Abstract
To better evaluate the health effects and indoor air quality impacts of nanoparticles generated by laser printers, measurements were made to characterize the number concentration, size distribution, morphology, and chemical composition of the emitted nanoparticles as a function of printer distance, idle time, “cold start” state, cartridge states, and number of pages printed. Emitted ion concentrations, nanoparticles, volatile organic compound (VOC) emissions, and toner particles were characterized using multiple analytical techniques. Finally, particle generation mechanisms and emission control strategies are discussed.
Emission measurements were conducted using a commonly used black and white office printer (HP LaserJet4100) operating in a small (12 m2) office conference room environment and a stainless steel environmental chamber of similar size. Particle concentrations and size distributions were monitored using condensation particle counter (CPC) and scanning mobility particle sizer (SMPS) directly above the printer and at distances of 1–2 m away. Emitted ion concentrations and VOCs were measured by ion density meter and thermal desorption gas chromatography/mass spectrometry (GC/MS), respectively. Aerosols emitted from the printer were collected using an electrostatic precipitator (ESP) sampler and analyzed with transmission electron microscopy/energy-dispersive X-ray spectroscopy (TEM/EDS). Toner particles from the LaserJet printer cartridge were analysis using thermogravimetric analyzer (TGA), differential scanning calorimetry (DSC), inductively coupled plasma/atomic emission spectroscopy (ICP/AES), scanning electron microscopy (SEM)/EDS, and TEM/EDS.
Repeated measurements revealed that the LaserJet printer emitted nanoparticles: (1) with a concentration from 104 to 106 #/cc and single-mode peak size between 20 and 150 nm, depending on the number of printed pages and aerosol age, (2) primarily during the cold power up of the printer and at the start of printing, or under conditions when the printer was allowed sufficient time to cool down the internal surfaces (about 30–90 min depending on the number of pages printed), (3) likely generated from ion-induced nucleation of vapor-phase organic compounds, which are released from the toner powder during the rapid heating of internal printer surfaces. TGA and ICP analysis revealed that about 0.064% (wt) of the toner materials (iron oxide coated with copolymer resin) are evaporated at the printer operating temperature, which allowed evaluation of the upper limit of nanoparticle formation by condensation of the vapor phase VOCs. SEM/EDS and TEM/EDS results suggest that fugitive toner particles and paper coatings were not important aerosol sources. Reducing toner solvent content, preventing the internal printer surfaces from cooling down, or increasing preheating time before printing may be helpful in reducing the VOC emissions and the generation of these polymer nanoparticles, which may represent a potential human health hazard.
INTRODUCTION
In modern industrialized societies, people spend most of their time in the indoor environment. Recent research has shown that the risks to health may be greater from exposure to indoor air pollution sources than outdoor air (Hetes et al. Citation1995). Because of the wide use of electronic products and computers in the home and office, the emissions from office machines such as copy machines and printers have become a health concern. Studies have shown that some office machines not only emit volatile organic compounds (VOCs) and ozone, but also submicrometer particles (Lee et al. Citation2001; He et al. Citation2007; Kagi et al. Citation2007; Destaillats et al. Citation2008; Koivisto et al. Citation2010). For poorly ventilated office buildings and homes with frequently operating printers, VOCs and ultrafine particles could be a serious health threat. However, there is still not enough detailed knowledge about the characteristics of emissions from laser printers to determine the associated health effects and viable control strategies.
An important area requiring further study is the formation mechanisms of nanoparticles emitted from laser printers. Kagi et al. (Citation2007) and Wensing et al. (Citation2008) pointed out that the ultrafine particles generated from laser printers were not the toner particles themselves, but rather a product of secondary formation due to volatile organic compounds (VOCs) and water mists emitted during the printer operations (Kagi et al. Citation2007; Wensing et al. Citation2008). More recently, Morawska et al. (Citation2009) suggested that these submicrometer-sized particles appear to be formed by complex reactions between different VOCs in the presence of both heat and ozone, a promising hypothesis requiring more direct evidence. In the same study, Morawska et al. further suggested that semivolatile organic compounds (SVOC) could be the main source of particle formation. Wang et al. (Citation2008) and Schripp et al. (Citation2008) observed initial bursts of nanoparticles and decreases in concentration after short printing durations, but the mechanism for this phenomenon remains unclear.
Recently, He et al. (Citation2010) further studied the relationship between fuser roller temperature and laser printer emissions, but did not find a consistent relationship between them. In fact, the heat transfer efficiency, the toner powder temperature while passing the roller, and the properties of the toner cartridge materials may be more important than the roller temperature, so more studies of these parameters are needed.
The objective of this study is to determine potential generation mechanisms and associated control strategies for laser printer-emitted nanoparticles by (1) considering laser printer operation principles and likely sources, (2) determining the physical and chemical properties of the materials used in the printing process, and (3) characterizing nanoparticles and VOCs emitted under various printing and ambient room conditions.
Most commercially available black and white laser printers utilize similar operating principles and toner compositions. Generally, the toner powder particles consist of an iron oxide core material to carry charges and copolymer outer layers to bind the electrostatically transferred toner particles to the paper when heated. In this study, experiments were conducted using an HP LaserJet 4100, a common office printer in the same series previously reported to be high emitters of nanoparticles (Hetes et al. Citation1995). Measurements were conducted in the real-world environment of a small conference room in an office building and in the laboratory setting of a well-controlled stainless steel environmental chamber of similar size. The magnitudes of the particle emission rate may vary for printer models with different operating temperatures and toner powder compositions, but the particle generation principles should be the same.
EXPERIMENTAL
Experiments were conducted in a small office suite conference room with standard painted drywall and an environmental chamber of similar size with stainless steel walls. The conference room size was 4.5 m × 3.3 m × 2.7 m (length, width, and height), with an average air exchange rate of 6.3/h, which is normal for this office building during working hours. The conference room temperature was 21–24°C and the relative humidity was 40–60%. The stainless steel chamber size was 3.35 m × 3.35 m × 2.43 m with an air exchange rate of 5.6/h and relative humidity controlled at 40%. Two fans were used to ensure uniform mixing of the stainless steel chamber air. The conference room tests were used primarily to assess concentrations at various distances from the printer. All other experiments were conducted in the chamber.
FIG. 1 Sampling locations for VOC and particulate matter sampling. (Figure provided in color online.)
The HP LaserJet 4100 was equipped with a HP C8061X toner cartridge, which is the cartridge specified by the manufacturer. Each page was printed with full page of small text, which had typical toner deposit coverage of 8% of the white page. The print coverage was calculated using image processing software (NIH Image J). The printer paper used in this study was Office Depot premium white copy paper (Item #348037). The paper density was 75 mg/cm2. Before and after printing, the temperature of the paper and the toner cartridge chamber inside the printer was measured using an infrared NIST traceable thermometer (Extech Instruments, HD500). The temperature of the cartridge chamber was measured by quickly removing the toner cartridge and aiming the thermometer sensor at the internal surface of the chamber.
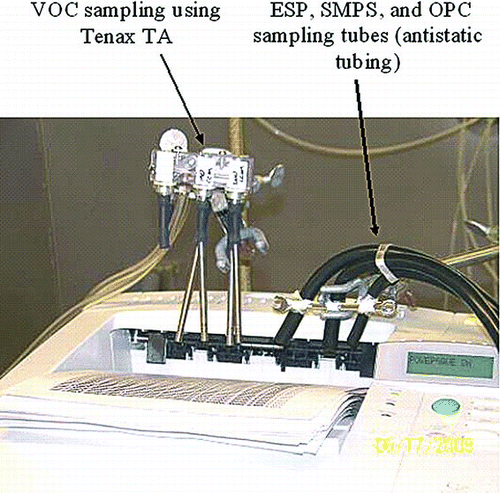
The test equipment set up is shown in . In contrast to well-mixed chamber tests, in which real-time source emissions are calculated by modeling the changes in particle size due to transport, dilution, and aging, this study focused on the direct measurement of source emissions. Particle and VOC emissions were sampled in the immediate vicinity of the top of the printer via tubing at the printed page discharge outlet. The paper outlet was chosen because it is a major discharge point for the printer cooling air, which becomes a carrier of emitted particles and VOCs.
Particle concentrations were monitored in real-time using condensation particle counters (CPCs, TSI model 3010 and 3775). Particle size distributions were monitored using a scanning mobility particle sizer (SMPS, TSI model 3080 electrostatic classifier incorporated with CPC, TSI 3010). The SMPS parameters were: sampling time of 120 s and scan range from 9.4 to 400 nm.
Particles were collected on transmission electron microscopy (TEM) grids during printing using an electrostatic precipitator (ESP) Aerosol Sampler (TSI model 3100). The ESP sampling flow rate was 5 l/min and the charging current was 18 μA. The samples were analyzed using TEM (FEI Tecnai 12) equipped with an energy-dispersive X-ray spectrometer (EDS) and 30 mm2 SiLi detector (Noran Vantage System, Thermo Fisher Scientific) to characterize the morphology and elemental composition of the emitted nanoparticles.
The air surface velocity at the printed paper discharge outlet on the top of the printer was 0.28 m/s, as measured using an anemometer (TSI VelociCalc 9555-A). This air flow rate was more than 20 times higher than at the outlet holes at the back of the printer. Both positive and negative ion concentrations were monitored using an ion density meter (Air Ion Counter, Standard Version, AlphaLab Inc.). The monitoring position was on the back of the printer, approximately adjacent to the fuser above the rear paper tray.
VOCs were sampled using stainless steel thermal desorption tubes packed with Tenax TA and sampled at a flow rate of 0.2 L/min. During each VOC test, 130 pages were continuously printed per episode. The waiting time between each printing episode was about 1 h to allow the printer to cool down to room temperature. Two types of VOC sampling were conducted. In the first, VOCs were only sampled while printing. The total sampling time was 70 min, with approximately 1000 pages printed. The second type of sampling was continuous, including printing and nonprinting times, for a total of 346 min. The samples were analyzed by ion trap gas chromatography/mass spectroscopy (GC/MS) (Varian Saturn 2200) using automated thermal desorption, (Perkin-Elmer ATD-400 or TurboMatrix ATD), a DB1701 column (Agilent J&W), and EPA Method TO-17. The desorption temperature was 200°C and the column temperature increased between 30 and 120°C at the rate of 10°C/min. Compounds within the range of volatility of n-Pentane (C5H12) to n-pentadecane (C15H32) were within the quantitative range for the method.
The cartridge toner materials were analyzed by several different techniques to determine the relationship between the toner powder and the composition of the generated aerosol particles. To estimate the total VOCs released at different temperatures, the toner powder mass change as a function of temperature was measured using a thermogravimetric analyzer (TGA) (TA Instruments, TGA 5000), using a sample heating rate of 5°C/min under N2 or O2. X-ray diffraction (XRD) spectra were acquired for the toner cartridge powder before and after heating to reveal any crystal phase changes (Bruker Advance D8). A phase transition analysis of the toner was also performed using a differential scanning calorimeter (DSC) (TA instruments, DSC Q500) with a heating rate of 5°C/min under N2.
Scanning electron microscopy (SEM) was used to determine the morphology and binding conditions of the toner and printed page samples. The SEM used was an environmental SEM (FEI XL30) with back-scattered electron detector and 30 mm2 silicon drift detector (Noran System Six, Thermo Fisher Scientific) to determine elemental composition by EDS.
Toner usage per page was calculated using measurements of the iron content of the toner and the average iron content per printed page area. The iron contents of the toner cartridge powder and the printed page were determined using inductively coupled plasma (ICP) atomic emission spectroscopy (AES) (Thermo Jarred Ash, IRIS/AP). Triplicate analyses of the iron content of the printed paper were conducted on randomly punched circles (D = 1.6 cm) from the printed area, which were dissolved in nitric acid overnight and then diluted to 50 mL before analysis by ICP/AES. Three sample blanks from the unprinted area on the page yielded negligible iron levels compared to the printed regions. The average iron mass per printed area was then multiplied by the total printed area to give the average iron mass per page. Dividing this number by the fraction of iron in the toner yielded the toner usage for each printed page. Then, using this toner mass per page and the toner mass loss from the TGA measurement at the printer fuser operating temperature, the maximum VOC emissions per page could be determined.
RESULTS AND DISCUSSION
Proposed Nanoparticle Formation Mechanisms and Emission Characteristics
To better understand the nanoparticle formation mechanisms for laser printers, it is helpful to review the principles of laser printer operation (Hewlett-Packard Company 2001; Wang et al. Citation2008). When the laser printer receives a print command, the laser beam draws the print image (text, figures, etc.) on a negatively charged photosensitive drum producing a neutral discharged print image. The print image on the drum then picks up the negatively charged toner powder from a developing cylinder. Paper sheets, often manufactured with particulate coatings to enhance their appearance and durability, are then introduced and given a positive charge. The drum with negatively charged toner is then pressed onto the positively charged paper to transfer the image. After passing over “static charge eliminator teeth” which discharge the paper to ground state, the paper and adhering toner particles pass over a heated fuser, where the toner copolymer is melted to fix the print image to the paper. Finally, the printed pages are transported out of the printer to the user. Thus, possible sources for nanoparticle generation in this process include: 1) fugitive toner powder agglomerates released directly from the printer cartridge, 2) loose particulate coatings released from the paper, and 3) evaporated VOCs produced in the high-temperature fuser region, emitted from the polymer coating of the toner powder on the page, and residue from previous printings.
is a typical total particle number concentration measured by CPC at the paper discharge point for a continuous printing of 140 pages from a cold (room temperature) start-up. During this print process (printing time = 8 min), the particle emissions reached a peak concentration value of 1.6 × 106 #/cc fairly rapidly, only 10–15 s after the printing started. After this peak value, the particle number concentration decreased sharply, even though the printer was still printing. After about 4 min, the particle number concentration decreased nearly to the background concentration level until the end of printing. This phenomenon has been observed previously by several research groups. (Schripp et al. Citation2008; Wang et al. Citation2008; Wensing et al. Citation2008)
FIG. 2 Typical measured CPC particle number concentration at the paper discharge point versus time for cold (room temperature) start-up, showing a rapidly decaying emission level.
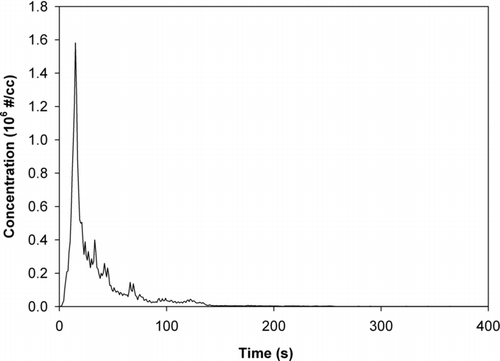
This sharp increase and decay of the nanoparticle number concentration is consistent with rapid nucleation from the high concentration of toner VOCs released by the heated fuser. In a “cold” start print, the high-temperature toner particle VOCs evaporated from the heated fuser encounter relatively colder air inside the printer, which has yet to reach operating temperature. This temperature gradient, combined with the presence of a high concentration of air ions produced by the printing process, suggests a homogeneous, ion-induced nucleation pathway to nanoparticle formation. As noted in the review of the principle of laser printer operation, there are abundant positive and negative charges generated in the printing process, and these free air ion charges may not be completely neutralized by collisions at the beginning of a cold start-up print. As the printer reaches stable operating conditions during continuous printing, a combination of the following mechanisms could lead to a rapid decrease in the particle number concentration: 1) neutralization of free charges by enhanced bipolar air ion collisions with the increasing interior printer temperature; 2) Suppressed nucleation due to elevated interior printer temperatures in the vicinity of the fuser where the VOCs are emitted, and thus a smaller temperature gradient; 3) enhanced dilution effect of VOCs with increasing interior printer air temperature; 4) thermally enhanced agglomeration of the nanoparticles () into fewer, larger particles, and 5) reduced emission of VOCs as printing progresses with a hot fuser, relative to a cold fuser that may have a residue of adhering of toner particles.
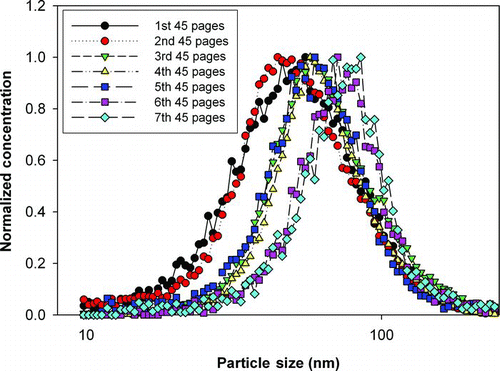
FIG. 3 Normalized size distributions for 7 consecutive sets of 45 page print jobs, demonstrating particle size increases with the number of pages printed, likely due to increasing agglomeration. (Figure provided in color online.)
The ion concentrations measured at the cooling air outlet support the proposed, ion-induced nucleation mechanism, as well as the proposed decrease mechanism (1) in the preceding paragraph. For a 5-page printing job, the average, peak positive ion concentration after printing was (700 ± 120) ions/cc and the average, peak negative ion concentration was (630 ± 110) ions/cc. The air ion concentration increased with the number of printed pages. For a 45-page printing job, the ion concentration reached 1200 ± 130 ion/cc. The air ion peak time coincided with the particle number concentration peak, approximately 14–15 s after the printing started, after which it then decreased. This coincidence of the ion and particle concentration peaks is consistent with the hypothesis that decreasing ion concentrations could cause a decrease in nanoparticle nucleation. It should be noted that these ion concentrations were monitored outside of the printer, where they likely experienced some ion charge neutralization, so the original ion concentrations generated from the fuser and other elements of the printer could be much higher.
Greatly elevated printer interior temperatures were measured after printing relative to their initial cold start temperatures, which supports the proposed thermal dependence of the nanoparticle formation process. Before printing, the measured internal printer temperatures were approximately the same as ambient air temperature, 21.6°C inside the printer toner cartridge bay (cartridge removed) and 20.3°C at the surface of the paper in the standard 1 ream capacity feed tray. After a continuous 45-page print job, the internal printer temperature increased to 37.2°C in the cartridge bay, and the paper surface at the printed page outlet reached 71.1°C. After a 90-page print job, the paper surface at the printed page outlet rose to 91.1°C. These elevated temperatures are feasible, given the fuser temperature of 195°C cited by the manufacturer (Hewlett-Packard Company 2001). The measured increases in paper and cartridge chamber temperatures as particle concentrations decrease is consistent with the hypothesis that temperature gradients between the fuser and surroundings initially drive high VOC nucleation rates, which decrease over time as the temperature gradient diminishes.
Effect of Distance, Idle Time, Cold Starts, Cartridge State, and Number of Pages Printed
Particle number concentration measurements were conducted as a function of distance from the printer to assess exposure in the 4.5 m × 3.3 m × 2.7 m conference room. Three sets of 45 pages were printed with one CPC sampling directly above the printer and another CPC sampling at the same vertical height at distances of 1 and 2 m away from the printer. Peak particle concentrations decreased by over an order of magnitude between the average peak value measured directly above the printer (9.62 × 105 #/cc) to that measured 1 m away (5.08 × 104 #/cc) and 2 m away (3.30 × 103 #/cc). Therefore, at a distance of only 1 m away from the printer, the particle number concentration was reduced to about 5% of the concentration at the source. Accordingly, increasing distance from the printer by even a small amount can substantially reduce a printer user's exposure to the high concentration of nanoparticles emitted, and should reduce exposures to the more dispersive gas-phase VOCs even further.
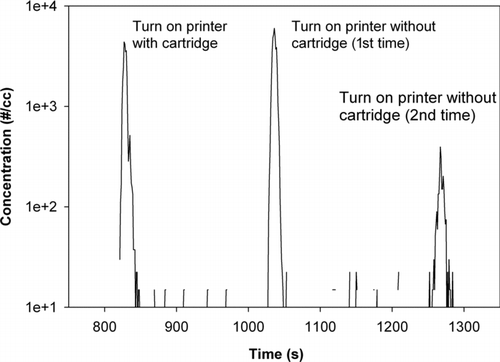
FIG. 5 Nanoparticle emissions produced without printing from fuser heating during repeated printer power up cycles, with and without toner cartridge inserted.
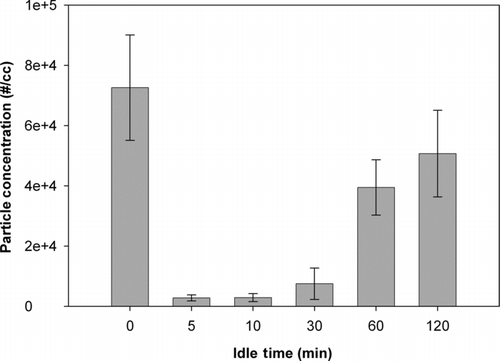
shows the peak nanoparticle concentration vs. printer cool down (idle) time between 45-page print jobs. After only a 10-min idle time, the particle number concentration during the next print job was suppressed to a very low level. After an idle time of 30 min, the particle number concentration was higher, but still only 1/10 of the initial concentration of the particles generated from the “cold start.” Even after 2-h idle time, the particle emission concentration was still not equal to the concentration of the first printing of the day. These suppressed particle emissions corresponded to measured internal printer temperatures which had not yet come down to their initial, cold start ambient room temperatures. However, after 15 h (overnight), the inside temperature of printer returned to the original temperature (around 22°C), and the printer emission concentration fully recovered to the original rate. Additional tests were conducted in which just one page was printed from a cold start. In this case, the minimum idle time to generate repeatable concentration peaks was only 5 min. In general, the more pages printed, the longer the idle time that was needed to reproduce the initial number concentration.
Without printing, particle concentration peaks were observed immediately after the printer was turned on for the first time on a given day (cold start). Particle emissions were monitored during printer and fuser power up without printing, with and without the toner cartridge inserted. As shown in , there was no obvious difference for particle emissions with or without the toner cartridge in place, similarly observed by Wensing et al. (Citation2008). However, the particle emissions after turning on the printer a second time within 5 min were much lower (about factor of 2) than during the first power-up event. This substantial difference suggests that residual toner particles deposited on the fuser from previous print jobs contribute to nanoparticle emissions, but only when the fuser is rapidly heated at power up from room temperature. It should be noted that the concentration peak levels generated during power-up are much less than the peak levels generated while printing (Figures , , and ). This implies that evaporation of the toner powder on the paper, rather than residual toner on the fuser, is the major source of nanoparticle emissions.
FIG. 6 Number particle size distributions measured by SMPS for printing different numbers of pages from a cold start-up. (Figure provided in color online.)
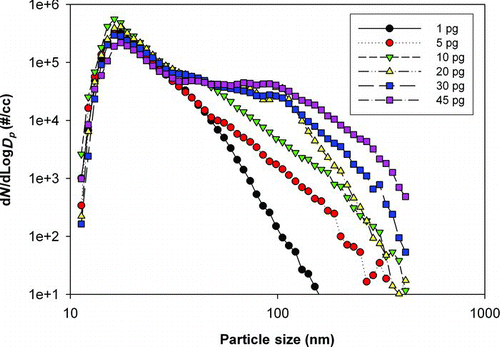
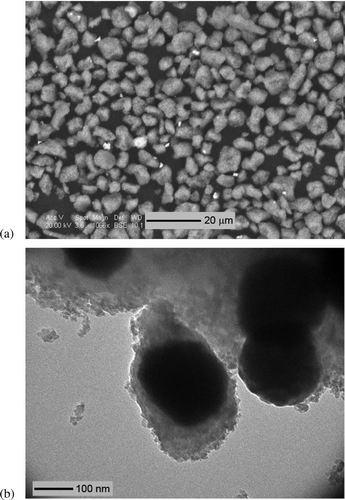
FIG. 7 (a) 2000× SEM image of toner powder, dominated by 3–8 μm clumps of 25–400 nm agglomerates, each composed of carbon, iron, and oxygen. Also present are 1–3 μm strontium–titanium–oxygen particles (bright particles). (b) 97,000× TEM image of the 25–400 nm agglomerates, each composed of an iron–oxygen core (dark shapes) coated with 5–15 nm carbon–oxygen particles.
Measurements were also conducted to compare the difference in particle emissions between a used and a freshly opened new toner cartridge. Particle emissions with the used cartridge were approximately five times lower than with the new cartridge (peak concentrations of 1.4 × 105 L/cc and 5.3 × 105 L/cc, respectively), as reported in a former study (He et al. Citation2007); however, this difference become relatively small if the old cartridge was shaken to redistribute the toner particles before printing (both peak concentrations = 4.5 × 105 L/cc). Shaking the cartridge may break up powder clumps, exposing fresh polymer- and solvent-coated surfaces similar to those readily available in freshly opened toner cartridges.
Particle size distributions were found to undergo marked changes with the number of pages printed as shown in . While printing from a cold start, the peaks in particle size number distribution (modes) occurred at the same diameter (about 30 nm) regardless of the number of continuously printed pages; however, the particle size distribution peak heights and tail shapes, and thus the mean particle sizes, were different. The more pages printed (and thus the longer the printing time), the larger was the mean size of the particle size distributions, which is characteristic of small particle agglomeration inside the printer. Note that each data set in is from measurements conducted during a cold start print event after a long overnight cool down period. Although the overnight cool down period prevented more than one set of measurements per day, this was critical to directly compare the particle number size distributions under the same fuser heat-up conditions, a primary controlling parameter for particle production characteristics. The difficulty in obtaining reproducible printer emitted particle concentrations and size distributions reported by several research groups likely stems from the inconsistent thermal cycling of the fuser when the printer is not allowed to undergo the lengthy overnight cool down to ambient temperature.
Chemical Characterization of Toner Particles and Printer Emissions
The material safety data sheet (MSDS) for the standard HP C8061X toner cartridge for the HP 4100 LaserJet Printer employed in this study states that the toner powder contains about 50% iron oxide and 50% copolymers by mass. The copolymers possibly include styrene, as well as other organic components and solvents. The measured iron content determined using acid digestion and ICP-AES was 33.7%, which corresponds to 46.8% iron oxide, and is in close agreement with the MSDS listing of 50% iron oxide.
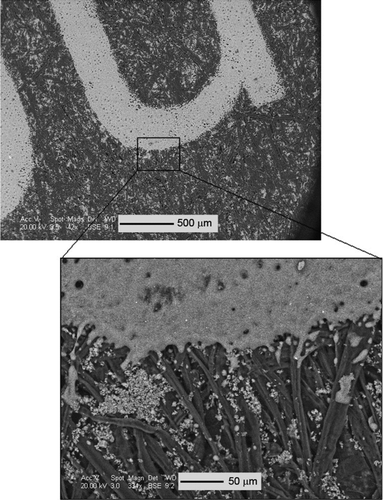
is an SEM image of large, 3–8 μm toner powder clumps, themselves made up of smaller agglomerates 25–400 nm in size. SEM/EDS analysis of the toner powder primarily yielded carbon, iron, oxygen, plus small amounts of distinct titanium–strontium–oxygen particles. is a TEM image of the 25–400 nm toner agglomerates, each consisting of a 20–300 nm iron core coated with 5–15 nm, amorphous, carbon–oxygen particles. The coating particles were fairly beam-insensitive, consistent with a polymer coating. shows the melted toner particles in the form of printed text on the paper, which exhibited the same carbon, oxygen, and iron peaks as the toner particles in . Also visible in are the paper coating particles, which were composed of calcium and oxygen, rod-shaped, and 2–3 μm long.
FIG. 8 SEM image of printed page, showing melted toner in the shape of printed letters and paper coating (nonmelted particles attached to the paper fibers).
TEM images of laser printer-emitted particles collected using the ESP sampler exhibited particles ranging in size from 10 to 30 nm, consistent with the peak particle sizes observed with the SMPS measurements. These semivolatile nanoparticles apparently did not evaporate under ambient room sampling conditions or the high vacuum of the TEM (approximately 10−6 Pa), but they did evaporate rapidly when heated by the electron beam, necessitating very short electron micrograph set-up and acquisition times, and magnifications below 100,000×. As a result, these observations were qualitative only; future work should be conducted to better characterize these volatile particles. The measured EDS peaks from these particles were generally not above background levels, though their volatility suggests they are composed of organic species. The volatility and likely organic composition of the majority of the particles suggests that the nanoparticles emitted during printing mainly consist of nucleation products from the evaporated toner copolymer, rather than other potential particle sources which would have had inorganic, nonvolatile components, such as fugitive toner particles with iron cores, or the calcium-based paper coating. The volatility of the emitted particles is also inconsistent with the 10 nm polymer coatings on the toner particles (), which were fairly stable under the beam. Larger, nonvolatile carbonaceous particles, iron-containing toner agglomerates, and calcium-based paper coating particles were also observed in the air samples, but only rarely.
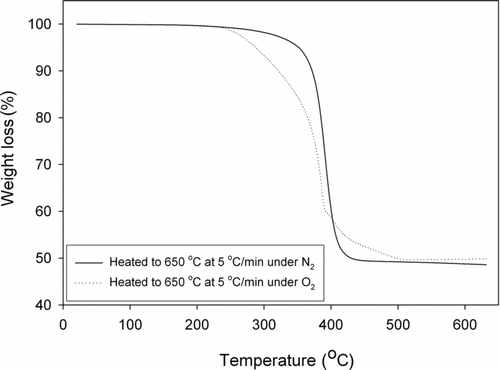
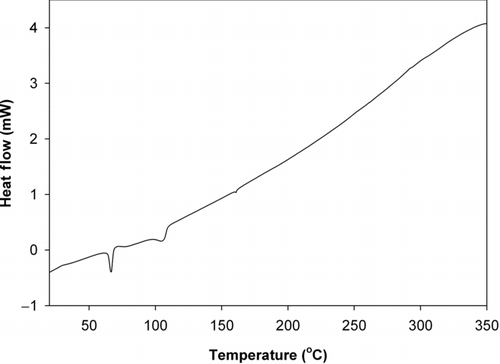
The TGA results, MSDS data, and DSC results all suggest that the temperature at which the toner powder is fused onto the printed paper is in the range of 110–150°C. This finding is based on the assumption that the melting point of toner copolymer coating must be achieved, at a minimum, to fuse the toner powder to the paper. TGA analysis () shows that the toner started to substantially lose mass at approximately 150°C. Similarly, according to the toner MSDS, the polymer softening point is 100–150°C. Above 200°C, the weight loss under oxygen was more rapid than that under nitrogen due to increased oxidation rates. However, the end weight loss percentage was similar under both gases, about 50%. The DSC results for the toner powder given in imply a melting point of 110°C at the second heat transfer inflection point. The first heat release peak at 70°C is well below the melting point for the copolymer and is likely due to the evaporation of the binding solvent. Since the detailed composition of the toner is unknown, further experiments would be needed to refine these peak identifications in the DSC results. Regardless, from the smooth DSC curve (and thus the lack of activity) above 180°C, it is reasonable that the fuser would not be designed to heat the toner above 180°C. According to the service manual of HP 4100 laser printer, the fuser's normal operating temperature is around 195°C (Hewlett-Packard Company 2001). Considering a heat transfer coefficient of less than 1 between the fuser and the paper and a contact time <0.1 s, the above analysis for the toner temperature profile is reasonable.
XRD analysis for the powders before and after heating revealed the presence of the same phase, gamma-Maghemite (Fe2O3, cubic, P 4(3)32, a = 8.3420 Å). The decrease in peak intensity after heating suggests a decrease in the crystal phase and an increase in the amorphous phase or a decrease in crystal size.
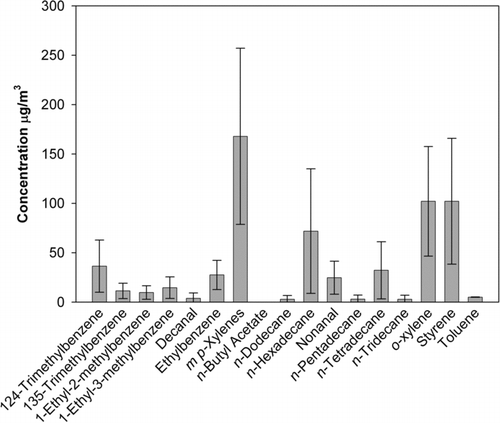
FIG. 11 GC/MS results for VOCs emitted from LaserJet printer, for sampling conducted only during printing cycles.
The iron oxide mass per printed page was determined to be 31.1 mg based on the ICP/AES results. Using the iron oxide content in the toner (46.8% from ICP analysis), the toner powder used for each printed page was calculated to be 66.5 mg. Using the TGA weight loss results (0.064%) for an assumed toner temperature of 110°C, the estimated maximum VOC emissions produced by the heating of the fuser would be 42.76 μg per printed page. The calculated VOC emissions for five other fuser temperatures within the softening point range of the copolymer are listed in . If one assumes these VOCs are initially emitted into 1 m3 of air surrounding the printer, the theoretical maximum level of VOCs emitted for a 1-page printing is 42.76 μg/m3/page. Assuming 100% of the emitted VOCs nucleated into particles, the concentration of 30 nm particles that would be produced from 42.76 μg/m3 in the vapor phase would be 2.75×106 #/cc per page. This estimate is higher than the measured particle concentration (), which suggests that not all of the evaporated VOCs were actually consumed in particle formation. Accordingly, substantial VOC emissions from the printer may also represent an exposure risk in addition to nanoparticles.
TABLE 1 Maximum VOC emissions, estimated using TGA test results for expected copolymer melting temperature range
VOC Emission Measurements
shows the GC/MS results for the VOC samples taken only while printing. Over 10 different VOCs were detected. Among them, m, p-xylene had the highest concentration, approximately 160 μg/m3, and o-xylene and styrene were the second highest, with concentrations of approximately 100 μg/m3. For the samples taken over the entire testing period, including printing and idle time, the concentrations of all VOCs were approximately 30 times lower, but the concentration ratios between these VOCs remained unchanged. The lower VOC concentrations measured during the entire testing period may have been further reduced by the room air pulled through the Tenax tubes during the nonprinting times, which may have acted as a desorbing agent. For the samples taking only when printing, the total concentration of all measured VOCs was 894.2 μg/m3. This concentration corresponds to 1000 pages printed. Using the measured surface air flow velocity above the printer of 7.62 m/min, an assumed air plume cross-section at the top of the printer of 22.8 × 7.62 cm, and a printing duration of 8 min, the total emitted plume volume would be 2.39 m3. Then, assuming a uniform concentration within the plume, the total mass of VOCs emitted would be 2.14 mg. This measured total VOC mass is much lower than the estimated maximum emission of VOCs determined from TGA/ICP, 1000 pages × 42.76/page = 42.76 mg. The lower measured value could be due to moisture in the toner cartridge or nonideal adsorption/desorption efficiency in the GC/MS analysis.
CONCLUSION
An HP 4100 LaserJet printer was found to generate nanoparticle emissions with a concentration of 104–106 #/cc and a mode of 30–100 nm, depending on the number of printed pages, operation conditions, and aerosol age. Nanoparticles were primarily emitted during cold power up of the printer or at the cold start of printing, but only if the LaserJet had been idle long enough for internal surfaces to cool to ambient room temperature. The emitted aerosol mainly consisted of carbon-based nanoparticles, likely generated by ion-induced homogeneous nucleation and condensation from the vapor-phase VOCs evaporated from copolymer toner particles rapidly heated by the fuser. Few iron-based or calcium-based particles were found in the printer emissions, suggesting that the unevaporated toner particles and paper coatings were not important nanoparticle sources. Several VOCs including xylene, styrene, and ethylbenzene have been identified as printer emissions. The maximum possible VOC emissions were estimated from TGA and ICP measurement results to be about 42.8 μg per printed page.
Increasing preheating time before printing and/or reducing the ion concentration during the printing process may be helpful for reducing nanoparticle generation, which may represent a human health concern. Also, adding a filter or adsorbent to the cooling air outlets may be helpful in reducing VOC releases. Finally, staying more than 1 m away from the printer can greatly reduce exposure to high concentration of nanoparticles generated while printing. To better evaluate the health effects caused by nanoparticles generated by laser printers, further real world health surveys and exposure assessments should include both the chemical compositions of the VOCs and the ultrafine particles released from the printers.
Acknowledgments
The authors would like to acknowledge Dr. Aude Demessence and Professor Jeffrey R. Long from the Chemistry Department of UC Berkeley for their help with thermogravimetric analyzer (TGA) and differential scanning calorimetry (DSC) analysis; Robert Miller, from Indoor Air Quality (IAQ), Calilfornia Department of Public Health (CDPH) for helping with the gas chromatography/mass spectroscopy (GC/MS) analysis; Dr. Kunning Zhu from Indoor Air Quality (IAQ) for the chamber operation; and Paul Wong from Outdoor Air Quality (OAQ), CDPH for the inductively coupled plasma (ICP) analysis.
REFERENCES
- Destaillats , H. , Maddalena , R. L. , Singer , B. C. , Hodgson , A. T. and McKone , T. E. 2008 . Indoor Pollutants Emitted by Office Equipment: A Review of Reported Data and Information Needs . Atmos. Environ. , 42 ( 7 ) : 1371 – 1388 .
- He , C. , Morawska , L. and Taplin , L. 2007 . Particle Emission Characteristics of Office Printers . Environ. Sci. Technol. , 41 ( 17 ) : 6039 – 6045 .
- He , C. R. , Morawska , L. , Wang , H. , Jayaratne , R. , McGarry , P. , Johnson , G. R. , Bostrom , T. , Gonthier , J. , Authemayou , S. and Ayoko , G. 2010 . Quantification of the Relationship Between Fuser Roller Temperature and Laser Printer Emissions . J. Aerosol Sci. , 41 ( 6 ) : 523 – 530 .
- Hetes , R. , Moore , M. and Northeim , C. 1995 . Project Summary: Office Equipment: Design, Indoor Air Emissions, and Pollution Prevention Opportunities , U.S : Environmental Protect Agency: Research Triangle Park .
- Kagi , N. , Fujii , S. , Horiba , Y. , Namiki , N. , Ohtani , Y. , Emi , H. , Tamura , H. and Kim , Y. S. 2007 . Indoor Air Quality for Chemical and Ultrafine Particle Contaminants from Printers . Build. Environ. , 42 ( 5 ) : 1949 – 1954 .
- Koivisto , A. J. , Hussein , T. , Niemela , R. , Tuomi , T. and Hameri , K. 2010 . Impact of Particle Emissions of New Laser Printers on Modeled Office Room . Atmos. Environ. , 44 ( 17 ) : 2140 – 2146 .
- Lee , S. C. , Lam , S. and Fai , H. K. 2001 . Characterization of VOCs, Ozone, and PM10 Emissions from Office Equipment in an Environmental Chamber . Build. Environ. , 36 ( 7 ) : 837 – 842 .
- Morawska , L. , He , C. R. , Johnson , G. , Jayaratne , R. , Salthammer , T. , Wang , H. , Uhde , E. , Bostrom , T. , Modini , R. , Ayoko , G. , McGarry , P. and Wensing , M. 2009 . An Investigation into the Characteristics and Formation Mechanisms of Particles Originating from the Operation of Laser Printers . Environ. Sci. Technol. , 43 ( 4 ) : 1015 – 1022 .
- Schripp , T. , Wensing , M. , Uhde , E. , Salthammer , T. , He , C. and Morawska , L. 2008 . Evaluation of Ultrafine Particle Emissions from Laser Printers Using Emission Test Chambers . Environ. Sci. Technol. , 42 ( 12 ) : 4338 – 4343 .
- Wang , Z.-M. , Wagner , J. and Wall , S. Characterization of Nanoparticles and Emission Variation from a LaserJet Under Different Printing Conditions . AAAR 27th Annual Conference , Orlando, FL
- Wensing , M. , Schripp , T. , Uhde , E. and Salthammer , T. 2008 . Ultra-Fine Particles Release from Hardcopy Devices: Sources, Real-Room Measurements and Efficiency of Filter Accessories . Sci. Total Environ. , 407 ( 1 ) : 418 – 427 .