Abstract
Microfiltration (MF) and ultrafiltration (UF) membrane filtration and separation of insoluble particles and dissolved solid in water, aerosolization, and subsequent sizing and counting of airborne particles were used to determine the number concentration of insoluble particles (#/ml) and mass concentration of dissolved solids (ppm) in water. By using a variety of solutions and their mixtures, we determined the penetration of insoluble particles and dissolved solids via the MF and UF membranes, as well as established a relationship between particle quantity in water and air. Both sub-500 nm insoluble particles and most dissolved solids passed through the MF membrane, while only dissolved solids passed through the UF membrane, enabling separation of waterborne insoluble particles and dissolved solids. Accordingly, we estimated the submicrometer particle number concentration and dissolved species mass concentrations via the proposed technique with 15% and 16% accuracy, respectively. Further, we examined the effects of mixing insoluble particles and dissolved solids with aerosolized particles by using a mixture of silica particles (insoluble particles) and artificial seawater (25,000 ppm) containing various dissolved ions. Afterward, we applied the proposed technique to quantify insoluble particles and dissolved solids in seawater. Concentrations of insoluble particles in MF membrane-filtrated seawater sampled from the cities of Pohang, Taean and Yeosu in Korea were 1.16 × 1012, 5.45 × 1011, and 4.82 × 1011 #/ml, respectively; mass concentrations of dissolved solids were 24362, 23358, and 24640 ppm, respectively, concurring closely with values measured by the Total Dissolved Solids (TDS) meter. Our quantification technique will be useful in monitoring both insoluble submicrometer particles and dissolved solids to examine water quality and primary marine aerosol formation in seawater more effectively.
INTRODUCTION
Quantification of both insoluble particles and dissolved solids has great import on water quality, water filtration, membrane fouling during filtration or desalination via reverse osmosis, wastewater treatment, and liquid filtration (CitationSchippers and Verdouw 1980; CitationZhu and Elimelech 1997; CitationSong et al. 2004). For example, waterborne insoluble particles cause permeate flux decline, pressure increase, as well as decreased membrane longevity during membrane filtration and reverse osmosis desalination (CitationSakol and Konieczny 2004; CitationKumar et al. 2006). Moreover, dissolved solids, i.e., initially waterborne ionic phases, can cake membrane surfaces, clog pores, reduce pore diameters due to absorption, as well as crystallize into particle phases, which affects membrane filtration (CitationHowe and Clark 2002; CitationNing and Troyer 2007). Further, both insoluble particles and dissolved solids in seawater play an important role in marine aerosol formation, leading to climate change (CitationLatham and Smith 1990). For example, bubble bursting due to wave breaking from the sea surfaces, coupled with enriched inorganic sea salts and organic matter, can contribute to primary marine aerosol production (CitationAndreas 1998; CitationMårtensson et al. 2003). Since mixtures of dissolved ions and insoluble particles form primary marine aerosols, determining dissolved ion and insoluble particle amounts in seawater before aerosolization is critical.
A quite common method for quantifying insoluble particles in water is light scattering, i.e., dynamic light scattering, directly determining particle size and quantity by utilizing scattered particle light. However, accurate nanoparticle quantification is limited due to low scattered light intensity, as well as the effects of bubbles and dissolved solids in water (CitationLenggoro et al. 2002; CitationIto et al. 2004). Although requiring extensive calibration to account for particle and membrane interaction, field-flow fractionation (FFF) is used to determine waterborne nanoparticle size by utilizing corresponding diffusion behavior and membrane systems (CitationCho et al. 2006). To determine waterborne dissolved solids, a Total Dissolved Solid (TDS) meter is utilized (CitationGustafson and Behrman 1939), measuring conductivity to determine ionic phase quantity (CitationFinlayson 1979). However, if a mixture of insoluble particles and dissolved solids exit simultaneously in water, TDS meter accuracy will deteriorate (CitationPatten 1901). Recently, measurements of insoluble particle size and quantity have been attempted by means of aerosol measurement techniques (CitationWiedensohler et al. 1991; CitationGreenwald et al. 2005). For example, an atomizer or electrospary (CitationEninger et al. 2009) can convert particles in water or other solvents into airborne particles (CitationMikuška 2004; CitationMahurin and Cheng 2007), allowing airborne particle sizing and quantification via differential mobility analyzers (DMA) and condensation particle counters (CPC; CitationLenggoro et al. 2002, Citation2007; CitationLing et al. 2010). With the use of an electrospary, researchers have dispersed nanometer-sized colloidal particles into small droplets—followed by solvent evaporation—and have determined size by the DMA and CPC. Although attempted for determining waterborne particle quantity, regarding complex mixtures like seawater, researchers struggle to determine both particle size and quantity (e.g., number concentration of particles in water) as well as quantity of dissolved species using such techniques. In particular, dissolved solids crystallize into airborne particles during drying, affecting insoluble particle quantification.
Accordingly, we developed a technique relying on a membrane filtration-differential mobility analyzer (MF-DMA) to examine both waterborne insoluble particles and dissolved solids (CitationPark et al. 2009a, 2009b). Such a technique was based not only on membrane filtration, separation, and aerosolization, but aerosol sizing and counting as well. In particular, the technique initially separated insoluble particles and dissolved solids using serial membrane filtration, quantifying each separately. The removal of large insoluble particles (>500 nm) was then rendered possible after microfiltration (MF), leaving only dissolved solids after ultrafiltration (UF). Here, we evaluated the MF-DMA technique for separation and penetration of various insoluble particles and dissolved solids through membrane filtration, establishing a relationship of water and air with regards to quantity. To study the mixing state of particles aerosolized from waterborne insoluble particles and dissolved solids, we used the tandem differential mobility analyzer (TDMA; CitationRader and McMurry 1986; Hameri et al. 2002; Mikhailov et al. 2004). Afterward, we applied the technique to quantify insoluble particles and dissolved solids in actual seawater.
EXPERIMENTAL METHODS
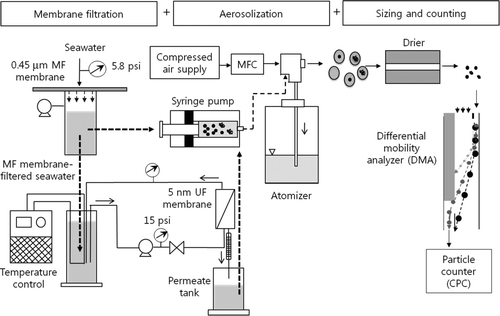
shows a schematic of the proposed MF-DMA technique, being described in detail in our previous paper (CitationPark et al. 2009a, 2009b). To separate waterborne insoluble particles and dissolved solids, we filtered the water solution by serially employing MF and UF membranes. A dead-end filtration unit was used for MF membrane filtration (0.45 μm pore size, mixed cellulose ester membrane; Advantec, Tokyo, Japan). Operating pressure was controlled at 5.8 Ψ during filtration, the MF membrane-filtered solution being further filtered with a polyethersulfone (PES) UF membrane (10,000 Da; GE Osmonics, Le Mee Sur Seine, France) via a laboratory scale cross-flow filtration unit with an active area of 56.8 cm2. Before UF membrane filtration, high purity deionized (DI) water was filtered through the membranes for 4–5 h to stabilize pressure and flow rate and remove possible impurities from the membrane surface. Solution temperature was maintained at 24 ∼ 25 °C using a chiller in the solution tank. Operating pressure, as well as the permeate and cross-flow rate of the cross-flow filtration unit, were 15 Ψ, 7.5 ∼ 8.3 ml/min, and 300 ml/min, respectively. Subsequently, each MF and UF membrane-treated solution was aerosolized. The MF membrane-filtered solution contained both insoluble particles and dissolved solids, while the UF membrane-filtered solution had only dissolved solids since all insoluble particles were removed due to small UF membrane pore sizes, i.e., ∼5 nm. Insoluble particles of known sizes, i.e., PSL, and dissolved solids of known concentrations, i.e., known mass concentrations of dissolved solids in artificial seawater, were used to evaluate insoluble particle and dissolved solid penetration through MF and UF membranes.
We pumped each MF and UF membrane-filtered solution into a constant output atomizer (TSI 3075, USA) by a syringe pump. The constant output atomizer produced a constant number of droplets that were subsequently dried out by a series of diffusion driers. We maintained a constant feed rate to the atomizer and a volumetric flow rate, i.e., output flow, from the atomizer. Further, with high purity DI water filtered by a series of UF and reverse osmosis (RO) membranes, i.e., non-porous, we minimized the effects of impurity solids in DI water. We observed very little peak for impurity, i.e., residue, particles from measured size distributions of commercial PSL particles. (Impurity particles were usually observed with the use of regular DI water.) Measurement was completed within the initial 15 min, and the solution was not recirculated into the reservoir in order to minimize any change in solution concentration during atomization. A current solution concentration range of 0.001 ∼ 0.2% wt and a droplet size of ∼0.35 μm ensured the presence of single particles in each droplet, preventing doublets, i.e., two particles in one droplet (CitationLing et al. 2010). After drying, we introduced particles into the DMA and CPC system. The DMA separated the particles according to electrical mobility, quantities of certain sized particles being counted by the CPC in real time. Accordingly, a size distribution within 20–600 nm was provided. By integrating the size distribution, we determined that the total particle concentration fell within 20–600 nm. Particle mass concentration was also calculated using particle density and size information.
Airborne particles aerosolized from the MF membrane-filtered solution included both insoluble particles and dissolved solids, while for the UF-membrane filtered solution, airborne particles were formed from droplets containing only dissolved solids. Thus, aerosol output differences between the MF and UF membrane-filtered solutions were used to estimate waterborne insoluble particle concentration after taking into account the effects of mixing insoluble particles and dissolved solids with aerosolized particles, as well as the predetermined relationship between water and air particle quantity. In addition, airborne particle mass concentration produced from the UF-membrane filtered solution, i.e., particles formed from droplets containing only dissolved solids, were converted to dissolved solid mass concentration in water via a predetermined relationship between air and water. To establish such a relationship, diverse particles and mixtures were used. PSL particles of 60 nm, 90 nm, 135 nm, 300 nm, 430 nm, and 500 nm (Duke Scientific, Fremont, CA, USA); silica particles (SILNOS-3M, ABC Nanotech, Daejeon, Korea, Korea); CaCO3 (Sigma-Aldrich, St. Louis, MO, USA); and MgCO3 (Sigma-Aldrich, St. Louis, MD, USA) were used to represent waterborne insoluble particles, while NaCl (Sigma-Aldrich, St. Louis, MO, USA), CaCl2 (Sigma-Aldrich, St. Louis, MO, USA), KCl (Sigma-Aldrich, St. Louis, MO, USA), MgCl2 (Sigma-Aldrich, St. Louis, MO, USA), and artificial seawater (Sigma-Aldrich, St. Louis, MD, USA) were used to represent waterborne dissolved solids. Mixtures of insoluble particles and/or dissolved solids with various mixing ratios were prepared in DI water for 15 min via dispersion. As to natural seawaters comprised of complex mixtures of insoluble particles and dissolved solids, we sampled such mixtures at three different cities in Korea (Yeosu on the South Sea, Taean on the West Sea, and Pohang on the East Sea of Korea). The seawater was filtered by MF and UF membranes into storage bottles immediately after sampling, to minimize biological activity and insoluble particles, and dissolved solid interaction. We used a TDS meter (OAKTON, Singapore) to determine dissolved solid mass concentrations.
We used the TDMA to examine the mixing state, i.e., external and internal, of airborne particles by measuring the hygroscopic growth factor of particles (CitationRader and McMurry 1986). We were able to separate hygroscopic and nonhygroscopic particles by means of various hygroscopic species particle properties. Moreover, particles were collected on a transmission electron microscopy (TEM) grid using a nanometer aerosol sampler (TSI 3089, Shoreview, MN, USA) for further mixing state analysis, morphology, and elemental composition via TEM (JEOL JEM-2100F) and energy dispersive spectroscopy (EDS) (Oxford INCA x-sight).
RESULTS AND DISCUSSION
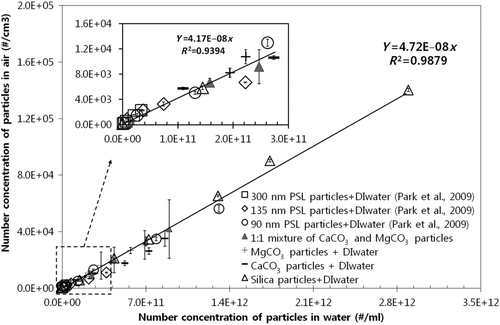
In our previous study (Park et al. 2009a), we demonstrated the linear relationship between airborne particle concentration (#/cm3) generated from water solutions and waterborne particles (#/ml) by utilizing various PSL particle sizes (90 nm, 135 nm, and 300 nm) within a solution concentration range of 0.001% ∼ 0.05% wt. In particular, we observed that airborne particle sizes were in substantial agreement, i.e., within ∼12%, with those provided by the manufacturer. In this study, we tested more diverse insoluble particles such as silica, CaCO3, MgCO3, and PSL, as well as corresponding mixtures. The solution was filtered by a MF membrane to focus the scope at sub-500 nm particles. The use of RO membrane-filtered DI water enabled us to minimize residue particle effects. A solution concentration range of 0.001% ∼ 0.2% wt and a small droplet size of ∼0.35 μm ensured the presence of single particles in each droplet. We identified a single linear relationship, i.e., one universal line, between air (#/cm3) and water (#/ml) particle concentration for different types of insoluble particles and corresponding mixtures, as shown in . Moreover, regardless of particle composition, such a linear relationship was applicable in determining unknown waterborne insoluble submicrometer particle concentration by measuring airborne particle concentration for a solution concentration range of 0.001% ∼ 0.2% wt. The data obtained from waterborne particles of known sizes and concentrations, e.g., CaCO3, MgCO3, and a 1:1 mixture of CaCO3 and MgCO3 particles, revealed a concentration predictability within 15% for unknown waterborne submicrometer particles via such a relationship. Excluded here, the data obtained from a 90 nm and 300 nm 1:1 mixture of waterborne PSL particles also confirmed that clear separation of such particles occurred according to airborne particle size distribution, peak sizes corresponding with predetermined values of ∼3.7%. Our results suggested that MF-membrane filtration for ultra-500 nm particle removal, the use of RO membrane-filtered DI water for residue removal, constant droplet generation, as well as a specified concentration range, effectively determined waterborne submicrometer particle concentration and size.
Figure 2 A single linear relationship between particle concentration in air (#/cm3) and water (#/ml), i.e., one universal line, for various types of insoluble particles and their mixture.
We measured diverse dissolved waterborne solid mass concentrations, e.g., artificial seawater, NaCl, KCl, CaCl2, and MgCl2, and corresponding mixtures, e.g., a 1:1 mixture of NaCl and MgCl2, a 1:1 mixture of NaCl and KCl, and a 1:4 mixture of NaCl and MgCl2, within a concentration range of 200–4000 ppm by using the MF-DMA technique in question. One such example is shown in (particle number size distribution acting as a function of solution concentration). As shown, concentration and peak size gradually increased as the solution concentration increased from 200 to 3500 ppm. The upper solution concentration limit, in which linear and solution concentrations ceased to increase, was found at ∼3500 ppm. Since dissolved species in droplets evaporated through a series of driers—being crystallized into particles—the final airborne particle size depended on both composition and dissolved solid quantity. We found that the size of airborne particles crystallized through the drying process, i.e., dissolved species, was related to the ionic radius and solute conductivity. The geometric mean diameters (GMDs) of airborne particles formed from the waterborne dissolved species, including solution conductivity and ionic radius, are summarized in . Solution conductivity was related to molecular weight (CitationIto et al. 1987) and ionization energy of the dissolved species (CitationFukushima et al. 1998). We found that the GMD of airborne CaCl2 solution particles was larger than that of the NaCl solution at an identical solution concentration—possibly occurring due to higher CaC12 solution conductivity—leading to higher ion-presence in droplets. Although NaCl and KCl solutions were similar, the KCl solution particles demonstrated higher conductivity than those of the NaCl solution—possibly occurring because the KC1 ionic radius was larger than that of NaCl—leading to larger particles. Thus, our data showed that airborne particle size via dissolved waterborne species was dependent on both conductivity and the solute ionic radius; further, a higher solution conductivity and solute ionic radius led to larger airborne particle formation after drying. In addition, as the solution's concentration increased, the resultant particle size increased, i.e., higher concentrated droplets produced larger particles after drying. Since sea spray droplets with various ions dissolved in seawater are emitted directly from the sea surface in marine environments, the size of produced particles are likewise affected by such properties, thereby playing an important role in marine aerosol formation (CitationO’Dowd et al. 1997).
Table 1 The geometric mean diameters (GMDs) of airborne particles formed from dissolved solids (NaCl, KCl, MgCl2, and CaCl2) in DI water
Figure 3 Size distributions of particles aerosolized from the CaCl2 water solution as a function of solution concentration (ppm).
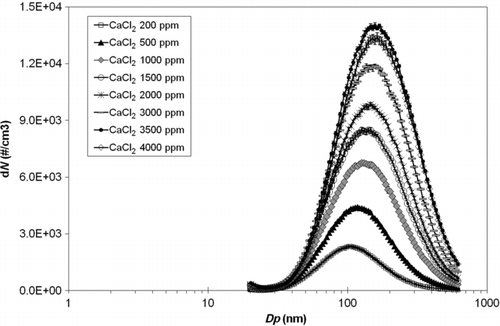
Figure 4 (a) Size distribution as a function of solution concentration; and (b) the relationship between the particle mass concentration in air (ug/m3) and water (ppm) for artificial seawater at various concentration levels.
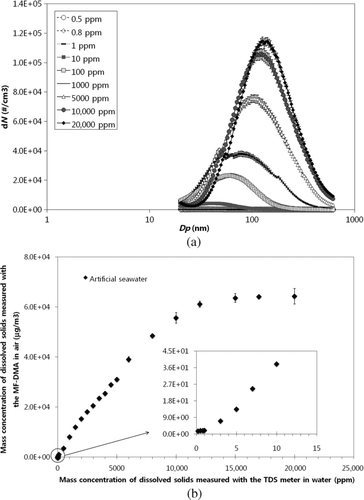
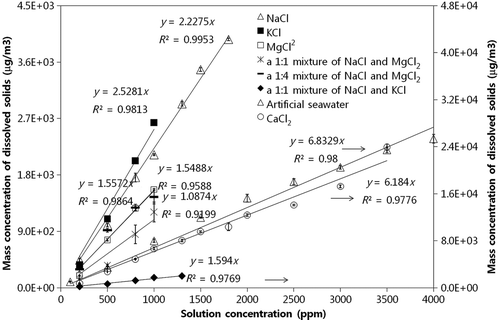
Figure 5 A relationship between particle mass concentration in air (ug/m3) and water (ppm) for various dissolved solids (artificial seawater, NaCl, KCl, CaCl2, and MgCl2) and their mixtures (a 1:1 mixture of NaCl and MgCl2, a 1:1 mixture of NaCl and KCl, and a 1:4 mixture of NaCl and MgCl2).
Explanations for the proportional relationship between increased airborne particle and solid solution concentration (ppm)—in respect of increased particle size and solution concentration—were unclear within the current concentration range. One hypothesis is that larger droplets have higher amounts of absolute mass (droplet GMD and coefficient of variation (COV) via the current constant output atomizer reported as 0.35 μm and 1.5, respectively) under constant droplet production, i.e., constant normal droplet distribution and homogeneous solute concentration. Thus, small droplets may not have enough solute mass during evaporation to produce particles that are detectable—minimum DMA size being about 20.2 nm—by the DMA. However, as the solute concentration increased, small droplets possessed enough solute mass for particle crystallization, leading to sufficient airborne particles for detectability via the proposed method. To test the hypothesis, we decreased the solution concentration to 0.2 ∼ 1 ppm to determine if adequate solute absolute mass existed for crystallized particle detection within droplets. shows the size distribution of artificial seawater for various solution concentration ranges (0.5 ∼ 20,000 ppm). We found that if dissolved species concentration was less than 1 ppm, detectable particle crystallization would be difficult using the proposed system. At 100, 1000, and 10,000 ppm, the dissolved species crystallized into detectable airborne particles, the number of detectable particles proportionally increasing as the concentration increased. At 12,000 ppm, all the droplets were saturated with the dissolved airborne crystallized species, i.e., all the droplets with the dissolved species changed into solid particles. Of note, the proportional relationship ceased above such a limit, as shown in .
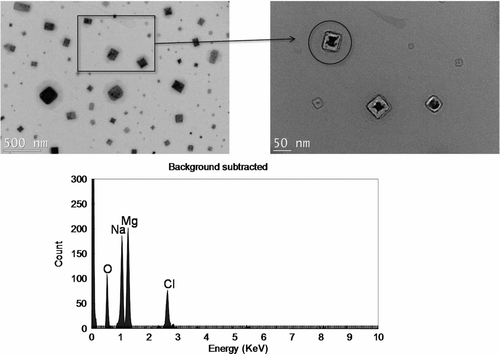
In order to determine the mass concentration of dissolved solids in water, we first determined the relationship between airborne (μg/m3) and waterborne (ppm) particle mass concentration. Calibration lines for various dissolved solids and corresponding mixtures are shown in . Although obtaining one universal line—as in the case of insoluble particles—was not possible due to a relationship dependence on solute type, determining mass concentration of dissolved solids and mixture in water was possible with dissolved species type information via such calibration lines. We observed that measured mass concentrations of various dissolved species via the current technique were consistent, i.e., within ∼16%, with those determined by the TDS meter. shows TEM/EDS analysis for airborne particles from a mixture of NaCl and MgCl2 solution. Particles have a cubic shape and contain Na, Mg, Cl, and O elements in each particle (CitationLi et al. 2003), suggesting that internally-mixed particles with water-dissolved ions were produced during drying. For quantification of seawater borne dissolved solids, we established a calibration line by using artificial seawaters of various concentrations, subsequently used to determine mass concentration of such dissolved solids, as shown in . The artificial seawater contained proportional amounts of major sea salt compounds such as chloride (55%), sodium (31%), sulfate (8%), magnesium (4%), potassium (1%), and others (2%; CitationMårtensson et al. 2003).
Next, we turned our attention to the mixing of waterborne insoluble particles and dissolved solids. As discussed before, the separation of both dissolved solids and insoluble particles through MF and UF membranes was included in the proposed method. Thus, particle and dissolved solid penetration through the MF and UF membranes was initially addressed. Such penetration was accomplished through the proposed MF and UF membrane system using the MF-DMA technique and TDS meter. The penetration of insoluble 60 nm, 90 nm, 135 nm, 300 nm, 430 nm, and 500 nm PSL particles through 0.45 μm sized pores is shown in . Data revealed that the ultra-500 nm particles were not able to pass through the MF membrane primarily due to pore plugging. Furthermore, we confirmed both the insoluble particle and dissolved solid penetration, i.e., a mixture of artificial seawater and 90 nm PSL particles, through the proposed 5 nm pore UF membrane. For dissolved solids, we tested artificial seawater in a 32,000 ppm concentration, corresponding to a typical mass concentration of dissolved salts in actual seawater. The degree of dissolved solid penetration was 99.4% and 98.9% through the MF and UF membrane, respectively; the degree of 90 nm PSL particle penetration was 98.6% through the MF membrane. Of note, the proposed membranes were identified as displaying the best dissolved solid penetration. Data suggested that both sub-500 nm insoluble particles and most dissolved solids were able to pass through the MF membrane, while only dissolved solids were able to pass through the UF membrane, leading to waterborne insoluble particle and dissolved solid separation.
Figure 7 Penetration of insoluble particles (60 nm, 90 nm, 135 nm, 300 nm, 430 nm, and 500 nm PSL particles) through the MF membrane with 0.45 μm pores.
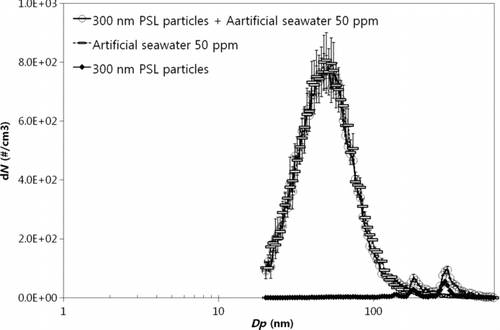
Since the UF membrane-filtered solution contained only dissolved solids, the airborne particle mass concentration was convertible to waterborne mass concentration using the predetermined relationship. However, in the case of the MF membrane-filtered solution, i.e., before UF membrane filtration, the existence of both waterborne insoluble particles and dissolved solids would simultaneously affect airborne particle size and quantity, complicating independent quantification. Some dissolved species could possibly be crystallized into insoluble particles, i.e., coating, during drying. The addition of dissolved solids to insoluble particles would lead to underestimation—compared to the independent production of airborne particles from waterborne insoluble particles and dissolved solids—of total airborne particles. Although the addition of dissolved species to insoluble particles would naturally lead to increased size, the number of insoluble particles would remain constant. Notably, insoluble particle quantity is determined by the difference in airborne particle quantity between a dissolved solid and insoluble particle mixture, i.e., MF filtered solution and dissolved solids, i.e., UF filtered solution. Thus, the total particle underestimation via addition of dissolved solids to insoluble particles would inevitably lead to insoluble particle underestimation. Airborne particle size distribution from an artificial seawater (50 ppm) and insoluble 300-nm PSL particle mixture is shown in . As shown, the size of PSL mixture particles was found to be higher than that of pure PSL particles without dissolved species due to the addition of dissolved solids to insoluble particles. The assertion that dissolved solids can be added to insoluble particles was tested by measuring hygroscopic growth factors of particles produced from pure silica solution (300 ppm), pure artificial seawater (25,000 ppm), and a silica and artificial seawater mixture at increased RH (∼85%) via the TDMA technique. The hygroscopic growth factors of pure silica, pure artificial seawater, and a silica and artificial seawater mixture were found to be 1.00, 2.33, and 2.19, respectively. Although pure silica particles did not grow, the particles produced from the silica and artificial seawater mixture grew significantly, indicating that the silica particles contained a significant amount of hygroscopic species from the artificial seawater during aerosolization process. We estimated the dissolved solids added to insoluble silica particles during drying by increasing silica particle concentration (100–2000 ppm) in fixed artificial seawater (25,000 ppm), determined to be 26% on an average. Although membrane filtration was manipulated to be short, i.e., ∼30 min, to minimize significant insoluble particle and dissolved solid accumulation on the UF membrane surface, insoluble particles nevertheless accumulated, causing dissolved solid penetration through the UF membrane and eventual deterioration due to caking on the membrane surface, i.e., pore blocking. Such an occurrence would lead to mass underestimation of dissolved solid concentration, as well as overestimation of insoluble particle concentration. Such an effect counteracted the one caused by adding dissolved solids to insoluble particles during drying, as previously mentioned. Both effects were taken into account to determine waterborne insoluble particle concentration and dissolved solid mass concentration.
Figure 8 Size distribution of airborne particles from artificial seawater (50 ppm), insoluble 300-nm PSL particles in DI water, and their mixture.
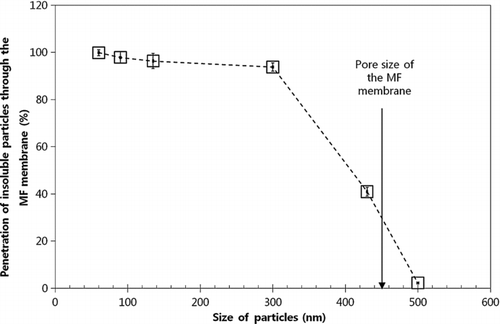
Figure 9 (a) Concentrations of insoluble submicrometer particles (#/ml); and (b) mass concentrations of dissolved solids (ppm) in seawater sampled in several locations in Korea.
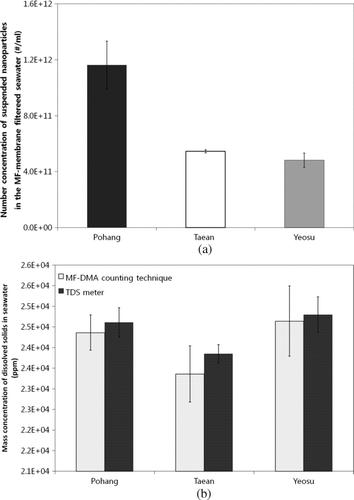
By using calibration lines from insoluble particles and dissolved solids ( for insoluble particles and for dissolved solids [artificial seawater]), we determined the insoluble submicrometer particle concentration (#/ml) and dissolved solid mass concentration (ppm) in seawater sampled in several locations in Korea. Of note, the seawater was initially filtered by the MF membrane to remove ultra-500 nm particles. shows the comparison among insoluble submicrometer particle concentrations in MF membrane-filtered seawater sampled from Pohang, Taean, and Yeosu in Korea, representing 1.16 × 1012, 5.45 × 1011, and 4.82 × 1011 #/ml, respectively. We determined that the insoluble submicrometer particle concentration in seawater sampled from Pohang was ∼2.3 times higher than that from Taean or Yeosu, representing the first insoluble submicrometer particle measurement in seawater to our knowledge. Moreover, we determined the mass concentration of dissolved solids in MF membrane-filtered seawater sampled from Taean, Yeosu, and Pohang, as shown in . For comparison, the dissolved solid mass concentration measured by the TDS meter was included, revealing excellent concurrence of ∼2%. More significant variation in insoluble particle concentration—compared to that of dissolved species—was found among sampling sites. Chemical and biological characterization, as well as seasonal variations in insoluble particle concentrations and dissolved solids in seawater, currently proceed in order to better understand the source and formation mechanisms.
CONCLUSIONS
We developed a membrane filtration and aerosolization measurement method to quantify waterborne insoluble submicrometer particles and dissolved species. Regardless of particle composition, we were able to determine a single linear relationship between particle concentration in air (#/cm3) and water (#/ml)—useful in determining unknown waterborne insoluble submicrometer particle concentrations—by using various types of waterborne insoluble particles, e.g., silica, CaCO3, MgCO3, and PSL particles, and corresponding mixtures. In addition, we established a relationship between the mass concentration of dissolved solids in air and water by using various dissolved solids and mixtures within a solution concentration range of 20–4000 ppm. The data showed that the size of airborne particles—crystallized and dried from droplets containing dissolved species—was dependent on both solution conductivity and the solute ionic radius. For a mixture of waterborne insoluble particles and dissolved solids, we performed a dissolved solid and insoluble particle separation via the proposed membrane system, showing that dissolved solid penetration was 99.4% and 98.9% through the MF and UF membranes, respectively; while, insoluble submicrometer particle penetration was 98.6% through the MF. By using relationships between air and water for insoluble particle and dissolved solids, we determined insoluble submicrometer particle number concentration (#/ml) and dissolved solid mass concentration (ppm) in seawater sampled in several locations in Korea after accounting for mixing effects on the quantitative performance of the proposed technique. Insoluble submicrometer particle concentration passed through the MF membrane in seawater sampled from Pohang, Taean, and Yeosu, measuring 1.16 × 1012, 5.45 × 1011, and 4.82 × 1011 #/ml, respectively. The measured mass concentrations of dissolved solids strongly concurred with TDS meter measurements.
Acknowledgments
The research described in this paper was supported by the Center for Seawater Desalination (B01-05-03) and by the National Research Foundation of Korea (NRF). Grant funded by the Korean Government (MEST) (NRF-2010-013-D00034).
Notes
*(Atkins and Jones 2008).
REFERENCES
- Andreas , E. L. 1998 . A New Sea Spray Generation Function for Wind Speeds up to 32 m s−1 . J. Phys. Oceanogr. , 28 ( 11 ) : 1945 – 1948 .
- Atkins , P. and Jones , L. 2007 . Chemical Principles: The Quest for Insight , 4th ed. , New York : W. H. Freeman .
- Cho , J. , Park , Y. J. , Sun , H. , Kim , S. and Yoon , Y. 2006 . Measurements of Effective Sizes and Diffusivities of Nano-Colloids and Micro-Particles . Colloid Surf A: Physicochem. Eng. Asp. , 274 ( 1–3 ) : 43 – 47 .
- Eninger , R. M. , Hogan , C. J. , Biswas , P. , Adhikari , A. , Reponen , T. and Grinshpun , S. A. 2009 . Electrospray versus Nebulization for Aerosolization and Filter Testing with Bacteriophage Particles . Aerosol Sci. Technol. , 43 ( 4 ) : 298 – 304 .
- Finlayson , B. 1979 . Electrical Conductivity: A Useful Technique in Teaching Geomorphology . J. Geogr. High Educ. , 3 ( 2 ) : 68 – 87 .
- Fukushima , M. , Hamada , Y. , Tabei , E. , Aramata , M. , Mori , S. and Yamamoto , Y. 1998 . Effects of Dopants and Polymer Structures on Electrical Conductivity of Organosilicon Polymers . Synthetic Met. , 94 ( 3 ) : 299 – 306 .
- Greenwald , R. , Bergin , M. H. , Carrico , C. M. and Grant , D. 2005 . New Real-Time Technique to Measure the Size Distribution of Water-Insoluble Aerosols . Environ. Sci. Technol. , 39 ( 13 ) : 4967 – 4973 .
- Gustafson , H. and Behrman , A. S. 1939 . Determination of Total Dissolved Solids in Water by Electrical Conductivity . Ind. Eng. Chem. , 11 ( 7 ) : 355 – 357 .
- Hameri , K. , Charlson , R. and Hansson , H. C. 2002 . Hygroscopic Properties of Mixed Ammonium Sulfate and Carboxylic Acids Particles . AIChE J. , 48 ( 6 ) : 1309 – 1316 .
- Howe , K. J. and Clark , M. M. 2002 . Fouling of Microfiltration and Ultrafiltration Membranes by Natural Waters . Environ. Sci. Technol. , 36 ( 16 ) : 3571 – 3576 .
- Ito , T. , Sun , L. , Bevan , M. A. and Crooks , R. M. 2004 . Comparison of Nanoparticle Size and Electrophoretic Mobility Measurements Using a Carbon-Nanotube-based Coulter Counter, Dynamic Light Scattering, Transmission Electron Microscopy, and Phase Analysis Light Scattering . Langmuir , 20 ( 16 ) : 6940 – 6945 .
- Ito , Y. , Kanehori , K. , Miyauchi , K. and Kudo , T. 1987 . Ionic Conductivity of Electrolytes Formed from PEO-LiCF3SO3 Complex Low Molecular Weight Poly (ethylene glycol) . J. Mater. Sci. , 22 ( 5 ) : 1845 – 1849 .
- Kumar , M. , Adham , S. S. and Pearce , W. R. 2006 . Investigation of Seawater Reverse Osmosis Fouling and Its Relationship to Pretreatment Type . Environ. Sci. Technol. , 40 ( 6 ) : 2037 – 2044 .
- Latham , J. and Smith , M. H. 1990 . Effect on Global Warming of Wind-Dependent Aerosol Generation at the Ocean Surface . Nature , 347 ( 6291 ) : 372 – 373 .
- Li , J. , Anderson , R. J. and Buseck , P. R. 2003 . TEM Study of Aerosol Particles from Clean and Polluted Marine Boundary Layers over the North Atlantic . J. Geophys. Res. , 108 ACH 8–1–ACH 8–14
- Ling , T. Y. , Wang , J. and Pui , D. Y. H. 2010 . Measurement of Retention Efficiency of Filters against Nanoparticles in Liquids Using an Aerosolization Technique . Environ. Sci. Technol. , 44 ( 2 ) : 774 – 779 .
- Lenggoro , I. W. , Widiyandari , H. , Hogan , C. J. Jr. , Biswas , P. and Okuyama , K. 2007 . Colloidal Nanoparticle Analysis by Nanoelectrospray Size Spectrometry with a Heated Flow . Anal. Chim. Acta , 585 ( 2 ) : 193 – 201 .
- Lenggoro , I. W. , Xia , B. and Okuyama , K. 2002 . Sizing of Colloidal Nanoparticles by Electrospray and Differential Mobility Analyzer Methods . Langmuir , 18 ( 12 ) : 584 – 4591 .
- Mahurin , S. M. and Cheng , M. D. 2007 . Generating Nanoscale Aggregates from Colloidal Nanoparticles by Various Aerosol Spray Techniques . Nanotoxicology , 1 ( 2 ) : 130 – 138 .
- Mårtensson , E. M. , Nilsson , E. D. , de Leeuw , G. , Cohen , L. H. and Hansson , H. C. 2003 . Laboratory Simulations and Parameterization of the Primary Marine Aerosol Production . J. Geophys. Res. , 108 ( 9 ) AAC 15-1-AAC 15–12
- Mikhailov , E. , Vlasenko , S. , Niessner , R. and Po˙schl , U. 2004 . Interaction of Aerosol Particles Composed of Protein and Salts with Water Vapor: Hygroscopic Growth and Microstructural Rearrangement . Atmos. Chem. Phys. , 4 ( 2 ) : 323 – 350 .
- Mikuška , P. 2004 . Generator of Fine Polydisperse Aerosol . Collect. Czechoslov. Chem. Commun. , 69 ( 7 ) : 1453 – 1463 .
- Ning , R. Y. and Troyer , T. L. 2007 . Colloidal Fouling of RO Membranes Following MF/UF in the Reclamation of Municipal Wastewater . Desalination , 208 ( 1–3 ) : 232 – 237 .
- O’Dowd , C. D. , Smith , M. H. , Consterdine , I. E. and Lowe , J. A. 1997 . Marine Aerosol, Sea Salt, and the Marine Sulphur Cycle: A Short Review . Atmos. Environ. , 31 ( 1 ) : 73 – 80 .
- Park , J. Y. , Cho , J. and Park , K. 2009a . Evaluation of Quantitative Performance of the Membrane Filtration-Differential Mobility Analyzer (MF-DMA) Counting Technique to Determine Suspended Particles and Dissolved Solids in Water . Desalination , 247 ( 1–3 ) : 316 – 325 .
- Park , K. , Park , J. Y. , Lee , S. and Cho , J. 2009b . Measurements of Size and Number of Suspended and Dissolved Nanoparticles in Water for Evaluation of Colloidal Fouling in RO Membrane . Desalination , 238 ( 1–3 ) : 79 – 89 .
- Patten , H. E. 1901 . Influence of the Solvent in Electrolytic Conduction . J. Phys. Chem. , 6 ( 8 ) : 554 – 600 .
- Rader , D. J. and McMurry , P. H. 1986 . Application of the Tandem Differential Mobility Analyzer to Studies of Droplet Growth or Evaporation . J. Aerosol Sci. , 17 ( 5 ) : 771 – 787 .
- Sakol , D. and Konieczny , K. 2004 . Application of Coagulation and Conventional Filtration in Raw Water Pretreatment Before Microfiltration Membranes . Desalination , 162 ( 1–3 ) : 61 – 73 .
- Schippers , J. C. and Verdouw , J. 1980 . Modified Fouling Index, a Method of Determining the Fouling Characteristics of Water . Desalination , 32 : 137 – 148 .
- Song , L. , Chen , K. L. , Ong , S. L. and Ng , W. J. 2004 . A New Normalization Method for Determination of Colloidal Fouling Potential in Membrane Processes . J. Colloid Interface Sci. , 271 ( 2 ) : 426 – 433 .
- Wiedensohler , A. , Krämer , M. and Hansson , H. C. 1991 . A New Method for Measurement of Insoluble Submicrometer Particles in Water . J. Aerosol Sci. , 22 ( Suppl. 1 ) : S329 – S330 .
- Zhu , X. and Elimelech , M. 1997 . Colloidal Fouling of Reverse Osmosis Membranes: Measurements and Fouling Mechanisms . Environ. Sci. Technol. , 31 ( 12 ) : 3654 – 3662 .