Abstract
An electrodynamic balance was used to examine the effect of the presence of particle-phase organics on the acid-catalyzed reactive uptake of nonanal (NL) vapor. Uptake experiments were conducted by using sulfuric acid (SA) particles, oleic acid/SA (hydrophobic), and levoglucosan/SA (hydrophilic) mixed particles with 6 ppm (approximately) gas-phase NL at about 3% relative humidity. SA reacted with the mixed organics prior to NL uptake to form organic products, denoted as OleA* and Levo*, and with NL to form hydrophobic NL* (particle-phase organics). Fresh SA particles had small mass increases (5%–13%) at the start of NL exposure (0–40 min) even though they are highly acidic. However, OleA*/SA mixed particles of about 30–70 wt% of OleA* took up NL swiftly during the first 40 min. For example, the mass increase of a 33 wt% OleA* particle jumped to 120%. As the organic product, NL*, accumulated, the uptake rate of SA particle increased and the mass increase surged to 150% at 100 min. Afterwards, the mass increase started to level off which yielded a sigmoid uptake curve. For OleA*/SA particles, the uptake rate gradually slowed down resulting in physical-absorption-like uptake kinetics. The physical uptake of NL by a pure OleA* surrogate was negligible (<1%) showing that the large uptake of OleA*/SA particles were attributed to the enhanced reactive uptake of NL in the presence of hydrophobic OleA*. Conversely, the hydrophilic Levo*/SA particles were incompatible with NL, and they showed insignificant enhanced uptake compared with the SA particles. Overall, the acidic uptake of NL is highly dependent on the chemical nature and weight percentages of particle-phase organics in mixed particles. Presence of hydrophobic organic materials in particles enhanced the reactive uptake of NL.
INTRODUCTION
Organic compounds are recognized as a major constituent of atmospheric aerosols, making up 20%–90% of aerosol mass (CitationKroll and Seinfeld 2008). This organic fraction consists of thousands of individual organic species with a wide range of properties (CitationJacobson et al. 2000). Because of their abundance and diverse functional characteristics, organic components can have significant impacts on both physical and chemical properties of atmospheric aerosols and consequently on the global climate and regional air quality (CitationKanakidou et al. 2005). A number of laboratory studies have demonstrated that the presence of organics can modify the hygroscopicity and the cloud condensation nucleation (CCN) activity of inorganic particles significantly (CitationCruz and Pandis 2000; CitationChoi and Chan 2002; CitationAbbatt et al. 2005). The health effects of aerosol are another issue of concern, and it is well established that exposure to ambient aerosols is associated with damaging effects on the respiratory and cardiovascular systems (CitationPope and Dockery 2006). All these impacts of organic aerosols (OA) depend on their composition, sizes, as well as their physical and chemical properties. In fact, the chemical compositions and the properties of OA keep changing throughout their lifetimes, due to a number of processes such as the condensation of gas-phase oxidation products of volatile organic compounds (VOCs) (CitationOdum et al. 1996), the oxidation of OA in the particle phase (CitationRudich et al. 2007), and the physical gas-to-particle partitioning (CitationPankow 1994) of volatile and semivolatile organic vapors.
The reactive uptake of VOCs into acidic particles (CitationJang et al. 2004) may also contribute to the altering of OA properties but the issue is currently under open debate due to inconsistent and sometimes even conflicting results in the literature. CitationJang et al. (2002) first reported that the uptake of aldehydes by sulfuric acid (SA) particle was much higher than the neutral ammonium sulfate particles. CitationGarland et al. (2006) also demonstrated that reactions of a high concentration of hexanal vapor (0.01 atm) with highly concentrated SA seed particles produce aerosols with up to 88 wt% of organics. Other groups have also shown that enhanced uptake into acidic particles occurs to commonly found VOCs that possess reactive functional groups (susceptible to proton addition), such as olefins (CitationLimbeck et al. 2003; CitationLiggio et al. 2007; CitationLiggio and Li 2008), hydroxyls (CitationLevitt et al. 2006; CitationNoziere et al. 2006), epoxides (CitationSurratt et al. 2008; CitationMinerath and Elrod 2009), and alkylamines (CitationWang et al. 2010). It is proposed that the acidic medium in the particle phase acts as a catalyst (CitationJang et al. 2002, 2004; Liggio and Li 2006a) in particle-phase reactions of carbonyls, forming high-molecular-weight products that tend to stay in the particle phase.
The reactive uptake of carbonyls onto acidic particles leads to a substantially higher secondary organic aerosol (SOA) yield (CitationJang et al. 2004) than those by only physical uptake when neutral seed particles were used. CitationJang et al. (2004) proposed that carbonyl compounds undergo (1) hydration, (2) hemiacetal/acetal formation, (3) polymerization, and (4) aldol condensation under acidic conditions. Aldol condensation of carbonyls has been extensively studied (CitationNoziere and Riemer 2003; CitationJang et al. 2004; CitationZhao et al. 2005; CitationGarland et al. 2006; Liggio and Li 2006a) because it not only nearly doubles the carbonyl's molecular weight by accretion reactions (CitationBarsanti and Pankow 2004), but also forms a conjugated α, β-unsaturated aldehyde, which alters the light absorption ability of the precursor (CitationNoziere and Esteve 2007).
On the other hand, some studies have found that some of these mechanisms, particularly for carbonyls, may not be kinetically (CitationNoziere and Riemer 2003; CitationEsteve and Noziere 2005; CitationCasale et al. 2007) or thermodynamically (CitationBarsanti and Pankow 2004; CitationTong et al. 2006) favorable to have large effects on the properties of aerosols under atmospheric conditions. The results from some field measurements (CitationZhang et al. 2005, Citation2007; CitationTakahama et al. 2006) also raised questions on the hypothesis that acid-catalyzed reactions of organics significantly increase the organic mass of aerosol particles. From laboratory experiments, CitationKroll et al. (2005) did not observe any significant enhancement in particle growth due to the reactive uptake of most carbonyls (except glyoxal) by SA particles compared with neutral ammonium sulfate particles, which is in contrast with the findings reported by CitationJang et al. (2002). CitationZhao et al. (2005) reported that octanal was physically absorbed by SA particles without undergoing irreversible reactions, although they observed irreversible reactive uptake of 2,4-hexadienal. The large discrepancy reported in the literature warrants effort to examine the importance of reactive uptake of VOCs into acidic particles regarding SOA formation.
Yet, most of these laboratory studies only focused on how particle acidity affected the SOA yield (CitationNoziere and Riemer 2003; CitationJang et al. 2004; CitationEsteve and Noziere 2005; CitationZhao et al. 2005; CitationGarland et al. 2006; Liggio and Li 2006a; CitationCasale et al. 2007; CitationSurratt, Lewandowski et al. 2007; CitationLee et al. 2008; CitationOffenberg et al. 2009; CitationConnelly and Tolbert 2010). Sulfuric acid and its mixtures with ammonium sulfate seed particles were employed as the surrogates for simulating the composition of ambient aerosols with different acidities. In atmosphere, however, organic components are ubiquitous and they are often accompanied with sulfate in fine aerosols (CitationSaxena et al. 1995; CitationHallquist et al. 2009). Depending on the compatibilities, preexisting organics may aid the physical absorption of VOCs (CitationPankow 1994) that in turn alter their particle-phase concentrations and, hence, their contributions to atmospheric processes. The combined effects of physical absorption of VOC into SA/organics mixtures and subsequent reactions in particle phase, if any, should be considered simultaneously for a more complete understanding of the impact of reactive uptake on SOA formation.
CitationSong et al. (2007) reported that some SOA species, e.g., oxidation products of α-pinene, are not miscible with dioctyl-phthalate, a primary organic aerosols (POA) surrogate (CitationVaden et al. 2010). Consequently, the POA mass is not fully available for the partitioning of oxidation products, and thus the measured SOA yield is lowered compared to the prediction using the assumption of well-mixed organic phase. Using this paradigm, one would expect that the SOA yield of reactive uptake was lowered if the condensable organics are not miscible with or are only sparingly soluble in the inorganic acidic particles. Bulk kinetic studies (CitationCasale et al. 2007) have shown that the solubility of aldehydes in aerosol phase critically controls the reactive uptake since the rate of acid-catalyzed reactions is of second order with respect to the aldehyde concentrations in the liquid/aerosol phase. By using a Knudsen cell, CitationWilliams et al. (2010) reported that the solubility (nonreactive) of acetaldehyde in SA/water is enhanced by approximately ten times in the presence of ethanol or acetone which suggested that particle-phase organics are important to the uptake of aldehyde vapor.
The electrodynamic balance (EDB) is very useful for determining the water uptake of aerosols (CitationPeng et al. 2001). Recently, we extended the use of an EDB to examine the gas-particle partitioning of volatile and semivolatile alcohol vapors on levitated oleic acid particles (CitationChan et al. 2010). The measured partitioning coefficients agreed well with Pankow's absorptive partitioning model (CitationPankow 1994), suggesting that the EDB is a reliable tool for organic uptake experiments. Using the EDB, our group has also demonstrated a strong uptake of octanal vapors by SA particles (CitationLee et al. 2008) at 10% relative humidity (RH). The uptake was slow at the beginning (0–6 h) when the fresh SA particles were exposed to octanal vapor. Interestingly, the uptake rates increased by about three to eleven times when the particles had accumulated about 6–12 wt% of organics. We speculated that the increased uptake rate was due to the presence of particle-phase organic reaction products.
In this study, we first investigated the uptake of nonanal (NL) by pure SA particles and found that the reaction products (particle-phase organics) enhanced the uptake and led to a subsequent exponential growth of particle mass before the uptake started to level off. To examine the effect of the presence, chemical nature, and abundance of organics on reactive uptake, we studied the uptake of NL by hydrophobic oleic acid/SA mixed particles and hydrophilic levoglucosan/SA mixed particles at various initial organic/SA weight percentages. The former organic chosen is abundant in emissions from cooking (Schauer et al. 1999a), while the latter is commonly found in biomass burning (CitationSimoneit et al. 1999). It is expected that both oleic acid and levoglucosan would react with SA to form a mixture of products, including organosulfates (OSs) (Liggio and Li 2006b; CitationIinuma et al. 2007; CitationSurratt et al. 2008). Although chemical speciation is important to identify reaction products and pathways, the potentially identifiable products in general could contribute only a small fraction of the organics and cannot explain the overall properties of the resultant mixtures completely. In fact, a fully resolved reaction system is rarely found in literature (CitationHallquist et al. 2009). As a compromise, we characterize the overall hydrophilicities of these two reaction mixtures as surrogates of hydrophobic and hydrophilic organic materials. Using the EDB, the mass increases as a result of the reactive uptake of NL by these mixtures are directly measured.
Aldehydes are ubiquitous in the atmosphere due to direct emission sources (Schauer et al. 1999b) and photodegradation of organics (CitationSurratt et al. 2010). NL was chosen as a model aldehyde and all uptake experiments were performed at a vapor concentration of 6.20 ± 0.25 ppm and a RH of 3% ± 2%. The particle-phase organics contents were varied in two ways: (1) the addition of different initial amounts of oleic acid or levoglucosan and their resulted reaction products with SA and (2) the formation of particle-phase organics due to the reactive uptake of NL. The uptake coefficient (γ), which quantifies the uptake ability of particles at a given organic composition, was plotted against the wt% of organics in particle phase so as to elucidate the compositional dependence of reactive uptake. It was found that the uptake coefficient is the maximum for NL uptake by acid particles with about 50 wt% oleic acid derived organics. Levoglucosan did not enhance the uptake. To the best of our knowledge, this is the first study devoted to examining the enhanced reactive uptake of an aldehyde by SA in the presence of particle-phase organics.
EXPERIMENTAL SETUP
Single Particle Generation
Oleic acid (99%, Sigma-Aldrich) was dissolved in ethanol (≥99.9%, Merck), while levoglucosan (99%, Sigma-Aldrich) and SA (95–98 wt%, Mallinckrodt Chemicals) were dissolved in deionized water. A small amount of the mixed solution (either SA or its organic mixtures) was introduced to a piezoelectric particle generator (MicroFab Tech., Inc., SN: B10-02-02) and a single droplet of 15–35 μm in diameter was produced by applying an electric pulse to the generator. The particle was charged as it passed through the induction plate mounted at the top entrance of the EDB.
EDB Mass Measurements
The EDB has been used extensively for direct measurements of particle mass changes (CitationDavis 1997; CitationPeng et al. 2001) and is used in this study. In brief, a charged particle is trapped and levitated at the null point of the EDB through a combination of AC and DC electric fields surrounding the particle. Assuming that there is no loss of charge, the weight of the single particle is proportional to the balancing DC voltage. The relative mass change of a particle during NL vapor uptake can be determined by monitoring the change in balancing DC voltage. The uptake data obtained were used to calculate the mass ratio (α), defined as α = mt /mo and the percentage mass increase, defined as (α − 1) × 100%, where mt is the mass of the particle after NL uptake and mo is the initial mass of the particle (reference state). To evaporate the solvent of the levitated particle, the EDB was purged with clean compressed air overnight prior to the initial mass measurement. This also allowed the completion of reactions between the added organics and SA, as confirmed by the invariant particle mass.
Generation and Detection of NL Vapor
NL vapor was generated by bubbling dry compressed air into a liquid NL (95%, Sigma-Aldrich) reservoir. The gas-phase NL concentration was controlled by diluting the NL-vapor-laden air with a stream of dry clean air (about 3% RH) by using mass flow controllers (Smart-Trak Series 100) and stainless steel metering valves (swagelok). The diluted NL vapor was split into two streams: one for EDB experiments (EDB stream), which was fixed at 180 cm3 min−1, and the other was the monitoring stream connecting to a portable VOC detector (PhoCheck 5000+, Ion Science, Inc.). The detector was calibrated by evaporating (bubbling) known amounts of NL at controlled flow rates. For all uptake experiments, the EDB stream was turned on when the monitoring stream became stable and the NL vapor concentration was kept between 5.95 and 6.45 ppm throughout the experiments.
Time Scale for Stabilizing Vapor Concentration in the EDB
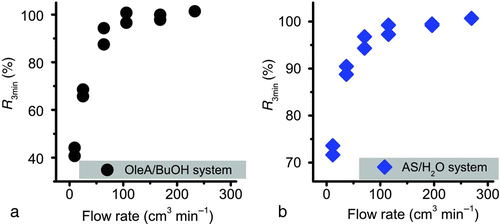
The measured mass uptakes of levitated single particles in an EDB chamber depend on both the vapor concentration in the neighborhood of the particle and the reactive uptake of the gas-phase species into the particle phase. Since the EDB was initially an organic-vapor-free environment after introducing the levitated particles, it is important to ensure that the EDB can equilibrate to a stable vapor concentration in a short time compared with that of the uptake experiments. The time scale for equilibrating the vapor concentration in the EDB can be reduced by increasing the flow rate of the NL stream to the EDB, which has an approximate volume of 60 cm3. We performed pure uptake (without reaction) experiments to evaluate the suitable flow rate to stabilize the vapor concentration at a short time. shows the percentage of mass uptake of the particles at 3 min after vapor was introduced to the equilibrium mass (at circa 30 min), abbreviate to R 3min, as a function of flow rate for (a) butanol vapor uptake by oleic acid particles and (b) water vapor uptake by ammonium sulfate particles. It is apparent that R 3min reaches 100% when the vapor stream flow rate is larger than 100 cm3 min−1, suggesting both the organic and water vapors can attain a steady state concentration within 3 min at a flow rate larger than 100 cm3 min−1. In the study of reactive uptake of NL, the EDB stream was fixed at 180 cm3 min−1 to ensure that the mass measurements over hundreds of minutes reflect the transients of the uptake but not the transient of vapor concentrations.
RESULTS AND DISCUSSION
Pure SA Particles
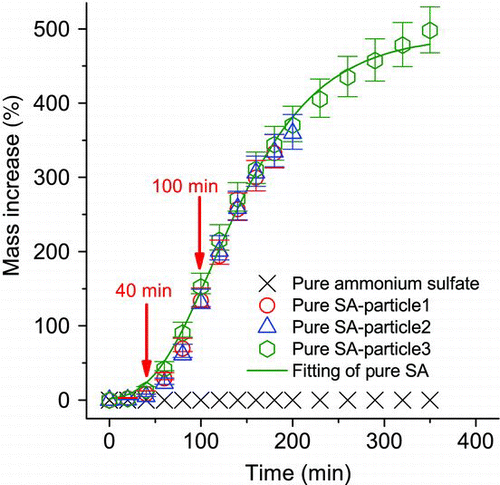
shows the percentage mass increase of levitated ammonium sulfate and SA particles during their exposure to 5.95–6.45 ppm of NL vapor at 180 cm3 min−1 and about 3% RH for 180 to 350 min, respectively. The mass increase of ammonium sulfate particle (crosses) was negligible, showing negligible NL uptake by neutral solid particles. All three SA particles showed a similar uptake trend and the data were fitted by a sigmoidal curve empirically, which will be used to describe the uptake kinetics of pure SA in later discussions. The main sources of errors included the ±5% fluctuation of NL vapor concentration and the ±1% reading error in mass-balancing DC voltage. Moreover, the unstable concentration of NL inside the EDB chamber in the first 3 min may cause a delay of uptake. Assuming the NL uptake has a 3-min delay, the error was estimated by the extrapolation of a linear fit of two successive data that bounded the data point of interest.
Although the fresh pure SA particles were highly acidic initially, the uptake of NL was extremely small and the particles had mass increases of only 5%–13% during the first 40 min. The initial 3-min uptake decay was estimated to have reduced the mass increase by 3%–4%, but it caused insignificant increase to the data point at 40 min. The uncertainty of such delay may also affect the initial uptake data of organic/SA mixed particles and more discussion will be given in the next section. After this lag phase (0–40 min), the SA particles experienced an exponential growth phase where the mass increase surged to 131%–152% at 100 min. This large uptake of mass is attributed to the low-volatility NL products that were formed in strong acidic medium through hydration, hemiacetal/acetal formation, polymerization, and aldol condensation (CitationJang et al. 2004). In general, these catalytic reactions would not consume SA in the particles phase. However, as reported recently (CitationLiggio and Li 2006b; CitationIinuma et al. 2007; CitationSurratt, Kroll et al. 2007), a certain portion of SA may be lost through the formation of OS. CitationSurratt, Kroll et al. (2007) reported that OS contributed to about 4% of the mass of secondary organic carbon, formed from the photooxidation of isoprene, when highly acidic pure SA is used as initial seeds. On the other hand, CitationLiggio and Li (2006b) found that the SOA formed by the reactive uptake of pinonaldehyde by less acidic ammoniated SA particles was composed of 22%–65% OS. Using ammoniated SA particles, CitationIinuma et al. (2007) found that the OS contributed to 19%–64% of the organic mass formed from the uptake of monoterpene oxides. Overall, less OS relative to other SOA were formed in more acidic particles based on these literature data. The secondary organic mass appears to be resulted from acid-catalyzed reaction mechanisms such as oligomerization (CitationJang et al. 2004) and aldol condensation (Li et al. 2010). In the current study, highly acidic particles were employed and the absolute amount of SA is assumed to be constant during the NL uptake. shows that, during the first 100 min, SA particles took up NL vapor at an increasing rate while the absolute amount of SA is not expected to change. This trend suggested that particle acidity may not be the only parameter governing the reactive uptake. The organic fraction may be responsible for promoting the acid-catalyzed reactive uptake, for instance, by increasing the availability (solubility) of gaseous NL in the particle phase. Afterwards, the mass of particles kept on increasing but at a decreasing rate and reached 500% at 350 min. At 500% mass increase, the approximate wt% of SA (including the associated water at ∼3% RH) in the reacted particle is about 17 wt%, which may be higher than that found in most ambient aerosols (CitationPathak et al. 2004). However, highly acidic SA particles do exist in the atmosphere, albeit at short durations. For example, freshly nucleated SA particles (CitationKulmala and Kerminen 2008) and aerosol plumes from power plants (CitationBrock et al. 2002) can also be very acidic. Hence the findings of this work can be important to the understanding of organic uptake and growth of these fresh particles.
OleA*/SA Mixed Particles
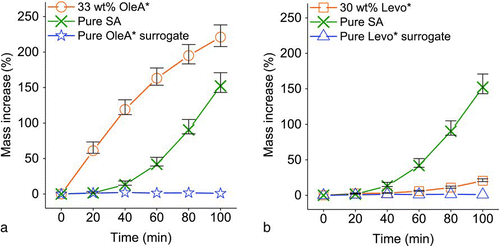
To show that the reactive uptake can be enhanced in the presence of particle-phase organics, the NL uptake experiments were repeated with the same reaction conditions as that of the pure SA particles using oleic acid/SA mixed particles of different initial oleic acid wt% (on a dry weight basis). Oleic acid reacted with concentrated SA to form a hydrophobic orange–brown solution, which turned turbid when mixed with water (Figures S1a and S1b). We term the resulting hydrophobic organics OleA*, which represents the initial organic content prior to NL uptake. Details on the determination of OleA* wt% are given in supplementary information. It is noted that both oleic acid and levoglucosan (see next section) would react with strong SA to form a mixture of products with a significant fraction of unresolved organic matters (CitationHallquist et al. 2009) such as oligomers and OSs. The limited chemical information would not offer a complete prediction to overall properties of the resultant mixture. Alternatively, we tested the overall hydrophilicities of the initial reaction mixtures (OleA*/SA and Levo*/SA) by examining their miscibility with water. The two organics served as proxies of hydrophobic and hydrophilic organics for reactive uptake. The related chemistry about the mixtures is given in the supplementary information. In , the uptake kinetics of a 33 wt% OleA*/SA mixed particle, the pure SA particle, and a 96 wt% OleA*/SA mixed particle, which served as a surrogate of pure OleA* particle for control, were compared. The pure OleA* surrogate particle (stars) only had a very small mass change (<1% at 100 min) during the course of NL exposure that suggested that the mass uptake owing to physical partitioning of NL was negligible. On the other hand, the mass increase of the 33 wt% OleA*/SA mixed particle (circles) was much higher than the pure SA particle (crosses) during the 100-min NL exposure. In particular, OleA* aided the early absorption of gaseous NL for particle-phase reactions and the mass increase of 33 wt% OleA* particle surged to 120% at 40 min, whereas the pure SA was suffered from the initial lag phase during the first 40 min, presumably due to the low solubility of NL in SA. The 33 wt% OleA* particle responded to NL vapor swiftly even at the first 20 min and the error bar of this point was small, suggesting that the high uptake ability of the 33 wt% OleA* particle overwhelmed the particle mass increase and the unstable NL vapor concentration at start (3-min uptake delay) did not affect the measurements significantly. The NL uptake rate of the 33 wt% OleA* particle gradually slowed down. On the other hand, the pure SA particle experienced an exponential uptake phase that in turn narrowed the difference between their mass increases to about 69% at 100 min. At 100 min, the reacted SA particle was composed of 60 wt% of organics after a 150% of organic mass increase. The significant uptake of organics greatly modified the chemical and physical properties of the original SA particle, and the high uptake ability of this reacted SA should not be attributed solely to SA. Rather, we believe that it is due to the presence of the reaction products, which promote NL solubility, like OleA* did. In later sections, the reactive uptake abilities of particles with different organic wt% were presented using the uptake coefficient at that particular organic wt%.
FIG. 3 The uptake kinetics of (a) pure SA, 33 wt% OleA*/SA, and pure OleA* surrogate and (b) pure SA, 30 wt% Levo*/SA, and pure Levo* surrogate.
shows the temporal mass uptake of OleA*/SA particles of different wt% OleA*. The OleA*/SA mixed particles showed a continuous increase in mass with two distinct trends, a physical absorption-like type (thick lines) and a sigmoidal type (thin lines), depending on the initial OleA* wt%. Mixed particles of high initial OleA* wt% (33–85 wt%, circles for example) belonged to the former type. These particles had a relatively large initial (0–40 min) uptake rate, which decreased with increasing initial OleA* wt%, and the subsequent uptake rate of each particle decreased gradually against time as NL reaction products accumulated. The shape of their uptake curves was similar to a physical absorption curve but the magnitude of mass increases was much higher. For instance, at 100 min, the physical uptake of NL by the pure OleA* surrogate (stars, control) increased the particle mass by less than 1% only, whereas the reactive uptake of NL by 41 wt% OleA* particle (triangles) caused a 167% increase in mass. A high OleA* wt% aided the early absorption of NL for particle-phase reactions and, hence, the initial uptake rate was high. However, the amount of SA (H+) available for reactions in these particles was limited, and, hence, increasing the amount of available particle-phase organics by NL uptake and by using higher initial OleA* wt% resulted in the saturation of particle growth and a decreasing initial uptake rate, respectively. At low OleA* wt% (9–28 wt%, squares for example), on the other hand, the mixed particles showed a sigmoidal trend of mass increase that is qualitatively similar to that of pure SA particles shown in , although the uptake of these mixed particles was significantly higher at the same uptake time. There was a relatively slow mass increase initially (0–20 min) due to the limited particle-phase organics for NL absorption. It was followed by a period (20–60 min) of accelerated uptake during which the accumulation of particle-phase organics increased the availability of NL for reaction. The mass increase rate started to decrease at about 60 min when the mixed particle had accumulated a large amount of organics that severely reduced the particle acidity.
FIG. 4 The uptake kinetics of OleA*/SA particles of 9–85 wt% OleA* (data points with lines), pure OleA* surrogate (stars with a dotted line), and the fitting of pure SA (dotted line).
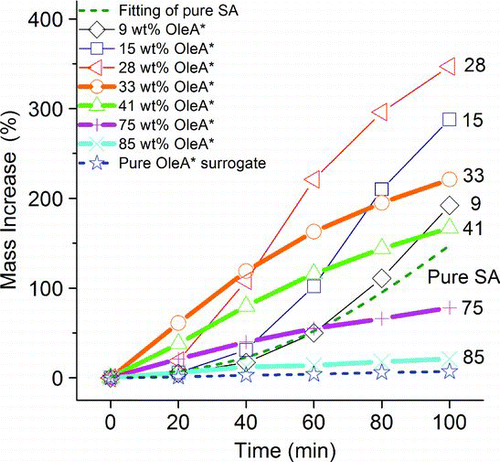
To summarize, OleA* enhanced the initial absorption of NL for acid-catalyzed reactions. For high OleA* wt% particles, the low SA fraction limited the reactions and the uptake rate decreased with the duration of NL uptake, which resulted in uptake trends that are similar to those of physical absorption in shape but with a larger percentage mass uptake. The limiting SA also resulted in the trend of a decreasing initial uptake rate with increasing wt% of OleA* (decreasing wt% of SA). Particles of low OleA* wt%, on the other hand, had excess SA initially and exhibited sigmoidal uptake trends. The initial uptake was slow with little organics present. Overall, the uptake of NL was governed by both the availability of reactants in the particle phase and the particle acidity, which are highly dependent on the wt% of organics.
Levo*/SA Mixed Particles
To examine the role of the chemical nature of organics in NL uptake, we investigated the uptake of NL by hydrophilic levoglucosan/SA mixed particles. The bulk mixture appeared as a dark-brown solution due to reactions between levoglucosan and SA (Figure S2a). We term this mixture Levo*/SA hereafter. Levo* is hydrophilic and mixes well with water (Figure S2b). In , the uptake kinetics of a 30 wt% Levo*/SA mixed particle, the pure SA (same as ), and a 94 wt% Levo*/SA mixed particle, which served as a surrogate of pure Levo* particle, were compared. After the 100-min NL exposure, the pure Levo* surrogate particle (triangles) had only little mass increase (<1% at 100 min), showing that the physical uptake of NL by Levo* was negligible. Unlike the hydrophobic 33 wt% OleA* particle (, circles), the hydrophilic 30 wt% Levo* particle (, squares) showed no enhanced initial NL uptake. The 30 wt% Levo* particle took up mass gradually during the NL exposure, and the mass increase reached 21% at 100 min which is only one seventh of that of the pure SA particle. The hydrophobic NL vapor is incompatible with the hydrophilic Levo* and the retained water in mixed Levo*/SA particle, and hence the partitioning and reactive uptake of NL are suppressed.
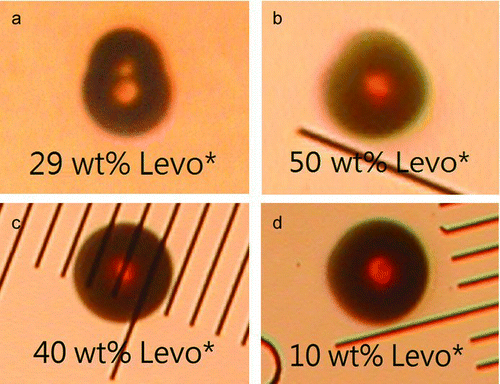
In , the uptake curves of Levo*/SA mixed particles with initial Levo* wt% from 10% to 78% and the sigmoidal fitting of the pure SA are compared. Note that the NL exposure time for the Levo*/SA particles was 200 min, double that of OleA*/SA particles (, 100 min), to achieve a mass increase of about 400%. In general, all Levo*/SA particles exhibited slow mass increase initially because the hydrophilic Levo* has a limited ability to promote the dissolution of gaseous NL for particle-phase reactions. The uptake rate increased after the reaction products of NL had accumulated in the particle phase. For the Levo*/SA particles of high Levo* wt% (60–78 wt%), the mass increased by 3%–7% and 3%–34% at 100 min and 200 min, respectively. These increases were much smaller than that of OleA*/SA particles of comparable OleA* wt% and that of SA particles at the same NL uptake time. Levo* is hydrophilic and its absorption of water even at low RH may further dilute SA in the Levo*/SA particles. The Levo*/SA particles of low Levo* wt% (10–18 wt%) have slightly higher uptake than the pure SA particles. They ended up with mass increases of about 400% after 200 min of NL exposure (circles) whereas some OleA*/SA particles took only 100 min to undergo similar mass increase (e.g., 28 wt% OleA*, ). For the Levo*/SA particles of medium Levo* wt% (29–50 wt%), the percentage of mass increase at 200 min ranged from 100% to 194%, in between that of the low (10–18 wt%) and high Levo* wt% (60–78 wt%) particles. Interestingly, and b show that phase separation occurred in 29 wt% (stars) and 50 wt% (diamonds) Levo* particles, resulting in morphologies and uptake trends that are different from other particles. These two particles were shaped like a sphere with a hemispherical extrusion after the NL uptake, which was presumably caused by the formation of a new phase of reaction products on the surface of the parent Levo*/SA particles. However, the majority of the Levo*/SA mixed particles ended up with a spherical shape ( and d, 40 and 10 wt% Levo*), indicating that phase separation may not have occurred. The 50 and 29 wt% Levo* particles showed significantly reduced uptake rates at mass increases of 150% and 100%, respectively (). It appears that phase separation was related to the observed inhibited uptake of NL, although the Levo* wt% alone cannot explain the separation. Oligomerization of levoglucosan in strong SA (CitationHolmes and Petrucci 2006) could increase its glass transition temperature and result in the formation of a viscous glassy organic phase in the mixed particle (CitationZobrist et al. 2008). It is possible that this viscous fraction inhibited the dissolution of NL and severely reduced the NL uptake rate. Unfortunately, the current technique does not offer a complete explanation and evidence for supporting the observation.
FIG. 5 The uptake kinetics of Levo*/SA particles of 10–78 wt% Levo* (data points with lines), pure Levo* surrogate (triangles with a dotted line), and the fitting of pure SA (dotted line).
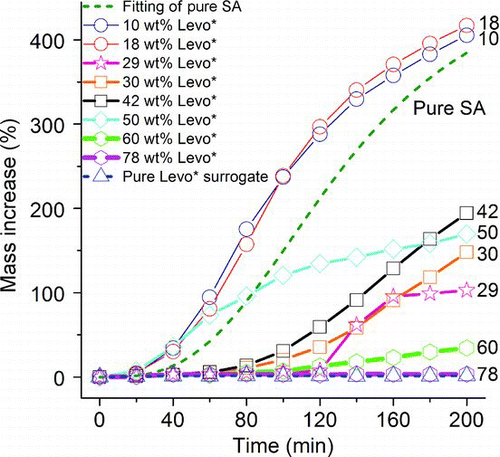
FIG. 6 Photo images of Levo*/SA particles with (6a and 6b) and without (6c and 6d) phase separation.
To summarize, OleA*/SA particles showed a significantly enhanced uptake compared with SA particles at the beginning of NL exposure (0–40 min), whereas Levo*/SA particles did not. As reactions proceeded, accumulation of NL reaction products in the particle phase enhanced further the uptake of NL for both OleA*/SA and Levo*/SA particles, narrowing the difference between the two types of mixed particles. However, the most reactive OleA*/SA particles required about 50% less time to reach a mass increase of about 400% compared with Levo*/SA particles.
Uptake Coefficients
The rate of NL mass uptake (dm/dt) by pure SA particles, OleA*/SA, and Levo*/SA mixed particles was highly dependent on the wt% and the chemical nature of particle-phase organics. Here, we utilize the net uptake coefficient, γ, to describe the rate of NL uptake by these particles at different organic wt%. In general, the uptake of gaseous reactant by liquid aerosol can be described by γ as follows:
The estimated error of γ was ±21% (supplementary information).
Uptake Coefficients at Different Organic wt%
The uptake coefficient at different average organic wt% of the uptake data of SA particles, OleA*/SA, and Levo*/SA mixed particles was used to examine the effect of changing organic wt% on the rate of NL mass uptake of particles (). The average organic wt% is the average wt% of organic (on a dry weight basis) between the two successive mass measurements during uptake where the corresponding γ is calculated. The abscissa error bars show the range of wt% of organics in successive mass measurements. Depending on the particle type, the organic mass can be composed of OleA*, Levo*, and reaction products of NL (NL*). NL* is the only contributor to the average organic wt% of SA particles, but it contributes to the average organic wt% in OleA*/SA and Levo*/SA particles to different degrees as reaction proceeds. NL* is defined to include the reaction products of SA and NL in all three types of particles studied and products of NL and OleA* or Levo* if they react in the presence of SA.
FIG. 7 (a) Plot of uptake coefficients against average organic wt% for fresh SA and SA/NL*. (b) OleA*/SA, (c) Levo*/SA mixed particles with low β (<0.3), (d) OleA*/SA, and (e) Levo*/SA particles with medium β (0.3–0.7) and high β (>0.7).
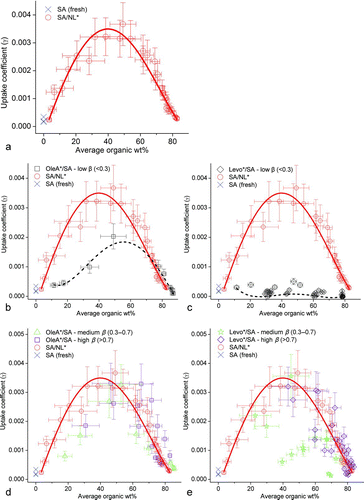
We now discuss the enhanced uptake effect of OleA*, Levo*, and NL* over fresh SA particles (without any organics). We categorized the data of SA particles into two groups: fresh SA particles (initial particles with low uptake) and SA particles with NL* (SA/NL*). The properties of SA/NL* were different from those of fresh SA particles, especially when a large amount of NL* was present (up to 83 wt%). In , the γ of fresh pure SA particles was 1.6−3.4 × 10−4 (crosses), which is within the range of reported values for aldehyde uptake (CitationLiggio and Li 2006a). Accumulation of NL* made the SA particles more hydrophobic and they absorbed more gaseous NL for reactions. The γ of SA/NL* particles increased with the wt% of organics (circles) up to about 40–50 wt% of organics, at which the γ of SA/NL* particles increased to 3.1–3.7 × 10−3, fourteen times higher than the average γ of the parent fresh SA particles. When the wt% of organics increased beyond 50%, the γ decreased because the acidity of particle had significantly weakened. Overall, the γ of SA particles distributed over a bell-shaped curve where the minima occur at both ends and the maximum peaks at 40–50 wt% organics.
The γ of OleA*/SA and Levo*/SA mixed particles of different average organic wt% are shown in . NL* was formed during the uptake of NL by these mixed particles and, hence, NL* also contributed to the average organic wt%. We defined β as the ratio of NL* mass to the total particle-phase organic mass. The particle-phase organics in SA/NL* particles were solely NL* and therefore they had a β of unity. Depending on the amount of initially added organics (OleA* and Levo*) and the extent of NL* formation, the mixed particle had β ranged from zero to unity. We classified γ of these mixed particles according to the following: β < 0.3 was low (squares or diamonds, ), β = 0.3–0.7 was medium (triangles or stars, ) and β > 0.7 was high (squares or rhombuses, ). To show the enhanced uptake effects of OleA* and Levo*, their γ at low β ratios, at which the effect of OleA* or Levo* were dominant, were compared in . The γ of OleA*/SA particles peaked at about 50 wt% organics and tailed at both ends, whereas those of Levo*/SA particles are much smaller, flatter, and comparable to those of pure SA particles (fresh) at all average organic wt%. The highest γ of OleA*/SA particles at low β was about 2.0 × 10−3, approximately sixteen times the average of Levo*/SA particles. This shows that hydrophobic OleA* enhanced reactive uptake but hydrophilic Levo*, which is not compatible with NL, did not. In , the fitted curve (dotted line) of OleA*/SA particles was well under the fitted curve (solid line) of SA/NL* particles suggesting that the enhancement effect of hydrophobic NL* was higher than the OleA*; yet, the highest γ of OleA*/SA particles at low β ratios was still about nine times higher than the average of fresh pure SA particles (crosses). show the γ of different average organic wt% at medium and high β ratios. In general, the γ of OleA*/SA and Levo*/SA particles at high β was comparable to that of the SA/NL* particle. The γ of OleA* at medium β ratio was also similar to that of the SA/NL*. On the other hand, the addition of hydrophilic Levo* suppressed the uptake of NL, and as a result the γ of Levo*/SA at medium β ratio was smaller than that of the SA/NL particles.
Overall, the most effective organic enhanced uptake of NL compared with fresh SA occurred at about 50 wt% of organics. Among the three particle-phase organics, NL* had the highest enhancement effect while OleA* came second and Levo* offered negligible enhancement.
CONCLUSION AND ATMOSPHERIC IMPLICATIONS
We determined the uptake coefficients (γ) of fresh pure SA particles (without organics) and organics/SA mixed particles of different wt% of organics and hydrophobicity. The organics in the mixed particles were either added initially (OleA* and Levo*) or formed during the reactive uptake of NL, i.e., NL*. The hydrophobic SA/NL* and OleA*/SA mixed particles had higher γ than the fresh pure SA particle even though they had lower wt% of SA, suggesting that the acidity of particle is not the only parameter that governs the reactive uptake. Particle-phase organics of a hydrophobic chemical nature enhanced the solubility of NL vapor and more NL was available for particle-phase reactions. Consequently, the rates of mass increases of the mixed particles increased and the highest γ of OleA*/SA (at low β) and NL*/SA of about 50 wt% organics were six times and sixteen times higher than the fresh pure SA particles, respectively. However, particle-phase organics like hydrophilic Levo*/SA particles were incompatible with NL vapor, and the γ of Levo*/SA (at low β) was similar to that of the fresh pure SA particles. Overall, the uptake of NL was determined by two factors, namely, the availability of reactants in particle phase and the particle acidity. The most effective uptake of NL occurred at about 50 wt% of organics (hydrophobic), which is a common organic wt% in ambient aerosols (CitationKanakidou et al. 2005; CitationHallquist et al. 2009).
The uptakes of NL by organic/SA mixtures have a synergistic effect. If SA and OleA* in a mixed OleA*/SA particle take up NL independently, the γ of the mixed particle can be roughly approximated by the linear combination of the pure components. Using the 50 wt% OleA*/SA (at low β) as an example, the predicted γ using the uptake data of fresh SA and pure OleA* is about six times lower than the actual value. This article demonstrates the role of particle-phase organics in the reactive uptake. It is suggested that acid-catalyzed reactive uptake should be examined with the explicit consideration of the role of particle-phase organics that are either initially present or accumulated as a reaction product. This finding indicates that simpler models (based on organic uptake on inorganic aerosols) might underestimate organic uptake on actual ambient aerosol. It is also possible that the synergy between organics and SA in reactive uptake can promote the growth of nucleated particles (CitationZhang and Wexler 2002; CitationZhang et al. 2004; CitationKulmala and Kerminen 2008).
uast_a_567314_sup_18025182.zip
Download Zip (207.3 KB)Acknowledgments
This work was supported by the Research Grants Council of the Hong Kong Special Administrative Region, China (GRF 600208 and GRF 610909).
[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Abbatt , J. P. D. , Broekhuizen , K. and Kumal , P. P. 2005 . Cloud Condensation Nucleus Activity of Internally Mixed Ammonium Sulfate/Organic Acid Aerosol Particles . Atmos. Environ. , 39 ( 26 ) : 4767 – 4778 .
- Barsanti , K. C. and Pankow , J. F. 2004 . Thermodynamics of the Formation of Atmospheric Organic Particulate Matter by Accretion Reactions—Part 1: Aldehydes and Ketones . Atmos. Environ. , 38 ( 26 ) : 4371 – 4382 .
- Brock , C. A. , Washenfelder , R. A. , Trainer , M. , Ryerson , T. B. , Wilson , J. C. , Reeves , J. M. , Huey , L. G. , Holloway , J. S. , Parrish , D. D. , Hubler , G. and Fehsenfeld , F. C. 2002 . Particle Growth in the Plumes of Coal-Fired Power Plants . J. Geophys. Res.—Atmos. , 107 ( D12 ) : 4155 doi:4110.1029/2001JD001062
- Casale , M. T. , Richman , A. R. , Elrod , M. J. , Garland , R. M. , Beaver , M. R. and Tolbert , M. A. 2007 . Kinetics of Acid-Catalyzed Aldol Condensation Reactions of Aliphatic Aldehydes . Atmos. Environ. , 41 ( 29 ) : 6212 – 6224 .
- Chan , L. P. , Lee , A. K. Y. and Chan , C. K. 2010 . Gas-Particle Partitioning of Alcohol Vapors on Organic Aerosols . Environ. Sci. Technol. , 44 ( 1 ) : 257 – 262 .
- Choi , M. Y. and Chan , C. K. 2002 . The Effects of Organic Species on the Hygroscopic Behaviors of Inorganic Aerosols . Environ. Sci. Technol. , 36 ( 11 ) : 2422 – 2428 .
- Connelly , B. M. and Tolbert , M. A. 2010 . Reaction of Isoprene on Thin Sulfuric Acid Films: Kinetics, Uptake, and Product Analysis . Environ. Sci. Technol. , 44 ( 12 ) : 4603 – 4608 .
- Cruz , C. N. and Pandis , S. N. 2000 . Deliquescence and Hygroscopic Growth of Mixed Inorganic-Organic Atmospheric Aerosol . Environ. Sci. Technol. , 34 ( 20 ) : 4313 – 4319 .
- Davidovits , P. , Kolb , C. E. , Williams , L. R. , Jayne , J. T. and Worsnop , D. R. 2006 . Mass Accommodation and Chemical Reactions at Gas-Liquid Interfaces . Chem. Rev. , 106 ( 4 ) : 1323 – 1354 .
- Davis , E. J. 1997 . A History of Single Aerosol Particle Levitation . Aerosol. Sci. Tech. , 26 ( 3 ) : 212 – 254 .
- Esteve , W. and Noziere , B. 2005 . Uptake and Reaction Kinetics of Acetone, 2-Butanone, 2,4-Pentanedione, and Acetaldehyde in Sulfuric Acid Solutions . J. Phys. Chem. A , 109 ( 48 ) : 10920 – 10928 .
- Garland , R. M. , Elrod , M. J. , Kincaid , K. , Beaver , M. R. , Jimenez , J. L. and Tolbert , M. A. 2006 . Acid-Catalyzed Reactions of Hexanal on Sulfuric Acid Particles: Identification of Reaction Products . Atmos. Environ. , 40 ( 35 ) : 6863 – 6878 .
- Hallquist , M. , Wenger , J. C. , Baltensperger , U. , Rudich , Y. , Simpson , D. , Claeys , M. , Dommen , J. , Donahue , N. M. , George , C. , Goldstein , A. H. , Hamilton , J. F. , Herrmann , H. , Hoffmann , T. , Iinuma , Y. , Jang , M. , Jenkin , M. E. , Jimenez , J. L. , Kiendler-Scharr , A. , Maenhaut , W. , McFiggans , G. , Mentel , T. F. , Monod , A. , Prevot , A. S. H. , Seinfeld , J. H. , Surratt , J. D. , Szmigielski , R. and Wildt , J. 2009 . The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues . Atmos. Chem. Phys. , 9 ( 14 ) : 5155 – 5236 .
- Holmes , B. J. and Petrucci , G. A. 2006 . Water-Soluble Oligomer Formation from Acid-Catalyzed Reactions of Levoglucosan in Proxies of Atmospheric Aqueous Aerosols . Environ. Sci. Technol. , 40 ( 16 ) : 4983 – 4989 .
- Iinuma , Y. , Muller , C. , Berndt , T. , Boge , O. , Claeys , M. and Herrmann , H. 2007 . Evidence for the Existence of Organosulfates from Beta-Pinene Ozonolysis in Ambient Secondary Organic Aerosol . Environ. Sci. Technol. , 41 ( 19 ) : 6678 – 6683 .
- Jacobson , M. C. , Hansson , H. C. , Noone , K. J. and Charlson , R. J. 2000 . Organic Atmospheric Aerosols: Review and State of the Science . Rev. Geophys. , 38 ( 2 ) : 267 – 294 .
- Jang , M. , Czoschke , N. M. and Northcross , A. L. 2004 . Atmospheric Organic Aerosol Production by Heterogeneous Acid-Catalyzed Reactions. . Chemphyschem , 5 ( 11 ) : 1647 – 1661 .
- Jang , M. S. , Czoschke , N. M. , Lee , S. and Kamens , R. M. 2002 . Heterogeneous Atmospheric Aerosol Production by Acid-Catalyzed Particle-Phase Reactions . Science , 298 ( 5594 ) : 814 – 817 .
- Kanakidou , M. , Seinfeld , J. H. , Pandis , S. N. , Barnes , I. , Dentener , F. J. , Facchini , M. C. , Van Dingenen , R. , Ervens , B. , Nenes , A. , Nielsen , C. J. , Swietlicki , E. , Putaud , J. P. , Balkanski , Y. , Fuzzi , S. , Horth , J. , Moortgat , G. K. , Winterhalter , R. , Myhre , C. E. L. , Tsigaridis , K. , Vignati , E. , Stephanou , E. G. and Wilson , J. 2005 . Organic Aerosol and Global Climate Modelling: A Review . Atmos. Chem. Phys. , 5 : 1053 – 1123 .
- Kroll , J. H. , Ng , N. L. , Murphy , S. M. , Varutbangkul , V. , Flagan , R. C. and Seinfeld , J. H. 2005 . Chamber Studies of Secondary Organic Aerosol Growth by Reactive Uptake of Simple Carbonyl Compounds. . J. Geophys. Res.—Atmos. , 110 D23):D23207, doi: 23210.21029/22005JD006004.
- Kroll , J. H. and Seinfeld , J. H. 2008 . Chemistry of Secondary Organic Aerosol: Formation and Evolution of Low-Volatility Organics in the Atmosphere . Atmos. Environ. , 42 ( 16 ) : 3593 – 3624 .
- Kulmala , M. and Kerminen , V. M. 2008 . On the Formation and Growth of Atmospheric Nanoparticles . Atmos. Res. , 90 ( 2–4 ) : 132 – 150 .
- Lee , A. K. Y. , Li , Y. J. , Lau , A. P. S. and Chan , C. K. 2008 . A Re-Evaluation on the Atmospheric Significance of Octanal Vapor Uptake by Acidic Particles: Roles of Particle Acidity and Gas-Phase Octanal Concentration . Aerosol. Sci. Tech. , 42 ( 12 ) : 992 – 1000 .
- Levitt , N. P. , Zhao , J. and Zhang , R. Y. 2006 . Heterogeneous Chemistry of Butanol and Decanol with Sulfuric Acid: Implications for Secondary Organic Aerosol Formation . J. Phys. Chem. A , 110 ( 49 ) : 13215 – 13220 .
- Liggio , J. and Li , S. M. 2006a . Organosulfate Formation During the Uptake of Pinonaldehyde on Acidic Sulfate Aerosols . Geophys. Res. Lett. , 33 ( 13 ) L13808, doi: 13810.11029/12006GL026079.
- Liggio , J. and Li , S. M. 2006b . Reactive Uptake of Pinonaldehyde on Acidic Aerosols . J. Geophys. Res.—Atmos. , 111 ( D24 ) D24303, doi: 24310.21029/22005JD006978.
- Liggio , J. and Li , S. M. 2008 . Reversible and Irreversible Processing of Biogenic Olefins on Acidic Aerosols . Atmos. Chem. Phys. , 8 ( 7 ) : 2039 – 2055 .
- Liggio , J. , Li , S. M. , Brook , J. R. and Mihele , C. 2007 . Direct Polymerization of Isoprene and Alpha-Pinene on Acidic Aerosols . Geophys. Res. Lett. , 34 ( 5 ) L05814, doi: 05810.01029/02006GL028468.
- Limbeck , A. , Kulmala , M. and Puxbaum , H. 2003 . Secondary Organic Aerosol Formation in the Atmosphere via Heterogeneous Reaction of Gaseous Isoprene on Acidic Particles . Geophys. Res. Lett. , 30 ( 19 ) : 1996 doi: 1910.1029/2003gl017738.
- Minerath , E. C. and Elrod , M. J. 2009 . Assessing the Potential for Diol and Hydroxy Sulfate Ester Formation from the Reaction of Epoxides in Tropospheric Aerosols . Environ. Sci. Technol. , 43 ( 5 ) : 1386 – 1392 .
- Noziere , B. and Esteve , W. 2007 . Light-Absorbing Aldol Condensation Products in Acidic Aerosols: Spectra, Kinetics, and Contribution to the Absorption Index . Atmos. Environ. , 41 ( 6 ) : 1150 – 1163 .
- Noziere , B. and Riemer , D. D. 2003 . The Chemical Processing of Gas-Phase Carbonyl Compounds by Sulfuric Acid Aerosols: 2,4-Pentanedione . Atmos. Environ. , 37 ( 6 ) : 841 – 851 .
- Noziere , B. , Voisin , D. , Longfellow , C. A. , Friedli , H. , Henry , B. E. and Hanson , D. R. 2006 . The Uptake of Methyl Vinyl Ketone, Methacrolein, and 2-Methyl-3-Butene-2-ol onto Sulfuric Acid Solutions . J. Phys. Chem. A , 110 ( 7 ) : 2387 – 2395 .
- Odum , J. R. , Hoffmann , T. , Bowman , F. , Collins , D. , Flagan , R. C. and Seinfeld , J. H. 1996 . Gas/Particle Partitioning and Secondary Organic Aerosol Yields . Environ. Sci. Technol. , 30 ( 8 ) : 2580 – 2585 .
- Offenberg , J. H. , Lewandowski , M. , Edney , E. O. , Kleindienst , T. E. and Jaoui , M. 2009 . Influence of Aerosol Acidity on the Formation of Secondary Organic Aerosol from Biogenic Precursor Hydrocarbons . Environ. Sci. Technol. , 43 ( 20 ) : 7742 – 7747 .
- Pankow , J. F. 1994 . An Absorption-Model of Gas-Particle Partitioning of Organic-Compounds in the Atmosphere . Atmos. Environ. , 28 ( 2 ) : 185 – 188 .
- Pathak , R. K. , Yao , X. H. and Chan , C. K. 2004 . Sampling Artifacts of Acidity and Ionic Species in PM2 . 5 , 38 ( 1 ) : 254 – 259 .
- Peng , C. , Chan , M. N. and Chan , C. K. 2001 . The Hygroscopic Properties of Dicarboxylic and Multifunctional Acids: Measurements and UNIFAC Predictions . Environ. Sci. Technol. , 35 ( 22 ) : 4495 – 4501 .
- Pope , C. A. and Dockery , D. W. 2006 . Health Effects of Fine Particulate Air Pollution: Lines that Connect . J. Air Waste Manage. , 56 ( 6 ) : 709 – 742 .
- Poschl , U. , Rudich , Y. and Ammann , M. 2007 . Kinetic Model Framework for Aerosol and Cloud Surface Chemistry and Gas-Particle Interactions—Part 1: General Equations, Parameters, and Terminology . Atmos. Chem. Phys. , 7 ( 23 ) : 5989 – 6023 .
- Rudich , Y. , Donahue , N. M. and Mentel , T. F. 2007 . Aging of Organic Aerosol: Bridging the Gap Between Laboratory and Field Studies . Annu. Rev. Phys. Chem. , 58 : 321 – 352 .
- Saxena , P. , Hildemann , L. M. , Mcmurry , P. H. and Seinfeld , J. H. 1995 . Organics Alter Hygroscopic Behavior of Atmospheric Particles . J. Geophys. Res.—Atmos. , 100 ( D9 ) : 18755 – 18770 .
- Schauer , J. J. , Kleeman , M. J. , Cass , G. R. and Simoneit , B. R. T. 1999a . Measurement of Emissions from Air Pollution Sources. 1. C-1 Through C-29 Organic Compounds from Meat Charbroiling . Environ. Sci. Technol. , 33 ( 10 ) : 1566 – 1577 .
- Schauer , J. J. , Kleeman , M. J. , Cass , G. R. and Simoneit , B. R. T. 1999b . Measurement of Emissions from Air Pollution Sources. 2. C-1 Through C-30 Organic Compounds from Medium Duty Diesel Trucks . Environ. Sci. Technol. , 33 ( 10 ) : 1578 – 1587 .
- Simoneit , B. R. T. , Schauer , J. J. , Nolte , C. G. , Oros , D. R. , Elias , V. O. , Fraser , M. P. , Rogge , W. F. and Cass , G. R. 1999 . Levoglucosan, a Tracer for Cellulose in Biomass Burning and Atmospheric Particles . Atmos. Environ. , 33 ( 2 ) : 173 – 182 .
- Song , C. , Zaveri , R. A. , Alexander , M. L. , Thornton , J. A. , Madronich , S. , Ortega , J. V. , Zelenyuk , A. , Yu , X. Y. , Laskin , A. and Maughan , D. A. 2007 . Effect of Hydrophobic Primary Organic Aerosols on Secondary Organic Aerosol Formation from Ozonolysis of Alpha-Pinene . Geophys. Res. Lett. , 34 ( 20 ) L20803, doi: 20810.21029/22007GL030720.
- Surratt , J. D. , Chan , A. W. H. , Eddingsaas , N. C. , Chan , M. N. , Loza , C. L. , Kwan , A. J. , Hersey , S. P. , Flagan , R. C. , Wennberg , P. O. and Seinfeld , J. H. 2010 . Reactive Intermediates Revealed in Secondary Organic Aerosol Formation from Isoprene . Proc. Nat. Acad. Sci. U.S.A. , 107 ( 15 ) : 6640 – 6645 .
- Surratt , J. D. , Gomez-Gonzalez , Y. , Chan , A. W. H. , Vermeylen , R. , Shahgholi , M. , Kleindienst , T. E. , Edney , E. O. , Offenberg , J. H. , Lewandowski , M. , Jaoui , M. , Maenhaut , W. , Claeys , M. , Flagan , R. C. and Seinfeld , J. H. 2008 . Organosulfate Formation in Biogenic Secondary Organic Aerosol . J. Phys. Chem. A , 112 ( 36 ) : 8345 – 8378 .
- Surratt , J. D. , Kroll , J. H. , Kleindienst , T. E. , Edney , E. O. , Claeys , M. , Sorooshian , A. , Ng , N. L. , Offenberg , J. H. , Lewandowski , M. , Jaoui , M. , Flagan , R. C. and Seinfeld , J. H. 2007 . Evidence for Organosulfates in Secondary Organic Aerosol . Environ. Sci. Technol. , 41 ( 2 ) : 517 – 527 .
- Surratt , J. D. , Lewandowski , M. , Offenberg , J. H. , Jaoui , M. , Kleindienst , T. E. , Edney , E. O. and Seinfeld , J. H. 2007 . Effect of Acidity on Secondary Organic Aerosol Formation from Isoprene . Environ. Sci. Technol. , 41 ( 15 ) : 5363 – 5369 .
- Takahama , S. , Davidson , C. I. and Pandis , S. N. 2006 . Semicontinuous Measurements of Organic Carbon and Acidity During the Pittsburgh Air Quality Study: Implications for Acid-Catalyzed Organic Aerosol Formation . Environ. Sci. Technol. , 40 ( 7 ) : 2191 – 2199 .
- Tong , C. H. , Blanco , M. , Goddard , W. A. and Seinfeld , J. H. 2006 . Secondary Organic Aerosol Formation by Heterogeneous Reactions of Aldehydes and Ketones: A Quantum Mechanical Study . Environ. Sci. Technol. , 40 ( 7 ) : 2333 – 2338 .
- Vaden , T. D. , Song , C. , Zaveri , R. A. , Imre , D. and Zelenyuk , A. 2010 . Morphology of Mixed Primary and Secondary Organic Particles and the Adsorption of Spectator Organic Gases During Aerosol Formation . Proc. Nat. Acad. Sci. U.S.A. , 107 ( 15 ) : 6658 – 6663 .
- Wang , L. , Lal , V. , Khalizov , A. F. and Zhang , R. Y. 2010 . Heterogeneous Chemistry of Alkylamines with Sulfuric Acid: Implications for Atmospheric Formation of Alkylaminium Sulfates . Environ. Sci. Technol. , 44 ( 7 ) : 2461 – 2465 .
- Williams , M. B. , Michelsen , R. R. H. , Axson , J. L. and Iraci , L. T. 2010 . Uptake of Acetone, Acetaldehyde and Ethanol in Cold Sulfuric Acid Solutions Containing Organic Material: Carbon Accretion Mechanisms . Atmos. Environ. , 44 ( 9 ) : 1145 – 1151 .
- Zhang , K. M. and Wexler , A. S. 2002 . A Hypothesis for Growth of Fresh Atmospheric Nuclei . J. Geophys. Res.—Atmos. , 107 ( D21 ) 4577, doi: 4510.1029/2002JD002180.
- Zhang , Q. , Alfarra , M. R. , Worsnop , D. R. , Allan , J. D. , Coe , H. , Canagaratna , M. R. and Jimenez , J. L. 2005 . Deconvolution and Quantification of Hydrocarbon-Like and Oxygenated Organic Aerosols Based on Aerosol Mass Spectrometry . Environ. Sci. Technol. , 39 ( 13 ) : 4938 – 4952 .
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Allan , J. D. , Coe , H. , Ulbrich , I. , Alfarra , M. R. , Takami , A. , Middlebrook , A. M. , Sun , Y. L. , Dzepina , K. , Dunlea , E. , Docherty , K. , DeCarlo , P. F. , Salcedo , D. , Onasch , T. , Jayne , J. T. , Miyoshi , T. , Shimono , A. , Hatakeyama , S. , Takegawa , N. , Kondo , Y. , Schneider , J. , Drewnick , F. , Borrmann , S. , Weimer , S. , Demerjian , K. , Williams , P. , Bower , K. , Bahreini , R. , Cottrell , L. , Griffin , R. J. , Rautiainen , J. , Sun , J. Y. , Zhang , Y. M. and Worsnop , D. R. 2007 . Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes . Geophys. Res. Lett. , 34 ( 13 ) L13801, doi: 13810.11029/12007GL029979.
- Zhang , Q. , Stanier , C. O. , Canagaratna , M. R. , Jayne , J. T. , Worsnop , D. R. , Pandis , S. N. and Jimenez , J. L. 2004 . Insights into the Chemistry of New Particle Formation and Growth Events in Pittsburgh Based on Aerosol Mass Spectrometry . Environ. Sci. Technol. , 38 ( 18 ) : 4797 – 4809 .
- Zhao , J. , Levitt , N. P. and Zhang , R. Y. 2005 . Heterogeneous Chemistry of Octanal and 2,4-Hexadienal with Sulfuric Acid . Geophys. Res. Lett. , 32 ( 9 ) L09802, doi: 09810.01029/02004GL022200.
- Zobrist , B. , Marcolli , C. , Pedernera , D. A and Koop , T. 2008 . Do Atmospheric Aerosols form Glasses? . Atmos. Chem. Phys. , 8 ( 17 ) : 5221 – 5244 .