Abstract
A new particle size magnifier (PSM) for detection of nano-CN as small as ∼1 nm in mobility diameter was developed, calibrated and tested in atmospheric measurements. The working principle of a PSM is to mix turbulently cooled sample flow with heated clean air flow saturated by the working fluid. This provides a high saturation ratio for the working fluid and activates the seed particles and grows them by condensation of the working fluid. In order to reach high saturation ratios, and thus to activate nano-CN without homogeneous nucleation, diethylene glycol was chosen as the working fluid. The PSM was able to grow nano-CN to mean diameter of 90 nm, after which an ordinary condensation particle counter was used to count the grown particles (TSI 3010). The stability of the PSM was found to be good making it suitable for stand-alone field measurements. Calibration results show that the detection efficiency of the prototype PSM + TSI 3010 for charged tetra-alkyl ammonium salt molecules having mobility equivalent diameters of 1.05, 1.47, 1.78, and 2.57 nm are 25, 32, 46, and 70%, respectively. The commercial version of the PSM (Airmodus A09) performed even better in the smallest sizes the detection efficiency being 51% for 1.47 nm and 67% for 1.78 nm.
1. INTRODUCTION
Condensation Particle Counters (CPCs) are commonly used to measure number concentrations of aerosol particles. These instruments can detect sub-micrometer particles that cannot be detected by optical counters (CitationMcMurry 2000). The working principle of CPCs consists of three consecutive processes: supersaturating the aerosol with the vapor of a working fluid, growth of the particles by condensation of the supersaturated vapor on the particle surface and optical detection of the particles after their growth. Several methods can be used to generate the supersaturation: adiabatic expansion of an aerosol–vapor mixture (CitationMetnieks and Pollak 1959), conductive cooling (CitationBricard et al. 1976; CitationSem 2002) or heating (Hering et al. 2005) and mixing of cool and warm saturated air flows (CitationKousaka et al 1982; CitationMavliev 2002; CitationWang et al. 2002). In applications using CPCs a constant sample flow rate is desirable, which can be provided by the last three methods. Even the expansion CPC with a quasi-continuous sample flow is available (Kürten et al. 2005). The main advantage of a CPC in comparison to electrical detection is its ability to count single particles without charging the particles.
The lowest cut-off diameter (defined here as the diameter of particles of which 50% are counted for) of modern commercial CPCs lie at around 3 nm in diameter (CitationLiu et al. 2006; CitationHermann et al. 2007; CitationMordas et al. 2008). A recent progress in the field of atmospheric aerosol science, especially in understanding of the new particle formation (CitationKulmala et al. 2004), as well as the bloom of nanoscience and -technology have created a need to develop CPCs for detection of smaller and smaller particles. Mixing type particle size magnifiers (PSM) developed by CitationGamero-Castaño and Fernandez de la Mora (2000), CitationKim et al. (2000), and CitationSgro and Fernández de la Mora (2004) has shown a superior performance in the sub-3nm size range. However, no reports on their applicability in, e.g., atmospheric studies exist. CitationSipilä et al. (2009) modified a commercial ultrafine CPC (TSI-3025A) and applied it to study sub-3 nm and even sub-2 nm particle concentrations in the atmosphere (CitationSipilä et al. 2008) and in laboratory conditions (CitationSipilä et al. 2010). CitationIida et al. (2009) used the UCPC (TSI-3025) prototype (Stolzenburg and McMurry 1991) with different working fluids, and succeeded in activating particles below 2 nm in diameter using diethylene glycol or oleic acid as the condensing vapor.
The largest problem associated to the condensation particle counting in the size range approaching molecular dimensions (∼1 nm) has been the onset of homogeneous nucleation of the working fluid at highly supersaturated conditions. However, in principle homogeneous nucleation will not occur before activation of existing particles (CitationKulmala et al. 2007a). Sophisticated experiments performed by Winkler et al. (2008a,b) have shown that with a proper instrument design the problems related to the occurrence of homogeneous nucleation can be overcome even with a non-ideal working fluid, like propanol. CitationIida et al. (2009), on the other hand, solved the problem of the homogenous nucleation by a choice of the working fluid, while CitationSipilä et al. (2009) used pulse height analysis (CitationWeber et al. 1998) to distinguish between droplets formed by homogeneous nucleation of butanol vapor and droplets formed by heterogeneous nucleation on a nano-sized condensation nuclei (nano-CN).
The objective of this study is to present an evaluation of a recently developed continuous flow Particle Size Magnifier, which combines mixing and dual stage growth towards detectable sizes. The aerosol particles are activated and grown by the PSM and after that the droplets are counted by an external CPC. This is due to the low saturation vapor pressure of the working fluid used in the PSM, which prevents the droplets to be easily grown to optically detectable size. The operation principle is studied both theoretically and by experiments. The detection efficiencies of the prototype PSM, as well as the commercial version (Airmodus A09) are verified with selected mobility standards, silver particles, and classified charger ions. The PSM is compared to a suite of state-of-the-art commercial and as well as with a pulse-height-analyzing condensation particle counter (PHA-UCPC) in field measurements.
2. INSTRUMENT DESCRIPTION
2.1. Particle Size Magnifier (PSM)
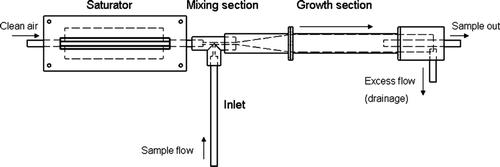
A schematic view of the PSM is presented in . Filtered air is introduced into a saturator, which consists of sintered stainless steel tubing surrounded by the working fluid (diethylene glycol, DEG) inside an aluminum casing. The porosity of the sintered tubing was chosen so that the surface tension of DEG prevented it to flow through the pores as a liquid. The saturation ratio of DEG can be assumed to be near unity at the inner wall of the tubing. By using the sintered tubing, the filling of the saturator can be done passively by using only hydrostatic pressure. Thus, no liquid pumps are needed.
The supersaturation inside the PSM is generated by rapidly mixing the heated and saturated DEG–air mixture with a cooler aerosol laden air. The mixing section of the PSM is constructed from anti-static plastic (Sustarin C ESD 90 POM-AS) to prevent losses of ions. Plastic was chosen to avoid heat transfer between the heated saturator and the cooled aerosol inlet. The inner diameter of the mixing section was chosen to be similar to Sgro and Fernández de la Mora (2004, 1.7 mm). After mixing, the flow is introduced into a growth section of which the first 5 cm is composed of conical tubing wherein the diameter of the tube changes from 1.7 to 16 mm, and the remaining 10 cm is stainless steel tubing having a 16 mm inner diameter. From the growth section the sample was then led to the counter (TSI 3010) via 6 mm tubing. The flow through the saturator and the excess flow were controlled with needle-valves and their difference determined the inlet flow rate (). The inlet flow rate was kept at 2.5 l/min, and the saturator flow rate was varied between 0.05 and 0.6 l/min. The flow rates were measured with pressure difference gauges (Sensirion SDP1000-R). Temperatures were measured from the surfaces of the saturator, the inlet and the growth section, and also from the flows through the inlet and the saturator with thermistors (BetaTherm 1K2A1B). The temperature of the saturator was controlled by using heating-resistors connected to a proportional-integral-derivative (PID) controller. The temperature of the saturator was chosen to be 75.5°C. A set of Peltier-elements and a PID-controller were used to control the temperatures of the inlet and the growth section. The inlet temperature was chosen so that the temperature of the flow prior the mixing section was 16°C. The growth section was cooled down to 3°C to enhance the cooling after the mixing and thus amplify the condensational growth of the activated particles. Also CitationSgro and Fernández de la Mora (2004) concluded that a proper cooling of the flow at the mixing or just after the mixing is essential.
A new version of the PSM (Airmodus A09) was constructed in 2010. The only main difference to the prototype is the saturator design. Due to this, different temperatures were used: saturator 72.5°C, growth tube 0°C and 8°C. Also the saturator flow range was extended to 1 lpm. Calibration results will be shown in section 3.2 for both instruments.
The Peclét number (Pe), which describes the heat conductivity was between 1549 and 1128 at the above mentioned flows, and the Reynolds number (Re) describing the turbulence between 5587 and 3835. This indicates that the flow inside the T-part after mixing is turbulent and in theory the conduction of heat can be neglected. However, previously CitationGamero-Castaño and Fernandez de la Mora (2000) showed that this assumption can result errors in calculating the saturation ratios inside the PSM. In Generally, increasing turbulence and preventing heat conduction increase the saturation ratio achieved inside the mixing T-part (Sgro and Fernándes de la Mora 2004).
2.2. Theoretical Considerations
The basic principle of a PSM is to produce high saturation ratio by mixing turbulently heated clean air saturated by the working fluid with aerosol sample having a lower temperature than the saturated air. This creates a saturated mixture for the working fluid. The saturation ratio S is defined by the ratio of actual vapor pressure pv and the equilibrium vapor pressure peq at temperature T.
TABLE 1 Physical properties of diethylene glycol
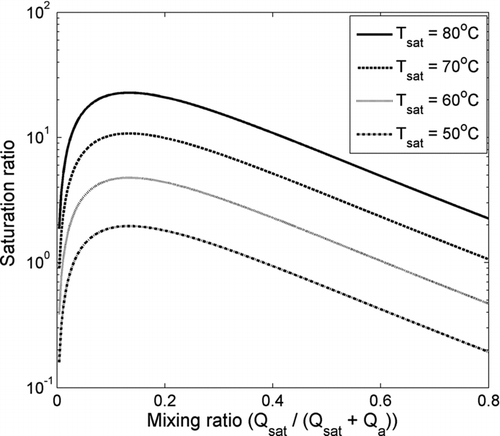
In the saturation ratio calculated according to the Equations (1)–(3) is presented as a function of mixing ratio for four different temperatures of the saturator. The mixing ratio is defined as the ratio between the flows through the saturator and the total flow after the mixing. After the saturator the air stream is considered to be fully saturated. Temperature of the aerosol sample was kept at 16°C. The saturation ratio increases steeply as the mixing ratio increases and presents a maximum at around the mixing ratio of 0.15, after which it starts to decrease. Also, generally at higher temperatures the saturation ratio is elevated. Thus, the saturation ratio of the working fluid in the PSM can be adjusted both by adjusting the mixing ratio and by altering the temperature difference between the saturated flow and the sample flow. As Gamero-Castaño and Fernández de la Mora (2000) pointed out, the assumption of adiabatic mixing might cause errors in calculating the saturation ratios. Due to this, mixing ratio is used instead of saturation ratio to describe the performance of the PSM in this work.
FIG. 2 Saturation ratio inside the PSM as a function of mixing ratio with different saturator temperatures. Temperature of the aerosol sample was kept at 16°C.
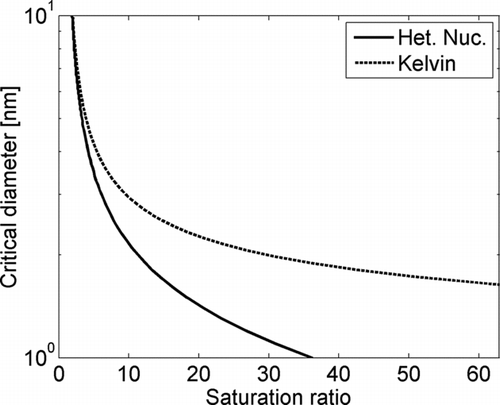
The activation probability of an aerosol particle is mainly dependent on the size of the particle. The smaller the particle is, the higher is the saturation ratio needed for activation. Also chemical composition (O’Dowd et al. 2002; CitationKulmala et al. 2007a) and charge (CitationWinkler et al. 2008a) affect the activation probability, especially for the small particles (<3 nm in diameter). If only size is taken into account the critical diameter in a certain saturation ratio S is defined by the Kelvin equation (CitationSeinfeld and Pandis 2006). Though, for small particles the heterogeneous nucleation probability has to be taken into account (CitationKulmala et al. 2007a; CitationWinkler et al. 2008b). In the critical diameters calculated from the Kelvin equation and from the heterogeneous nucleation theory (CitationKulmala et al. 2007a) are presented as a function of the saturation ratio of DEG. Flow rates and temperatures were set to 2.5 l min−1 and 16°C for the aerosol flow, and 0.6 l min−1 and 75.5°C for the saturator flow. Temperature (28.1°C) used in the calculations was calculated by using Equation (2). Zero contact angle was assumed in the calculations. It can be seen that the Kelvin equation overestimates the critical diameter at high saturation ratios in comparison to heterogeneous nucleation theory which has been shown to match the measurements in previous studies (CitationWinkler et al. 2008a). Therefore, for small particles, heterogeneous nucleation theory has to be used to calculate the critical diameter, as expected.
FIG. 3 Theoretical critical diameters as a function of saturation ratio of diethylene glycol determined using Kelvin equation (dashed line) and heterogeneous nucleation theory (solid line).
To achieve high enough saturation ratios for activation and growth of nano-sized particles without homogeneous nucleation, a proper working fluid has to be used (CitationMagnusson et al. 2003; CitationIida et al. 2009). CitationIida et al. (2009) performed model calculations of homogeneous nucleation rates and corresponding Kelvin diameters by using different condensing vapors. The results suggested that by using working fluids of low vapor pressure and high surface tension, high saturation ratios can be achieved without homogeneous nucleation. Based on their theoretical findings accompanied with laboratory tests CitationIida et al. (2009) suggested that the best compounds for activating nano-sized particles are diethylene glycol and oleic acid. Therefore, diethylene glycol was chosen as the working fluid in the present study.
2.3. Laboratory Verification Setup
The PSM was calibrated using tetra-alkyl ammonium salts as mobility standards (CitationUde and Fernández de la Mora 2005). The mobility standards are relatively large charged molecules (tetra-alkyl ammonium halide salts A+B−) that can act as condensation nuclei with a known mobility. The compounds used in the calibrations and their mobilities and corresponding particle sizes are presented in . For TMAI monomer the mobility diameter is 1.05 nm, TPAI monomer 1.16 nm, for THAB monomer and dimer 1.47 and 1.78 nm, respectively, and for TDDAB monomer and tetramer 1.7 and 2.57 nm, respectively. All the ions are positively charged, TDDAB tetramer being doubly charged. Ions were produced by means of electrospray and the correct ion was chosen by using a high flow and a high resolution Herrmann-type differential mobility analyzer (HDMA, CitationHerrmann et al. 2000). This way a highly monodisperse aerosol was achieved. An electrometer (TSI 3068B) was used as a reference for the concentration.
TABLE 2 Mobilities of selected ions used in the calibrations
Silver nanoparticles produced in a tube furnace and Tungsten oxide (WOx) particles generated in a Grimm Nano Aerosol Generator Model #7.860 were also used to calibrate the PSM. A distinct mobility diameter was chosen either with Herrmann DMA or with a short Hauke-type DMA (CitationWinklmayr et al. 1991). Sample flow of 2.0 l min−1 and sheath flow of 20 l min−1 were used in the Hauke-type DMA. Calibrations for the PSM were conducted for both positively and negatively charged particles from mobility equivalent diameter 1.6 to 5 nm using Herrmann DMA and 3 to 8 nm using Hauke-type DMA. The same TSI-3068B electrometer was used as a reference for the concentration. The electrometer flow rate was set to 8 l min−1 in order to reduce losses due to diffusion. Temperature of the furnace varied between 950 and 1100°C, and the nitrogen flow through the furnace was set to 2 l min−1. More specific description of the silver particle calibration setup can be found in CitationPetäjä et al. (2006).
2.4. Ancillary Field Measurements
The field measurements were performed at the SMEAR II station in Hyytiälä, Southern Finland (61°51′N, 24°17′E, 170 asl, CitationHari and Kulmala 2005) during April 27 and August 5, 2009. The station is surrounded by Scots pine dominated boreal forest and is equipped with extensive facilities to measure continuously various ecosystem-atmosphere interactions. The measurement set-up consists of complementary instruments for observing the total number concentration of aerosol particles. The measurements within this study focused on the intercomparison with several commercial and prototype condensation particle counters. The evolution of ambient aerosol particle population was measured with a Differential Mobility Particle Sizer (DMPS, CitationAalto et al. 2001) from mobility equivalent diameter of 3 to 1000 nm. A constant mixing ratio of 0.19 was used with the protype PSM.
The Condensation Particle Counter Battery (CPCB, CitationKulmala et al. 2007a) used in this study consists of two CPCs: one ultrafine butanol- and water-CPC pair having a cut-off diameter at about 3 nm. The operation principle of the CPCB relies on the differences between the water and butanol solubilities of the sampled nanoparticles, which lead to different concentration readings of the CPCs. According to the laboratory calibrations, the CPC battery is able to observe aerosol particles as small as 2 nm in diameter (CitationKulmala et al. 2007a). The working principle is described in more detail in CitationRiipinen et al. (2009). The time resolution of the CPCB data was 19 s.
The pulse-height CPC (PH-CPC) comprises a TSI-3025A ultrafine butanol CPC with modified optics (CitationDick et al. 2000) and a multichannel analyzer. For minimizing the transportation losses of nanoparticles the aerosol flow through the CPC capillary was increased by ∼30% from nominal, and for maximizing the activation probability the supersaturation was increased by increasing the temperature difference between the CPC saturator and condenser until homogenous nucleation appeared. The pulse height analysis technique (CitationSaros et al. 1996) allows distinguishing homogenous nucleation from activation of nanoparticles and resolving the size distribution between ∼1.3–5 nm (CitationSipilä et al. 2008, 2009). In this study, the PH-CPC was used as a reference-CPC for total particle concentration without taking the detection efficiency into account.
3. RESULTS AND DISCUSSION
3.1. Particle Size after the PSM
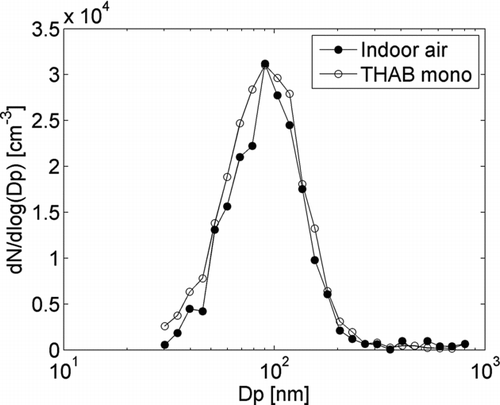
The size distribution of the droplets after the prototype PSM was measured by using a DMPS (CitationAalto et al. 2001). THAB monomer and indoor air aerosol particles were used as seed particles. The measured size distribution after the PSM is presented in . For both seed particles the final droplet mode had a maximum at a mobility diameter of 90 nm. This means that the droplets are still below the optical detection limit (∼1 μm) and an additional growth section has to be used in order to detect the particles. A commercial TSI 3010 CPC is used as a counter. Because the cut-off size of the TSI-3010 is about 10 nm (CitationMertes et al. 1995), it can be assumed that all the droplets from the PSM are counted.
3.2. Detection Efficiency
presents the detection efficiency of the PSM as a function of electrical mobility equivalent diameter, by using constant mixing ratio of 0.19 for the prototype PSM, and 0.33 for the commercial version (Airmodus A09). The detection efficiency was measured for mobility standards () and both negatively and positively charged silver nanoparticles as well as WOx particles. Also size classified ions that were produced in Am241 bipolar charger were used as calibration particles even though their chemical composition is not known. Results for TSI-3776 ultrafine CPC are also shown in for comparison.
Generally, the detection efficiency of a CPC can be presented as a product of sampling, activation, and counting efficiencies (Stolzenburg and McMurry 1991). The sampling efficiency is mainly governed by the diffusion losses, which are presented in as a function of particle size calculated according to CitationGormley and Kennedy (1949) and taking into account turbulent losses in the mixing section (CitationLee and Gieseke 1994). Theoretical losses are for ideal case and are probably underestimated. The counting efficiency depends on the ability of the TSI 3010 to count the activated particles after the PSM as well as the diffusion losses between the PSM and the CPC. A nominal cut-off size for a TSI 3010 is 10 nm (CitationMertes et al. 1995) and thus all the particles can be considered to be accounted for as long as the total particle number concentration remains low enough for single particle counting, i.e., below 104 cm−3. The larger concentrations up to 3·104 cm−3 can be measured taking into account the coincidence in the optics of the CPC. By using a different counter after the PSM this limit can be exceeded. The diffusion losses after the PSM can be considered negligible as the modal particle size after the PSM is approximately 90 nm ().
The detection efficiencies of the prototype PSM for the mobility standards TMAI monomer (d = 1.05 nm), THAB monomer (d = 1.47 nm), THAB dimer (d = 1.78 nm), and TDDAB trimer (d = 2.57 nm) are 25, 32, 46, and 70%, respectively. For the successor PSM (Airmodus A09) the performance was found to be even better: 51% for THAB monomer and 67% for THAB dimer. The results are consistent for both silver and WOx particles and mobility standards in the area where their size ranges overlap. We found that the PSM was able to detect even 1 nm charger ions with detection efficiency of tens of percents, whereas the TSI-3776 detection efficiency was close to zero for particles smaller than 2 nm. However detection efficiency for positive and negative charger ions differed considerably. Similar behavior was also observed by CitationSipilä et al. (2009) using a PHA-UCPC. No clear sign preference was observed for silver or WOx particles.
FIG. 5 Detection efficiency of both prototype and Airmodus A09 PSM as a function of electrical equivalent mobility diameter. The detection efficiencies were measured with mobility standards (MS), charger ions and both negatively and positively charged silver and WOx nanoparticles. A constant mixing ratio of 0.19 and 0.33 were used for prototype and Airmodus A09 PSM, respectively.
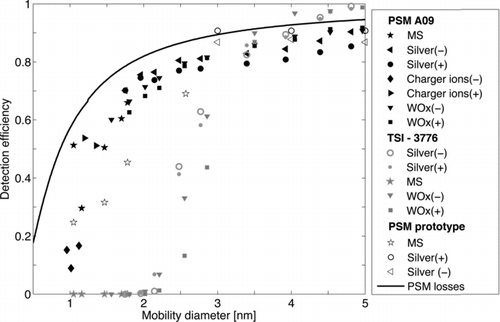
FIG. 6 Activated fraction as a function of the mixing ratio (Qsat/(Qsat+Qaer)) for TMAI monomer, THAB monomer, THAB dimer and doubly charged TDDAB trimer with mobility diameters of 1.05, 1.47, 1.78, and 2.57 nm, respectively.
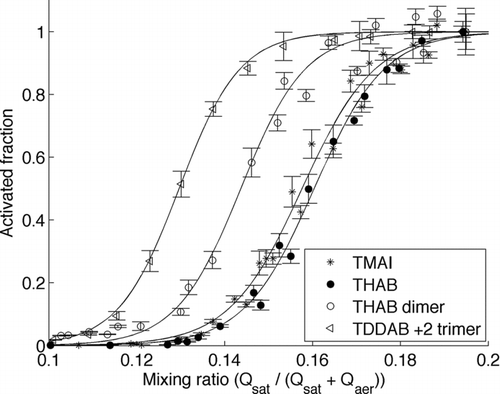
FIG. 7 Detection efficiencies as a function of the mixing ratio (Qsat/(Qsat+Qaer)) for THAB monomer for two different days by using the same calibration setup.
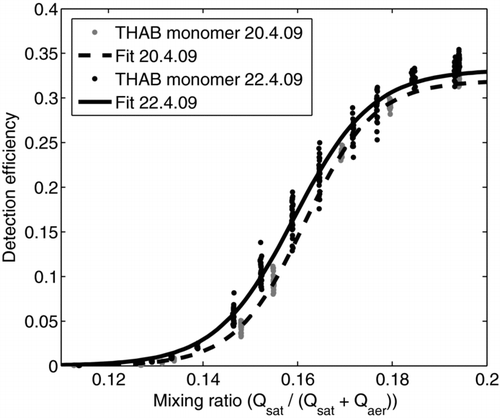
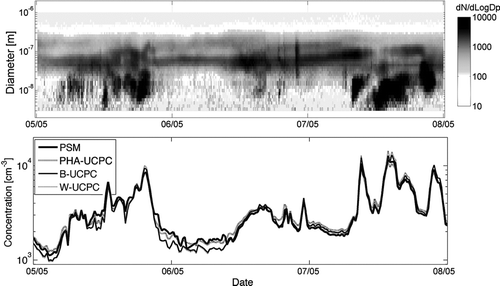
FIG. 8 Aerosol size distributions measured with the DMPS (upper panel) and comparison of total particle number concentration (lower panel) measured with the prototype PSM, PHA-UCPC, TSI 3776 (B-UCPC), and TSI 3786 (W-UCPC) at the boreal forest measurement station in Hyytiälä, Finland for three consecutive days in May 2009.
The detection efficiency of the PSM for nanoparticles larger than 5 nm is saturated to 90%. This indicates that all the particles larger than 5 nm that reach the saturation peak inside the PSM are accounted for. The 100% detection efficiency cannot be reached due to the losses inside the mixing section.
For the smallest particles the diffusion losses inside the inlet dominate the total detection efficiency (). In comparison, CitationMordas et al. (2008) reported 50% cut-off diameters for the commercial ultrafine CPCs TSI-3776 (B-UCPC), TSI-3025 (B-UCPC), and TSI-3786 (W-UCPC) of 3.2 nm, 3.6 nm, and 3.9 nm, respectively. CitationSipilä et al. (2009) measured the detection efficiency of the PHA-UCPC (CitationDick et al. 2000), which is a modified TSI-3025 ultrafine CPC, to be less than 10% for the sub–2 nm particles, for THAB monomer the detection efficiency was ∼5%. The detection efficiency of the PSM saturates to 90%, which might be due to inlet losses or imperfect sampling from the growth tube to the CPC.
represents the activated fraction as a function of mixing ratio. The activated fraction is calculated by assuming that the sampling efficiency depicts the maximum possible counting efficiency (unity), i.e., the detection efficiency is normalized to the penetration efficiency of the prototype PSM. A similar approach was used also by CitationIida et al. (2009). The onset mixing ratio is defined as the mixing ratio where the activated fraction is 50% of its peak value. It increases with decreasing mobility diameter as expected when comparing the TDDAB tetramer, THAB dimer, and THAB monomer. The onset mixing ratio for TMAI is about the same and even a bit smaller than for the THAB monomer even though mobility equivalent diameter of the TMAI monomer is smaller than the THAB monomer. This indicates that the onset mixing ratio might not reflect the mobility equivalent size of the particle acting as a condensation nucleus when measuring particles smaller than 2 nm. The ability of the particle to act as a condensation nucleus can be dominated by the charge (Gamero-Castaño and Fernández de la Mora 2002) or some chemical parameter for example wettability or solubility (CitationKulmala et al. 2007a) of the particle in the working fluid. The THAB monomer is hydrophobic whereas the TMAI monomer is hydrophilic, which might affect the activation behavior when using hydrophilic diethylene glycol as the working fluid. Also Gamero-Castaño and Fernández de la Mora (2000) showed similar behavior of critical supersaturations for THAB and TMAI monomer.
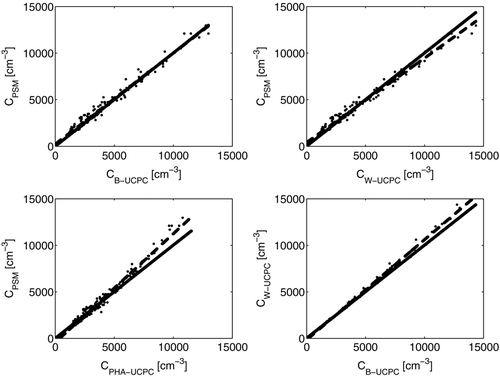
FIG. 9 Number concentration measured with the prototype PSM in boreal forest measurement station in Hyytiälä Finland in May 2009, presented as a function of concentrations measured with (a) TSI 3776 (B-UCPC), (b) TSI 3786 (W-UCPC), and (c) PHA-UCPC. In panel (d) the concentrations measured with TSI 3776 and TSI 3786 are compared.
The PSM can be used as a size spectrometer for the smallest aerosol particles as the mixing ratio is scanned and subsequently the particles of different size are detected with the PSM. This provides a nice tool to investigate aerosol particle number size distribution below 3 nm size range. Though, it has to be noted that the size determined by measuring the onset of heterogeneous nucleation does not necessarily convert easily to a mobility equivalent diameter achieved from, e.g., DMA classification.
When comparing and it can be seen that the mixing ratio does not convert to saturation ratio by using the adiabatic assumption. The activated fraction increases in up to the mixing ratio of 0.2 while according to the theory it should start to decrease after mixing ratio of 0.15. This might be due to the low temperature of the growth tube (3°C) compared to the inlet temperature (16°C). Because heat diffuses faster than the DEG, the highest saturation ratio might be achieved at the entry of the growth tube rather than in the mixing region.
Reproducibility of the prototype PSM was tested by measuring the detection efficiency for THAB monomer in two different days with the same measurement setup. In , the detection efficiency is presented as a function of the mixing ratio for both days. The agreement between the measurements is good. There is a 3.6% difference in the detection efficiency at the highest mixing ratio used. Though the shape of the slope is similar there is a difference of 1.5% in the onset mixing ratio (where the detection efficiency is 50% of the maximum detection efficiency).
3.3. Ambient Measurements
The prototype PSM was compared with a suite of commercial CPCs (TSI 3776 and TSI 3786) and with a Pulse-Height-Analysing UCPC (CitationDick et al. 2000; CitationSipilä et al. 2009) by measuring atmospheric aerosol particles for three consecutive days at a boreal forest station in Hyytiälä, Finland (CitationHari and Kulmala 2005). A constant mixing ratio of 0.19 was used in the measurements. The results are shown in . The total concentration measured with the PSM follows nicely the commercial CPCs. The only difference arises during first two nights when the particle concentration is relatively low. During those nights both the PSM and the PHA-UCPC observe more particles than the commercial CPCs. This can be due to the lower detection limit of both PSM and PHA-UCPC compared to commercial CPCs. Previously CitationLehtipalo et al. (2009) observed small (<3 nm) condensation nuclei during night time at Hyytiälä with the PHA-UCPC.
In the number concentrations measured with the PSM are presented as a function of number concentrations measured with the commercial CPCs TSI 3776 and TSI 3786, and with the PHA-UCPC. Concentrations measured with the PSM using a TSI 3010 as a counter are coincidence corrected according to the TSI 3010 manual. The solid line represents the 1:1 line and the dashed line the fit to the data. The agreement between the PSM + TSI 3010 with the TSI 3776 butanol CPC is really good. Comparisons with the TSI 3786 water CPC show that there is a slight difference at higher concentrations for both the TSI 3776 butanol CPC and the PSM + TSI 3010. The PHA-UCPC seems to underestimate the concentration at high aerosol particle concentrations as stated also by CitationSipilä et al. (2009).
4. CONCLUSIONS
We have developed and constructed a robust particle size magnifier for detection of airborne particles and molecular clusters down to ∼1 nm in mobility equivalent diameter. This instrument uses diethylene glycol as a working fluid as suggested by CitationIida et al. (2009). The supersaturation is generated by turbulently mixing a cool sample flow containing the nanoparticles with a heated air flow saturated with the working fluid as presented in Sgro and Fernandez de la Mora (2004). The combined flow is subsequently cooled. A secondary CPC is used to count the particles, which grow inside the PSM to approximately 90 nm in diameter.
Laboratory calibrations showed that for positively charged mobility standards of different tetra-alkyl ammonium halide salts with corresponding mobility diameters of 1.05, 1.47, 1.78, and 2.57 nm the prototype instrument's detection efficiencies were 25, 32, 46, and 70%, respectively. Even better performance was found for the commercial PSM (Airmodus A09) which is based on the prototype instrument. The detection efficiency of the PSM–TSI-3010 combination was dominated by the diffusion losses in the sampling lines and by the turbulent diffusion losses in the mixing part.
The detection efficiency of the PSM depends on the selected temperatures as well as on the mixing ratio between the saturator and the sample flows. By scanning the mixing ratio the PSM can also be used as a size spectrometer, which is able to detect single particles. The PSM thus provides a tool to probe the number size distribution at sizes below 3 nm, which is where the gas-to-particle conversion occurs (CitationKulmala et al. 2007b).
The laboratory comparison with a suite of commercially available CPCs showed that the instrument's stability and reproducibility is good. Initial ambient measurement experiments showed that it can be operated as a stand-alone device in atmospheric field stations. Integrating the PSM with the capability to detect nanoparticles and their number size distribution below 3 nm into the long-term measurement network in various atmospheric environments would provide gap-filling information on the formation of new particles in the atmosphere.
Acknowledgments
This work was partially funded by European Commission 6th Framework programme 13 project EUCAARI, contract no 036833–2 (EUCAARI). Financial support from Kone foundation, Väisälä foundation, Otto Malm foundation, The Academy of Finland and European Research Council is acknowledged.
REFERENCES
- Aalto , P. , Hämeri , K. , Becker , E. , Weber , R. , Salm , J. , Mäkelä , J. M. , Hoell , C. , O’Dowd , C. D. , Karlsson , H. , Hansson , H.-C. , Väkevä , M. , Koponen , I. K. , Buzorius , G. and Kulmala , M. 2001 . Physical Characterization of Aerosol Particles during Nucleation Events . Tellus. , 53B : 344 – 358 .
- Bricard , J. , Delattre , P. , Madelaine , G. and Pourprix , M. 1976 . “ Detection of Ultra-Fine Particles by Means of a Continuous Flux Condensation Nuclei Counter ” . In Fine Particles: Aerosol Generation, Measurement, Sampling, and Analysis , Edited by: Liu , B. Y. H. 565 – 580 . New York : Academic Press .
- Dick , W. D. , McMurry , P. H. , Weber , R. J. and Quant , R. 2000 . White-Light Detection for Nanoparticle Sizing with the TSI Ultrafine Condensation Particle Counter . J. Nanoparticle Res. , 2 : 85 – 90 .
- Gamero-Gastaño , M. and Fernández de la Mora , J. 2000 . A Condensation Nucleus Counter (CNC) Sensitive to Singly Charged Sub-Nanometer Particles . J. Aerosol Sci. , 31 : 757 – 772 .
- Gamero-Gastaño , M. and Fernández de la Mora , J. 2002 . Ion-Induced Nucleation: Measurements of the Effect of Embryo's Size and Charge State on the Critical Supersaturation . J. Chem. Phys. , 117 : 3345 – 3353 .
- Gormley , P. G. and Kennedy , M. 1949 . Diffusion from a Stream Flowing through a Cylindrical Tube . Proc. R. Irish Acad. , 52 ( A ) : 163 – 169 .
- Hari , P. and Kulmala , M. 2005 . Station for Measuring Ecosystem-Atmosphere Relations (SMEAR II) . Boreal Environ. Res. , 10 : 315 – 322 .
- Hering , S. , Stolzenburg , M. , Quant , F. , Oberreit , D. and Keady , P. 2005 . A Method for Particle Size Amplification by Water Condensation in a Laminar Thermally Diffusive Flow . Aerosol Sci. Technol. , 39 : 428 – 436 .
- Hermann , M. , Wehner , B. , Bischof , O. , Han , H.-S. , Krinke , T. , Liu , W. , Zerrath , A. and Wiedensohler , A. 2007 . Technical Note: Particle Counting Efficiencies of New TSI Condensation Particle Counters . J. Aerosol Sci. , 38 : 674 – 682 .
- Herrmann , W. , Eichler , T. , Bernardo , N. and Fernández de la Mora , J. 2000 . Turbulent Transition Arises at Reynolds Number 35,000 in a Short Vienna Type DMA with a Large Laminarization Inlet. [Abstract 15B5] . AAAR Conference. ,
- Iida , K. , Stoltzenburg , M. R. and McMurry , P. H. 2009 . Effect of Working Fluid on Sub-2nm Particle Detection with a Laminar Flow Ultrafine Condensation Particle Counter . Aerosol Sci. Technol. , 43 : 81 – 90 .
- Kim , C. S. , Okuyama , K. and Fernández de la Mora , J. 2000 . Performance Evaluation of an Improver Particle Size Magnifier for Single Nanoparticle Detection . Aerosol Sci. Technol. , 37 : 791 – 803 .
- Kousaka , Y. , Niida , T. , Okuyama , K. and Tanaka , H. 1982 . Development of Mixing Tyoe Condensation Nucleus Counter . J. Aerosol Sci. , 13 : 231 – 240 .
- Kulmala , M. , Vehkamäki , H. , Petäjä , T. , Dal Maso , M. , Lauri , A. , Kerminen , V.-M. , Birmili , W. and McMurry , P. H. 2004 . Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations . J. Aerosol Sci. , 35 : 143 – 176 .
- Kulmala , M. , Mordas , G. , Petäjä , T. , Grönholm , T. , Aalto , P. P. , Vehkamäki , H. , Hienola , A. I. , Herrmann , E. , Sipilä , M. , Riipinen , I. , Manninen , H. E. , Hämeri , K. , Stratmann , F. , Bilde , M. , Winkler , P. M. , Birmile , W. and Wagner , P. E. 2007a . The Condensation Particle Counter Battery (CPCB): A New Tool to Investigate the Activation Properties of Nanoparticles . J. Aerosol Sci. , 38 : 289 – 304 .
- Kulmala , M. , Riipinen , I. , Sipilä , M. , Manninen , H. E. , Petäjä , T. , Junninen , H. , Dal Maso , M. , Mordas , G. , Mirme , A. , Vana , M. , Hirsikko , A. , Laakso , L. , Harrison , R. M. , Hanson , I. , Leung , C. , Lehtinen , K. E. J. and Kerminen , V.-M. 2007b . Towards Direct Measurement of Atmospheric Nucleation . Science. , 318 : 90 – 92 .
- Kürten , A. , Curtius , J. , Nillius , B. and Borrmann , S. 2005 . Characterization of an Automated, Water-Based Expansion Condensation Nucleus Counter for Ultrafine Particles . Aerosol Sci. Technol. , 39 : 1174 – 1183 .
- Lee , K. W. and Gieseke , J. A. 1994 . Deposition of Particles in Turbulent Pipe Flows . J. Aerosol Sci. , 25 : 699 – 709 .
- Lehtipalo , K. , Sipilä , M. , Riipinen , I. , Nieminen , T. and Kulmala , M. 2009 . Analysis of Atmospheric Neutral and Charged Molecular Clusters in Boreal Forest Using Pulse-Height CPC . Atmos. Chem. Phys. , 9 : 4177 – 4184 .
- Lide , D. R. and Frederikse , H. P. R. 1998 . CRC Handbook of Chemistry , 79th ed. , Edited by: Lide , D. R. and Frederikse , H. R. P. Boca Raton, FL : CRC Press .
- Liu , W. , Kaufman , S. L. , Osmondson , B. L. , Sem , G. J. , Quant , F. R. and Oberreit , D. R. 2006 . Water-Based Condensation Particle Counters for Environmental Monitoring of Ultrafine Particles . J. Air Waste Manage. Assoc. , 56 : 444 – 455 .
- Magnusson , L.-E. , Koropchak , J. A. , Anisimov , M. P. , Poznjakovskiy , V. M. and Frenández de la Mora , J. 2003 . Correlations for Vapor Nucleating Critical Embryo Parameters . J. Phys. Chem. Ref. Data. , 32 : 1387 – 1409 .
- Mavliev , R. 2002 . Turbulent Mixing Condensation Nucleus Counter . Atmos. Res. , 62 : 302 – 314 .
- McMurry , P. H. 2000 . The History of CPCs . Aerosol Sci. Technol. , 33 : 297 – 322 .
- Mertes , S. , Schröder , F. and Wiedensohler , A. 1995 . Technical Note: The Particle Detection Efficiency Curve of the TSI-3010 CPC as a Function of the Temperature Difference between Saturator and Condenser . Aerosol Sci. Technol. , 23 : 257 – 261 .
- Metnieks , A. L. and Pollak , L. W. 1959 . “ Introduction for Use of Photo-Electric Condensation Nucleus Counter ” . In Geophysical Bulletin, School of Cosmic Physica , Vol. 16 , Dublin Institute for Advanced Studies .
- Mordas , G. , Manninen , H. E. , Petäjä , T. , Aalto , P. P. , Hämeri , K. and Kulmala , M. 2008 . On Operation of the Ultra-Fine Water-Based CPC TSI 3786 and Comparison with Other TSI Models (TSI 3776, TSI 3772, TSI 3025, TSI 3010, TSI 3007) . Aerosol Sci. Technol. , 42 : 152 – 158 .
- O’Dowd , C. D. , Aalto , P. A. , Hämeri , K. , Kulmala , M. and Hoffmann , T. 2002 . Atmospheric Particles from Organic Vapours . Nature. , 416 : 497 – 498 .
- Okuyama , K. , Kousaka , Y. and Motouchi , T. 1984 . Condensational Growth of Ultrafine Aerosol Particles in New Aerosol Size Magnifier . Aerosol Sci. Technol. , 3 : 353 – 366 .
- Petäjä , T. , Mordas , G. , Manninen , H. , Aalto , P. P. , Hämeri , K. and Kulmala , M. 2006 . Detection Efficiency of a Water-Based TSI Condensation Particle Counter 3785 . Aerosol Sci. Technol. , 40 : 1090 – 1097 .
- Riipinen , I. , Manninen , H. E. , Yli-Juuti , T. , Boy , M. , Sipilä , M. , Ehn , M. , Junninen , H. , Petäjä , T. and Kulmala , M. 2009 . Applying the Condensation Particle Counter Battery (CPCB) to Study the Water-Affinity of Freshly-Formed 2–9 nm Particles in Boreal Forest . Atmos. Chem. Phys. , 9 : 3317 – 3330 .
- Saros , M. , Weber , R. J. , Marti , J. and McMurry , P. H. 1996 . Ultra Fine Aerosol Measurement Using a Condensation Nucleus Counter with Pulse Height Analysis . Aerosol Sci. Technol. , 25 : 200 – 213 .
- Seinfeld , J. H. and Pandis , S. N. 2006 . Atmospheric Chemistry and Physics—From Air Pollution to Climate Change , 2nd Ed. , 519 – 523 . New York : John Wiley & Sons .
- Sem , G. J. 2002 . Design and Performance Characteristics of Three Continuous-Flow Condensation Particle Counters: A Summary . Atmos. Res. , 62 : 267 – 294 .
- Sgro , L. A. and Fernández de la Mora , J. 2004 . A Simple Turbulent Mixing CNC for Charged Particle Detection Down to 1.2 nm . Aerosol Sci. Technol. , 38 : 1 – 11 .
- Sipilä , M. , Berndt , T. , Petäjä , T. , Brus , D. , Vanhanen , J. , Stratmann , F. , Patokoski , J. , Mauldin , R. L. III , Hyvärinen , A.-P. , Lihavainen , H. and Kulmala , M. 2010 . The Role of Sulphuric Acid in Atmospheric Nucleation . Science. , 327 : 1243 – 1246 .
- Sipilä , M. , Lehtipalo , K. , Attoui , M. , Neitola , K. , Petäjä , T. , Aalto , P. P. , O’Dowd , C. D. and Kulmala , M. 2009 . Laboratory Verification of PH-CPC's Ability to Monitor Atmospheric Sub-3 nm Clusters . Aerosol Sci. Technol. , 43 : 126 – 135 .
- Sipilä , M. , Lehtipalo , K. , Kulmala , M. , Petäjä , T. , Junninen , H. , Aalto , P. P. , Manninen , H. E. , Kyrö , E.-M. , Asmi , E. , Riipinen , I. , Curtius , J. , Kürten , A. , Borrmann , S. and O’Dowd , C. D. 2008 . Applicability of Condensation Particle Counters to Measure Atmospheric Clusters . Atmos. Chem. Phys. , 8 : 4049 – 4060 .
- Stoltzenburg , M. R. and McMurry , P. H. 1991 . An Ultrafine Aerosol Condensation Nucleus Counter . Aerosol Sci. Technol. , 14 : 48 – 65 .
- Ude , S. and Fernández de la Mora , J. 2005 . Molecular Monodisperse Mobility and Mass Standards from Electrosprays of Tetra-alkyl Ammonium Halides . J. Aerosol Sci. , 36 : 1224 – 1237 .
- Wang , J. , McNeill , V. F. , Collins , D. R. and Flagan , R. C. 2002 . Fast Mixing Condensation Nucleus Counter: Application to Rapid Scanning Differential Mobility Analyzer Measurements . Aerosol Sci. Technol. , 36 : 678 – 689 .
- Weber , R. J. , Stolzenburg , M. R. , Pandis , S. N. and McMurry , P. H. 1998 . Inversion of Ultrafine Condensation Nucleus Counter Pulse Height Distributions to Obtain Nanoparticle (∼3–10 nm) Size Distributions . J. Aerosol Sci. , 29 : 601 – 615 .
- Winkler , P. M. , Steiner , G. , Vrtala , A. , Vehkamäki , H. , Noppel , M. , Lehtinen , K. E. J. , Reischl , G. P. , Wagner , P. E. and Kulmala , M. 2008a . Heterogeneous Nucleation Experiments Bridging Scale from Molecular Ion Clusters to Nanoparticles . Science. , 319 : 1374 – 1377 .
- Winkler , P. M. , Vrtala , A. and Wagner , P. E. 2008b . Condensation Particle Counting Below 2 nm Seed Particle Diameter and the Transition from Heterogeneous to Homogeneous Nucleation . Atmos. Res. , 90 : 125 – 131 .
- Winklmayr , W. , Reischl , G. , Lindner , A. and Berner , A. 1991 . A New Electromobility Spectrometer for the Measurement of Aerosol Size Distribution in the Size Range from 1 to 1000 nm . J. Aerosol Sci. , 22 : 289 – 296 .