Abstract
In this study, biological collection efficiencies and culturable bacterial and fungal aerosol diversities were investigated when different bioaerosol sampling tools and culturing methods were applied. The samplers included Reuter centrifugal sampler (RCS) High Flow, BioSampler, electrostatic sampler, gelatin filter, BioStage impactor, mixed cellulose ester (MCE) filter as well as gravitational settling methods. For culturable bacterial aerosol diversity, the colony-forming units (CFUs) were washed off from the agar plates, and further went through polymerase chain reaction- and denaturing gradient gel electrophoresis (PCR–DGGE). For culturable fungal aerosol diversity, microscopic identification method was applied.
In general, the BioStage impactor, MCE filter, and the BioSampler remained robust when sampling culturable bioaerosols. The PCR–DGGE study revealed that the use of different samplers and culturing methods resulted in different culturable bioaerosol diversity. For indoor bacterial aerosols, the MCE filter with direct culturing on agar plates and the BioSampler resulted in the highest number of visible bands on DGGE gels, followed by the gravitational settling (26°C), the RCS High Flow, electrostatic sampler, and the MCE filter extracted. However, for outdoor bacterial aerosols, the BioStage impactor obtained the highest number of visible bands followed by the BioSampler, MCE filter, electrostatic sampler, the RCS High Flow, and gravitational settling at 26°C. When sampling fungal aerosols, the electrostatic sampler was observed to produce the highest diversity. Similar to previous studies, Alternaria, Cladosporium, and Aspergillus species were found to dominate the fungal community in most samples collected here.
The results obtained here suggested that the sampler design, sampling environments, and culturing methods should be considered together when assessing the biological aerosol exposure.
INTRODUCTION
Aerosols of biological origin including airborne bacteria, fungi, viruses, and their derivatives are ubiquitous in the environment. In general, the health effects associated with bioaerosol exposure include lung impairments (Trout Citation2001; Douwes et al. Citation2003), asthma exacerbation (Gavett and Koren Citation2000), and infectious diseases (Christiani et al. Citation1986; Hsu and Zee Citation2004; Ayres et al. Citation2009). Fungal species are often identified as the cause for allergic diseases (Gravesen Citation1979), headaches, eye irritation, epistaxis, nasal and sinus congestion, and cough (Kuhn and Ghannoum Citation2003). A recent study showed that the microbial aerosol is diverse, including many possible human pathogens in respirable fine particle fraction (<3 μm; Frohlich-Nowoisky et al. Citation2009). Many fungal species could colonize indoor walls, possibly be released into the environments, thus negatively affecting the human health (Pieckova and Jesenska Citation1999). In addition, human tuberculosis caused by Mycobacterium tuberculosis is cited as the one of the most prevalent and deadly airborne bacterial infectious diseases worldwide (Murray et al. Citation1990; Rodrigues and Smith Citation1990; Zhang et al. Citation1992). Among many other adverse effects, sick building syndrome (SBS), which impacts a great percentage of population (Finnegan et al. Citation1984; Marmot et al. Citation2006; Wong et al. Citation2009), is another growing indoor health issue related to the biological exposure. To well quantify the airborne biological agents, it is important to develop robust sampling and detection techniques.
Among the bioaerosol samplers, the BioSampler (SKC, Inc.), the BioStage impactor (SKC, Inc.), the electrostatic samplers, and also the filter sampling have been widely investigated. In a previous study, the biological collection efficiencies of seven portable microbial samplers including the BioStage impactor, the Reuter centrifugal sampler (RCS) High Flow, and gelatin filter were studied when sampling three different bacterial species (Bacillus subtilis, Pseudomonas fluorescens, and Escherichia coli) and three fungal species (Cladosporium cladosporioides, Aspergillus versicolor, and Penicillium melinii; Yao and Mainelis Citation2006a). In their study, it was indicated that the biological collection efficiency depended on both physical (impaction velocity, cutoff size, and jet-to-plate distance) and biological parameters (the sensitivity and size of the microorganisms). In another study, the performances of a portable BioStage impactor (SKC, Inc.) and the RCS High Flow when sampling environmental bacteria and fungi aerosols have been investigated (Zhen et al. Citation2009). Their results indicated that the sampling time, particle bounce, and desiccation play major roles in the observed sampling differences among the samplers (Zhen et al. Citation2009). In another study, the performance of the RCS High Flow was also compared with the BioSampler when sampling aerosolized Bacillus subtilis var. niger and environmental bioaerosols (An et al. Citation2004). Their results indicated that the RCS High Flow obtained lower culturable counts than that of the BioSampler due to its higher desiccation factors (An et al. Citation2004). Among various bioaerosol samplers, the electrostatic sampler is increasingly being applied to detect biological aerosols (Yao and Mainelis Citation2006c; Yao et al. Citation2009b; Han et al. Citation2010). It was shown that when the electrostatic sampler was used, the culturable bioaerosol concentrations could be 5–9 times higher than those obtained by the BioStage impactor (Yao and Mainelis Citation2006c). It was suggested that the electrostatic sampler introduced less impaction and desiccation stress than the BioStage impactor (Yao and Mainelis Citation2006c). In the meantime, filter sampling including gelatin filter was also investigated for collecting bioaerosols in numerous studies (Wang et al. Citation2001; Burton et al. Citation2005; Chen and Li Citation2005; Nehme et al. Citation2008; Droogenbroeck et al. Citation2009; Yao et al. Citation2009a; Wu et al. Citation2010). These studies suggested that the filter materials and the sampling stress might affect its overall performance.
These investigations showed that the performances of the bioaerosols varied with the operating parameters (sampling flow rate and sampling time), microbial species, and the sampling environments. The differences observed among different bioaerosol samplers are largely due to their differences in physical collection efficiencies, sampling stress, impaction velocity, degree of embedding if applicable, and the desiccation factor of the samplers as summarized in . In addition, it was also shown that the sampling environments also play important roles in the biological collection efficiencies of the sampling devices (Yao and Mainelis Citation2007; Zhen et al. Citation2009). For different environments, bioaerosol size, source, composition, atmospheric irradiation, and humidity level could be very different, and these factors together could affect the biological performance of the sampling devices. The overall biological performance of the sampler depends on the interplay of environmental factors and sampler characteristics. Although these samplers have been widely applied to detect culturable bioaerosols, fewer studies have been conducted to study the culturable bioaerosol diversity obtained by these bioaerosol samplers. As observed in , different bioaerosol samplers have different types and degrees of sampling stress (impaction, desiccation, and degree of embedding). For a given particular sampler, one type of the stress, e.g., desiccation or impaction stress, might be more pronounced. Among the samplers, the BioSampler has the highest impaction velocity, while the RCS High Flow has the highest desiccation factor. The airborne bioaerosols composed of many types of microorganisms could respond differently to the sampling stress caused by each of the bioaerosol samplers. Thus, it is possible that the culturable bioaerosol diversity obtained by different samplers could be very different, and certain species might not be detected when a particular sampler is used. This type of information is significantly lacking in the literature.
TABLE 1 The operating parameters for different bioaerosol samplers investigated in this study
In this study, the biological collection efficiencies of six different bioaerosol samplers including filtration, impaction, liquid impingement, and electrostatic as listed in were investigated when sampling both indoor and outdoor bacterial and fungal aerosols. The culturable bacterial aerosol diversity obtained by the samplers was analyzed using the polymerase chain reaction and denaturing gradient gel electrophoresis (PCR–DGGE) method. In addition, the culturable fungal aerosol diversity was also analyzed using microscopy methods. This study is the first to report the culturable bacteria and fungal aerosol diversity obtained when different bioaerosol samplers are used.
MATERIALS AND METHODS
Bioaerosol Samplers Investigated
In this study, six different bioaerosol samplers coupled with different culturing methods were investigated when analyzing the culturable bioaerosol concentration and diversity in both indoor and outdoor environments. The bioaerosol samplers included button aerosol sampler (SKC, Inc., Eighty Four, PA, USA) in conjunction with mixed cellulose ester (MCE; SKC, Inc.) with a pore size of 0.45 μm and gelatin filter (SKC, Inc.), BioStage impactor (SKC, Inc.), BioSampler (SKC, Inc.), electrostatic sampler (Yao et al. Citation2009b), and RCS High Flow (Biotest Diagnostics, Denville, NJ, USA). In addition, gravitational settling was also investigated in culturing bioaerosols in this study. Their sampling mechanisms, impaction velocity, relevant cutoff sizes, degree of embedding, and desiccation factors are summarized in . The degree of embedding and the desiccation factor here were calculated using the equation developed in a previous study (Zhen et al. Citation2009). Among these samplers, the MCE and gelatin filters are widely applied to collect bioaerosols (Chen and Li Citation2005; Burton et al. Citation2007; Yao et al. Citation2009c; Wu et al. Citation2010). The BioStage impactor is generally used as a standard agar-plate-based bioaerosol detection method in many studies including the investigation of the anthrax event in 2001 (Weis et al. Citation2002). The BioSampler, electrostatic sampler, and RCS High Flow were also utilized in numerous studies for detecting bioaerosols (An et al. Citation2004; Han and Mainelis Citation2008; Vianelli et al. Citation2006; Yao and Mainelis Citation2006a, Citation2006b; Yao et al. 2009b, 2009c; Zhen et al. Citation2009). Accordingly, these samplers were selected in sampling bioaerosols in both indoor and outdoor environments in this study.
EXPERIMENTAL PROCEDURES
Bioaerosol Sampling
The samplings were performed for airborne bacteria and fungi in both indoor (a university office environment) and outdoor environments (outside of three-story building) within the 4th Ring of Beijing. The air samples were collected using the samplers with their operating parameters (different sampling flow rates and sampling times) shown in . The button aerosol sampler with either gelatin or MCE filter was operated at 5 L/min with a total of 75 L air collected. The BioStage impactor was operated at its standard sampling flow rate of 28.3 L/min with a total of 424.5 L air collected. The BioSampler was also operated at its standard sampling flow rate of 12.5 L/min with 500 L of air collected. For the electrostatic sampler, the sampling was conducted at the flow rate of 5 L/min and an applied electrostatic field strength of 5.4 kV/cm for 60 min. The RCS High Flow was operated at 100 L/min for 5 min. For the gravitational settling, the agar plates were placed on the ground in open air for a period of 5 min for both indoor and outdoor environments. The samplings were alternated among the bioaerosol samplers in a random order, and three independent air samples were taken for each of the samplers investigated in this study. During indoor air sampling, the range of temperature was 18.1°C–18.9°C and the relative humidity was 36%–38%, and for outdoor air sampling, the range of temperature was 19.1°C–19.9 °C and the relative humidity was 34%–38%. All sampling flow rates were calibrated using a Mini-Buck calibrator in this study (AP Buck Inc., Orlando, FL, USA).
TABLE 2 The sampling flow rates and sampling times used for bioaerosol samplers
After the sampling, for the BioStage impactor, the RCS High Flow, and the electrostatic sampler, the air samples were directly incubated at 26°C. For the BioSampler, 20 mL distilled (DI) water was used as the sampling medium. After the sampling, 2 mL of the sample was filtrated through an MCE filter, which was further placed on agar plates for culturing. For gravitational settling, the agar plates were incubated directly both at 26°C and 37°C. For MCE filter samples, two culturing methods were employed: directly placed on agar plates (MCE-placed) and cultured after extraction (MCE-extracted). For the extraction, the MCE filter was first extracted in 2 mL DI water using a sonicator (Kunshan Ultrasonic Instruments Co. Ltd., Shanghai, China) for about 20 min, and 100-μL extraction liquid was then used for plate culturing. In this study, gelatin filters with collected air samples were directly dissolved into 2 mL DI water, and similar to the MCE filter technique, 100-μL extraction liquid was also taken for plate culturing. The colony-forming units (CFUs) were manually counted after the culturing (2 days for bacteria with tryptic soy agar and 3 days for fungi with malt extract agar). The culturable bacterial and fungal concentrations were calculated as CFU/m3. For the BioStage impactor samples, the CFUs were statistically corrected according to the equation described by Feller (Citation1968). The biological collection efficiencies of the samplers investigated here were compared when sampling bacterial and fungal aerosols both indoors and outdoors.
The biological collection efficiency of a sampler is a function of many factors including its physical collection efficiency, the bioaerosol size, composition, and sampler's characteristics such as its desiccation effects, impaction stress, and degree of embedding factors. Particle size distribution alone would not be able to dictate the biological collection efficiency of a sampler. Nonetheless, it is desirable to have the bioaerosol size distribution in the environments, but such information is changing dynamically, and it is difficult to obtain a representative one. Owing to the equipment restrictions, this type of information was not obtained in this study.
CULTURABLE BIOAEROSOL DIVERSITY
The DGGE analysis was applied to analyze the culturable bacterial aerosol communities in the air samples collected. For the air samples collected by each of the samplers in either indoor or outdoor environments, all the CFUs were washed off the agar plates (three replicates) and pooled together using DI water to a total liquid volume of 10 mL in a 50-mL corning tube. Then, 2 mL of the bacterial suspension for each sample was taken out for DNA extraction by a bacteria DNA extraction kit (Tiangen, Beijing, China) following the manufacturer's instruction. The extracted DNA samples were further resuspended into 50 μL DI water. The V3 region of the 16S rRNA gene was amplified using primers P2 (5′-ATTACCGCGGCTGCTGG-3′) and P3 (5′-GCclamp-CCTACGGGAGGCAGCAG-3′) as described by Muyzer et al. (Citation1993). The PCR reaction mixture (total volume was 50 μL) included 5 μL DNA template, 2 μL primer P2 (10 μM), 2 μL primer P3 (10 μM), 25 μL 2 × master mix [10× Taq Buffer, dNTP Mixture, Taq (2.5 U/μL); Tiangen, Beijing, China] and 16 μL DI water. The cycle conditions were as follows: 94°C for 3 min, 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and 72°C for 5 min. DI water was used as the negative control in the PCR experiments. DGGE was performed with the Bio-Rad DCode mutation-detection system (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. In details, approximately 20-μL PCR product was transferred to each well of 8% polyacrylamide gels containing a vertical gradient of denaturant from 30% to 65%. The electrophoresis was performed for 600 min at a constant voltage of 75 V at 60°C. After the electrophoresis, gels were stained with GelRed solution (10,000× diluted with DI water; Biotium, Hayward, CA, USA) and photographed (Molecular Imager Gel Doc XR System, Bio-Rad) under ultraviolet lamp at the wavelength of 254 nm. Here, the bands were visually scored as either present or absent on the gel labeled as letters, and all lanes were compared with each other. The DGGE bands for the samples obtained by each of the samplers investigated were further compared by using dendrograms obtained using the BioRad built-in software functions “band detection” and “unweighted pair group method with arithmetic mean (UPGMA)” clustering, and the similarity of the culturable microbial aerosol diversities was analyzed using the scale bar produced by the methods.
For culturable fungal aerosols, microscopy method was used for the identification of fungal genus. Fungal colonies were first washed off from the agar plates, and about 10 μL of fungal suspension was pipetted onto the glass slide. A magnification of 100× was achieved using the immersion oil for Olympus CX 41 to obtain the images of the fungi recognized. The fungal genuses were identified through the visual comparison of the images taken and existing fungal morphologies according to some reference books such as one written by Bold et al. (Citation1980).
Statistical Analysis
The differences in the culturable microbial aerosol concentrations obtained by different bioaerosol samplers were analyzed by paired t-test via the statistical component of SigmaPlot 10 (Systat Software, Inc.) and analysis of variance (ANOVA). A p value of less than 0.05 indicated a statistically significant difference at a confidence level of 95%.
RESULTS AND DISCUSSION
In this study, the overall biological collection efficiencies of six different bioaerosol samplers when collecting environmental bacterial and fungal aerosols were studied. At the same time, the culturable bacterial and fungal bioaerosol diversity obtained by different bioaerosol samplers were also investigated and analyzed.
Comparison of Culturable Bioaerosol Concentrations Obtained by Different Samplers and Culturing Methods
shows the culturable bacterial aerosol concentrations (in log scale) obtained by different samplers in indoor and outdoor environments. As observed in , the methods with centrifugal force (the RCS High Flow) and filtration (gelatin and MCE) obtained higher biological collection efficiencies when compared with other methods. The ranks for indoor bacterial aerosol sampling were as follows: RCS High Flow > gelatin dissolved > MCE filter extracted > BioSampler > electrostatic sampler > BioStage impactor > MCE filter placed > gelatin placed. For outdoor bacterial aerosols, the ranks were as follows: RCS High Flow > gelatin dissolved > BioStage impactor > MCE filter extracted > MCE filter placed > BioSampler > electrostatic sampler > gelatin placed.
FIG. 1 Comparison of culturable bacterial aerosol concentrations (in log scale) obtained by different samplers and culturing methods in both indoor and outdoor environments. Error bar stands for the standard deviation from three independent samples. The operating parameters for the samplers were shown in . The differences among the biological collection efficiencies of the samplers were statistically significant as indicated by ANOVA analysis (p value < 0.0001 for both environments).
For the BioSampler, when sampling the particles whose size are less than 0.5 μm, its collection efficiency decreased by approximately 11% (Willeke et al. Citation1998). Therefore, the potential loss of some airborne microorganisms with smaller sizes might have lowered the culturable counts. The results from the RCS High Flow were higher than those of gelatin dissolved (with paired t-test: p value = 0.0005 for indoor results, and 0.0009 for outdoor results).
In a previous study, it was also shown that the RCS High Flow sampler performed better than the gelatin filter when sampling indoor bacteria (Yao and Mainelis Citation2007). In this study, the performance of the BioStage impactor was shown to be lower in relation to other samplers when compared with previous studies (Yao and Mainelis Citation2007). The RCS High Flow and button aerosol sampler with MCE filter extraction, and gelatin dissolved obtained higher culturable aerosol concentrations than the BioStage impactor as observed in .
The differences between the RCS High Flow and the BioStage impactor were statistically significant (p value = 0.00001). This difference could result from the significant desiccation effects of the BioStage impactor. In this study, 15-min sampling time was used for the BioStage impactor, while 5-min sampling time was used in the previous study (Yao and Mainelis Citation2007). The sampling time is an important factor that influences the performance of the BioStage impactor in preserving the bacterial culturability. Increasing the sampling time would lead to the quicker drying of the agar surface, which correspondingly could result in severe particle bounce problem. A previous study has shown that the total bacteria concentration obtained by the RCS High Flow was increased if the sampling time was decreased from 5 min to 1 min (Zhen et al. Citation2009). This was largely due to the fact that the RCS High Flow has a higher desiccation factor as shown in , thus resulting in the quicker drying of the agar surface. In addition, the sampling environment was also observed to play a role in the biological collection efficiencies of the bioaerosol samplers (Zhen et al. Citation2009).
It was shown that the collection differences for airborne bacteria were not statistically significant when sampling in airport and riverfront by the portable BioStage impactor and the RCS High Flow; however, they were statistically significant when sampling in other environments (train station, hotel, and subway; Zhen et al. Citation2009). Here, the electrostatic sampler was operated at 5 L/min and 5.4 kV/cm, which might have resulted in lower physical collection efficiency compared with those obtained at a lower sampling flow rate of 1.2 L/min (Yao and Mainelis Citation2006c). Nonetheless, the overall trends about the differences in collection efficiencies between the electrostatic sampler and the BioStage impactor observed in this study were similar to those found in a previous study when sampling both bacterial and fungal aerosols (Yao and Mainelis Citation2006c). In a previous study, the bioaerosols with both charge polarity were collected (Yao and Mainelis Citation2006c).
Although the cutoff size of the BioStage impactor (0.65 μm) is smaller than that of the RCS High Flow (1.2 μm), when sampling natural environments, microbial cells might appear as aggregates or harbor on larger inert particles, correspondingly resulting in higher collection efficiencies for the RCS High Flow (Zhen et al. Citation2009). In addition, the embedding degree of the RCS High Flow was only one third of the BioStage impactor (Zhen et al. Citation2009), which might have also played a role in the difference observed. A study showed that too deep embedding could result in the inhibition of the bacterial growth (Reponen et al. Citation1998). However, for too little embedding, the microorganisms might have limited access to the nutrients and moisture for their growth (Reponen et al. Citation1998).
For the button aerosol sampler, two types of filters were used in this study: MCE filter and gelatin filter. The biological collection differences observed between the gelatin filter placed and MCE filter placed were statistically significant for both indoor and outdoor samplings (p value = 0.014 for indoor, and 0.009 for outdoor). When the samples from both filters were transferred to liquid form (extraction or dissolution), the differences were also found statistically significant (p value = 0.0018 for indoor and 0.0039 for outdoor). Generally, when sampling with the MCE filter, the culturable bioaerosol concentrations were found higher than those by the gelatin filters.
For the MCE filter, there were statistically significant differences between the placed and dissolved culturing methods for the outdoor sampling (p value = 0.0049), but not significant for the indoor sampling (p value = 0.37). Although for the gelatin filter, two methods resulted in statistically significant differences for both indoor and outdoor samplings (p value = 0.0116 and 0.001). The results suggested that the sampling environments, materials, and culturing methods played roles in the enumeration of culturable microorganisms.
shows the biological collection efficiencies of different bioaerosol samplers when sampling culturable fungal aerosols in both indoor and outdoor environments. In general, when sampling indoor fungal aerosols, the RCS High Flow and the BioSampler remained robust. For outdoor fungal aerosols, the RCS High Flow, gelatin dissolved, and MCE filters extracted seemed to perform better than other samplers. In a previous study, when sampling outdoor fungal aerosols, the RCS High Flow was shown to perform better than the BioStage impactor and the gelatin filter (Yao and Mainelis Citation2007). When sampling indoor fungal aerosols, the BioStage impactor was shown to perform better (Yao and Mainelis Citation2007). It was suggested that the sampling environment, which is likely to have different fungal compositions, played a role in the sampling efficiency. In this study, the longer sampling time (15 min) used for the BioStage impactor might have resulted in its lower biological collection efficiencies because of desiccation and particle bounce problem. In a previous study, it was shown that the BioStage impactor performed better than the RCS High Flow and the gelatin filter when sampling Cladosporium cladosporioides, a relatively sensitive fungal species (Yao and Mainelis Citation2006a). However, when sampling hardy species Aspergillus versicolor and Penicillium melinii, the gelatin filter was shown to perform better than the BioStage impactor and the RCS High Flow (Yao and Mainelis Citation2006a). When the sampling is conducted in natural environments, the fungal aerosols being collected could include both sensitive and hardy species. Accordingly, in this study, the gelatin filter was found to obtain lower culturable fungal counts possibly due to its desiccation effects for those sensitive fungal species. In addition, the longer sampling time (40 min) used for the gelatin filter in this study might have also contributed to its lower performance. The electrostatic sampler, operated at 5 L/min and 5.4 kV/cm, was found to perform reasonably well compared with the BioSampler when sampling both indoor and outdoor culturable fungal aerosols in this study. The collection of the biological aerosols by using the electrostatic collection is based on the amount of the charges carried by the microorganisms, which however could vary with time and environments.
FIG. 2 Comparison of culturable fungi aerosol concentrations (in log scale) obtained by different samplers and culturing methods in both indoor and outdoor environments. Error bar stands for the standard deviation from three independent samples. The operating parameters for the samplers were shown in . ANOVA tests indicated that the differences among the biological collection efficiencies of the samplers were statistically significant for all tests shown in (p value < 0.0001 for both environments).
In a previous study, the electrostatic sampler (5 L/min and 5 kV/cm) was reported to obtain higher culturable fungal counts, about 1.6–3.7 times those obtained by the BioStage impactor (Yao and Mainelis Citation2006a). In this study, the electrostatic sampler was also shown to produce higher indoor culturable fungal counts than the BioStage impactor under similar operating conditions for both samplers. However, when sampling outdoor fungal aerosols, the BioStage impactor was shown to produce slightly higher culturable counts than those obtained by the electrostatic sampler in this study. This was possibly due to the fungal composition and their charge-level variations in geophysically different sampling environments. In addition, the amount of agar used for the plate might also pay a role, which might affect the aerosol residence time. When the gelatin filter was used to sample outdoor fungal aerosols, significantly higher culturable counts were obtained in this study. This might be due to the fact that the outdoor fungal aerosols might have better desiccation resistant ability than those indoors. In addition, the fungal compositions could be also different for indoor and outdoor environments. For most samplers investigated here, the outdoor culturable fungal aerosol concentrations were observed to be higher than those in indoor environments. Similar finding was also observed in another study (Shelton et al. Citation2002). Similar to the bacterial aerosols, for the MCE filter, there were statistically significant differences between the placed and dissolved culturing methods for outdoor fungal aerosol sampling (p value = 0.011), but not significant for the indoor sampling (p-value = 0.07). For the gelatin filter, two methods resulted in statistically significant differences for both indoor and outdoor fungal aerosol samplings (p value = 0.008 and 0.00076).
Comparison of Culturable Bioaerosol Diversity Obtained by Different Samplers and Culturing Methods
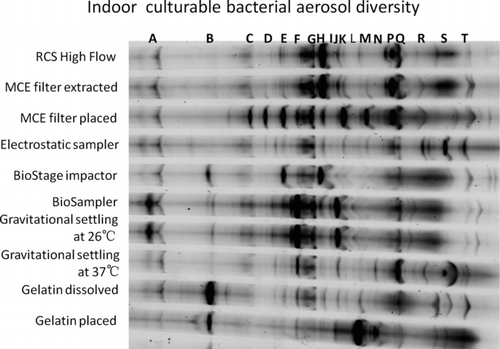
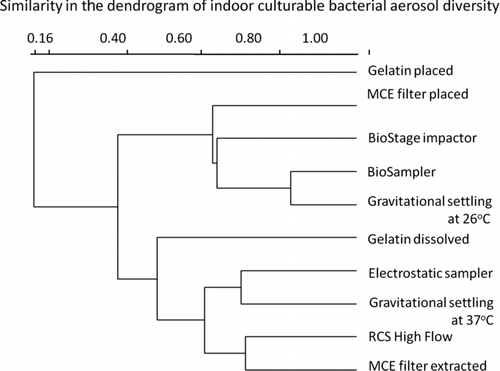
shows the DGGE profile of indoor culturable bacterial aerosol diversity. In general, the MCE filter placed obtained the highest number of visible bands (C, D, E, F, G, H, K, M, P, Q) followed by the BioSampler (A, E, F, G, H, J, Q, R), gravitational settling at 26°C (A, E, G, J, Q, S), RCS High Flow (E, F, G, P, Q, S), electrostatic sampler (G, P, Q, R, S, T), and MCE filter extracted (F, G, H, J, P, S) as shown in . For other samplers, there were less than five visible bands. The results from indicate that the use of different bioaerosol samplers could result in different culturable bacterial aerosol diversity. For electrostatic sampling, due to its lower sampling stress, e.g., lower impaction velocity and lower desiccation, as shown in , the culturable bacterial aerosol diversity obtained was higher than those obtained by the gravitational setting at 37°C, gelatin filter methods, and the BioStage impactor. shows the similarity in the dendrograms of indoor culturable bacterial aerosol diversity. For most samplers, the similarity was in the range of 40%–80%. As observed in the figure, the BioSampler and the gravitational settling at 26°C had about 80% similarity among the bands obtained, suggesting similar culturable bioaerosol species recovery. It is possible that the BioSampler and the gravitational settling method had similar degree of damages to the bioaerosols sampled here. However, the diversity is different from the biological collection efficiency. The biological collection efficiency could be higher (collection of most dominant species), while the diversity could be low because of stronger culturability damages. On the contrary, the diversity could be also very high (gentle collection of bioaerosols), while the biological collection efficiency could be low. For different aerosol sample replicates, the culturable counts could vary, but the overall diversity possibly remained the same. The microbial agents, in general, have an optimal cultivation temperature, e.g., P. fluorescens at 26°C, and B. subtilis var. niger at 30°C, at which they grow better. Likewise, environmental aerosol samples contain diverse forms of biological agents, most of which could grow better at 26°C, thus with higher diversity at this cultivation condition.
shows the DGGE profile of outdoor culturable bacterial aerosol diversity. In general, the BioStage impactor obtained the highest number of visible bands (d, f, g, h, j, k, d, r, o) followed by the BioSampler (d, e, f, i, j, p, r, t), MCE placed (b, d, g, j, l, o, q), MCE filter extracted (d, f, h, m, p, r, t), electrostatic sampler (d, f, i, j, n, p), RCS High Flow (a, c, e, g, j, p), and gravitational settling at 26°C (d, f, i, j, p, q). Other samplers or methods had the least number of bands. The electrostatic sampler was shown to perform reasonably well when sampling outdoor culturable bacterial aerosols. In this study, the electrostatic sampler only collected negatively charged bacterial aerosols by electrical force. However, in natural environments, the bacteria could carry both negative and positive charges. For other samplers, they collected both types of bacteria. Different from the indoor environment, the BioStage impactor performed best in preserving the culturable bioaerosol diversity. shows the similarity in the dendrograms of outdoor culturable bacterial aerosol diversity. In general, the similarity in diversity was higher among the samplers when collecting outdoor culturable bioaerosols than indoors. From the results shown in Figures –, we observed that the performance of the BioStage impactor is more sensitive to the sampling environments, and MCE filter placed and the BioSampler remained robust regardless of the environments. In this study, we have performed several DGGE experiments among which most of the DGGE profiles appeared to be similar. The DGGE profiles shown in Figures and for indoor and outdoor environments are the representative ones. The results from Figures – imply that the culturable bioaerosol diversity obtained varies with the sampler design, sampling environments, and culturing methods. The actual diversity obtained could be also negatively impacted through the possible diversity masking by the fast growing microbial species on agar plates.
FIG. 5 DGGE profiles of outdoor culturable bacterial aerosol diversity obtained by using different samplers and culturing methods. The operating parameters for the samplers are shown in .

FIG. 6 The similarity analysis of outdoor culturable bioaerosol diversity using BioRad software; scale bar on the top indicates the percentage of similarity between the bioaerosol diversity () obtained using different methods. The operating parameters for the samplers are shown in .
The DGGE is increasingly being used in bioaerosol studies (Nehme et al. Citation2008; Maki et al. Citation2008; Li et al. Citation2010). However, the identification of bacteria by DGGE has a number of potential problems. The DGGE profiles as shown in this study could have many bands, which are difficult to purify, and the intraspecies operon heterogeneities can significantly contribute to complex genetic profiles in microbial community analysis (Schmalenberger et al. Citation2001). It has been suggested that the effect of operon heterogeneities could be reduced by choosing appropriate variable regions with less intraspecies diversity, e.g., V4 and V5 (Schmalenberger et al. Citation2001). The results presented here could thus possibly be affected by the relevant limitations of DGGE.
In this study, the dominant culturable fungal aerosol diversity obtained by different samplers was also investigated using microscopy methods. As observed in , the dominant culturable fungal aerosol diversity varied with sampler and culturing methods. The electrostatic sampler (5 L/min and 5.4 kV/cm) was shown to produce the highest culturable fungal aerosol diversity among the samplers investigated in this study. The gelatin filter, RCS High Flow, and gravitational settling were shown to report comparably higher fungal aerosol diversity. For indoor samplings, Cladosporium, Aspergillus, Alternaria, and Ascospores genuses were observed to dominate the culturable fungal aerosols collected by the samplers investigated under the experimental conditions described in . The culturable fungal aerosols collected by the RCS High Flow were of Alternaria and Cladosporium genuses. For filter samplings, Alternaria, Cladosporium, and Aspergillus species were found to dominate the culturable fungal aerosols. For outdoor samplings, Ascospores, Aspergillus, Ascospores, and Cladosporium species were found to dominate the culturable fungal aerosols obtained by the samplers investigated in this study. Lee et al. (Citation2006) also found that Cladosporium, Aspergillus, and Alternaria genuses were detected among the predominant fungal species in six family homes. In other studies, these genuses were also shown to dominate the fungal community (Ren et al. Citation1999; Shelton et al. Citation2002; Adhikari et al. Citation2003). In this study, Wallemia species were detected by the gravitational settling methods both indoors and outdoors. This indicates that the culturability of Wallemia genus might be sensitive to the sampling stress induced by other sampling methods as shown in . It was suggested that the increased culturability of fungi inside the homes might result in a potential increase in the release of allergens from viable spores and the pathogenicity of viable fungi on immune-compromised individuals (Lee et al. Citation2006). The use of microscopy methods in this study could possibly result in fungal morphology analysis bias; thus, the results obtained here might be affected by the method's limitations. Nonetheless, the morphology identification of fungi was used in numerous studies (Ren et al. Citation1999; Pieckova and Jesenska Citation1999; Shelton et al. Citation2002; Adhikari et al. Citation2003; Lee et al. Citation2006). In this study, 3-day incubation time used for the fungal species could result in an inadequate identification of certain species that require longer culturing time. In addition, low air volume and small filter area might also prevent the detection of certain infrequently occurring fungal species. The larger the air volume, the more likely the rarely occurring fungal types will be detected. Likewise, the larger the area where the colonies are deposited, the less likely the rarely occurring fungal types will be masked by the more common ones.
TABLE 3 Dominant culturable fungi aerosols obtained by different samplers and culturing methods in both indoor and outdoor environments
The results from this study indicated that both bioaerosol concentrations and diversity varied with samplers and culturing methods. The differences were largely due to the different sampling mechanisms, the sampling stress, and bioaerosol growth conditions as shown in . In addition, it was also shown that the sampler's performance varied with the sampling environments including both indoors and outdoors (Yao and Mainelis Citation2007). In different environments, the bacterial compositions, e.g., Gram-negative and Gram-positive fractions, are different (Whyte et al. Citation2007); thus, they could respond to the sampling stress differently as the Gram-positive bacteria are more stress resistant than the gram-negative ones (Stewart et al. Citation1995). Among the samplers, the electrostatic sampler had the least impaction and desiccation stress (Zhen et al. Citation2009). However, the electrostatic sampling might have resulted in less embedding of the microorganisms, which could affect their growth. In a previous study, it was indicated that too little of embedding has resulted in lower culturability (Stewart et al. Citation1995). It was hypothesized that the microorganisms collected on the top of the collection surface had a limited ability to obtain the nutrients, moisture, and warmth needed for their survival. For larger microorganisms such as fungal spores, the problem of insufficient embedding might be minimal. This was manifested by the fact that the highest culturable fungal aerosol diversity was obtained by the electrostatic sampling in outdoor environments. When sampling outdoor particles, the collection efficiencies of the electrostatic sampler were shown to be higher than those for indoor particles (Yao and Mainelis Citation2006a).
The BioSampler and the BioStage impactor are two standard bioaerosol samplers and have been investigated in numerous studies. As shown in , the BioSampler has the highest impaction velocity of about 265.2 m/s, followed by the BioStage impactor, the RCS High Flow, and the filter sampling (MCE and gelatin filters). In a previous study, the increasing impaction velocity was shown to lead to increased metabolic and structural injuries to Pseudomonas fluorescens and Micrococcus luteus cells (Stewart et al. Citation1995). In addition, higher impaction velocity could result in a higher degree of embedding, e.g., the BioStage impactor. The RCS High Flow, as shown in , has an impaction velocity of 8 m/s (Yao and Mainelis Citation2006a), thus resulting in 3 times less of embedding according to a recent study (Zhen et al. Citation2009). However, the RCS High Flow exhibited the highest desiccation factors about 7 times that of the BioStage impactor among the samplers investigated as shown in . Another important factor, which is associated with desiccation, is the particle bounce. During the bioaerosol sampling, the agar surface could become harder due to the desiccation, which correspondingly results in a higher degree of particle bounce. A recent study has shown that when the sampling time was reduced from 5 min to 1 min, the physical collection efficiency of the RCS High Flow, quantified by the quantitative PCR (qPCR) technique, was shown to increase significantly, which was mainly due to the particle bounce problem (Zhen et al. Citation2009). For the gravitational settling and the electrostatic sampling, the particle bounce problem, if any, could be minimal due to their low impaction velocity. The BioSampler is an improvement over the AGI-30 impinger, by minimizing the particle bounce and reaerosolization problems. A study indicated that a significant inlet loss of larger particles, e.g., the fungal species, could be expected due to the inertia (Yao et al. Citation2009b). This might help to explain that the BioSampler obtained limited fungal aerosol diversity as shown in .
Filter sampling is widely applied to detect bioaerosols due to its high physical collection efficiency. In this study, the MCE filter was found to perform reasonably well when collecting culturable bacterial and fungal aerosols. The detection of biological particles depends on numerous parameters. In general, the physical collection efficiencies of the samplers should be an important parameter to consider when sampling biological aerosols. It was shown that the impaction velocity and the jet-to-plate distance also play roles in the enumeration of biological aerosols (Yao and Mainelis Citation2006a; Grinshpun et al. Citation2007). In addition, the sampling time was shown to play an important role in the enumeration of culturable bioaerosols (Wang et al. Citation2001; Zhen et al. Citation2009; Mainelis and Tabayoyong Citation2009). Basically, a shorter sampling time is recommended for biological aerosol sampling when possible. As observed in this study, the culturing method also played a role in the culturable microorganism counts. The extraction of MCE samples could result in additional stress, which causes additional culturability loss. In addition, the culturing temperature was also observed to affect the culturing of bacteria and fungi collected.
CONCLUSION
Traditionally, bioaerosol samplers are applied to detect the total culturable counts or the total amount when combined with qPCR in many bioaerosol studies. However, no studies were conducted to investigate their sampling effects on the culturable biological diversity. It is important to develop a sensible protocol to identify the potential bioaerosol health hazard, especially when culturing methods are applied. This study was the first to report the culturable bacterial and fungal aerosol diversity obtained by different bioaerosol samplers that are widely used. The results from this study indicated that different samplers resulted in different culturable bacterial bioaerosol diversity in different environments. The electrostatic sampling was shown to report the highest outdoor fungal aerosol diversity in this study. The differences in bioaerosol diversity could be due to the sampling mechanism, sampling time, sampling environments, desiccation, impaction stress, degree of embedding, and the particle bounce. The overall biological sampling performance of a bioaerosol sampler is influenced by the interplay of these factors, and the results here might be affected by different environmental matrix other than those investigated in this work. The samples were collected only at two locations, which is a limitation of this study. Nonetheless, the information provided here not only describes the variation of sampler's stress to the microorganisms, but also provides the information for selecting the best bioaerosol sampler and the sampling parameters for obtaining the highest airborne bioaerosol biodiversity for health-related investigations.
Acknowledgments
This study was supported by the National Science Foundation of China (Grant 20877004), and National High Technology Research and Development Program of China (Grant 2008AA062503).
REFERENCES
- Adhikari , A. , Martuzevicius , D. , Reponen , T. , Grinshpun , S. A. , Cho , S. H. , Sivasubramani , S. K. , Zhong , W. , Levin , L. , Kelley , A. L. , Clair , H. G. and Lemastersa , G. 2003 . Performance of the Button Personal Inhalable Sampler for the Measurement of Outdoor Aeroallergens . Atmos. Environ. , 37 : 4723 – 4733 .
- An , H. R. , Mainelis , G. and Yao , M. 2004 . Evaluation of a High-Volume Portable Bioaerosol Sampler in Laboratory and Field Environments . Indoor Air , 14 : 385 – 393 .
- Ayres , J. G. , Forsberg , Annesi-Maesano , B. , Dey , I. , Ebi , R. , Helms , K. L. , Medina-Ramon , P. J. , Windt , M. , Forastiere , M. and F., and Environmental Health Commission European Respiratory . 2009 . Climate Change and Respiratory Disease: European Respiratory Society Position Statement . Eur. Respir. J. , 34 : 295 – 302 .
- Bold , H. C. , Alexopoulos , C. J. and Delevoryas , T. 1980 . Morphology of Plants and Fungi , 4th ed. , New York : Harper & Row .
- Burton , N. C. , Adhikari , A. , Grinshpun , S. A. , Hornung , R. and Reponen , T. 2005 . The Effect of Filter Material on Bioaerosol Collection of Bacillus subtilis Spores Used as a Bacillus anthracis Simulant . J. Environ. Monit. , 7 : 475 – 480 .
- Burton , N. C. , Grinshpun , S. A. and Reponen , T. 2007 . Physical Collection Efficiency of Filter Materials for Bacteria and Viruses . Ann. Occup. Hyg. , 51 : 143 – 151 .
- Chen , P. S. and Li , C. S. 2005 . Quantification of Airborne Mycobacterium Tuberculosis in Health Care Setting Using Real-Time qPCR Coupled to an Air-Sampling Filter Method . Aerosol Sci. Technol. , 39 : 371 – 376 .
- Christiani , D. C. , Eisen , E. A. , Wegman , D. H. , Ye , T. T. , Lu , P. L. , Gong , Z. C. and Dai , H. L. 1986 . Respiratory Disease in Cotton Textile Workers in the People's Republic of China. I. Respiratory Symptoms . Scand. J. Work Environ. Health , 12 : 40 – 45 .
- Douwes , J. , Thorne , P. , Pearce , N. and Heederik , D. 2003 . Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects . Ann. Occup. Hyg. , 3 : 187 – 200 .
- Droogenbroeck , V. C. , Van Risseghem , M. , Braeckman , L. and Vanrompay , D. 2009 . Evaluation of Bioaerosol Sampling Techniques for the Detection of Chlamydophila psittaci in Contaminated Air . Vet. Microbiol. , 135 : 31 – 37 .
- Feller , W. 1968 . An Introduction to Probability Theory and Its Applications , New York : Wiley .
- Finnegan , M. J. , Pickering , C. A. C. and Burge , P. S. 1984 . The Sick Building Syndrome: Prevalence Studies . British Med. J. , 289 : 1573 – 1575 .
- Frohlich-Nowoisky , J. , Pickersgill , D. A. , Despres , V. R. and Poschl , U. 2009 . High Diversity of Fungi in Air Particulate Matter . Proc. Natl. Acad. Sci. USA , 106 : 12814 – 12819 .
- Gavett , S. H. and Koren , H. S. 2000 . The Role of Particulate Matter in Exacerbation of Atopic Asthma . Int. Arch. Allergy Immunol. , 124 : 109 – 112 .
- Gravesen , S. 1979 . Fungi as a Cause of Allergic Disease . Allergy , 34 : 135 – 154 .
- Grinshpun , S. A. , Adhikari , A. , Cho , S.-H. , Kim , K.-Y. , Lee , T. and Reponen , T. 2007 . A Small Change in the Design of a Slit Bioaerosol Impact or Significantly Improves Its Collection Characteristics . J. Environ. Monit. , 9 : 855 – 861 .
- Han , T. , An , H. R. and Mainelis , G. 2010 . Performance of an Electrostatic Precipitator with Superhydrophobic Surface When Collecting Airborne Bacteria . Aerosol Sci. Technol. , 44 : 339 – 348 .
- Han , T. and Mainelis , G. 2008 . Design and Development of an Electrostatic Sampler for Bioaerosols with High Concentration Rate . J. Aerosol Sci. , 39 : 1066 – 1078 .
- Hsu , S. and Zee , A. 2004 . Global Spread of Infectious Diseases . J. Biol. Syst. , 12 : 289 – 300 .
- Kuhn , D. M. and Ghannoum , M. A. 2003 . Indoor Mold, Toxigenic Fungi, and Stachybotrys chartarum: Infectious Disease Perspective . Clin. Microbiol. Rev. , 16 : 144 – 172 .
- Lee , T. , Grinshpun , S. A. , Martuzevicius , D. , Adhikari , A. , Crawford , C. M. and Reponen , T. 2006 . Culturability and Concentration of Indoor and Outdoor Airborne Fungi in Six Single-Family Homes . Atmos. Environ. , 40 : 2902 – 2910 .
- Li , K. , Dong , S. , Wu , Y. and Yao , M. 2010 . Comparison of the Biological Content of Air Samples Collected at Ground Level and at Higher Elevation . Aerobiologia , 26 : 233 – 244 .
- Lundin , J. I. and Checkoway , H. 2009 . Endotoxin and Cancer . Environ. Health Perspect. , 117 : 1344 – 1350 .
- Mainelis , G. and Tabayoyong , M. 2009 . The Effect of Sampling Time on the Overall Performance of Portable Microbial Impactors . Aerosol Sci. Technol. , 44 : 75 – 82 .
- Maki , T. , Susuki , S. , Kobayashi , F. , Kakikawa , M. , Yamada , M. , Higashi , T. , Chen , B. , Shi , G. , Hong , C. , Tobo , Y. , Hasegawa , H. , Ueda , K. and Iwasaka , Y. 2008 . Phylogenetic Diversity and Vertical Distribution of a Halobacterial Community in the Atmosphere of an Asian Dust (KOSA) Source Region, Dunhuang City . Air Qual. Atmos. Health , 1 : 81
- Marmot , A. F. , Eley , J. , Stafford , M. , Stansfeld , S. A. , Warwick , E. and Marmot , M. G. 2006 . Building Health: An Epidemiological Study of “Sick Building Syndrome” in the Whitehall II Study . Occup. Environ. Med. , 63 : 283 – 289 .
- Murray , C. J. , Styblo , K. and Rouillon , A. 1990 . Tuberculosis in Developing Countries: Burden, Intervention and Cost . Bull. Int. Union Tuberc. Lung Dis. , 65 : 6 – 24 .
- Muyzer , G. , Dewaal , E. C. and Uitterlinden , A. G. 1993 . Profiling of Complex Microbial-Populations by Denaturing Gradient Gel-Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes-Coding for 16S Ribosomal-RNA . Appl. Environ. Microbiol. , 59 : 695 – 700 .
- Nehme , B. , Letourneau , V. , Forster , R. J. , Veillette , M. and Duchaine , C. 2008 . Culture-Independent Approach of the Bacterial Bioaerosol Diversity in the Standard Swine Confinement Buildings, and Assessment of the Seasonal Effect . Environ. Microbiol. , 10 : 665 – 675 .
- Pieckova , E. and Jesenska , Z. 1999 . Microscopic Fungi in Dwellings and Their Health Implications in Humans . Ann. Agric. Environ. Med. , 6 : 1 – 11 .
- Ren , P. , Jankun , T. M. and Leaderer , B. P. 1999 . Comparisons of Seasonal Fungal Prevalence in Indoor and Outdoor Air and in House Dusts of Dwellings in One Northeast American County . J. Expo. Anal. Environ. Epidemiol. , 9 : 560 – 568 .
- Reponen , T. A. , Gazenko , S. V. , Grinshpun , S. A. , Willeke , K. and Cole , E. C. 1998 . Characteristics of Airborne Actinomycete Spores . Appl. Environ. Microbiol. , 64 : 3807 – 3812 .
- Rodrigues , L. C. and Smith , P. G. 1990 . Tuberculosis in Developing Countries and Methods for Its Control . T. Roy. Soc. Trop. Med. H. , 84 : 739 – 744 .
- Schmalenberger , A. , Schwieger , F. and Tebbe , C. C. 2001 . Effect of Primers Hybridizing to Different Evolutionarily Conserved Regions of the Small-Subunit rRNA Gene in PCR-Based Microbial Community Analyses and Genetic Profiling . Appl. Environ. Microbiol. , 67 : 3557 – 3563 .
- Shelton , B. G. , Kirkland , K. H. , Flanders , W. D. and Morris , G. K. 2002 . Profiles of Airborne Fungi in Buildings and Outdoor Environments in the United States . Appl. Environ. Microbiol. , 68 : 1743 – 1753 .
- Stewart , S. L. , Grinshpun , S. A. , Willeke , K. , Terzieva , S. , Ulevicius , V. and Donnelly , J. 1995 . Effect of Impact Stress on Microbial Recovery on an Agar Surface . Appl. Environ. Microbiol. , 61 : 1232 – 1239 .
- Trout , D. B. 2001 . Bioaerosol Lung Damage: Trout's Response . Environ. Health Perspect. , 109 : A516 – A517 .
- Vianelli , N. , Giannini , M. B. , Quarti , C. , Sabattini , M. A. B. , de Fiacchini , M. , Vivo , A. , Graldi , P. , Galli , S. , Nanetti , A. , Baccarani , M. and Ricci , P. 2006 . Resolution of a Pseudomonas aeruginosa Outbreak in a Hematology Unit with the Use of Disposable Sterile Water Filters . Haematol.-Hematol. J. , 91 : 983 – 985 .
- Wang , Z. , Reponen , T. , Grinshpun , S. A. , Gorny , R. L. and Willeke , K. 2001 . Effect of Sampling Time and Air Humidity on the Bioefficiency of Filter Samplers for Bioaerosol Collection . J. Aerosol Sci. , 32 : 661 – 674 .
- Weis , C. P. , Intrepido , A. J. , Miller , A. K. , Cowin , P. G. , Durno , M. A. , Gebhardt , J. S. and Bull , R. 2002 . Secondary Aerosolization of Viable Bacillus anthracis Spores in a Contaminated US Senate Office . J. Am. Med. Assoc. , 288 : 2853 – 2858 .
- Whyte , W. , Green , G. and Albisu , A. 2007 . Collection Efficiency and Design of Microbial Air Samplers . J. Aerosol Sci. , 38 : 97 – 110 .
- Willeke , K. , Lin , X. J. and Grinshpun , S. A. 1998 . Improved Aerosol Collection by Combined Impaction and Centrifugal Motion . Aerosol Sci. Technol. , 28 : 439 – 456 .
- Wong , S. K. , Lai , L. W. C. , Ho , D. C. W. , Chau , K. W. , Lam , C. L. K. and Ng , C. H. F. 2009 . Sick Building Syndrome and Perceived Indoor Environmental Quality: A Survey of Apartment Buildings in Hong Kong . Habitat Int. , 33 : 463 – 471 .
- Wu , Y. , Shen , F. and Yao , M. 2010 . Use of Gelatin Filter and BioSampler in Detecting Airborne H5N1 Nucleotides, Bacteria and Allergens . J. Aerosol Sci. , 41 : 869 – 879 .
- Yao , M. and Mainelis , G. 2006a . Effect of Physical and Biological Parameters on Enumeration of Bioaerosols by Portable Microbial Impactors . J. Aerosol Sci. , 37 : 1467 – 1483 .
- Yao , M. and Mainelis , G. 2006b . Investigation of Cut-Off Sizes and Collection Efficiencies of Portable Microbial Samplers . Aerosol Sci. Technol. , 40 : 595 – 606 .
- Yao , M. and Mainelis , G. 2006c . Utilization of Natural Electrical Charges on Microorganisms for Their Collection by Electrostatic Means . J. Aerosol Sci. , 37 : 513 – 527 .
- Yao , M. and Mainelis , G. 2007 . Analysis of Portable Impactor Performance for Enumeration of Viable Bioaerosols . J. Occup. Environ. Hyg. , 4 : 514 – 524 .
- Yao , M. , Wu , Y. , Zhen , S. and Mainelis , G. 2009a . A Comparison of Airborne and Dust-Borne Allergens and Toxins Collected from Home, Office and Outdoor Environments Both in New Haven, United States and Nanjing, China . Aerobiologia , 25 : 183 – 192 .
- Yao , M. , Zhang , H. , Dong , S. , Zhen , S. and Chen , X. 2009b . Comparison of Electrostatic Collection and Liquid Impinging Methods When Collecting Airborne House Dust Allergens, Endotoxin and (1,3)-[Beta]-D-Glucans . J. Aerosol Sci. , 40 : 492 – 502 .
- Yao , M. , Zhu , T. , Li , K. , Dong , K. , Wu , Y. , Qiu , X. , Jiang , B. , Chen , L. and Zhen , S. 2009c . Onsite Infectious Agents and Toxins Monitoring in 12 May Sichuan Earthquake Affected Areas . J. Environ. Monit. , 11 : 1993 – 2001 .
- Zhang , Y. , Heym , B. , Allen , B. , Young , D. and Cole , S. 1992 . The Catalase Peroxidase Gene and Isoniazid Resistance of Mycobacterium-Tuberculosis . Nature , 358 : 591 – 593 .
- Zhen , S. Q. , Li , K. J. , Yin , L. H. , Yao , M. , Zhang , H. L. , Chen , L. S. , Zhou , M. H. and Chen , X. D. 2009 . A Comparison of the Efficiencies of a Portable Biostage Impactor and a Reuter Centrifugal Sampler (RCS) High Flow for Measuring Airborne Bacteria and Fungi Concentrations . J. Aerosol Sci. , 40 : 503 – 513 .