Abstract
The extent of an individual's sensitivity to inhaled bronchoconstrictors has historically been attributed to biochemical sensitivities, without regarding the dose delivered to airway surfaces. Yet computational models of the lung indicate that when individuals with different lung-airway morphometry receive the same inhalation exposure, they will not receive the same delivered dose. For example, by using a typical path geometric lung model for the first 6 tracheobronchial airway generations, previous work has shown that between-subject differences in sensitivity to bronchoconstrictors could mainly be due to airway-surface dosimetry. However, such dosimetry calculations ignore both the possible impact of the complex parallel pathways formed by lung airways and the consequence of including in the models within-lung averaging of airway dimensions. The purpose of this work was to compare the impact of several dosimetry models in predicting sensitivity to bronchoconstrictors. Two commonly used strains of laboratory mice (Balb/c and B6C3F1) were used, and dosimetry calculations for inhaled methacholine were based on average or individual tracheobronchial airway dimensions as well as on the assignment of airflow distribution by distal airway volume or airway cross-sectional area. For these two strains of laboratory mice, the dosimetry calculations made by using individual airway dimensions and assigning airflow by distal airway volume predicted the greatest difference in sensitivity to bronchoconstrictors. The comparison of lung dosimetry models also showed that airway-surface dosimetry and molecular bio-sensitivity may drive pulmonary hyperresponsiveness.
INTRODUCTION
The extent of an individual's sensitivity to inhaled bronchoconstrictors has historically been attributed to biochemical sensitivities, without regarding the dose delivered to airway surfaces (CitationLevitt and Mitzner 1989; CitationTankersley et al. 1994; CitationLeme et al. 2010). Yet, all lungs are not the same. In mice, for example, recent reports quantify strain differences in both tracheobronchial airway dimensions (CitationOldham and Phalen 2002) and alveolar size (CitationSoutiere et al. 2004). Strain-specific lung-morphometry impacts the airway surface dose. For example, CitationSarpong et al. (2003) showed that even when by intubation the same mass is delivered to the lungs of mice, strain-specific lung-morphometry (due to tracheobronchial airway dimensions or number or size of alveoli) results in different doses to airway surfaces. This observation is supported by previous work (CitationMoss and Oldham 2006) using dosimetry predictions based upon a typical path anatomical model (all airways of each airway generation are given the same mean airway dimensions). Their work shows that, for the first 6 tracheobronchial airway generations in two strains of mice, dosimetric differences could account for all or the majority of the sensitivity to bronchoconstrictors.
Because of their geometric simplicity, typical path anatomical models are commonly used in dosimetry calculations, especially for population risk assessment (CitationWeibel 1963; CitationYeh and Schum 1980; CitationOldham et al. 1994; International Commisions on Radiological Protection (ICRP) 1994; National Council on Radiological Protection and Measurements (NCRP) 1997; CitationOldham and Phalen 2002; CitationNational Institute for Public Health and the Environment and Chemical Industry Institute of Toxicology 2002; CitationOldham and Robinson 2007; CitationAsgharian and Price 2009). Such dosimetry calculations ignore the possible impact of the complex parallel pathways formed by lung airways and the consequence of differences in morphometry between different human subjects.
Contrary to the variability in human tracheobronchial airway dimensions (CitationOldham 2010), laboratory animals of the same species and strain that are age and weight matched have fairly consistent tracheobronchial airway dimensions (CitationOldham et al. 1994; CitationOldham and Phalen 2002). This consistency, when applied to inhaled particle dosimetry calculations, enables use of individual tracheobronchial airway dimensions instead of typical path tracheobronchial airway dimensions. Recent work has also highlighted that airflow distribution within tracheobronchial airways, especially during resting ventilation, correlates better with distal airway volume than airway cross-sectional area (CitationAsgharian and Price 2006).
The purpose of this work was to determine the impact of different dosimetry-modeling approaches in predicting sensitivity to bronchoconstrictors. Two commonly used strains of laboratory mice (Balb/c and B6C3F1) were used, and dosimetry calculations for inhaled methacholine were based on average or individual tracheobronchial airway dimensions as well as on the assignment of airflow distribution by airway cross-sectional area or distal airway volume. For these two strains of laboratory mice, the dosimetry calculations made by using individual airway dimensions and assigning airflow by distal airway volume resulted in predicting the greatest difference in sensitivity to bronchoconstrictors.
MATERIALS AND METHODS
The measurements and calculations reported here extend the results of previous research on the dosimetry of pulmonary hyperresponsiveness in mice (CitationMoss and Oldham 2006). Basically five experimental and/or theoretical efforts were needed in order to reanalyze this existing data. The morphometry tables used by CitationMoss and Oldham (2006) were redone to include, for lung-airway generations 1–6, measurements made on each individual airway and also to include, for the thirty-two airways of airway generation 6, the number of distal terminal bronchioles. Deposition of methacholine was calculated three ways. First, in airway generations 1–6, methacholine deposition was calculated using the typical path tracheobronchial airway model with airflow rate at each bifurcation partitioned between daughter airways based on cross-sectional area. Second, deposition was calculated using the dimensions of each individual airway with airflow rate based on cross-sectional area. Third, deposition was calculated using individual airways with airflow rate at each bifurcation partitioned between daughter airways based on distal lung volume.
As before, the methacholine deposition curves and individual airway deposition were calculated for minute ventilations and aerosol size distributions specific to exposures producing a 200% change in resistance to breathing. For completeness, along with descriptions of the computational approaches for these additional efforts, the materials and methods for the morphometry measurements and methacholine exposures are also briefly repeated here.
Animals
Specific pathogen-free male mice (6 weeks old) obtained from commercial suppliers (Balb/c—Charles River, Wilmington, MA; B6C3F1—Harlan Sprague Dawley, Inc., Indianapolis, IN) were used in the morphometry measurements. These mice were housed in isolator cages and provided food and water ad libitum. All animal use in procedures for mophometry measurement was approved by the Institutional Animal Care and Use Committee of the University of California.
Lung Casts
Tracheobronchial morphometry data on the first six generations (trachea = generation 1) were obtained from morhphometric measurements of three replical silicone lung casts from each variety of mouse. Silicone rubber (Silastic E; Dow-Corning, Midland, MI) replica lung casts were produced in-situ by using the saline replacement technique of CitationPhalen et al. (1973) adapted for casting the mouse tracheobronchial tree (CitationOldham et al. 1994; CitationOldham and Phalen 2002). These methods are consistent with the recent guidance by the American Thoracic Society and European Respiratory Society (CitationHsia et al. 2010).
Morphometric Measurements
A unique binary identification number was assigned to each airway (CitationPhalen et al. 1978). For each airway in generations 1–6, length, diameter, and branch angle were measured. The inclination of the airway to the gravity force vector was measured whenever possible for each airway with the trachea being defined as 90°. To aid in calculating particle deposition, the airway lengths are defined so that adding the lengths along the airflow path traversing several generations yields an estimate of the total path length. For the three mice in each mouse variety, the individual airway dimensions were taken as the average of the three airways having the same identification number. For these same three mice, the typical path model was created after the scheme used for humans by CitationWeibel (1963). For each individual mouse, the dimensions of all airways comprising an airway generation were averaged together to obtain the dimensions of the airway of average size (the typical airway for that airway generation in that mouse). Then, for the three mice of each variety, the dimensions of the typical airway for each airway generation were averaged to obtain the average typical path.
The total surface area per airway generation was calculated two ways: first, by calculating the average diameter and length for each airway generation and multiplying the resulting surface area by the number of airways and, second, by adding together the surface area of each individual airway.
Although all airways from the trachea to the terminal bronchi (TB) were assigned a unique binary identification number (CitationPhalen et al. 1978), dimensional measurements for airways distal to the sixth airway generation were not performed. In order to estimate the lung volume distal to these airways, we assumed that for mice of the same variety, each TB ended in an alveolus of equal size. The distribution of lung volume between lobes was then based on the distal lung volume being directly proportional to the number of TB. For example, in , on the basis of this method of counting TB, we compare lobar volumes.
TABLE 1 Lobar distribution of distal lung volume (as % of total; mean ± SE; n = 3 lungs): comparison between mice (B6C3F1 and Balb/c)
Methacholine Challenge
All dosimetry calculations were based on the results of the mechacholine challenges reported in CitationDeLorme and Moss (2002) and discussed in CitationMoss and Oldham (2006) and CitationMoss (2010). When the methacholine challenge is given to rodents, the degree of difficulty in breathing (airway resistance) is measured indirectly as a lag time, Δt, between the start of a breath (in the thorax) and the movement of air (in the nose). As an indicator of change in airway resistance, this lag-time method is sufficiently reproducible to allow the ranking of mouse varieties according to hyperresponsiveness: And this rank ordering is assumed to be indicative of similar degrees of hyperresponsiveness in humans.
Briefly, the procedure used by CitationDeLorme and Moss (2002) was as follows: Methacholine (acetyl-b-methacholine bromide, molecular weight 240.14 g/mole, Sigma A-2126; Sigma-Aldrich, St. Louis, MO) was used to conduct bronchonstrictor tests in mice. Each mouse was placed into an individual plethysmograph (model PLY 3211-unrestrained; Buxco Electronics, Sharon, CT), and, over a 3-min period, a baseline physiological measurement (Penh; the enhanced pause between exhalation and inhalation) was averaged. These measurements were continued into a second 3-min period during which the mouse was challenged with a methacholine aerosol that was produced by nebulizing a solution of phosphate buffered saline (PBS) containing methacholine at concentrations of either 0, 2.5, 20, 80, or 320 mg/mL. The baseline and challenge were repeated at incremental increases in methacholine concentration until the highest concentration was reached or until the increase in airway resistance was greater than 200%. From the concentration-response curves, the provocative concentration of methacholine (PC200R) was determined as that concentration (of methacholine in the nebulized PBS solution) which produced a 200% increase in airway resistance.
Dose Calculations
In the methacholine challenge protocol and analysis, the underlying assumption is that the provocative concentration (in the nebulized PBS solution) is an accurate indicator of the effective dose that produces the 200% change in difficulty to breathe. Unfortunately this assumption was not supported by the reanalysis of the dosimetry (CitationMoss and Oldham 2006; CitationMoss 2010). In the reanalysis, published aerosol dosimetry computer codes (NCRP 1997) were used to calculate, for airway generations 1–6, the amount of mechacholine deposited on airway epithelium (surface dose, ng/cm2). In this current work, the same approach was used to calculate surface dose in three different ways:
| 1. | TP_xA: methacholine deposition was calculated using the typical path model (i.e., average airway dimensions) with airflow rate at each bifurcation partitioned between daughter airways based on cross-sectional area. | ||||
| 2. | IA_xA: deposition was calculated using the dimensions of each individual airway with airflow rate based on cross-sectional area. | ||||
| 3. | IA_dV: deposition was calculated using individual airways with airflow rate at each bifurcation partitioned between daughter airways based on distal lung volume. | ||||
As discussed in the previous analysis (CitationMoss and Oldham 2006), for the Balb/c and B6C3F1 mice, we assumed that, in the extrathoracic region, methacholine deposition would be equivalent and pressure drop change would be minimal.
Analysis of Dose Response
The response to an inhaled bronchoconstrictor is impacted by three dose-response factors: airway surface dose of methacholine; physiological relationship between airway circumference change and change in resistance to airflow; and molecular-biological sensitivity. In the methacholine challenge, the most accurate representation of dose is the mass of methacholine deposited in each airway of the lung—represented by the surface density of deposited methacholine (ng/cm2). However, larger airways require a greater change in circumference in order to produce the same percent change in resistance seen in smaller airways (CitationMoss and Oldham 2006). In the methacoline challenge, this is the airway physiology factor—accounting for the impact of smooth muscle constriction being inversely effected by initial airway circumference (cm−1). And, within the tissue, the bio-molecular component of the response to methacholine—the “bio-sensitivity”—is the degree that all remaining potential causes of mechacholine induced bronchoconstriction (including methacholine receptor-presence and -specificity) directly or indirectly relate the magnitude of smooth muscle constriction to surface density of methacholine, cm/(ng/cm2).
In comparing the Balb/c mouse response with the response of the B6C3F1 mouse to inhaled methacholine, the same approach was used as previously reported (CitationMoss and Oldham 2006) by calculating the following ratios:
| • | the ratio, R 1 [(Balb/c)/B6C3F1], of methacholine surface dose represented by the total mass (Xdep) deposited in airway generations 1–6 divided by the surface area (A) of airway generation 5. Airway generation 5 was used because (as observed in CitationMoss and Oldham 2006) this airway generation appeared to form that part of a respiratory isthmus where slight changes in airway circumference produced the greatest change in resistance to flow. | ||||
| • | The ratio, R 2 [(Balb/c)/B6C3F1], of the airway physiology factors inversely represented by the diameter of airway generation 5. | ||||
| • | The ratio, R3 , of the Balb/c and B6C3F1 bio-sensitivity factors, K (Bc) and K (B6), estimated, based on the observation (CitationMoss and Oldham 2006) that, for the two varieties of mice to have the same change in resistance to breathing, the product of the three ratios (the airway-surface dosimetry ratio, the airway physiology ratio, and the bio-sensitivity ratio) must equal one. | ||||
RESULTS
Morphometric Measurements
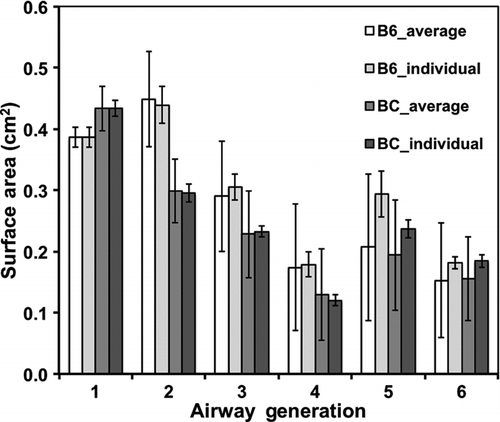
shows the mean airway surface areas for typical path and individual airway anatomical models for each mouse variety.
FIG. 1 Surface area per airway generation (mean ± SE; n = 3). For airway generations 1–6, comparison of surface area between mice (B6C3F1 or Balb/c) and between method of calculating airway surface area (based on average airway dimensions or based on individual airway dimensions). White columns represent surface area from average airway dimensions of B6C3F1 mice; light-grey columns represent area from individual airway dimensions of B6C3F1 mice; grey columns represent area from average airway dimension of Balb/c mice; and dark-grey columns represent area from individual airway dimensions of Balb/c mice.
Dose Calculations
For each mouse strain, the total mass of methacholine deposited in airway generations 1–6 was calculated for three dosimetry models: TP_xA, IA_xA, and IA_dV (). For comparison, includes the deposited mass reported by CitationMoss and Oldham (2006): TP_xA_2006. Although included as a model in , the standard methacholine challenge analysis (M_Ch_2002) (CitationDeLorme and Moss 2002) does not calculate the amount of methacholine deposited in the lung.
TABLE 2 Mass deposited in airway generations 1–6 (mean ± SE): Comparison between mice (B6C3F1 and Balb/c), deposition model (typical path, TP; individual airway, IA; or none, M_Ch), and method of calculating airflow (cross-sectional area, xA; distal volume, dV)
Analysis of Dose Response
A comparison of the three dose-response-factors ratios for the Balb/c and B6C3F1 mice (airway surface dose; airway physiology; bio-sensitivity) indicates that there are significant differences depending on which morphometric model is used (typical path vs. individual airway) as well as on which assumptions were used for estimating the airflow distribution (cross-sectional area vs. distal airway volume) ().
FIG. 2 Relative impact on response to methacholine: Comparison of dose-response-factor (DRF) ratios [DRF(Balb/c) vs. DRF(B6C3F1; mean ± SE)] of three DRFs [airway surface dose (vertical bar filled with tight stipple of small dots); airway physiology (relating airway circumference with pressure drop; vertical bar filled with open stipple of parallel 45-degree lines); and bio-sensitivity (vertical bar filled with open stipple of large dots)] as calculated by five dosimetry models: M_Ch_2002; TP_xA_2006; TP_xA(current); IA_xA(current); and IA_dV(current).
![FIG. 2 Relative impact on response to methacholine: Comparison of dose-response-factor (DRF) ratios [DRF(Balb/c) vs. DRF(B6C3F1; mean ± SE)] of three DRFs [airway surface dose (vertical bar filled with tight stipple of small dots); airway physiology (relating airway circumference with pressure drop; vertical bar filled with open stipple of parallel 45-degree lines); and bio-sensitivity (vertical bar filled with open stipple of large dots)] as calculated by five dosimetry models: M_Ch_2002; TP_xA_2006; TP_xA(current); IA_xA(current); and IA_dV(current).](/cms/asset/89c6af9c-b7d8-486c-ada6-870d322a3b86/uast_a_565822_o_f0002g.gif)
DISCUSSION
In contrast to our previous results (CitationMoss and Oldham 2006) in which differences in dosimetry alone accounted for the entire difference in overall methacholine sensitivity, this work indicates that, of the three dose-response factors (airway-surface dosimetry, airway physiology, and bio-sensitivity), airway-surface dosimetry and molecular biological sensitivity are responsible for the observed differences in response to methacholine between the B6C3F1 and Balb/c strains of mice. This conclusion is the same whether a typical path or individual tracheobronchial airway anatomical model is used. As expected, different tracheobronchial anatomical models yield different estimates of the contribution of dosimetry versus molecular biological sensitivity in the overall sensitivity to methacholine. However, for the Balb/c and B6C3F1 strains of laboratory mice, the dosimetry calculations made by using individual airway dimensions and assigning airflow by distal airway volume resulted in predicting the greatest difference in sensitivity to bronchoconstrictors.
It is interesting that utilizing distal airway volume for airflow distribution in individual airways provides results similar to results obtained from utilizing airway cross-section for airflow distribution in the typical path anatomical model (; compared IA_dV[current] with TP_xA[current]). This highlights one of the limitations of this work, which is that only two strains of mice were used. However, preliminary work has indicated that these results are applicable to a third mouse strain, the AJ mouse (CitationMoss and Oldham 2008).
The dose-response-factor ratios differed between the typical path deposition calculations made in 2006 and those made in this current work (; compared TP_xA_2006 with TP_xA[current]). The difference is attributed to the way the Balb/c mouse-lung airway dimensions were obtained. The Balb/c mouse typical path geometry used in CitationMoss and Oldham (2006) was that published in CitationOldham and Phalen (2002) and was derived from a sampling plan of Balb/c mouse airways. The individual airway calculations made in the current work required measurement of every airway in the first six generations. For some airway generations, this additional level of detail resulted in different typical path tracheobronchial airway dimensions.
This work is based on earlier measurements establishing a clear difference between Balb/c and B6C3F1 airway dimensions in the tracheobronchial airways of airway generations 1–6 (CitationOldham et al. 1994; CitationOldham and Phalen 2002). Our reanalysis of the individual airways supports this observation and is consistent with similar conclusions of differences in functional residual capacity among strains of mice (CitationMitzner et al. 2001), aerobic capacity (CitationLightfoot et al. 2001), alveolar size (CitationSoutiere et al. 2004), and baseline breathing patterns (CitationTankersley et al. 1997). Furthermore, our reanalysis points the way for the application of mathematical modeling in developing new methods of lung-related self-monitoring and treatment. The differences in B6C3F1 and Balb/c mouse-lung morphometry provide a basis for preliminary application of three dose-response factors for bronchoconstriction (airway- dosimetry, airway physiology, and bio-sensitivity). And, the differences in both lung morphometry and dose-response factors provide insights upon which to refine and apply mathematical models of allergy- and asthma-related pulmonary hypersensitivity.
REFERENCES
- Asgharian , B. and Price , O. T. 2006 . Airflow Distribution in the Human Lung and Its Influence on Particle Deposition . Inhal. Tox. , 18 : 795 – 801 .
- Asgharian , B. and Price , O. T. 2009 . Multiple Path Particle Deposition Model (MPPD Version 2.00). . Available online at: http://www.ara.com/products/mppd.htm (Accessed 9 March, 2011)
- DeLorme , M. P. and Moss , O. R. 2002 . Pulmonary Function Assessment by Whole-Body Plethysmography in Restrained Versus Unrestrained Mice . J. Pharmacol. Toxicol. Methods , 47 : 1 – 10 .
- Hsia , C. C. W. , Hyde , D. M. , Ochs , M. and Weibel , E. R. 2010 . An Official Research Policy Statement of the American Thoracic Society/European Respiratory Society: Standards for Quantitative Assessment of Lung Structure . Am. J. Respir. Crit. Care. Med. , 181 : 394 – 418 .
- International Commission on Radiological Protection (Task Group of Committee 2) . 1994 . Human Respiratory Tract Model for Radiological Protection, Publication 66 , New York : Pergamon Press .
- Leme , A. S. , Berndt , A. , Williams , L. K. , Tsaih , S. W. , Szatkiewicz , J. P. , Verdugo , R. , Paigen , B. and Shapiro , S. D. 2010 . A Survey of Airway Responsiveness in 36 Inbred Mouse Strains Facilitates Gene Mapping Studies and Identification of Quantitative Trait Loci . Mol. Genet. Genomics , 283 : 317 – 326 .
- Levitt , R. C. and Mitzner , W. 1989 . Autosomal Recessive Inheritance of Airway Hypereactivity to 5-Hydroxytrptamine . J. Appl. Physiol. , 67 : 1125 – 1132 .
- Lightfoot , J. T. , Turner , M. J. , Debate , K. A. and Kleeberger , S. R. 2001 . Interstrain Variation in Murine Aerobic Capacity . Med. Sci. Sports Exerc. , 33 : 2053 – 2057 .
- Mitzner , W. , Brown , R. and Lee , W. 2001 . In Vivo Measurements of Lung Volume in Mice . Physiol. Genomics , 4 : 215 – 221 .
- Moss , O. R. 2010 . “ Chapter 8, Particle Dosimetry in the Nose and Upper Airways of Humans, in Toxicology of the Nose and Upper Airways ” . In Series: Target Organ Toxicology, Vol. 30 , Edited by: Morris , J. B. and Shusterman , D. J. 480 New York : Informa Healthcare . ISBN: 9781420081879, 142008187X
- Moss , O. R. and Oldham , M. J. 2006 . Dosimetry Counts: Molecular Hypersensitivity may not Drive Pulmonary Hyperresponsiveness . J. Aerosol. Med. , 19 : 555 – 564 .
- Moss , O. R. and Oldham , M. J. 2008 . Mt. Laurel, NJ. : AAAR Annual Conference Abstracts, American Association for Aerosol Research (AAAR) . Abstract 3F.11, Available online at: http://aaar.conference2008.org/includes/Abstracts/abstracts_sessions.pdf Estimation of Regions of Sensitivity to Smooth-Muscle Agonists Inhaled by Balb/c, B6C3F1, and AJ Mice: Impact of Pulmonary Morphometry. 2008 (Accessed 9 March, 2011)
- National Council on Radiological Protection and Measurements . 1997 . Report 125—Deposition, Retention and Dosimetry of Inhaled Radioactive Substances , Bethesda, MD : National Council on Radiological Protection and Measurements .
- Oldham , M. J. 2010 . Dosimetry Implications of Uncertainties in Lung Architecture: Is Weibel Good Enough? in Respiratory Drug Delivery 2010 , Edited by: Dalby , R. N. , Byron , P. R. , Peart , J. , Suman , J. D. , Farr , S. J. and Young , P. M. Vol. 1 , 205 – 214 . Richmond, VA : Virginia Commonwealth University .
- Oldham , M. J. and Phalen , R. F. 2002 . Dosimetry Implications of Upper Tracheobronchial Airway Anatomy in Two Mouse Varieties . Anat. Rec. , 268 : 59 – 68 .
- Oldham , M. J. , Phalen , R. F. , Schum , G. M. and Daniels , D. S. 1994 . Predicted Nasal and Tracheobronchial Particle Deposition Efficiencies for the Mouse . Ann. Occup. Hyg. , 38 ( Supp. 1 ) : 135 – 141 .
- Oldham , M. J. and Robinson , R. J. 2007 . Dosimetry Predictions in an Animal Model of Emphysema . Anat. Rec. , 290 : 1309 – 1314 .
- Phalen , R. F. , Yeh , H. C. , Raabe , O. G. and Velasquez , D. J. 1973 . Casting the Lungs In-Situ . Anat. Rec. , 177 : 255 – 263 .
- Phalen , R. F. , Yeh , H. C. , Schum , G. M. and Raabe , O. G. 1978 . Application of an Idealized Model to Morphometry of the Mammalian Tracheobronchial Tree . Anat. Rec. , 190 : 167 – 176 .
- National Institute for Public Health and the Environment (RIVM) Chemical Industry Institute of Toxicology (CIIT) . 2002 . Multiple Path Particle Dosimetry Model (MPPD v.1.0): A model for Human and Rat Airway Particle Dosimetry. . RIVM, Bilthoven, the Netherlands, RIVM Report 650010030. Available online at: http://riun.openrepository.com/rivm/bitstream/10029/9272/1/650010031.pdf (Accessed 9 March, 2011)
- Sarpong , S. B. , Zhang , L. Y. and Kleeberger , S. R. 2003 . A Novel Mouse Model of Experimental Asthma . Int. Arch. Allergy Immunol. , 132 : 346 – 354 .
- Soutiere , S. E. , Tankersley , C. G. and Mitzner , W. 2004 . Differences in Alveolar Size in Inbred Mouse Strains . Resp. Physiol. Neurobi. , 140 : 283 – 291 .
- Tankersley , C. G. , Fitzgerald , R. S. and Kleeberger , S. R. 1994 . Differential Control of Ventilation Among Inbred Strains of Mice . Am. J. Physiol. 267 (Regulatory Integrative Comp. Physiol. 36) , : R1371 – R1377 .
- Tankersley , C. G. , Fitzgerald , R. S. , Levitt , R. C. , Mitzner , W. A. , Ewart , S. L. and Kleeberger , S. R. 1997 . Genetic Control of Differential Baseline Breathing Pattern . J. Appl. Physiol , 82 : 874 – 881 .
- Weibel , E. R. 1963 . Morphometry of the Human Lung , New York : Academic Press .
- Yeh , H. C. and Schum , G. M. 1980 . Models of Human Airways and Their Application to Inhaled Particle Deposition . Bul. Math. Biol. , 42 : 461 – 480 .