Abstract
In diffusion charging theory, it is assumed that each ion–particle collision leads to the transfer of charge from ion to particle, and that charge transfer will not occur upon collision between a vapor molecule and a charged particle. However, in chemical ionization, charge transfer can occur in two directions—from charge-donating ion to vapor molecule and back from charged vapor molecule to the original charge-donating species. Both aerosol diffusion charging and chemical ionization are collision-based charge transfer processes, and for particles only slightly larger than vapor molecules (aerosol clusters), the line between diffusion charging and chemical ionization becomes blurred. We examined the charge transfer from aerosol clusters (positively charged amino acid clusters) in the ∼1.0 nm size range to neutral vapor molecules (trimethylamine) at atmospheric pressure by using a combined experimental and theoretical approach. It was found that for singly charged amino acid cluster ions composed of 1, 2, and 3 amino acid molecules, the rate of charge transfer to trimethylamine vapor molecules was clearly observable, particularly for clusters composed of 1 and 2 molecules. The charge transfer rate for singly charged clusters with 4 or more amino acid molecules was consistently close to 0, indicating that the rate of charge transfer from clusters to vapor molecules is size dependent. The charge transfer rates also varied with cluster's chemical composition. Overall, this study demonstrates that small aerosol clusters (∼0.5 nm) can lose charge through collisions with vapor molecules, which is typically not considered in diffusion charging theories.
INTRODUCTION
A central paradigm in aerosol measurement is the determination of aerosol particle size distributions through the measurement and inversion of their mobility distributions (CitationHoppel 1978; CitationAlofs and Balakumar 1982; CitationAdachi et al. 1990). Proper inversion requires that particles reach a known charge distribution, which is often a steady-state charge distribution obtained via diffusion charging (particle collisions with ions in which charge is transferred from ion to particle) in a bipolar environment (CitationLiu and Pui 1974). For nanoparticles and submicrometer particles, particle charge distributions resulting from diffusion charging processes are typically well predicted by Fuchs’ limiting sphere theory (CitationFuchs 1963; CitationAdachi et al. 1985; CitationHoppel and Frick 1986). However, in Fuchs’ theory, the charging rate is not calculated explicitly; only the collision rates between aerosol particles and ions are determined as functions of particle size (in most case diameter), background gas properties (temperature and pressure), net particle charge, ion electrical mobility, and ion mass. It is thus assumed that upon collision, an ion will transfer charge to a particle, regardless of the collision frame of reference energy or the orientation of the ion and particle during collision.
Conversely, in traditional chemical ionization (CitationBowers et al. 1973; CitationSu and Bowers 1973), ions collide with vapor molecules, and the transfer of charge from ion to vapor molecule is highly dependent on the collision energy relative to the activation energy for charge transfer, as well as the orientation of ion and vapor molecule (steric effects) during collision. True equilibrium (as opposed to steady state) is achievable on experimentally relevant time scales in chemical ionization, as the transfer of charge can also occur from the ionized vapor molecule (charge acceptor) back to the original charge donor. Nonetheless, chemical ionization and diffusion charging are both collision-based ionization processes. In principle, they are both describable by unified charging theory, i.e., a single equation or set of equations. Despite the fact that these two charge exchange processes have been hitherto described by differing approaches (CitationFuchs 1963; CitationBowers et al. 1973), the only major difference between aerosol diffusion charging and chemical ionization lies in the size of the charge acceptor (particle or vapor molecule) relative to the donor ion. A governing theory for all collision-based ionization processes must (1) capture all the features of chemical ionization when the charge donor (ion) and charge acceptor (vapor molecule) are of similar size and (2) accurately describe aerosol particle diffusion charging when there is a considerable size difference between charge acceptor and charge donor. This requirement further suggests that for particles only slightly larger than ions (sub-2-nm diameter particles), the dynamics of ion–particle collisions will have features akin to chemical ionization, i.e., ions may not transfer charge to small particles during all ion–particle collisions, and small charged particles may transfer their charge to colliding vapor molecules when the latter are highly basic (for positive charges) or highly acidic (for negative charges).
As evidenced by a recent special issue of this journal, there is currently considerable interest in the measurement of sub-2-nm atmospheric aerosol clusters—particles composed of a limited number of molecules (CitationEisele et al. 2006; CitationKulmala et al. 2007; CitationWinkler et al. 2008; CitationIida et al. 2009; CitationJunninen et al. 2010; CitationZhao et al. 2010). Unfortunately, the potential for both collisions between ions and sub-2-nm aerosol clusters without charge transfer and collisions between charged clusters and neutral vapor molecules that lead to charge transfer is hitherto unexplored. The purpose of this study is to examine the latter of these two possibilities by observing the transfer of charge from sub-2-nm positively charged amino acid clusters to highly basic trimethylamine vapor molecules in the gas phase. We will refer to this charge transfer reaction as the back reaction, as more often an ionized vapor molecule transfers charge to a cluster/particle during diffusion charging. High back reaction rates for charged aerosol clusters and vapor molecules would have far-reaching effects on cluster diffusion charging kinetics. This subsequently would influence inversion routines to determine the size distributions of clusters from electrical mobility measurements, as well as the predicted rates of ion-induced nucleation in the atmosphere (CitationYu et al. 1998, Citation2008). While analyzing back reactions, we develop a kinetic relation that not only applies to the reactions examined here, but can better describe both chemical ionization as well as aerosol particle diffusion charging in the purely free molecular limit (both mass and momentum Knudsen numbers are infinitely large; CitationLushnikov and Kulmala 2004, Citation2005). Amino acids were chosen as the test clusters due to the ease of generating protonated amino acid cluster ions via electrospray ionization (ESI; CitationJulian et al. 2002; CitationMyung et al. 2006). Trimethylamine was chosen as the test vapor because it has an unusually large gas-phase basicity (GB = 908 kJ/mol; CitationWu and Fenselau 1993), thus investigation of back charging reactions with trimethylamine allows us to place an approximate upper limit on the size at which back charging is significant for positive ions in air. Furthermore, at present, amine vapors are suspected of playing a key role in new particle formation in the atmosphere, and therefore, during the bipolar charging of freshly nucleated atmospheric aerosol clusters (CitationBarsanti et al. 2009), amine vapors are possibly present in appreciable concentrations.
While the back reactions for charged aerosol clusters and neutral vapor molecules have not been well characterized, charge transfer reactions in the gas phase have been an active area of research over several decades. We make note of such studies, as they provide precedent for the experiments we performed in this study. Many of these studies focus on evaluating gas-phase basicities and proton affinities of molecules, as these parameters provide important information on the structure and stability of gas-phase ions. Two methods have been widely used to determine relative gas-phase basicities: (1) the measurement of the equilibrium constant for a reversible proton transfer reaction and (2) the kinetic method, which relies on the rates of competitive dissociations of mass-selected cluster ions (CitationCooks et al. 1994; CitationPapayannopoulos 1995; CitationCooks and Wong 1998). Both of these methods involve the production of protonated or deprotonated species by techniques such as ESI (CitationWhitehouse et al. 1985), matrix-assisted laser desorption ionization (CitationKaras and Hillenkamp 1988), or traditional chemical ionization (CitationTsang and Harrison 1976). Studies for these methods have been carried out extensively using systems such as ion cyclotron resonance (ICR; CitationAue et al. 1976), Fourier-transform (FT) ICR (CitationMarshall and Grosshans 1991; CitationDecouzon et al. 1996), and quadrupole ion trap mass spectrometers (MSs; CitationLouris et al. 1987; CitationBrodbelt-Lustig and Cooks 1989). Our study also involves the measurement of the rate of proton transfer from an electrospray-generated ion to a neutral vapor molecule with the charge transfer reaction monitored by an MS. However, unlike prior studies, the two colliding species are not confined together long enough in a reaction chamber for equilibrium to be attained. We also examine cluster–vapor interactions at atmospheric pressure and temperature, as opposed to a low-pressure environment where, in the presence of electric fields (e.g., inside a quadrupole), collisions between entities are often of significantly higher energy. Furthermore, we examined charge transfer reactions for different sized clusters in parallel, as ESI of amino acids gives rise to an array of cluster ions of varying size and charge.
EXPERIMENTAL METHODS
For experiments, trimethylamine (TMA, C3H9N), ammonium acetate (C2H3O2NH4), water (HPLC grade), ethanol (HPLC grade, C2H6O), and 5 different amino acids: l-leucine (C6H13NO2), l-serine (C3H7NO3), l-asparagine (C4H8N2O3), l-tyrosine (C9H11NO3), and l-threonine (C4H9NO3, all of reagent grade, ≥98%) were purchased from Sigma Aldrich (St. Louis, MO, USA) and used without any further purification. A schematic of the system in which these chemicals were used is shown in . The setup includes a neutral vapor molecule source composed of two nebulizers, an ESI source to produce cluster ions and a commercial quadrupole—time-of-flight MS (API QSTAR Pulsar, Sciex, Toronto, CA, USA). For neutral vapor (TMA vapor) production, a control solution consisting of a 50:50 (by volume) ethanol–water mixture and a TMA solution (42 mM TMA dissolved in a mixture of 31%–35% in ethanol and 65%–69% water by volume) were each nebulized in separate nebulizers using in-house filtered and dehumidified air. Syringe pumps (New Era Pump Systems, Inc., Farmingdale, NY, USA) were used to control the liquid flow rates of the TMA and control solutions, such that the total liquid flow rate was maintained constant at 10 μL/min while the flow rate of the TMA vapor source was varied. The independently atomized vapor streams were mixed together before being introduced into the reaction chamber via a copper tube of inner diameter 4.37 mm with its outlet modified to resemble a nozzle. The use of a constant total flow rate ensured that the relative humidity (RH) of the vapor stream and ethanol vapor concentration was approximately constant. The RH calculated based on the operating conditions (T = 300 K) was 29.5%, which ensured that all atomized droplets completely evaporated before exiting the tube (CitationFriedlander 2000).
Figure 1 Schematic of the system used to examine back charging reactions between TMA vapor and charged amino acid clusters. Amino acid clusters were produced by electrospray ionization, and TMA vapor was introduced into the gas phase at varying concentration by nebulizing TMA solution.
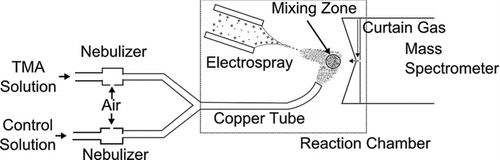
Within the reaction chamber, ESI was used to produce gas-phase cluster ions (aerosol particles). ESI solutions were made for each amino acid, which consisted of 10 mM ammonium acetate, 1 mM of the amino acid, 10 mM TMA, and 50:50 ethanol:water by volume. TMA was specifically added to ESI solutions to mitigate the potential effects of vapor absorption by ESI-generated droplets during the evaporation process. Although the outlet of the copper tube was directed away from the ESI source and when the droplets are micrometers in size, the Stefan flow created by evaporation drives gaseous species away from the droplets (CitationHuckaby and Ray 1989; CitationMartínez-Lozano and de la Mora 2009), it is difficult to guarantee that no vapor uptake occurred. Substantial vapor uptake could influence ion evaporation kinetics, which, in turn, could alter the size and charge distributions of the generated cluster ions (CitationGamero-Castano and de la Mora 2000; CitationGamero-Castaño and Fernández de la Mora 2000; CitationHogan et al. 2008; CitationHogan and de la Mora 2009). In these experiments, it was essential that the generated amino acid cluster size and charge distributions remained constant and independent of the TMA vapor concentration. The amount of TMA added to ESI solutions was much greater than a droplet could possibly absorb even if equilibrium conditions were reached (from Henry's law). Therefore, it prevented any adverse affects of vapor adsorption and ensured constant cluster generation. The additional TMA in the electrospray solution eventually gave rise to neutral TMA vapor molecules, which were accounted for in data analysis as described in the Results and Discussion section as well as in the supplemental information (available online). ESI solutions were dispensed from a 1.46-mm bore diameter syringe (Hamilton Company, Reno, NV, USA) with the flow rate controlled using a Harvard syringe pump. The ESI capillary needle was positioned facing the MS inlet orifice (), which served as the ground electrode for the ESI source. The amino acid cluster ions resulting from the ESI process collided with neutral vapor molecules in the mixing zone in front of the MS inlet. A small counterflow of curtain gas (clean N2 from an in-house N2 generator) was present at the MS inlet; thus, only charged species entered the MS and any charge exchange reactions between the cluster ions and TMA vapor were ceased once ions entered the MS.
At varying liquid flow rates of the TMA vapor generating nebulizer, with all other system parameters held constant, mass spectra of the amino acid cluster ions were measured using the time-of-flight section of the QSTAR Pulsar. MS instrument settings were as follows: declustering potential 1−0 V, focusing potential 100 V, declustering potential 2–10 V, and curtain gas 25. The declustering and focusing potentials create electric fields that serve to increase the transmission of ions through the MS inlet, resulting in an increase in the measured signal-to-noise ratio. Nitrogen curtain gas prevents nonionized species from entering the MS orifice. Prior to setting the declustering and focusing potentials, mass spectra for different values of these potentials were obtained to evaluate the tendency of amino acid clusters to fragment. Neither changes in signal intensity nor any shift in cluster ion size distribution for a given TMA vapor concentration were observed for a wide range of potentials around the chosen values.
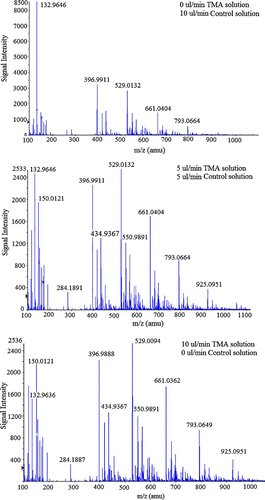
Figure 2 Selected mass spectra obtained when producing charged clusters of Asparagine with 3 different TMA vapor concentrations. The TMA concentration present during data collection is proportional to the flow rate of TMA solution, which is noted in the upper right corner of each plot. (Figure provided in color online.)
RESULTS AND DISCUSSION
Mass Spectra
As examples of the data obtained and subsequently analyzed in this work, shows the mass spectra recorded using asparagine solution and three different liquid flow rates of the TMA syringe pump. The signal intensity in these spectra is a monotonically increasing function of the number concentration of the ionic species at a particular mass to charge (m/z) ratio, and it is immediately evident that the signal intensity of the amino acid cluster ions decreases with increasing TMA liquid flow rate. A similar phenomenon is observed for all examined amino acid clusters. This suggests the occurrence of the back charging reaction wherein neutral TMA molecules “steal” charge from the amino acid cluster ions resulting in neutral clusters that do not contribute to the MS signal. The magnitude of the decrease in signal intensities with increasing TMA flow rates is largest for the smaller cluster ions, as compared with the larger ones. Using the MS data acquired by varying TMA flow rates for each ESI solution, we are able to infer information on the back reaction kinetics between neutral TMA molecules and [AA] x [H+], [AA] x [Na+], [AA] x [K+], [AA] x [TMAH+], [AA] x [H+]2, [AA] x [Na+]2, [AA] x [K+]2, and [AA] x [TMAH+]2 cluster ions (where AA denotes an amino acid molecule and x denotes the number of amino acids per cluster). The analysis performed, which is described in the following sections, is limited to examination of singly and doubly charged cluster reactions with vapor molecules. We have chosen to focus on clusters with these charge states, because in collision-based ionization of aerosol clusters, the collision rate of charged clusters and ions of the same polarity is essentially zero due to Coulombic repulsion (CitationFuchs 1963), and multiply charged clusters would be extremely rare in the gas phase.
Reaction Kinetics Model
We develop a model to better understand the decrease in signal intensity due to charge transfer from cluster to vapor molecule. The reaction occurring in the mixing zone () is represented as
For sufficiently small products of the neutral vapor concentration and residence time in the mixing zone (analogous to the ion concentration × residence time product in unipolar charging), the reionization of amino acid clusters by charged TMA molecules can be neglected. This results in the following solution to Equation (2):
Figure 3 Plots used in determination of the relative back reaction rate for protonated amino acid monomer, dimer, and trimer ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.
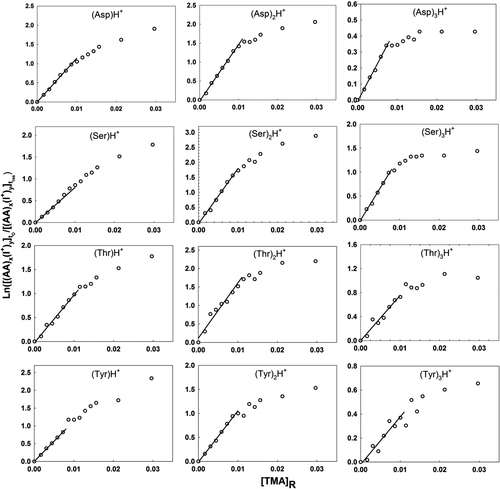
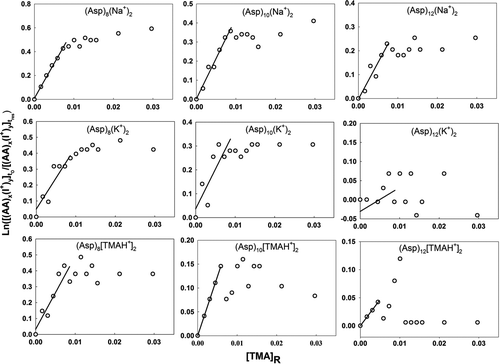
Figure 4 Plots used in determination of the relative back reaction rate for sodiated, potassiated, and trimethylammoniated amino acid monomer, dimer, and trimer ions with TMA vapor molecules. Negative slopes for the trimethylammoniated clusters indicate that TMA vapor molecules can stick to cluster ions upon collisions. The labels on the abscissa and ordinate are described in the text.
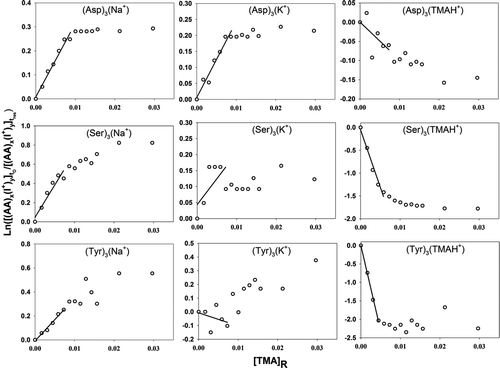
Figure 5 Plots used in determination of the relative back reaction rate for doubly protonated amino acid cluster ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.
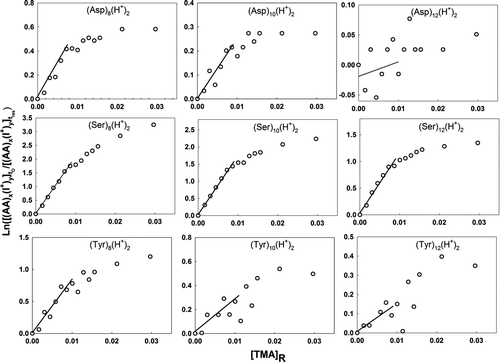
Figure 6 Plots used in determination of the relative back reaction rate for doubly sodiated, potassiated, and trimethylammoniated asparagine cluster ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.
To further analyze back charging reactions, the backward reaction rate constant of Equation (1), K B, may be decomposed as
Back Reaction Analysis
Equation (4) is plotted with ln([(AA) x (I+) y ] t 0/[(AA) x (I+) y ] t res) as the ordinate and [TMA]R as the abscissa for various cluster ions examined in this study in –. For all clusters where the back reaction was clearly prevalent, ln([(AA) x (I+) y ] t 0/[(AA) x (I+) y ] t res) was, as expected, linearly proportional to [TMA]R for small values of [TMA]R. Linear regression was therefore performed using the first several data points in each graph, with the value of A sys K B equivalent to the slopes of the regression lines (lines are drawn in each plot). At sufficiently high TMA vapor concentrations, the slopes decrease below A sys K B; at such high concentrations, the recharging of amino acid clusters by ionized TMA vapor is nonnegligible and the solution to Equation (2) given in Equations (3) and (4) is no longer valid. The experimentally determined value of A sys K B and the numerically calculated value of K phy were used to extract information on K chem using Equation (5). Again, however, with the undetermined A sys, only relative K chem values are reported here. Despite this ambiguity, the combined experimental–theoretical approach allows us to better understand the size and material dependence of charge transfer between cluster ions and neutral vapor molecules.
and and provide information on back reaction rates for collisions between TMA molecules and singly charged amino acid cluster ions. Small quantities of sodium and potassium salts are always present as impurities in commercially available amino acid salts as well as in high performance liquid chromotography (HPLC)-grade water. As ESI-generated droplets evaporate, some of the Na+ and K+ ions stick to amino acid clusters resulting in sodiated and potassiated cluster ions. The presence of positive slopes for the curves in each graph clearly indicates that a back charging reaction occurs. In the case of singly protonated amino acid monomers, the back reaction rates are highest for asparagine followed by tyrosine, threonine, serine, and leucine. However, this order is variable with cluster size and charge-carrying cation. The observations for singly charged (cationic species being any of H+, Na+, K+, TMAH+) amino acid cluster ions are similar, i.e., the rate constants for the monomers and dimers are much higher than that of the higher-order clusters, which are negligibly small. Comparison of the rate constants for singly charged clusters with the same number of amino acid molecules but with different charge-carrying cations indicates that the protonated amino acid clusters typically have the highest back reaction rates, followed by the sodiated and potassiated amino acid cluster ions. For potassiated serine and tyrosine trimers (), the absence of linearity may be interpreted as statistical variations about a rate constant value that is very close to zero (slope of the graph being horizontal). In the case of the trimethylammoniated species, the distinct negative slopes in the graphs can be explained by the fact that rather than mere charge transfer, some TMA molecules stick to amino acid clusters upon collision, increasing the signal intensity of trimethylammoniated clusters.
Table 1 Summary of the parameters used in the calculation of K phy, the collision rate between vapor molecules and cluster ions
Table 2 Summary of calculated and experimentally determined collision rate parameters for amino acid cluster ions with TMA vapor molecules
The mass spectra recorded were also used to extract information on the back reaction rates for doubly charged amino acid clusters ( and ). We note that the signal intensities of the doubly charged cluster ions were substantially lower than the singly charged clusters under all conditions. Therefore, although the back reaction for the doubly charged clusters leads to the formation of singly charged clusters, they appeared with such low signal intensity (and at larger masses than the singly charged clusters of interest), that they did not interfere with singly charged cluster analysis. Similarly, triply charged clusters were near-undetectable and did not interfere with doubly charged cluster analysis. However, quite often, a doubly charged cluster and a singly charged cluster were of the same m/z, and it was necessary to isolate singly charged cluster signal from doubly charged cluster signal. Distinguishing singly and doubly charged cluster ion signal intensities was possible by examining the isotopic distribution of all measured ions, which is described in detail in the supplemental information (available online).
Phenomena similar to those seen for singly charged clusters are observed for the doubly charged clusters wherein decreasing reaction rate constants are observed with increasing cluster size. Dependency of the reaction rate constant on the charge-carrying cation is evident as well. In general, the doubly protonated octamers have higher back reaction rate constants compared with other doubly charged clusters. Unlike the singly protonated clusters, for a particular doubly protonated cluster size, there is a strong variation in the reaction rate constant among different amino acids. The doubly protonated asparagine octamers have an AK B ∼ 57, while their threonine counterparts have an AK B ∼ 228.37. Similarly, a wide disparity between the highest and the lowest back reaction rate was found for the doubly protonated decamers and dodecamers. This can be contrasted to the singly protonated amino acid clusters where the differences in AK B values were less than 100 for all cases.
provides values of A sys K chem calculated for the various TMA and cluster ion reactions using Equation (5). The value of A sys K chem decreases with increasing cluster size for all examined amino acid cluster and charge-carrying cation combinations, implying that the influence of the coupled product of the steric factor and activation energy on the collision rate is less for larger clusters. Somewhat surprisingly, quite often, A sys K chem for dimer cluster ions is higher than that of monomer ions, indicating that the back reaction rate is the highest for amino acid dimers. Although K phy also decreases with increasing cluster size, the reduction of A sys K chem is more drastic, leading to a net reduction in the reaction rate constant. For singly protonated species, A sys K chem decreases by several orders of magnitude (note that some data for tetramer and pentamer ions are not shown, as there was no decrease in signal intensity observed for these clusters upon interaction with TMA vapor) for clusters greater than a size of ∼0.5 nm, indicating that above this size, the back reaction rate is negligibly small. As TMA vapor molecules are extremely basic in the gas phase (although we note that this does not necessarily ensure that back charging reactions with TMA vapor molecules would be faster than reactions with other vapor molecules), this size can be considered as an approximate threshold value below which back reactions need to be considered for positively charged cluster ions. We thus conclude that the rate of charge transfer from only the smallest cluster ions (with sizes close to that of the vapor molecules themselves) to vapor molecules is no longer negligible. Above this critical size, however, it is simply sufficient to calculate the forward reaction rate to determine aerosol particle charging rates. In the purely free molecule regime, the model described here [Equation (2) generalized for any cluster–vapor combination], in which the charge transfer rate constants are decomposed to K chem and K phy, effectively describes charge transfer processes in both the diffusion charging (large particle size, K chem → 0) and chemical ionization (K chem > 0) regimes.
We must strongly emphasize that the reported critical size of ∼0.5 nm is an estimate based on the data taken for amino acid clusters with charge removal via collisions with TMA vapor molecules. The chemicals chosen for analysis here were simply chosen for ease of use and merely to examine whether back charging occurs with a vapor of high gas-phase basicity. The considerable variation in back charging rate for different amino acid clusters suggests that there is a strong material dependency to this process. Therefore, to determine if a back reaction will occur between a particular charged particle and a vapor molecule (for example, atmospheric aerosol clusters), controlled studies such as the one described here need to be undertaken. Furthermore, with our available data, it appears that the rate of the back reaction decreases as a smooth function of size; thus, any definition of a critical size is somewhat qualitative. Finally, while the assessment of the back reaction rate performed here allows us to better understand the dynamics of aerosol cluster charging, a complete model of cluster charging needs to take into account the probability of ionization of an aerosol cluster by a vapor ion (K chem for the forward reaction) and further examination of aerosol cluster charging dynamics is necessary.
CONCLUSIONS
The occurrence and the size dependency of back charging reaction rates in collisions between cluster ions and gas molecules were determined for the first time. The procedure developed can be used to estimate a critical particle size below which back reaction rates have a considerable effect on charging kinetics for collision-based reactions between neutral vapor molecules and charged aerosol particles. In this study, measurement of back reaction rates for collisions between charged amino acid clusters ([(AA) x (I) y +], x denotes the cluster size and y = 1 or 2 in our study) and uncharged TMA molecules was carried out. The model was developed to describe this process in which the enhancement in the collision rate due to the presence of attractive potentials such as image and van der Waals potentials between charged clusters and uncharged TMA molecules were accounted for. The coupled product of this enhancement factor and the collision frequency, K phy, along with the measured relative rate constants, K B A sys, helped extract qualitative information on the chemical rate coefficient, K chem (where K chem = steric factor × activation energy).
On the basis of this study, we draw the following conclusions:
| 1. | Back reactions can occur during collisions between TMA molecules (neutral molecules) and both singly and doubly charged amino acid cluster ions (charged particles). | ||||
| 2. | The rate constants for these back charging reactions are dependent on the amino acid cluster size and the material of the cationic species charging the amino acid cluster. The reaction rates are surprisingly found to be highest for amino acid dimers. | ||||
| 3. | In the case of both singly and doubly charged amino acid clusters, the back reaction rate constants are higher for the smaller amino acid clusters compared with the larger ones. With increasing cluster size, the rate of the back charging reaction drops, indicating that the back reaction is energetically unfavorable. For a particular cluster size, the singly protonated cluster had the highest back reaction rate constants, but sodiated and potassiated clusters can also lose charge to vapor molecules. Likewise, in the case of doubly charged clusters, those that were doubly protonated ones had the highest back charging rate constants. | ||||
| 4. | An approximate critical size for collisions between TMA molecules and singly protonated amino acid clusters is ∼0.5 nm, implying that back charging reaction rates need to be accounted for when studying collision kinetics for cluster sizes below 0.5 nm. | ||||
| 5. | While a size dependency of the back charging rate was to be expected given the success of the limiting sphere theory alone in describing nanoparticle charging in the gas phase, our work here does little to elucidate the underlying reason for which the K chem for the back reaction decreases with size. A plausible explanation is that the electrostatic capacity of a particle is linearly proportional to its radius and thus a larger particle can more easily retain excess charge. This would also explain the occurrence of the back reaction for larger doubly charged clusters, which also decreases with increasing size. At present, however, this is only speculation. | ||||
uast_a_556683_sup_17468601.zip
Download Zip (90.3 KB)Acknowledgments
This work was partially supported by the National Science Foundation award CHE-1011810.
[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Adachi , M. , Kousaka , Y. and Okuyama , K. 1985 . Unipolar and Bipolar Diffusion Charging of Ultrafine Aerosol-Particles . J. Aerosol Sci. , 16 ( 2 ) : 109 – 123 .
- Adachi , M. , Okuyama , K. , Kousaka , Y. , Moon , S. W. and Seinfeld , J. H. 1990 . Facilitated Aerosol Sizing Using the Differential Mobilitiy Analyzer . Aerosol Sci. Technol. , 12 ( 2 ) : 225 – 239 .
- Alofs , D. J. and Balakumar , P. 1982 . Inversion to Obtain Aerosol Size Distributions from Measurements with a Differential Mobility Analyzer . J. Aerosol Sci. , 13 ( 6 ) : 513 – 527 .
- Aue , D. H. , Webb , H. M. and Bowers , M. T. 1976 . Quantitative Proton Affinities, Ionization Potentials, and Hydrogen Affinities of Alkylamines . J. Am. Ceram. Soc. , 98 ( 2 ) : 311 – 317 .
- Barsanti , K. C. , McMurry , P. H. and Smith , J. N. 2009 . The Potential Contribution of Organic Salts to New Particle Growth . Atmos. Chem. Phys. , 9 ( 9 ) : 2949 – 2957 .
- Bowers , M. T. , Su , T. and Anicich , V. G. 1973 . Theory of Ion-Polar Molecule Collisions—Kinetic-Energy Dependence of Ion-Polar Molecule Reactions—CH3OH++CH3OH− CH3OH+2+CH3O . J. Chem. Phys. , 58 ( 11 ) : 5175 – 5176 .
- Brodbelt-Lustig , J. S. and Cooks , R. G. 1989 . Determination of Relative Gas-Phase Bascities by the Proton-Transfer Equilibrium Technique and the Kinetic Method in a Quadrupole Ion-Trap . Talanta , 36 ( 1–2 ) : 255 – 260 .
- Cooks , R. G. , Patrick , J. S. , Kotiaho , T. and McLuckey , S. A. 1994 . Thermochemical Determinations by the Kinetic Method . Mass Spectrom. Rev. , 13 ( 4 ) : 287 – 339 .
- Cooks , R. G. and Wong , P. S. H. 1998 . Kinetic Method of Making Thermochemical Determinations: Advances and Applications . Accounts Chem. Res. , 31 ( 7 ) : 379 – 386 .
- CRC . Handbook of Chemistry and Physics , 80th ed. , 1999 – 2000 . Cleveland, OH : CRC Press .
- Decouzon , M. , Gal , J.-F. , Herreros , M. , Maria , P.-C. , Murrell , J. and Todd , J. F. J. 1996 . On the Use of the Kinetic Method for the Determination of Proton Affinities by Fourier-transform Ion Cyclotron Resonance Mass Spectrometry . Rapid Commun. Mass. Spectrom. , 10 ( 2 ) : 242 – 245 .
- Eisele , F. L. , Lovejoy , E. R. , Kosciuch , E. , Moore , K. F. , Mauldin , R. L. , Smith , J. N. , McMurry , P. H. and Iida , K. 2006 . Negative Atmospheric Ions and Their Potential Role in Ion-Induced Nucleation . J. Geophys. Res.-Atmos. , 111 : D04305
- Friedlander , S. K. 2000 . Smoke, Dust, and Haze , New York : Oxford .
- Fuchs , N. A. 1963 . On the Stationary Charge Distribution on Aerosol Particles in a Bipolar Ionic Atmosphere . Pure Appl. Geophys. , 56 ( 1 ) : 185 – 193 .
- Gamero-Castano , M. and de la Mora , J. F. 2000 . Kinetics of Small Ion Evaporation from the Charge and Mass Distribution of Multiply Charged Clusters in Electrosprays . J. Mass Spectrom. , 35 ( 7 ) : 790 – 803 .
- Gamero-Castaño , M. and Fernández de la Mora , J. 2000 . Mechanisms of Electrospray Ionization of Singly and Multiply Charged Salt Clusters . Anal. Chim. Acta. , 406 ( 1 ) : 67 – 91 .
- Hogan , C. J. , Carroll , J. A. , Rohrs , H. W. , Biswas , P. and Gross , M. L. 2008 . Charge Carrier Field Emission Determines the Number of Charges on Native State Proteins in Electrospray Ionization . J. Am. Ceram. Soc. , 130 ( 22 ) : 6926 – 6927 .
- Hogan , C. J. and de la Mora , J. F. 2009 . Tandem Ion Mobility-Mass Spectrometry (IMS-MS) Study of Ion Evaporation from Ionic Liquid-Acetonitrile Nanodrops . Phys. Chem. Chem. Phys. , 11 ( 36 ) : 8079 – 8090 .
- Hogan , C. J. and Fernandez de la Mora , J. 2010 . Ion Pair Evaporation from Ionic Liquid Clusters . J. Am. Soc. Mass Spectrom. , 21 : 1382 – 1386 .
- Hogan , C. J. , Li , L. , Chen , D. R. and Biswas , P. 2009 . Estimating Aerosol Particle Charging Parameters Using a Bayesian Inversion Technique . J. Aerosol Sci. , 40 ( 4 ) : 295 – 306 .
- Hoppel , W. A. 1978 . Determination of the Aerosol Size Distribution from the Mobility Distribution of the Charged Fraction of Aerosols . J. Aerosol Sci. , 9 ( 1 ) : 41 – 54 .
- Hoppel , W. A. and Frick , G. M. 1986 . Ion–Aerosol Attachment Coefficients and the Steady-State Charge Distribution on Aerosols in a Bipolar Ion Environment . Aerosol Sci. Technol. , 5 ( 1 ) : 1 – 21 .
- Huang , D. D. , Seinfeld , J. H. and Marlow , W. H. 1990 . BGK Equation Solution of Coagulation for Large Knudsen Number Aerosols with a Singular Attractive Contact Potential . J. Colloid. Interf. Sci. , 140 ( 1 ) : 258 – 276 .
- Huang , D. D. , Seinfeld , J. H. and Okuyama , K. 1991 . Image Potential between a Charged-Particle and an Uncharged Particle in Aerosol Coagulation Enhancement in all Size Regimes and Interplay with van der Waals Forces . J. Colloid. Interf. Sci. , 141 ( 1 ) : 191 – 198 .
- Huckaby , J. L. and Ray , A. K. 1989 . Absorption of Sulfur-Dioxide by Growing and Evaporating Water Droplets . Chem. Eng. Sci. , 44 ( 12 ) : 2797 – 2808 .
- Iida , K. , Stolzenburg , M. R. and McMurry , P. H. 2009 . Effect of Working Fluid on Sub-2 nm Particle Detection with a Laminar Flow Ultrafine Condensation Particle Counter . Aerosol Sci. Technol. , 43 ( 1 ) : 81 – 96 .
- Julian , R. R. , Hodyss , R. , Kinnear , B. , Jarrold , M. F. and Beauchamp , J. L. 2002 . Nanocrystalline Aggregation of Serine Detected by Electrospray Ionization Mass Spectrometry: Origin of the Stable Homochiral Gas-Phase Serine Octamer . J. Phys. Chem. B , 106 ( 6 ) : 1219 – 1228 .
- Junninen , H. , Ehn , M. , Petaja , T. , Luosujarvi , L. , Kotiaho , T. , Kostiainen , R. , Rohner , U. , Gonin , M. , Fuhrer , K. , Kulmala , M. and Worsnop , D. R. 2010 . A High-Resolution Mass Spectrometer to Measure Atmospheric Ion Composition . Atmos. Meas. Technol. , 3 : 1039 – 1053 .
- Karas , M. and Hillenkamp , F. 1988 . Laser Desorption Ionization of Proteins with Molecular Masses Exceeding 10,000 Daltons . Anal. Chem. , 60 ( 20 ) : 2299 – 2301 .
- Kulmala , M. , Riipinen , I. , Sipila , M. , Manninen , H. E. , Petajda , T. , Junninen , H. , Dal Maso , M. , Mordas , G. , Mirme , A. , Vana , M. , Hirsikko , A. , Laakso , L. , Harrison , R. M. , Hanson , I. , Leung , C. , Lehtinen , K. E. J. and Kerminen , V. M. 2007 . Toward Direct Measurement of Atmospheric Nucleation . Science , 318 : 89 – 92 .
- Kumar , G. R. , Raj , S. G. , Mathivanan , V. , Kovendhan , M. , Mohan , R. , Raghavalu , T. , Kanjilal , D. , Asokan , K. , Tripathi , A. , Sulania , I. and Kulriya , P. K. 2007 . Swift Ion Irradiation Effects on L-Threonine Amino Acid Single Crystals . J. Phys.-Condens. Mat. , 19 : 46
- Liu , B. Y. H. and Pui , D. Y. H. 1974 . Equilibrium Bipolar Charge Distribution of Aerosols . J. Colloid. Interf. Sci. , 49 ( 2 ) : 305 – 312 .
- Louris , J. N. , Cooks , R. G. , Syka , J. E. P. , Kelley , P. E. , Stafford , G. C. and Todd , J. F. J. 1987 . Instrumentation, Applications, and Energy Deposition in Quadrupole Ion-Trap Tandem Mass Spectrometry . Anal. Chem. , 59 ( 13 ) : 1677 – 1685 .
- Lushnikov , A. A. and Kulmala , M. 2004 . Charging of Aerosol Particles in the near Free-Molecule Regime . Phys. Rev. E , 70 : 046413
- Lushnikov , A. A. and Kulmala , M. 2005 . A Kinetic Theory of Particle Charging in the Free-Molecule Regime . J. Aerosol Sci. , 36 : 1069 – 1088 .
- Marlow , W. H. 1980a . Derivation of Aerosol Collision Rates for Singular Attractive Contact Potentials . J. Chem. Phys. , 73 ( 12 ) : 6284 – 6287 .
- Marlow , W. H. 1980b . Lifshitz-van der Waals Forces in Aerosol-Particle Collisions.1. Introduction—Water Droplets . J. Chem. Phys. , 73 ( 12 ) : 6288 – 6295 .
- Marshall , A. G. and Grosshans , P. B. 1991 . Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: The Teenage Years . Anal. Chem. , 63 ( 4 ) : 215A – 229A .
- Martínez-Lozano , P. and de la Mora , J. F. 2009 . On-Line Detection of Human Skin Vapors . J. Am. Soc. Mass Spectrom. , 20 ( 6 ) : 1060 – 1063 .
- Myung , S. , Fioroni , M. , Julian , R. R. , Koeniger , S. L. , Baik , M. H. and Clemmer , D. E. 2006 . Chirally Directed Formation of Nanometer-Scale Proline Clusters . J. Am. Ceram. Soc. , 128 ( 33 ) : 10833 – 10839 .
- Papayannopoulos , I. A. 1995 . The Interpretation of Collision-Induced Dissociation Tandem Mass Spectra of Peptides . Mass Spectrom. Rev. , 14 ( 1 ) : 49 – 73 .
- Su , T. and Bowers , M. T. 1973 . Ion-Polar Molecule Collisions—Effect of Molecular Size on Ion-Polar Molecule Rate Constants . J. Am. Ceram. Soc. , 95 ( 23 ) : 7609 – 7610 .
- Tsang , C. W. and Harrison , A. G. 1976 . Chemical Ionization of Amino Acids . J. Am. Ceram. Soc. , 98 ( 6 ) : 1301 – 1308 .
- Vincenti , W. G. and Kruger , C. H. Jr . 1965 . Introduction to Physical Gas Dynamics , New York : John Wiley .
- Whitehouse , C. M. , Dreyer , R. N. , Yamashita , M. and Fenn , J. B. 1985 . Electrospray Interface for Liquid Chromatographs and Mass Spectrometers . Anal. Chem. , 57 ( 3 ) : 675 – 679 .
- Winkler , P. M. , Steiner , G. , Vrtala , A. E. , Vehkamaki , H. , Noppel , M. , Lehtinen , K. E. J. , Reischl , G. , Wagner , P. E. and Kulmala , M. 2008 . Heterogenous Nucleation Experiments Bridging the Scale from Molecular Ion Clusters to Nanoparticles . Science , 319 : 1374 – 1377 .
- Wu , Z. and Fenselau , C. 1993 . Structural Determinants of Gas Phase Basicities of Peptides . Tetrahedron , 41 : 9197 – 9206 .
- Yu , F. , Wang , Z. , Luo , G. and Turco , R. 2008 . Ion-Mediated Nucleation as an Important Global Source of Tropospheric Aerosols . Atmos. Chem. Phys. , 8 ( 9 ) : 2537 – 2554 .
- Yu , F. Q. , Turco , R. P. , Karcher , B. and Schroder , F. P. 1998 . On the Mechanisms Controlling the Formation and Properties of Volatile Particles in Aircraft Wakes . Geophys. Res. Lett. , 25 ( 20 ) : 3839 – 3842 .
- Zhao , J. , Eisele , F. , Titcombe , M. , Kuang , C. and McMurry , P. H. 2010 . Chemical Ionization Mass Spectrometric Measurenents of Atmospheric Neutral Clusters Using the Cluster—CIMS . J. Geophys. Res. , 115 : D08205