a particle of high thermal conductivity is able to spread heat over the particle and reduce the local temperature gradient along the particle surface. The thermal creeping flow and thus the thermophoretic velocity are suppressed but, relatively, the thermal stress flow becomes dominant.
FIG. 5 Variation of dimensionless thermophoretic velocity with relative thermal conductivity of the particle at various conditions of Knudsen number.
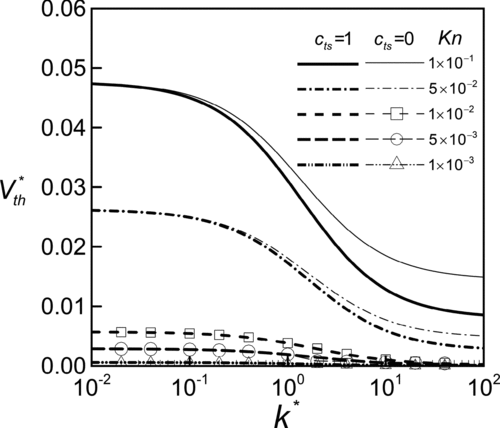
demonstrates that the influence of thermal stress slip is diminished as k* reduces but becomes more remarkable at higher values of k*. Since the thermal source in the field is the temperature gradient of the gas medium, the particle has the surface temperature gradient, (∂Tp /∂θ) w , in the same direction as that in gas field. As k* increases, the non-uniformity of the temperature distribution along the particle surface is smeared out and, through interactions at the particle-gas interface with temperature jump condition, the gas side temperature near the interface has the same tendency, i.e., reduction in (∂Tg /∂θ) w . This will raise the temperature on the cold side of the particle and increase the local value of the normal temperature gradient (∂Tg /∂r) w on the cold side. Therefore, it leads to an increase in the tangential variation of the gradient (∂Tg /∂r) w or an enhancement of the thermal stress slip ∂(∂Tg /∂r) w /∂θ. As to the thermal stress slip effect at various conditions of Kn, both Figures and demonstrate that the effect on V* th is more pronounced at high Kn or small radius of the particle just as that disclosed by the order-of-magnitude analysis.
4. CONCLUDING REMARKS
In the present study, we have modified the conventional thermophoresis theory with consideration of the thermal stress slip and developed novel analytical expressions for predictions of thermophoretic mobility. Through comparison with the measured data for various particles, the present theory has shown its capability in appropriate prediction of the thermophoretic mobility. Order-of-magnitude analysis as well as the theoretical expressions developed in the work both demonstrate enhancement of the thermal stress slip with reducing particle size. The present theory reveals that the thermal stress slip effect becomes more pronounced at conditions of high particle thermal conductivity and high Knudsen number as well. The theoretical expressions and the physical insights obtained in this work are useful in further understanding of the physical mechanism of the thermophoresis with strong interfacial thermal interactions.
This study was supported by National Science Council of the Republic of China (Taiwan) through the Grant NSC-98-2221-E-035-068-MY3 (2009-2012).
References
- Aoki , K. , Sone , Y. and Waniguchi , Y. 1998 . A Rarefied Gas Flow Induced by a Temperature Field: Numerical Analysis of the Flow between Two Coaxial Elliptic Cylinders with Different Uniform Temperatures. . Comput. Math. Appl. , 35 : 15 – 28 .
- Brock , J. R. 1962 . On the Theory of Thermal Forces Acting on Aerosol Particles. . J. Colloid Sci. , 17 : 768 – 780 .
- Chein , R. and Liao , W. 2005 . Thermophoretic Effects on Nano-Particle Deposition in Channel Flow. . Heat Mass Trans. , 42 : 71 – 79 .
- Davis , E. J. 2006 . “ Thermophoresis of Particles ” . In Encyclopedia of Surface and Colloid Science , 6274 – 6282 . New York : Marcel Dekker .
- Epstein , P. S. 1929 . Zur Theorie des Radiometers. . Z. Phys. , 54 : 537 – 563 .
- Jacobsen , S. and Brock , J. R. 1965 . The Thermal Force on Spherical Sodium Chloride Aerosols. . J. Colloid Sci. , 20 : 544 – 554 .
- Keh , H. J. and Tu , H. J. 2001 . Thermophoresis and Photophoresis of Cylindrical Particles. . Colloids Surf. A. , 176 : 213 – 223 .
- Keh , H. J. and Chen , P. Y. 2003 . Thermophoresis of an Aerosol Sphere Parallel to One or Two Plane Walls. . AIChE J. , 49 : 2283 – 2299 .
- Keh , H. J. and Ou , C. L. 2004 . Thermophoresis of Aerosol Spheroids. . Aerosol Sci. Tech. , 38 : 675 – 684 .
- Keh , H. J. and Chang , Y. C. 2006 . Thermophoresis of an Aerosol Sphere Perpendicular to Two Plane Walls. . AIChE journal , 52 : 1690 – 1704 .
- Keh , H. J. and Chang , Y. C. 2009 . Thermophoresis of an Aerosol Spheroid along Its Axis of Revolution. . Phys. Fluids. , 21 : 062001
- Kennard , E. H. 1938 . Kinetic Theory of Gases , New York : McGraw-Hill .
- Latyshev , A. V. , Popov , V. N. and Yushkanov , A. A. 2005 . Calculation of the Velocity of a Molecular Gas Slipping over a Sphere with Regard to Accommodation Coefficients. . Technical Physics , 50 : 1417 – 1422 .
- Li , W. and Davis , E. J. 1995 . The Effects of Gas and Particle Properties on Thermophoresis. . J. Aerosol Sci. , 26 : 1085 – 1099 .
- Lockerby , D. A. , Reese , J. M. , Emerson , D. R. and Barber , R. W. 2004 . Velocity Boundary Condition at Solid Walls in Rarefied Gas Calculations. . Phys. Rev. E , 70 : 017303
- Loyalka , S. K. 1992 . Thermophoretic Force on a Single Particle I. Numerical Solution of the Linearized Boltzmann Equation. . J. Aerosol Sci. , 23 : 291 – 300 .
- Lu , S. Y. and Lee , C. T. 2003 . Thermophoretic Motion of a Spherical Aerosol Particle in a Cylindrical Pore. . Aerosol Sci. Tech. , 37 : 455 – 459 .
- Maxwell , J. C. 1879 . On Stresses in Rarefied Gases Arising from Inequalities of Temperature. . Philos. Trans. R. Soc. Lond. , 170 : 231 – 256 .
- Ohwada , T. and Sone , Y. 1992 . Analysis of Thermal Stress Slip Flow and Negative Thermophoresis using the Boltzmann Equation for Hard-Sphere Molecules. . Eur. J. Mech. B/Fluids , 11 : 389 – 414 .
- Piazza , R. and Parola , A. 2008 . Thermophoresis in Colloidal Suspensions. . J. Phys. Condens. Matter , 20 : 153102
- Poddoskin , A. B. , Yushkanov , A. A. and Yalamov , Y. I. 2001 . Slip and Macroparameter Jumps of a Polyatomic Gas with Rotational Degrees of Freedom on a Weakly Curved Spherical Surface. . Fluid Dynamics , 36 : 843 – 850 .
- Santachiara , G. , Prodi , F. and Cornetti , C. 2002 . Experimental Measurements on Thermophoresis in the Transition Region. . J. Aerosol Sci. , 33 : 769 – 780 .
- Schadt , C. F. and Cadle , R. D. 1961 . Thermal Force on Aerosol Particles. . J. Phys. Chem. , 65 : 1689 – 1694 .
- Senchenko , S. and Keh , H. J. 2007 . Thermophoresis of a Slightly Deformed Aerosol Sphere. . Phys. Fluids. , 19 : 033102
- Sone , Y. 1972 . Flow Induced by Thermal Stress in Rarefied Gas. . Phys. Fluids. , 15 : 1418
- Sone , Y. and Aoki , K. 1981 . “ Negative Thermophoresis: Thermal Stress Slip Flow around a Spherical Particle in a Rarefied Gas ” . In Rarefied Gas Dynamics , 489 – 503 . New York : American Institute of Aeronautics and Astronautics .
- Soong , C. Y. , Li , W. K. , Liu , C. H. and Tzeng , P. Y. 2010 . Effect of Thermal Stress Slip on Micro-Particle Photophoresis in Gaseous Media. . Opt. Lett. , 35 : 625 – 627 .
- Talbot , L. , Cheng , R. K. , Schefer , R. W. and Willis , D. R. 1980 . Thermophoresis of Particles in Heated Boundary Layer. . J. Fluid Mech. , 101 : 737 – 758 .
- Williams , M. M. R. 1986 . Thermophoretic Forces Acting on a Spheroid. . J. Phys. D. Appl. Phys. , 19 : 1631 – 6142 .
- Yamamoto , K. and Ishihara , Y. 1988 . Thermophoresis of a Spherical Particle in a Rarefied Gas in the Transition Regime. . Phys. Fluids , 31 : 3618 – 3624 .
- Zheng , F. 2002 . Thermophoresis of Spherical and Non-Spherical Particles: A Review of Theories and Experiments. . Adv. Colloid Interface Sci. , 97 : 255 – 278 .