Abstract
Analytical expressions are provided for charged and uncharged particles in the perfect-wetting limit of Fletcher's classical heterogeneous nucleation theory with finite activation energy G*. A dimensionless logarithm of the critical supersaturation can be represented as a single universal curve versus a dimensionless nucleus radius for neutral particles, while a uniparametric family of curves is required for charged particles. Comparison with experimental critical supersaturations for nanoparticles and molecular ions shows qualitative agreement, with substantial quantitative disagreement. At increasing sizes the predicted critical diameter augmented by a fixed length ∼1 nm approaches the Kelvin limit. Predicted activation probability curves versus size (supersaturation) are steep, whence stringent experimental definitions of the thermodynamic state and the seed particle are required for meaningful comparison with theory. Activation curves for mobility-selected WOx particles are broader than predicted, even with a well-defined supersaturation, while those of mobility-purified molecular ions agree qualitatively with theory, even in a turbulent mixing nucleation chamber. Recent work by CitationWinkler (2009) based on the Vienna expansion chamber and mobility-purified molecular ions has anticipated these conclusions. The theory is combined with similar considerations on the onset of homogeneous nucleation, to draw conclusions on the smallest nucleus size that may be activated by heterogeneous nucleation. Water is predicted to be able to detect uncharged nuclei with diameters as small as 0.6 nm. The associated possibility to develop detectors for relatively small single neutral molecules is enticing.
1. INTRODUCTION
Following the dramatic advances introduced by CitationStolzenburg and McMurry (1991) in their Ultrafine Condensation Particle Counter (commercialized as TSI's UCPC 3025), the best condensation nucleus counters (CNCs) became capable of detecting particles with minimal diameters dmin slightly smaller than 3 nm. A common reference used to estimate lower detectable particle diameter dmin has been the critical Kelvin diameter dKH (Equation (1)), the diameter at which the capillary model predicts that a neutral nucleus surrounded by a supersaturated vapor will require no activation energy to grow into a large drop.
One goal of the present study is to compare several existing heterogeneous nucleation measurements with Fletcher's predictions. This task is still pending except for the data of CitationWinkler et al. (2008a), whose computational approach could of course be used to analyze prior data. However, the general trends of the theory have not yet been represented in the form of simple material independent graphs able to provide ready insights on whether or not a concrete measurement conforms to theory. In particular, we do not know if the excellent agreement between theory and measurements reported by CitationWinkler et al. (2008a) is applicable to other data available, either below 3 nm or above. An issue of particular interest is how fast the Fletcher curves approach the Kelvin and Thomson asymptotes at increasing nucleus size. For instance, does the experimental observation of CitationWinkler et al. (2008a) of a substantial reduction below the Kelvin-Thomson critical supersaturation even above 20 nm fit within Fletcher's theory? In order to easily capture these general trends, and compare available data to theory with minimal algebraic complexity, we have derived close form analytical expressions for the various dimensionless parameters of the classical heterogeneous nucleation problem with perfect wetting. In the case of neutral particles a single universal curve describes the critical supersaturation as a function of nucleus size, with only one additional activation energy parameter affecting the form of the activation curves. An additional parameter is required for charged particles. These expressions show that Fletcher's critical size approaches the Kelvin-Thomson values at diameters considerably smaller than 20 nm. The results from these simple formulae are also compared with existing data on the critical sizes and the activation probability for particles a few nm in diameter. We finally combine capillary theory results for heterogeneous and homogeneous nucleation to provide practical criteria to select optimal solvents to activate the smallest possible nuclei. A partial account of this study has previously been given by CitationFernandez de la Mora (2011).
Before proceeding, some remarks on the merits of pure analysis versus the numerical approach of Winkler et al. are appropriate. The first thing to note is that Fletcher's expressions are singular in the limit of zero wetting angle. This singularity can be avoided by assigning a small wetting angle, but the solution is then refractory to analytical inversion. For calculations involving zero wetting angle it is clearly preferable to write this limit directly, to obtain regular algebraic expressions. Their multiple roots can then be systematically analyzed, providing unambiguous choices for the correct ones. The process may appear as more complex than obtaining a numerical root; but the numerical method still has to decide which is the proper root. It is far simpler and safer to do this once by analysis than to leave it to a program whose choice of roots will tend to change from one machine to the other. Analysis has the additional advantage of minimizing the number of variables involved, and of providing universal (corresponding-state-like) representations valid for any material, where critical data for different substances can be compared in a single figure. The algebraic equations provided here are far more readily programmed than is the inversion of Fletcher's original equations.
2. THEORETICAL CONSIDERATIONS
We shall for simplicity consider first the case of neutral particles, to then analyze the algebraically more complex case of charged particles. The analysis will be restricted to classical nucleation theory (CNT) of insoluble nuclei with perfect wetting, though the method used is suited to incorporate also Fletcher's theory for imperfect wetting (numerically). The discussion includes precisions on the range of validity of the expressions for the activation energy, missing in CitationFernandez de la Mora (2011).
2.1. Neutral Particles
The excess free energy G(R,Ro,S) needed to form a perfectly wetting drop of radius R over a pre-existing spherical and neutral nucleus of radius Ro (<R) surrounded by its vapor is given by classical capillary theory as
TABLE 1 Parameters for conversion of the experimental data into dimensionless variables
The criterion for criticality can now be established by stating that half of the nuclei initially present be activated over the residence time tres in the nucleation region. In other words, we substitute N/N o = 1/2 and t = tres in (7) to find:
In order to cast the predictions of the theory in its simplest and most general (material property independent) form, we introduce a length Lo to define the dimensionless radii of Equation (10), and the supersaturation parameter β defined in Equation (11). For simplicity we have chosen Lo in Equation (12) such that the critical relation between S and Ro is universal for all materials (see below):
FIG. 1 Predicted activation characteristics of neutral particles at 9 different super-saturations (β = 0.44213; 0.40466; 0.37160; 0.34271; 0.31750; 0.295435; 0.27604; 0.25890; 0.24367, corresponding to xo* varying from 1 to 3 in 0.25 intervals), each at 4 values of C (10, 16, 40, 100, with slopes increasing with C). Reproduced from CitationFernandez de la Mora (2011).
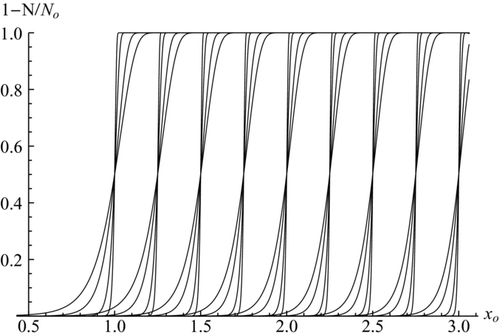
FIG. 2 Dimensionless critical supersaturation versus dimensionless seed nucleus diameter for neutral particles, showing the exact prediction Equation (15) (thick line peaking at xo = 0), and three approximations xo −1, (xo +1)−1 and Eq. (21) (lowest line going through a maximum). Reproduced from Figure 32.7a of CitationFernandez de la Mora (2011).
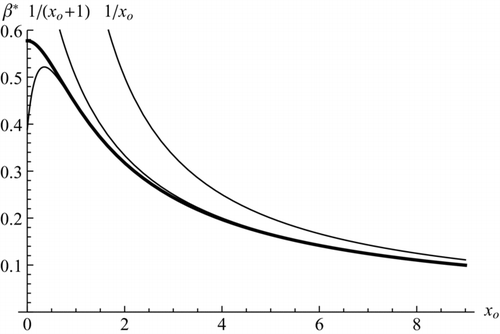
The critical relation is a cubic equation for both β* and xo , whose complicated relevant root may be simplified into:
2.2. Charged particles
The generalization of Equation (2) including a Coulombic term is
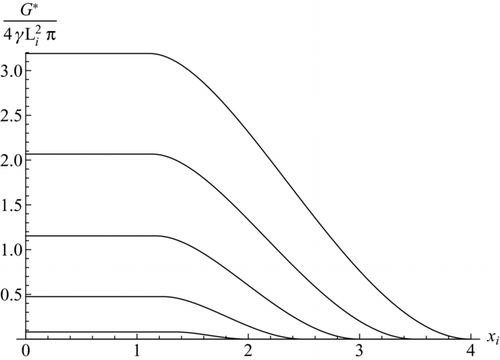
FIG. 3 Dimensionless activation energy versus ion size for α = 0.2461, 0.2790, 0.32099, 0.3744, 0.4375 (top to bottom), showing a constant value below xi = y+ (α).
Equation (30) leads to the following expression for the activation curve (13a)
The two constants C and c enter now explicitly in the expression (32) for the activation curves. The ratio C/c can be seen in Equation (33) to be substance-dependent, but independent of the somewhat ambiguous parameter Ktres . shows the predicted activation curves for the special case C = 15, c = 0.45, while fixing the supersaturation parameter α and varying xi (left), or vice-versa (right). The curves can be plotted directly as a function of xi , and parametrically as a function of α = 1/y–1/y 4 (taking the precaution of selecting the parameter y > 41/3). At high supersaturations one can see on the left figure that even the smallest ions have a substantial probability of activation (non-zero horizontal asymptote at small xi ). This size-independent behavior of the smallest ions is repeated on the right curve, where ions smaller than the ones corresponding to the curve farthest to the right fall on top of the same curve.
The critical supersaturation associated to the condition N/No = 1/2 is again obtained by cancelling the exponent in Equation (32):
FIG. 4 Activation curves for ions, with C = 15 and c = 0.45. Top: varying ion diameter at fixed supersaturation: α = 0.3804, 0.3744, 0.3627, 0.3409, 0.3210, 0.3030, 0.2866, 0.2718, 0.2584, 0.2461 (from left to right). The two leftmost curves continue horizontally at decreasing x o. Bottom: varying α and fixed ion diameter: xi = 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25, 3.5, 3.75, 4, 4.25, 4.5, 4.75, 5 (from right to left).
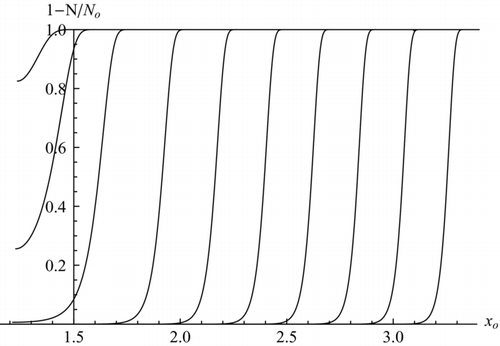
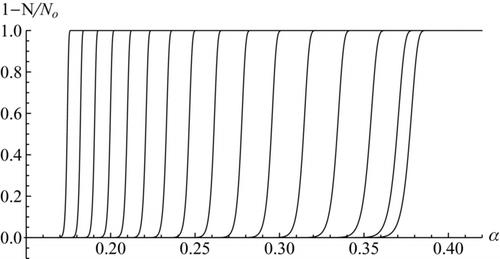
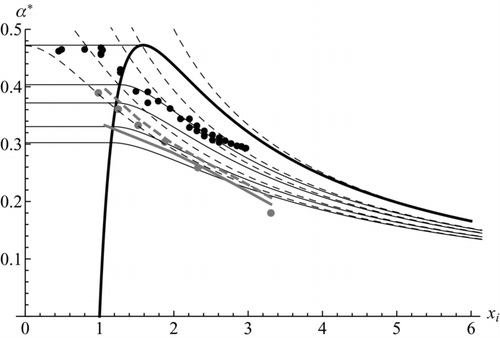
FIG. 5 Critical supersaturation of charged and neutral particles. Black continuous lines are for charged particles, showing predictions from Equation (35) at several values of the activation energy parameter c (1.5; 1; 0.5; 0.25; 0, from bottom to top). Dashed theoretical lines are for neutral particles. Thomson's curve, α* = 1/xi −1/xi 4 is shown in thicker trace. Its rising piece marks the boundary between dry (right) and solvated (left) ions; its decaying piece coincides with the curve for c = 0. Black data points (Gamero et al., 2002) are for cluster ions in DBP. The grey data points and the two thick grey segments near them are the data and theoretical calculations from CitationWinkler et al. (2008a). After Figure 32.8a of CitationFernandez de la Mora (2011).
3. COMPARISON BETWEEN THEORY AND EXPERIMENTS
Prior to the work of CitationWinkler et al. (2008a), the Thomson curve had been used as theoretical reference for comparison with the few size-resolved data available in this size range, all corresponding to charged particles (Gamero et al. 2000, 2002). For unexplained reasons their data for xi >1 fell substantially below the Thomson curve. The same phenomenon was reported by CitationWinkler (2004) for considerably larger singly charged particles with diameters going up to 24 nm. Winkler et al. (2008a,b) have provided new data for small charged and uncharged tungsten oxide (WOx) particles in n-propanol vapor, also showing activation well below the Thomson and the Kelvin curves. They provide the first explanation for this peculiarity in terms of Fletcher's theory for heterogeneous nucleation. Fletcher includes two effects: one associated to the finite value of G*, the other due to imperfect wetting of the nucleus by the vapor. CitationWinkler et al. (2008a) consider both, but in the end assume almost perfect wetting, so their calculations probably account simply for the finite activation energy. of CitationWinkler et al. (2008a) shows excellent agreement between theory and experiments for all the size ranges studied, and for charged and neutral particles. The calculation is strictly numerical, without details on the selection of branches. It also includes all the algebraic complexity of the imperfect wetting theory, which is singular near the limit of perfect wetting. collects both the earlier charged cluster data (black) and the new neutral WOx data (grey). The charged WOx data are not shown, as those for positive polarity fall on top of the neutral particle data, and those for negative charges are only slightly below. In order to permit comparison between charged and uncharged particles, the theory and the data for uncharged particles are made dimensionless with the same characteristic length Li used for ions. This turns Equation (14) into
In conclusion, the main discrepancy is not between the two theoretical curves, but between the theory and both sets of data. Particularly noteworthy is the lack of convergence of the data of Winkler et al. (2008a–c) at large particle sizes on the Fletcher or Kelvin curves (which in this region are simply slightly horizontally displaced from each other). Plotting these data as 1/α* versus xi yields a straight line for the larger particles, as expected. But its slope is 1/0.63, as if the group 2γvo /(kT) was a factor 0.63 below what is expected for n-propanol. A similar linear relation between lnS* and 1/dp with a non-Kelvin slope can be seen in of CitationWinkler et al. (2008c) for nonane vapors. Chen and Cheng (2007) have previously observed an analogous need to decrease the surface tension to fit their own data to the Kelvin curve, also at relatively large diameters (10–15 nm). Several prior studies with large particles have compared critical diameters for heterogeneous nucleation in water vapor with the corresponding Kelvin diameter, and found either agreement (i.e., CitationLiu et al. 1984 for dioctyl phthalate drops 18 and 56 nm in diameter in water vapor), or diameters larger than Kelvin's (i.e., CitationPorstendorfer et al. 1985 for Ag particles 6–19 nm in diameter, and CitationAlofs et al. 1995 for silver particles 16, 19, and 30 nm in diameter).
Another useful test of the capillary theory can be made by comparing it with measurements for the full activation curve rather than just the point at N/No = 1/2. Such comparisons often show considerably slower rises of the curve for the experiments than for the theory. This is not surprising because the predicted activation curves are quite sharp both upon varying the supersaturation (α) or the particle diameter (xi ). Therefore, no theoretical information can be drawn from the width of the experimental curves unless the experiment is carried with a sharply defined supersaturation, and with a highly monodisperse aerosol that must in addition be of uniform composition and shape. As a reference, taking c = 0.5, C = 15, near the condition when the ion becomes dry, (xi ∼ 1.22), α varies only by 1.9% as the activation probability rises from 20% to 80%. It is therefore apparent that ln(S) must be fixed experimentally within better than 2% for the experimental width of the activation curves to be theoretically relevant. It is presently unclear whether this sharp definition is possible with existing instrumentation. Probably the best devices from the point of view of fixing precisely the supersaturation in space and time are isentropic expansion CNCs. Although there are hot boundary layers near the walls, these can be avoided by restricting the optical detection to particles near the center of the nucleation region. Even so, most of the data obtained by even the most refined among such instruments have exhibited considerably wider activation curves than predicted here. This may be seen in the small gray data points shown in for negatively charged tungsten oxide nanoparticles in n-propanol (taken from of CitationWinkler et al. 2008a). Besides dispersion in the experimental S, other possible causes for this mismatch are lack of uniformity in ion mobility (associated to the finite resolving power of the DMA used for size selection), chemical composition (oxidation of tungsten even under the cleanest conditions produces a complex mixture of different oxides), charging ion (many different ions are produced in radioactive chargers, particularly those not using ultraclean standards), and particle shape.
FIG. 6 Comparison of predicted and measured activation curves for charged particles: small gray (cluster ions from CitationWinkler et al. 2011) and large gray data (negative WOx ions from CitationWinkler et al. 2008a) are in n-propanol. Black data (THAn+1Brn + with n = 2, 5, 8, 10, 12, right to left) are in DBP. Continuous lines are from Equation (32), with xi selected to match the data at N/No = 1/2, (c, C/c) taken to be (0.25, 25.9) for 2-propanol and (0.2, 25.4) for DBP.
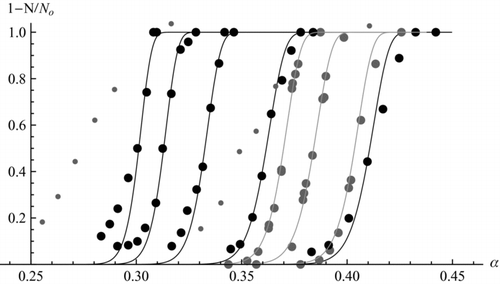
These inhomogeneities are difficult to avoid by conventional aerosol techniques, but they may be overcome by using DMA-purified molecular ions from electrosprayed solutions (CitationFenn et al. 1989; CitationRosell et al. 1996; CitationSeto et al. 1997). shows activation data in black for THAn+1Brn + clusters (n = 2, 5, 8, 10, 12) in DBP vapor, inferred from of Gamero et al. 2000. The calculations are for c = 0.2 (a factor over two below the estimated experimental value, as a larger c would preclude the observed dependence of critical supersaturation on ion size) and for C/c = 25.4 (appropriate for DBP), with xi chosen, irrespective of its experimental value, to match the experimental α 1/2. Interestingly, the observed activation curves are almost as sharp as those predicted. This is unexpected for a turbulent mixing CNC, where unit supersaturation at the walls coexists with S ∼ 1000 at the core. Some nonidealities are observed in the form of tails at the low α end of the distributions. This mismatch is almost surely due to the selection by the DMA of a small fraction of multiply charged ions with the same mobility as the main singly charged cluster. This problem is to be expected from the shoulders seen in the mobility distribution of the partially discharged aerosol from which the test ions were selected (see of Gamero et al. 2000). Because multiply charged ions are activated at reduced α, the sharp activation curves seen on the right side of the black curves confirms the fact that the supersaturation is indeed very well defined in this mixing instrument. This circumstance provides a first indication that the broad activation curves for WOx just discussed are due to lack of homogeneity in the nuclei rather than imperfect definition of S. This point is confirmed more directly by recent very narrow activation curves for ion clusters in n-propanol (black data points in ), taken in the same Vienna expansion CNC used for the broad WOx measurements. I am much indebted to Prof. Paul Wagner and his colleagues (CitationWinkler et al. 2011) for kindly letting me use these still unpublished data. CitationWinkler (2009) has previously noted the sharp rise of these data, and their eminent suitability to provide unique theoretical information. This singular finding provided our motivation to compare Gamero's data to theory.
We conclude that, although the experimental relation α 1/2(xi ) does not match the capillary theory in either DBP or n-propanol, the narrow widths and the general shape of the theoretical activation curves do generally match the theory. This trend provides qualitative confirmation of the fact that the supersaturation in the Vienna expansion CNC and in the turbulent mixing CNC of Gamero et al. is defined sharply enough to enable theoretical inferences based on the shape of the activation curve. Note however that the steepness of the curves depends on the choice of c, and doubling c leads in both data sets to a clearly sharper theoretical versus experimental curves. Recent work by CitationVanhanen et al. (2011) on a turbulent mixing CNC (the first commercial CNC sensitive to charged particles down to 1 nm) also reports relatively sharp activation curves for molecular ions.
TABLE 2 Characteristics of various working fluids relating to the smallest detectable size
Laminar diffusion CNCs normally have spatially dependent supersaturations, and would tend to generate a thermodynamic state less homogeneous than a turbulent mixing CNC. Nonetheless, one cannot preclude the possibility that, once tested with molecular ions, some laminar diffusion CNCs might also yield sharp activation curves. Hopefully, use of clean chargers and improved aerosol generation techniques will also provide sufficiently sharp activation curves for a wider class of aerosols than the molecular ions and ion clusters discussed here.
4. FLUID SELECTION CRITERIA BASED ON CAPILLARY MODELS
4.1. Homogeneous Nucleation
Homogeneous nucleation eventually takes place at a critical supersaturation SH at which drops of vapor form without the need for seed particles. The smallest nucleus that can be detected by vapor condensation must therefore be activated at a supersaturation S* < SH . Since both S* and SH depend on fluid properties, selection of an ideal fluid for small particle detection requires an ability to predict SH . The critical activation energy GH* for homogeneous nucleation is fixed similarly as before, now by the condition that fewer nuclei form homogeneously than heterogeneously, such as not to obstruct the observation of heterogeneously formed drops. The homogeneous nucleation rate nvKH exp[−GH*/kT] must then be smaller than, say, N/tres , where nv is the number concentration of vapor. We shall for simplicity ignore the differences between K and KH , since the effective collision diameter for a vapor molecules with a typical homogeneously nucleating cluster is quite comparable to the 1 nm assumed for the calculation of K. Associated errors are comparable to those introduced in the calculation of K, and affect the results only through small logarithmic terms (see Volmer 1979 for more refined expressions). Hence, ignoring the reduction of nv during nucleation, all the reasoning followed before for heterogeneous nucleation on neutral particles remains valid here, with the simple substitution of CH instead of C, and the selection of xo = 0 turning (14) into (42):
4.2. Heterogeneous Nucleation
We shall consider here only the more difficult case of neutral particles, previously discussed in less accessible form by CitationFernandez de la Mora (2011). This earlier study included also the simpler case of charged particles, which are much easier to activate (even at subnanometer sizes), apparently without the need for exceptional working fluids. The criterion (42) can be expressed as
In conclusion, the smallest neutral particle diameter detectable depends principally on (kT/γ)1/2 , favoring low T and high γ. There are other smaller but useful effects of other parameters through the quantities C and CH which may provide advantages for some fluids and need to be considered in each specific situation. CitationIida et al. (2009) have suggested that, besides γ, another key parameter favoring low Rmin is a low vapor pressure, which led them to study oleic acid and dioctyl sebacate (DOS) (dKH ∼ 1.87 nm for both). The role of vapor pressure is clear in C = ln(tresK/ln2), since K ∼ nv . It is even more important in CH = ln(Ktresnv /N) ∼ ln(tresnv 2/N). Since one seeks to reduce CH , it is plain that besides the benefits already described of increasing N, it is similarly useful to decrease t res, and twice as advantageous to reduce nv . These considerations confirm the views of CitationIida et al. (2009) about the interest of low volatility fluids.
5. CONCLUSIONS
Based on capillary theory for heterogeneous nucleation, closed form solutions have been obtained for the activation probability and the critical size of spherical perfectly wetting seed particles, charged and uncharged. As previously observed, the predicted critical nucleus is below the Kelvin-Thomson limit for very small nuclei. It approaches the Kelvin diameter at nucleus sizes large compared to a characteristic length Lo = (CkT/4πγ)1/2, where C ∼ 10. The activation curves are very steep, both upon varying the nucleus size and the supersaturation. Highly homogeneous nuclei and supersaturations are therefore required in meaningful measurements of the structure of activation probability curves. As expected, isentropic expansion devices are capable of achieving this sharp supersaturation requirement. Surprisingly, so is the turbulent mixing instrument of Gamero et al. (2000). In contrast, no seed particle produced by conventional aerosol generators has yet achieved the necessary uniformity in the seed nucleus, with the exception of mobility classified molecular ions produced by electrospray ionization of controlled solution ions. Theoretical considerations on the critical supersaturation for homogeneous nucleation are made, similarly to those previously made on heterogeneous nucleation. From these we draw criteria on the smallest neutral cluster that can be activated with the most favorable working fluid. This turns out to be water (or a high surface tension liquid) at low temperature, for which a smallest detectable diameter of 0.6 nm is expected. This implies the real possibility to detect single vapor molecules by condensation.
Acknowledgments
I thank M. Attoui, P.H. McMurry, S.V. Hering, M. Kulmala, and P. Winkler for a number of very useful discussions, and P. Wagner for many insights and for his critical reading of the manuscript. I am grateful to my SEADM colleagues for their support of this work, and thank particularly Guillermo Vidal and Roberto Alonso for sharing their ideas on the development of particle detectors. This article draws material from Fernandez de la Mora (copyright John Wiley & Sons, 2011), parts of which are reprinted with permission of John Wiley & Sons, Inc.
REFERENCES
- Alofs , L. D. , Lutrus , C. K. , Hagen , D. E. , Sem , G. J. and Blesener , J. L. 1995 . Intercomparison between Commercial CNCs and an Alternating Temperature Gradient Cloud Chamber . Aerosol Sci. Technol. , 23 : 239 – 249 .
- Fenn , J. B. , Mann , M. , Meng , C. K. , Wong , S. K. and Whitehouse , C. 1989 . Electrospray Ionization for Mass-Spectrometry of Large Biomolecules . Science , 246 : 64 – 71 .
- Fernandez de la Mora , J . 2011 . “ Electrical Classification and Condensation Detection of sub-3 nm Aerosols ” . In Aerosol Measurements , Edited by: Baron , P. , Kulkarni , P. and Willeke , K. New York : John Wiley & Sons .
- Fletcher , N. H. 1958 . Size Effect in Heterogeneous Nucleation . J. Chem. Phys. , 29 ( 3 ) : 572 – 576 .
- Fletcher , N. 1962 . The Physics of Rainclouds. , Cambridge University Press . chapter 3
- Gamero-Castaño , M. 1999 . Ph.D. Thesis , Yale University, Mechanical Engineering Dept .
- Gamero-Castaño , M. and Fernandez de la Mora , J. 2000 . A Condensation Nucleus Counter (CNC) Sensitive to Singly Charged Subnanometer Particles . J. Aerosol Sci. , 31 : 757 – 772 .
- Gamero-Castaño , M. and Fernandez de la Mora , J. 2002 . Ion-Induced Nucleation: Measurement of the Effect of Embryo's Size and Charge State on the Critical Supersaturation . J. Chem. Phys. , 117 : 3345 – 3353 .
- Henson , B. F. 2007 . An Adsorption Model of Insoluble Particle Activation: Application to Black Carbon . J. Geophys. Res. , 112 : D24S16 doi: 10.1029/2007JD008549
- Hering , S. V. , Stolzenburg , M. R. , Quant , F. R. , Oberreit , D. R. and Keady , P. B. 2005 . A Laminar-Flow, Water-Based Condensation Particle Counter (WCPC) . Aerosol Sci. Technol. , 39 : 659 – 672 .
- Iida , K. , Stolzenburg , M. R. and McMurry , P. H. 2007 . Edited by: O’Dowd , Colin D. and Wagner , Paul E. Netherlands : Springer . Detecting Below 3 nm Particles Using Ethylene Glycol-based Ultrafine Condensation Particle Counter, in Nucleation and Atmospheric Aerosols, 17th International Conference, Galway, Ireland, pp. 649–653.
- Iida , K. , Stolzenburg , M. R. , McMurry , P. H. , Smith , J. N. , Quant , F. R. , Oberreit , D. R. , Keady , P. B. , Eiguren-Fernandez , A. , Lewis , G. S. , Kreisberg , N. M. and Hering , S. V. 2008 . An Ultrafine, Water-Based Condensation Particle Counter and its Evaluation under Field Conditions . Aerosol Sci. Technol. , 42 : 862 – 871 .
- Iida , K. , Stolzenburg , M. R. and McMurry , P. H. 2009 . Effect of Working Fluid on Sub-2 nm Particle Detection with a Laminar Flow Ultrafine Condensation Particle Counter . Aerosol Sci. Technol , 43 : 81 – 96 .
- Kim , C. S. , Okuyama , K. and Fernández de la Mora , J. 2004 . Performance Evaluation of Improved Particle Size Magnifier (PSM) for Single Nanoparticle Detection . Aerosol Sci. Technol. , 37 : 791 – 803 . See correction on 38:409
- Kogan , Y. and Burnashova , Z. 1960 . Size Magnification and Measurement of Condensation Nuclei in the Continuous Flow . J. Phys. Chem. , 34 : 2630 – 2639 . (in Russian)
- Kogan , Ya. L . 1988 . Molecular Nuclei of Condensation, Conditions for Observance and Physicochemical Features . Russ. Chem. Bull. , 47 ( 2 ) : 203 – 208 .
- Köhler , H. 1936 . The Nucleus in and the Growth of Hygroscopic Droplets . Trans. Faraday Soc. , 32 ( 2 ) : 1152 – 1161 .
- Liu , B. Y. H. , Pui , D. Y. H. , McKenzie , R. L. , Agarwal , J. K. , Pohl , F. G. , Preining , O. , Reischl , G. , Zsymansky , W. and Wagner , P. E. 1984 . Measurements of the Kelvin-Equivalent Size Distribution of Well Defined Aerosols with Particle Diameters > 13 nm . Aerosol Sci. Technol. , 3 : 107 – 115 .
- Magnusson , L. E. , Koropchak , J. A. , Anisimov , M. P. , Poznjakovskiy , V. M. and Fernandez de la Mora , J. 2003 . Correlations for Vapor Nucleating Critical Embryo Parameters . J. Phys. Chem. Ref. Data. , 32 : 1387 – 1409 .
- Okuyama , K. , Kousaka , Y. and Motouchi , T. 1984 . Condensational Growth of Ultrafine Aerosol-Particles in a New Particle-Size Magnifier . Aerosol Sci. Technol. , 3 : 353
- Pichelstorfer , L. , Vrtala , A. , Winkler , P. M. and Wagner , P. E. 2010 . Experiments on Heterogeneous Nucleation for Polar and Nonpolar Compounds, Poster Presented at the International Aerosol Conference, Helsinky, Finland, August 30–Sept. 3, 2010 .
- Porstendorfer , J. , Scheibel , H. G. , Pohl , F. G. , Preining , O. , Reischl , G. and Wagner , P. E. 1985 . Heterogeneous Nucleation of Water Vapor on Monodispersed Ag and NaCl Particles with Diameters between 6 and 18 nm . Aerosol. Sci. Technnol. , 4 : 65 – 79 .
- Rosell , J. , Loscertales , I. G. , Bingham , D. and Fernández de la Mora , J. 1996 . Sizing Nanoparticles and Ions with a Short DMA . J. Aerosol Sci. , 27 : 695 – 719 .
- Seto , T. , Okuyama , K. and Fernández de la Mora , J. 1997 . Condensation of Supersaturated Vapors on Monovalent and Divalent Ions of Varying Size . J. Chem. Phys , 107 : 1576 – 1585 .
- Sgro , L. A. and Fernandez de la Mora , J. 2004 . A Simple Turbulent Mixing CNC for Charged Particle Detection Down to 1.2 nm . Aerosol. Sci. Technnol , 38 : 1 – 11 .
- Sipilä , M. , Lehtipalo , K. , Attoui , M. , Neitola , K. , Petäjä , T. , Aalto , P. P. , O’Dowd , C. D. and Kulmala , M. 2009 . Laboratory Verification of PH–CPC's Ability to Monitor Atmospheric Sub-3 nm Clusters . Aerosol Sci. Technol. , 43 : 126 – 135 .
- Stolzenburg , M. R. and McMurry , P. H. 1991 . An Ultrafine Aerosol CNC . Aerosol Sci. Technol. , 14 : 48 – 65 .
- Thomson , J. J. 1969 . Conduction of Electricity through Gases , New York : Dover . section 92
- Vanhanen , J. , Mikkila , J. , Sipila , M. , Manninen , H. E. , Lehtipalo , K. , Siivola , E. , Petaja , T. and Kulmala , M. 2011 . Particle Size Magnifier for Nano–CN Detection . Submitted to Aerosol Sci. Technol. , 45 : 533 – 542 .
- Van Luik , F. W. Jr. and Rippere , R. E. 1962 . Condensation Nuclei, a New Technique For Gas Analysis . Anal. Chem. , 34 ( 12 ) : 1617 – 1620 .
- Volmer , M. 1939 . Kinetik Der Phasenbildung , Dresden : Theodor Steinkopf .
- Wilson , C. T. R. 1897 . Condensation of Water Vapour in the Presence of Dust-Free Air and Other Gases, Philosophical Transactions of the Royal Society of London. Series A . 189 : 265 – 307 .
- Wilson , C. T. R. 1927 . “ On the Cloud Method of Making Visible Ions and the Tracks of Ionizing Particles ” . In Nobel Lectures, Physics , Amsterdam : Elsevier . (1965), pp. 194–214.
- Winkler , P. M. 2004 . University of Vienna . Experimental Study of Condensation Processes in Systems of Water and Organic Vapors Employing an Expansion Chamber, Ph.D. Thesis
- Winkler , P. M. , Steiner , G. , Vrtala , A. , H. , Vehkamäki , Noppel , M. , K. E. J. , Lehtinen , G. P. , Reischl , P. E. , Wagner and M , Kulmala . 2008a . Heterogeneous Nucleation Experiments Bridging the Scale from Molecular Ion Clusters to Nanoparticles . Science , 319 : 1374 – 1377 .
- Winkler , P. M. , Vrtala , A. and Wagner , P. E. 2008b . Condensation Particle Counting below 2 nm Seed Particle Diameter and the Transition from Heterogeneous to Homogeneous Nucleation . Atmos. Res. , 90 : 125 – 131 .
- Winkler , P. M. , Hienola , A. , Steiner , G. , Hill , G. , Vrtala , A. , Reischl , G. P. , Kulmala , M. and Wagner , P. E. 2008c . Effects of Seed Particle Size and Composition on Heterogeneous Nucleation of N-Nonane . Atmos. Res. , 90 : 187 – 194 .
- Winkler , P. M. 2009 . “ Heterogeneous Nucleation by Nanoparticles: Recent Experiments and some Applications ” . In Nucleation and Atmospheric Aerosols , Edited by: Smolik , J. and O’Dowd , C. 23 New York : Springer .
- Winkler , P. M. , Vrtala , A. , Steiner , G. , Wimmer , D. , Vehkamaeki , H. , Lehtinen , K. E. J. , Reischl , G. P. , Kulmala , M. and Wagner , P. E. 2011 . Manuscript in Preparation .