Abstract
Airborne mineral dust particles contribute a significant fraction to the total aerosol mass, thus they make a substantial contribution to the Earth's radiative budget by direct scattering and absorption of radiation. Quantifying their contribution is complicated by the variability of optical properties as a function of water uptake. To improve understanding, we directly measured the relative humidity (RH) dependence of extinction [fRHext(RH, Dry)] for three key silicate clay components (illite, kaolinite, and montmorillonite) of mineral dust aerosols through cavity ring-down spectroscopy at 532 nm. The three clays studied show significant differences in fRHext(RH, Dry) at three RH values, and reasons for this are explored. With 68% RH as an example, we used the fRHext(RH, Dry) and Mie theory to calculate a growth factor for comparison with other measurement techniques. Humidified tandem differential mobility analyzer and quartz crystal microbalance growth factors from the literature are larger than our optical measurements indicate. An apparent decrease in particle size calculated from optical measurements for illite and kaolinite was further investigated by determining the aerosol electrical mobility size distribution of 68% RH and dry clay particles at that indicated shrinkage of approximately 10% at elevated humidity. Direct optical measurement has advantages because the effects of irregular shape and internal voids are observed. Our calculated growth factors provide a lower limit and can be incorporated into climate models in conjunction with other results to reduce the uncertainty associated with the optical response to water uptake on clay aerosols.
INTRODUCTION
Mineral dust aerosol is associated with windblown dust soil and has an estimated global source strength of 1000–3000 Tg/yr, accounting for 45% of the total aerosol mass load (Caquineau et al. Citation2002; Zender et al. Citation2004). Larger particles will sediment out quickly due to gravitational settling, but particles smaller than 10 μm have longer atmospheric lifetimes of about a week. During this time, mineral dust can be transported tens of thousands of kilometers, depositing particles in areas that are located far away from the source (Biscaye et al. Citation1997; Grousset et al. Citation2003). For example, a large amount of Asian dust is subject to long-range transport over the Pacific Ocean, showing up as background dust aerosol on the West Coast of the United States, whereas African dust frequently gets deposited over the Caribbean (Evan and Mukhopadhyay; Perry et al. 2004).
Mineral dust is involved in many different atmospheric processes including deposition of micronutrients (Duce and Tindale Citation1991; Uematsu et al. Citation2003), providing surfaces for heterogeneous reactions (Bauer et al. Citation2004; Cwiertny et al. Citation2008; McNaughton et al. Citation2009) and direct (Carlson and Benjamin Citation1980; Sokolik and Toon Citation1996; Miller and Tegen Citation1998) and indirect radiative forcing (Sassen Citation2002; Lohmann and Diehl Citation2006). Each of these processes is complicated by the ability of mineral dust to take up water. For example, the more soluble a particle, the more likely it will be removed from the atmosphere via wet deposition. Further, if particle size and mass are affected by water uptake, it will influence the rate of gravitational settling (Herich et al. Citation2009). Reactivity toward other species is also affected by aerosol hygroscopicity. It has been shown that clay aerosols provide a sink for reactive gases and a substrate for chemical reaction, as a function of water content (Falkovich et al. Citation2004; Hallquist et al. Citation2003; Mashburn et al. Citation2006; Vlasenko et al. Citation2006; Navea et al. Citation2009). In particular, increased ambient relative humidity (RH) resulted in a greater concentration of short-chain hydrocarbons on the surface of mineral dust particles, indicating water-assisted uptake onto these particles (Falkovich et al. Citation2004). Mashburn et al. (2006) measured a greater nitric acid content on montmorillonite particles with increased RH as the particles were exposed to atmospherically relevant nitric acid concentrations. In contrast, kaolinite samples showed decreased uptake of two different methyl siloxanes, in conjunction with an increase in surface absorbed water at elevated RH (Navea et al. Citation2009). These studies show that uptake of gas-phase species on dust surfaces can be either increased or decreased as a function of the water concentration on the surface and in air.
Understanding the hygroscopicity of atmospheric particles is also important in assessing their direct and indirect effects. Mineral dust particles contribute to radiative forcing, changing the energy balance of the atmosphere by scattering incoming short-wave radiation (negative forcing) and absorbing outgoing infrared terrestrial radiation (positive forcing) (Carlson and Benjamin Citation1980; Miller and Tegen Citation1998; Lohmann and Diehl Citation2006). The size of the particle and its complex refractive index are a function of water content, and these two characteristics determine the extent of absorption and scattering of light by the particle. Owing to the complicated chemical makeup and irregular shape of the species, along with high loading and spatial and temporal variability, mineral dust aerosols represent the largest uncertainty associated with climate change having a net negative forcing reported as −0.1 ± 0.2 W/m2 (IPCC 2007). The Fourth Assessment Report of the Intergovernmental Panel on Climate Change reports “the level of scientific understanding” as very low for the radiative effects of mineral dust (IPCC 2007).
Mineral dust particles can also impact the radiative balance of the atmosphere indirectly, by acting as cloud condensation nuclei (CCN) or ice nuclei (IN). Previously studies have shown that mineral dust can act as efficient nuclei for cloud droplet formation and cirrus cloud formation affecting cloud albedo and cloud lifetime (DeMott et al. Citation2003; Hung et al. Citation2003; Koehler et al. Citation2009). The ability of the particle to act as an effective CCN or IN depends on its hygroscopicity. For mineral dust, an increase in nucleation with increased RH has been seen (Salam et al. Citation2006; Kumar et al. Citation2010). Therefore, understanding water uptake and specifically the optical effect of water uptake is crucial for understanding mineral dust's direct and indirect impact on climate change. Mie theory is typically used to model the climate impact from the interaction of light with aerosols because it provides a basic estimate using spherically shaped particles and approximate values for the refractive index (Bohren and Huffman Citation2004). However, these calculations are not assumed to be valid for assessing the optical properties of mineral dust, as the particles are irregular in shape and are composed of an inhomogeneous mix of minerals (Curtis et al. Citation2008; Hudson et al. Citation2008). A recent study investigated the agreement between Mie theory simulations for various shapes, including sphere and disk, and experimental extinction results for the major silicate resonance bands of mineral dust in the infrared region from 4000 to 10,000 cm−1. The results of the study show a large deviation from spherical Mie theory for band position, band shape, and peak intensity with the resonance peak red shifted 27–44 cm−1 (Hudson et al. Citation2008).
Generally, mineral dust is considered to be nonhygroscopic and therefore global climate models assume it to be fully insoluble. Recently, several studies of different clay components of mineral dust found that these particles are slightly hygroscopic and the extent of water uptake largely depends on the structure and chemical composition of the clay (Gustafsson et al. Citation2005; Seisel et al. Citation2005; Schuttlefield et al. Citation2007; Herich et al. Citation2009; Koehler et al. Citation2009). Measurements of the water uptake on mineral dust are highly uncertain and difficult to quantify as their hygroscopicity is small in comparison to other aerosol species such as inorganic salts. In addition, the water uptake by dust has been shown to enhance both the extinction and absorption, with a pronounced effect for ambient African dust that has been transported long distances and is therefore well aged (Lack et al. Citation2009). This effect will have implications on the single scattering albedo, an important parameter in assessing the global radiation balance. To date, laboratory quantification of water uptake on mineral dust particles has been investigated using a humidified tandem differential mobility analyzer (HT-DMA) and a quartz crystal microbalance (QCM). Physical growth factors (GF) from a HT-DMA are a measure of hygroscopicity, where GF are defined as follows:
In this study, we investigate water uptake on three clay minerals by quantifying optical growth upon humidification by using cavity ring-down spectroscopy (CRD). The three minerals considered, montmorillonite [(Na,Ca)(Al,Mg)6(Si4O10)3 (OH)6], kaolinite [Al2Si2O5(OH)4], and illite [(K,H3O) (Al,Mg,Fe)2(Si,Al)4O10(OH)2], are common components of mineral dust from different source regions (Usher et al. Citation2003). The relative abundance of these clays can be used as a tracer to assess the origin of mineral dust aerosols. For example, the illite/kaolinite ratio seems to be the most sensitive indicator of Saharan dust even after long-range transport (Caquineau et al. Citation1998). Kaolinite is indicative of dust from low latitudes in West Africa, whereas illite is more common in the northern regions toward the Mediterranean coast (Prospero Citation1999). Montmorillonite is less commonly used as a tracer but is frequently associated with volcanic rock (Sudo and Shimoda 1978).
Optical properties were determined by measuring the extinction cross section (σext) of size-selected clay aerosols as a function of RH, fRHext(RH, Dry), where fRHext is
CRD at 532 nm is used for these measurements and showed both decreases and increases in extinction with humidification depending on the clay and the humidity levels. Mie theory was used to convert the fRHext(RH, Dry) into a calculated growth factors (GFc) value at 68% RH for comparison with measured growth factors (GFm) from the literature. Further, the electrical mobility size distributions of clay particles at 68% RH and dry conditions were measured and used to indicate size change for all clays in this study. Direct optical measurements are able to capture the effects of the irregular shapes and internal voids possible in clay particles, but are difficult to include in models. Our GFc provides a lower limit and can be incorporated into climate models in conjunction with other results to improve the uncertainty associated with the optical response to water uptake on clay aerosols.
EXPERIMENTAL METHODS
Properties of Selected Clays and Aerosol Generation
The three clays investigated, illite (IMt-1), kaolinite (KGa-1b), and montmorillonite (STx-1b), were purchased from the Source Clays Repository. These clays have been previously well studied with other methods, and their chemical composition and structure are known. The structures of the three types of clay are useful in understanding the different optical response upon humidification. Previous studies by Cases et al. (1992) determined that all of the clays are composed primarily of layered aluminum and silicon held together by interlayer van der Waals forces, charge-countering cations, or hydrogen bonds. The layers are arranged with tetrahedrally coordinated silicon layers and octahedrally coordinated aluminum layers (Cases et al. Citation1992). The ability of these clays to swell upon hydration is based on the type of interlayer interactions. In addition, they differ in the number of hydroxyl groups and silicate groups on their surfaces that will influence the extent to which they can hydrogen bond with water vapor (Salam et al. Citation2006).
The layers of montmorillonite are composed of a 2:1 ratio of silicon to aluminum held together by van der Waals forces and charge-countering cations (Cases et al. Citation1992). Montmorillonite is classified as a “swelling clay” in which the extent of water uptake is largely dependent on the availability of cations in between the structural sheets and their hydration energies (Hensen and Smit Citation2002; Schuttlefield et al. Citation2007; Herich et al. Citation2009). There are six hydroxyl groups on its surface, and it can therefore easily hydrogen bond to water upon humidification. Illite has a similar structure to montmorillonite with a 2:1 silicon to aluminum ratio in its layers, but it is considered a “nonswelling” clay. The layers are held together by stronger electrostatic interactions with interlayer cations such as potassium, calcium, or magnesium. The degree of water uptake can be affected by isomorphic substitution of these interlayer ions for cations of lower charge causing a charge imbalance (Cases et al. Citation1992). There are only four hydroxyl groups on its surface leaving less sites for possible hydrogen bonding to water. The chemical structure of kaolinite is a 1:1 silicon to aluminum layered clay and is considered a “nonswelling” clay. Kaolinite has strong hydrogen bonding between the layers preventing cations and water molecules from entering the interlayer space forcing water to primarily absorb on the surface of the particle (Schuttlefield et al. Citation2007). It only has two hydroxyl groups on its surface for hydrogen bonding to water.
To generate aerosols, illite was ground using a Wig-L-Bug (Dentsply Rinn® Crescent®, Fischer Scientific) prior to being atomized, while the kaolinite and montmorillonite powders were used as received. Suspensions of the clays (10 wt% clay) in high-performance liquid chromatography (HPLC) grade water (J. T. Baker) were constantly mixed using a stir plate and atomized using a custom-built constant output atomizer. The polydisperse aerosol was subsequently dried in a diffusion dryer with a residence time of approximately 12.5 s so that the RH of the aerosol stream was decreased to <10%.
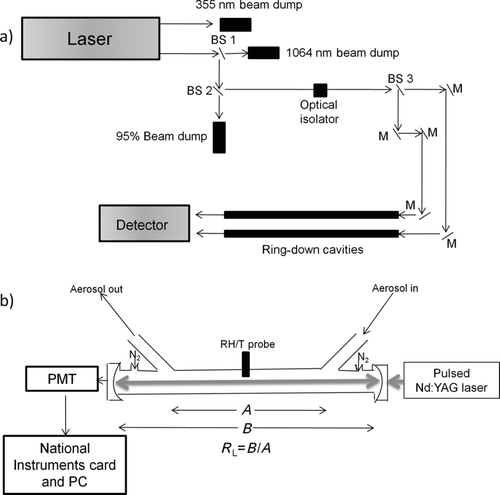
FIG. 1 (a) Optical layout of the cavity ring-down spectrometer. Laser light in the ultraviolet (355 nm) is immediately dumped. The other output is a combination of 1064 and 532 nm light. The first beam splitter (BS 1) is a long wave pass dichroic beam splitter that transmits the 1064 nm light and reflects 532 nm light at a 45° angle relative to the beam splitter. The 532 nm light then hits the second beam splitter (BS 2) that transmits 95% of the light (which is dumped) and reflects 5% of the light. An optical isolator sits in the beam path that prevents back reflection from damaging the laser head. The third beam splitter (BS 3) transmits 50% of the light and reflects 50% of the light, splitting the beam in two. The remaining optics are highly reflective mirrors (M) and simply serve to align the beam into one of the two sample cavities. (b) Cavity setup: Our light source is a pulsed Nd:YAG laser which enters the optical cavity and is reflected between two mirrors. The decay of the laser pulse is captured by a photomultiplier tube (PMT). R L, the ratio of the optical cavity length (B) to the sample length (A), is used in calculating the extinction (EquationEquation (3)).
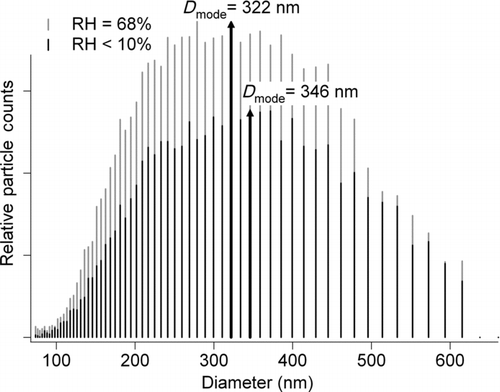
After atomization and drying, the clay particles were directed through a differential mobility analyzer (DMA, TSI 3081L) to select a monodisperse size population for optical measurements. The electrical mobility size distribution for each clay type was determined at wet (68% RH) and dry conditions by following the DMA with a condensation particle counter (CPC, TSI 3775) to function as a scanning particle mobility sizer (SMPS, TSI 3936L75). Atomization produced a lognormal distribution with a single mode for the diameter of montmorillonite, illite, and kaolinite at 157, 209, and 346 nm, respectively, under dry (RH < 10%) conditions. At elevated RH, the diameter mode from the size distribution is approximately 10% lower in each case, where differences seen between trials were considered. Owing to the limitations of the current setup, only 68% RH was selected for these measurements.
For optical measurements, montmorillonite, illite, and kaolinite aerosol populations were size selected for 200, 300, and 425 nm diameter particles, respectively. The particle size was chosen to be larger than the mode of the size distribution, which minimizes the contribution of multiply charged clay particles in the sample. The multiply charged particles result from the neutralizer of the DMA. A sufficient concentration of particles for optical measurements was still achieved. The DMA size selection has a reported uncertainty of approximately 10% which is included in uncertainty calculations (McMurry Citation2000). The aerosol sizes selected for this study can be compared with ambient atmospheric mineral dust particles, which span a size range of 100–5000 nm (Duce Citation1995). We have chosen to focus on smaller particles because larger particles have lifetimes on the order of a few hours and tend to settle out quickly from the atmosphere. Smaller particles will reside in the atmosphere longer and can be transported long distances (Tegen and Fung Citation1994).
Optical measurements are terminated at the CPC to count the population exiting the CRD and to determine the doublet concentrations by using the measured number concentration at the doublet size and the neutralizer charging distribution from the manufacturer for subsequent corrections. Since our measurements are reported as a function of particle size, the extinction contribution for particles with multiple charges is calculated and used to correct the extinction values of the size-selected particles. Only doublets were used in corrections; the number of higher charged particles is expected to be small and would have a negligible effect on the extinction (Wiedensohler Citation1988). It is important to note that the CPC is the largest source of error with a reported uncertainty of 10% in particle counts. This uncertainty is minimized by reporting the fRHext as a ratio between humidified extinction and dry extinction for the same sample.
Cavity Ring-Down Spectrometer
CRD has been used for a variety of optical measurements of both aerosol and gas-phase species and has been reviewed extensively in the literature (Smith and Atkinson Citation2001; Atkinson Citation2003; Baynard et al. Citation2007; Freedman et al. Citation2009; Lang-Yona et al. Citation2010). The system used in the present work is newly constructed and similar to that fabricated by Baynard et al. (2007) and is briefly described here. The optical layout and cavity setup is shown in and b, respectively. The cavities have an optical length of 68.9 cm and a sample length of 57.8 cm.
FIG. 2 Atomized particles are first passed through a diffusion dryer before entering the DMA where a polydisperse population of aerosols is size selected. The particles are then dried to a RH < 10% before entering the first optical cavity. After exiting the first cavity, the particles are humidified to a desired RH and enter the second optical cavity before being counted by the CPC.
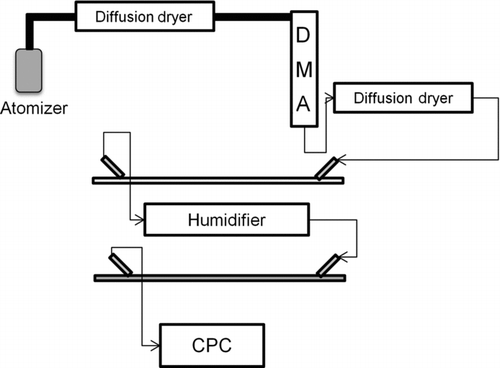
TABLE 1 fRHext for all three clays at select relative humidities measured with cavity ring-down spectroscopy. Errors in fRHext values are reported as the experimental standard deviation (1σ) of at least 18 trials at each RH for each clay type over three different experiments
Two concave mirrors 1″ in diameter with a 1 m radius of curvature and a reflectivity of >99.998% (Advanced Thin Films) are located at each end of the cavity. These are supplied with a constant flow of filtered nitrogen (0.02 L/min) to prevent deposition of particulate matter onto the mirror surface. The laser source is a solid state 20 Hz pulsed Nd:YAG laser (Big Sky Laser, now Quantel USA, Ultra). The second harmonic produces 532 nm light which is coupled into the cavity and reflected between the two mirrors. The light intensity decays exponentially in time due to losses through the two end mirrors with a time constant τ 0, known as the particle free cavity-ring down time. Typical, τ 0, values were on the order of 100 μs, which yields an effective path length of about 31 km. Particles in the cavity will absorb and scatter light, and their extinction will reduce the ring-down time to τ. The extinction of the particles, α ext (m−1), is calculated according to EquationEquation (3):
A schematic presentation of the full experimental setup is shown in . After atomization and drying, the RH is <10% and the RH of the monodisperse dry sample following the DMA is maintained by passing the aerosols through a second diffusion dryer to remove any water vapor that was introduced via the sheath flow of the DMA. The dry aerosol sample enters and exits the first cavity at a flow rate of 0.3 L/min, where the extinction, α ext(dry), is measured. After exiting the first cavity, the aerosol stream is humidified to 50%, 68%, or 90% RH using a custom-built temperature-controlled humidifier. The particles then enter the second cavity where the extinction, α ext(RH), is measured. Temperature and RH are monitored using Vaisala INTERCAP HMP50 probes (accuracy ± 3%) in both optical channels. The extinction results are converted to extinction cross sections by dividing by the particle number concentration. The dry and humidified extinction cross section values are then used to calculate fRHext, which describes the dependence of light extinction on RH by the aerosols. Errors in fRHext values are reported in as the experimental standard deviation (1σ) of at least 18 trials at each RH for each clay type over 3 different experiments.
Growth Factors
GFc were calculated using optical measurements in combination with Mie theory. These closure-based GFc use literature values for the refractive indices as an input for Mie theory to determine the effective optical diameter that corresponded to the dry and wet extinction cross section. The retrieved diameters were used to calculate a GFc (Garland et al. Citation2007).
RESULTS AND DISCUSSION
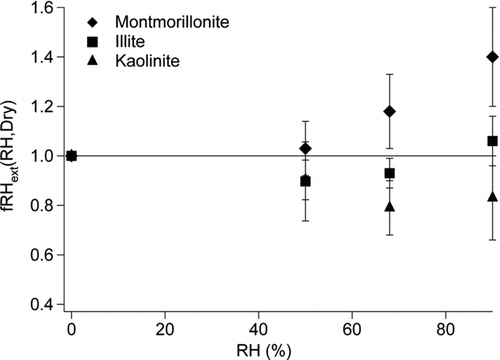
The fRHext of three clay components of mineral dust as measured by CRD at 50%, 68%, and 90% RH are shown in and . An fRHext of 1 is expected when there is no optical change in the particle upon humidification. Montmorillonite showed a steady increase in extinction as a function of RH, with an fRHext ranging from 1.03 at 50% to 1.4 at 90% RH. Illite showed a decrease in extinction upon humidification at 50% RH and 68% RH. At 90% RH, a moderate increase in extinction was observed when compared with dry illite. Kaolinite showed a decrease in extinction for all three RH values studied. We also monitored the aerosol electrical mobility diameter size distribution of 68% RH and dry clay particles and observed shrinkage of approximately 10% upon humidification for each clay type as illustrated in for kaolinite. A decrease in extinction upon humidification may be attributed to compaction of the particle leading to a decrease in optical diameter, a shape transition to a more spherical humidified particle than its dry counterpart, or a significant decrease in refractive index for the humidified particle (Vlasenko et al. Citation2005; Herich et al. Citation2009; Koehler et al. Citation2009). We will consider these explanations below.
FIG. 4 Size distributions of kaolinite clay under dry (RH < 10%) and wet conditions (RH = 68%) measured by the SMPS. A decrease in particle size upon humidification of approximately 10% is seen for this case and all other clays studied.
A comparison between closure-based GFc calculated from optical extinction measured in this study and GFm from the literature at 68% RH in all cases is shown in for all three clays investigated. The differences between the literature-derived GFm values for specific clay types illustrate the general challenge associated with GF measurements of clays.
TABLE 2 Comparison of our GFc with GFm presented in the literature for clay minerals. All GF were measured at 68% RH. The HTDMA study reported GF at comparable sizes and the QCM work reported mg water per gram of sample
Each of these techniques has some disadvantages. The optical measurements in conjunction with Mie theory used to calculate a GFc begins with a high-accuracy technique of CRD and accounts for the irregular shape and possible internal voids of the clay particles. However, these calculated GFc do not take into consideration the small decrease in refractive index for the clay particles that could accompany water uptake. Such a decrease leads to a larger calculated GFc. Further, the GFc calculated using Mie theory and optical measurements assumes that the particles are spherical. Recent work has shown that Mie theory underestimates extinction by clay particles, so sizes would have to be larger to reproduce the extinction intensity (Hudson et al. Citation2008). The impact of this on our calculations is minimized by reporting a ratio between two cases. These two complications mean that calculating the GFc using Mie theory represents a lower bound for the GF of clays in comparison with HT-DMA and QCM measurements.
The fRHext for montmorillonite is the largest of all the clays at each RH value studied. This can be explained by specific physical characteristics of montmorillonite which has a structure that is easily hydrated by water on the surface. Montmorillonite is also expected to have the greatest amount of swelling by incorporation of water between the layers after initial restructuring of the particles due to the weak van der Waals forces holding the layers together. At 68% RH, montmorillonite had an fRHext of 1.18 that corresponds to a calculated a GFc of 1.03. The increase in the GFc is in contrast to a decrease of approximately 10% in the mode of the particle size distribution. Typically increases in optical results should be mirrored by increases in size as water is taken up. This apparent discrepancy could be attributed to enhanced absorption by a scattering (water) shell formed around an absorbing core (Fuller et al. Citation1999). Our hypothesis is supported by the steady increase in the optically determined fRHext with increased RH corresponding to the addition of subsequent water layers which are expected to directly translate into enhanced absorption. The linearity of the montmorillonite trace from 50%–90% RH seen in could be indicative of the proportionality of the enhancement with increasing numbers of water monolayers (Nessler et al. Citation2005). At 90% RH, the fRHext of 1.4 could also be attributed to growth of the particle beyond its dry diameter in combination with an enhancement. The possibility of an increase in the mode of the particle size distribution could be confirmed with additional measurements at 90% RH; however, this is beyond the current capabilities of our equipment.
Using Mie theory to model experimental extinction results, the optical fRHext (68%, Dry) values measured can be converted to a GFc using the literature value for the refractive index of 1.523 + 0.0000382i for montmorillonite at 532 nm (Egan and Hilgeman Citation1979). An fRHext (68%, dry) of 1.18 corresponds to a calculated closure-based GFc of 1.03. This calculation assumed that the water associated with the particle, if any, does not change the refractive index. If this is not the case, the actual real portion of the refractive index of the humidified particle would be a composite, and it would decrease since the refractive index of water is 1.33. This potential decrease in refractive index would result in a higher calculated GFc from the measured fRHext (68%, Dry). Recent studies have used other methods to quantify the water uptake onto the clay components of mineral dust (Schuttlefield et al. Citation2007; Herich et al. Citation2009). Herich et al. (2009) reported a GFm for montmorillonite of approximately 1 using an HT-DMA technique, indicating no change of the EMD of the particle at 68% RH (Herich et al. Citation2009). Schuttlefield et al. (2007) measured water uptake on clays using a QCM at different RH values. For a similar montmorillonite clay, an uptake of 225 mg of water per gram of sample was measured at 68% RH (Schuttlefield et al. Citation2007). This corresponds to GFm of 1.15 using the literature value of 2.35 g/cm3 for the density of montmorillonite (Lide Citation2007). This QCM-based calculation, however, provides an upper limit of the GF due to the possibility that internal voids present in the clay particles could fill with water and increase the mass without causing particle growth as described previously.
Illite only reaches an fRHext (90%, Dry) of 1.06 in contrast to montmorillonite (1.4), probably due to its structure being less hygroscopic. The fRHext results for illite show the expected general trend, increasing extinction with increasing RH after an initial decrease, although the changes are less significant than montmorillonite. It is expected that a decrease in fRHext would translate to shrinkage of the particle. At 68% RH, the observed fRHext < 1 that corresponds to a calculated GFc of 0.99 can be compared with a 10% shrinkage seen when the mode of the size distributions are examined. These results are anticipated since a decrease in particle size should, based on physical structures, yield a decrease in extinction. It is also possible that water could be adding to the surface of the illite aerosols and an enhancement effect is being observed similar to montmorillonite. As more water is taken up (RH 90%), the fRHext increases greater than 1 and this could also be due to hydration of interlayers causing the particle to grow beyond its dry diameter (Schuttlefield et al. Citation2007).
Using Mie theory, a GFc can be calculated from the fRHext (68%, Dry) using the literature value of 1.414 + 0.000773i for the refractive index of illite at 532 nm (Egan and Hilgeman Citation1979). The calculated GFc is 0.99. Similar to montmorillonite, Herich et al. (2009) found a GFm of approximately 1 for illite at 68% RH and this is in good agreement with our results. According to the QCM measurements by Schuttlefield et al. (2007), illite particles take up approximately 225 mg of water per gram of sample. This corresponds to a GFm of 1.17 assuming the density of illite to be 2.75 g/cm3 as reported in the literature (Lide Citation2007). Again, the QCM measurements provide an upper limit to describe the particle GF.
Kaolinite displays decreases at 50% and 68% RH for the fRHext with no further significant change at 90% RH. The strong hydrogen bonding between the layers of kaolinite does not allow water to enter between the layers, so it primarily has to absorb on the surface of the particle. However, there are not many surface sites available and so after initial compaction, not much change is expected. As a result, this sample has a calculated GFc at 68% RH of 0.93 in good agreement with the approximately 10% shrinkage with humidification represented in the change of the mode of the particle size distributions measured at corresponding RH. At 68% RH, the particles have taken up the maximum amount of water onto the surface and between the layers, therefore further increasing the RH, even as high as 90%, has little effect on the extinction. It is also possible that as these particles are exposed to water vapor, partial dissolution of the surface layer in addition to compaction of particles can occur. When dry, the clay particles have an irregular shape and rough edges (Schuttlefield et al. Citation2007). The rough surface of the particle could smooth out during the redistribution and restructuring of the surface layer, which could help explain the decrease in extinction upon humidification. Specifically, it is known that particles with smooth surfaces scatter light less efficiently than particles with sharp edges (Xue et al. Citation2009).
A closure-based GFc of 0.93 for kaolinite was calculated at 68% RH by Mie theory and a refractive index of 1.493 + 0.0000477i (Egan and Hilgeman Citation1979). On the basis of HT-DMA measurements, Herich et al. (2009) reported a GFm near 1, which is similar to the other two clays studied in that work but does not represent the shrinkage observed optically in this study (Herich et al. Citation2009). The QCM study by Schuttlefield et al. (2007) reported water uptake of approximately 88 mg of water per gram of sample at 68% RH, which corresponds to a upper limiting GFm of 1.07 assuming the density of kaolinite is 2.6 g/cm3 (Lide Citation2007). Again, it is clear that GF measurements of clays are variable up to 20% using different measurement techniques.
CONCLUSIONS AND ATMOSPHERIC IMPLICATIONS
In this work, the fRHext was measured for three clay components of mineral dust by using CRD at 50%, 68%, and 90% RH in comparison with dry conditions (RH < 10%). The optical behavior varied considerably between different clay types indicating that different morphologies and chemical structures of the clays play an important role in water uptake. As mentioned previously, there are regional differences in the composition of mineral dust and the proportion of each clay type that can be an indicator of source region (Caquineau et al. Citation1998; Prospero Citation1999). Therefore, it is reasonable to assume that aerosols from different regions will exhibit different optical properties as they encounter elevated RH in transport due to the differences in water uptake between the three clays. Since the fRHext (RH, dry) differs from unity, neglecting the change in optical properties with variable RH and neglecting the difference in water uptake between different clays in radiative forcing models will lead to incorrect predictions for the direct effect of mineral dust on climate. Typically the change in optical properties due to water uptake is inferred on the basis of physically measured particle growth. However, on the basis of the deviations between physical GFm and optically based GFc results noted in this work, the inclusion of direct optical property measurements for clays in climate models is advantageous for reducing uncertainties.
Closure-based GFc were calculated from optical measurements using Mie theory. These GFc deviate from literature values highlighting the uncertainties in the techniques used to quantify water uptake and growth of mineral dust particles. Accurate measurements of water uptake for these particles are crucial for understanding their atmospheric lifetimes, their ability to act as CCN and IN, and their reactivity in the atmosphere. Using fRHext measurements to calculate GFc provides a lower limit for the physical GF and is an alternate method for assessing water uptake as compared with conventional measurements using an HT-DMA or a QCM. Converting the optical measurement to closure-based GFc by using our method initially accounts for the effect of the irregular shape and possible voids of clay aerosols to provide advantages over other techniques. The bounding of the GF using our closure-based calculations (lower bound) with QCM measurements (upper bound) may further reduce uncertainties associated with GF measurements.
On the basis of top of the atmosphere globally averaged direct aerosol radiative forcing (ΔF R) calculations previously described by Chylek and Wong Citation(1995) and Garland et al. (2007), we have performed calculations to estimate the impact of our results on radiative forcing in comparison with the assumption that clays do not take up water. ΔF R is determined by EquationEquation (4) (Chylek and Wong Citation1995):
As aerosols are emitted into the atmosphere, they can be transported over large distances, experiencing changes in temperature, pressure, and RH. For example, mineral dust emanated from warm, arid regions of the Saharan desert is lofted up beyond the marine boundary layer allowing for extended residence time and transport of mineral dust over long distances (Petit et al. Citation2005). At the source, the amount of water vapor in air is extremely low. However, as it is transported over the Atlantic, the particles will experience fluctuations in RH until reaching the warm, moist Caribbean, where they are usually deposited. It is important to take these RH changes into account when considering mineral dust aerosol because as our data show, especially for montmorillonite and illite, the amount of water vapor available will affect particle size as well as its optical properties.
In the future, experiments based on work by others should be pursued. For example, studies have measured the enhancement of the fRHabs (RH, Dry) to determine the change in single scattering albedo and therefore the global radiation balance (Nessler et al. Citation2005; Lack et al. Citation2009). While the fRH (RH, Dry) of scattering and absorption will be enhanced from particle growth due to water uptake, the fRH (RH, Dry) of absorption can also be enhanced due to a lensing effect as previously described. This effect can be counteracted by a decrease in the imaginary portion of the refractive index due to the mixing of water (Fuller et al. Citation1999; Nessler et al. Citation2005; Lack et al. Citation2009). Therefore it is necessary to not only measure how water uptake affects extinction as reported here but also how scattering and absorption are affected individually, due to the changes in single scattering albedo.
It is also necessary to consider a size dependence to the RH response and the physical size changes at other RH values. Further, it would be useful to consider how the optical properties of these systems change as a function of wavelength as the solar spectrum is broad but we have only considered a central wavelength of 532 nm. Further experiments, especially considering mineral dust mixed with common aerosol components, will improve understanding of realistic atmospheric conditions. For additional improvements in radiative forcing calculations, it is important to experimentally measure the fRH for extinction and absorption.
Acknowledgments
This work was funded by the University of New Hampshire (UNH) start up fund (Greenslade) and a UNH College of Engineering and Physical Sciences first year fellowship (Attwood). The authors would like to thank Dr. Courtney Hatch and Dr. Paula Hudson for their advice about clay aerosol generation and Donald Troop (UNH) for his assistance with the Labview software. We would also like to acknowledge the helpful suggestions of Dr. Carolyn Jordan in the preparation of this manuscript.
REFERENCES
- Atkinson , D. B. 2003 . Solving Chemical Problems of Environmental Importance Using Cavity Ring-Down Spectroscopy . Analyst , 128 : 117 – 125 .
- Bauer , S. E. , Balkanski , Y. , Schulz , M. , Hauglustaine , D. A. and Dentener , F. 2004 . Global Modeling of Heterogeneous Chemistry on Mineral Aerosol Surfaces: Influence on Tropospheric Ozone Chemistry and Comparison to Observations . J. Geophys. Res. , 109 : D02304
- Baynard , T. , Lovejoy , E. R. , Pettersson , A. , Brown , S. S. , Lack , D. Osthoff , H. 2007 . Design and Application of a Pulsed Cavity Ring-Down Aerosol Extinction Spectrometer for Field Measurements . Aerosol Sci. Technol. , 41 : 447 – 462 .
- Biscaye , P. E. , Grousset , F. E. , Revel , M. , Van der Gaast , S. , Zielinski , G. A. Vaars , A. 1997 . Asian Provenance of Glacial Dust (Stage 2) in the Greenland Ice Sheet Project 2 Ice Core, Summit, Greenland . J. Geophys. Res. , 102 ( C12 ) 26765–26781
- Bohren , C. F. and Huffman , D. R. 2004 . Absorption and Scattering of Light by Small Particles , Weinheim, , Germany : Wiley-VCH Verlag GmbH .
- Caquineau , S. , Gaudichet , A. , Gomes , L. and Legrand , M. 2002 . Mineralogy of Saharan Dust Transported Over Northwestern Tropical Atlantic Ocean in Relation to Source Regions . J. Geophys. Res. , 107 : D154251
- Caquineau , S. , Gaudichet , A. , Gomes , L. , Magonthier , M. and Chatenet , B. 1998 . Saharan Dust: Clay Ratio as a Relevant Tracer to Assess the Origin of Soil-Derived Aerosols . Geophys. Res. Lett. , 25 : 983 – 986 .
- Carlson , T. N. and Benjamin , S. G. 1980 . Radiative Heating Rates for Saharan Dust . J. Atmos. Sci. , 37 : 193 – 213 .
- Cases , J. M. , Berend , I. , Besson , G. , Francois , M. , Uriot , J. P. Thomas , F. 1992 . Mechanism of Adsorption and Desorption of Water Vapor by Homoionic Montmorillonite. 1. The Sodium-Exchanged Form . Langmuir , 8 : 2730 – 2739 .
- Chylek , P. and Wong , J. 1995 . Effect of Absorbing Aerosols on Global Radiation Budget . Geophys. Res. Lett. , 22 : 929 – 931 .
- Curtis , D. B. , Meland , B. , Aycibin , M. , Arnold , N. P. , Grassian , V. H. Young , M. A. 2008 . A Laboratory Investigation of Light Scattering from Representative Components of Mineral Dust Aerosol at a Wavelength of 550 nm . J. Geophys. Res. , 113 : D08210
- Cwiertny , D. M. , Young , M. A. and Grassian , V. H. 2008 . Chemistry and Photochemistry of Mineral Dust Aerosol . Annu. Rev. Phys. Chem. , 59 : 27 – 51 .
- DeMott , P. J. , Sassen , K. , Poellot , M. R. , Baumgardner , D. , Rogers , D. C. Brooks , S. D. 2003 . African Dust Aerosols as Atmospheric Ice Nuclei . Geophys. Res. Lett. , 30 : 1732
- Duce , R. A. 1995 . “ Sources, Distributions, and Fluxes of Mineral Aerosols and Their Relationship to Climate ” . In Dahlem Workshop on Aerosol Forcing of Climate , Edited by: Charlson , R. J. and Heintzenberg , J. 43 – 72 . Chichester, , UK : John Wiley .
- Duce , R. A. and Tindale , N. W. 1991 . Atmospheric Transport of Iron and Its Deposition in the Ocean . Limnol. Oceanogr. , 36 : 1715 – 1726 .
- Egan , W. G. and Hilgeman , T. W. 1979 . Optical Properties of Inhomogeneous Materials: Applications to Geology, Astronomy, Chemistry, and Engineering , New York : Academic Press .
- Evan , A. T. and Mukhopadhyay , S. 2010 . African Dust over the Northern Tropical Atlantic: 1955–2008 . J. Appl. Meteorol. Climatol. , 49 : 2213 – 2229 .
- Falkovich , A. H. , Schkolnik , G. , Ganor , E. and Rudich , Y. 2004 . Adsorption of Organic Compounds Pertinent to Urban Environments onto Mineral Dust Particles . J. Geophys. Res. , 109 : D02208
- Freedman , M. A. , Hasenkopf , C. A. , Beaver , M. R. and Tolbert , M. A. 2009 . Optical Properties of Internally Mixed Aerosol Particles Composed of Dicarboxylic Acids and Ammonium Sulfate . J. Phys. Chem. A , 113 : 13584 – 13592 .
- Fuller , K. A. , Malm , W. C. and Kreidenweis , S. M. 1999 . Effects of Mixing on Extinction by Carbonaceous Particles . J. Geophys. Res. , 104 : 15941 – 15954 .
- Garland , R. M. , Ravishankara , A. R. , Lovejoy , E. R. , Tolbert , M. A. and Baynard , T. 2007 . Parameterization for the Relative Humidity Dependence of Light Extinction: Organic-Ammonium Sulfate Aerosol . J. Geophys. Res. , 112 : D19303
- Grousset , F. E. , Ginoux , P. , Bory , A. and Biscaye , P. E. 2003 . Case Study of a Chinese Dust Plume Reaching the French Alps . Geophys. Res. Lett., , 30 : 1277
- Gustafsson , R. J. , Orlov , A. , Badger , C. L. , Griffiths , P. T. , Cox , R. A. and Lambert , R. M. 2005 . A Comprehensive Evaluation of Water Uptake on Atmospherically Relevant Mineral Surfaces: DRIFT Spectroscopy, Thermogravimetric Analysis and Aerosol Growth Measurements . Atmos. Chem. Phys., , 5 : 3415 – 3421 .
- Hallquist , M. , Stewart , D. J. , Stephenson , S. K. and Cox , R. A. 2003 . Hydrolysis of N2O5 on Sub-Micron Sulfate Aerosols . Phys. Chem. Chem. Phys., , 5 : 3453 – 3463 .
- Hensen , E. J. M. and Smit , B. 2002 . Why Clays Swell . J. Phys. Chem. B, , 106 : 12664 – 12667 .
- Herich , H. , Tritscher , T. , Wiacek , A. , Gysel , M. , Weingartner , E. Lohmann , U. 2009 . Water Uptake of Clay and Desert Dust Aerosol Particles at Sub- and Supersaturated Water Vapor Conditions . Phys. Chem. Chem. Phys., , 11 : 7804 – 7809 .
- Hudson , P. K. , Gibson , E. R. , Young , M. A. , Kleiber , P. D. and Grassian , V. H. 2008 . Coupled Infrared Extinction and Size Distribution Measurements for Several Clay Components of Mineral Dust Aerosol . J. Geophys. Res., , 113 : D01201
- Hung , H.-M. , Malinowski , A. and Martin , S. T. 2003 . Kinetics of Heterogeneous Ice Nucleation on the Surfaces of Mineral Dust Cores Inserted into Aqueous Ammonium Sulfate Particles . J. Phys. Chem. A, , 107 : 1296 – 1306 .
- IPCC. 2007 . “ Climate Change 2007 ” . In The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M. and Miller , H. L. 940 Cambridge : Cambridge University Press .
- Koehler , K. A. , Kreidenweis , S. M. , DeMott , P. J. , Petters , M. D. , Prenni , A. J. and Carrico , C. M. 2009 . Hygroscopicity and Cloud Droplet Activation of Mineral Dust Aerosol . Geophys. Res. Lett., , 36 : L08805
- Kumar , P. , Sokolik , I. N. and Nenes , A. 2010 . Measurements of Cloud Condensation Nuclei Activity and Droplet Activation Kinetics of Fresh Unprocessed Regional Dust Samples and Minerals . Atmos. Chem. Phys. Discuss., , 10 : 31039 – 31081 .
- Lack , D. A. , Quinn , P. K. , Massoli , P. , Bates , T. S. , Coffman , D. Covert , D. S. 2009 . Relative Humidity Dependence of Light Absorption by Mineral Dust After Long-Range Atmospheric Transport from the Sahara . Geophys. Res. Lett. , 36
- Lang-Yona , N. , Abo-Riziq , A. , Erlick , C. , Segre , E. , Trainic , M. and Rudich , Y. 2010 . Interaction of Internally Mixed Aerosols with Light . Phys. Chem. Chem. Phys., , 12 : 21 – 31 .
- Lide , D. 2007 . CRC Handbook of Chemistry and Physics, 88th Edition , Boca Raton, FL : CRC .
- Lohmann , U. and Diehl , K. 2006 . Sensitivity Studies of the Importance of Dust Ice Nuclei for the Indirect Aerosol Effect on Stratiform Mixed-Phase Clouds . J. Atmos. Sci., , 63 : 968 – 982 .
- Mashburn , C. D. , Frinak , E. K. and Tolbert , M. A. 2006 . Heterogeneous Uptake of Nitric Acid on Na-montmorillonite Clay as a Function of Relative Humidity . J. Geophys. Res., , 111 : D15213
- McMurry , P. H. 2000 . A Review of Atmospheric Aerosol Measurements . Atmos. Environ., , 34 : 1959 – 1999 .
- McNaughton , C. S. , Clarke , A. D. , Kapustin , V. , Shinozuka , Y. , Howell , S. G. Anderson , B. E. 2009 . Observations of Heterogeneous Reactions Between Asian Pollution and Mineral Dust Over the Eastern North Pacific During INTEX-B . Atmos. Chem. Phys. , 9 : 8283 – 8308 .
- Miller , R. L. and Tegen , I. 1998 . Climate Response to Soil Dust Aerosols . J. Climate, , 11 : 3247 – 3267 .
- Navea , J. G. , Xu , S. , Stanier , C. O. , Young , M. A. and Grassian , V. H. 2009 . Heterogeneous Uptake of Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5) onto Mineral Dust Aerosol Under Variable RH Conditions . Atmos. Environ., , 43 : 4060 – 4069 .
- Nessler , R. , Weingartner , E. and Baltensperger , U. 2005 . Effect of Humidity on Aerosol Light Absorption and Its Implications for Extinction and the Single Scattering Albedo Illustrated for a Site in the Lower Free Troposphere . J. Aerosol Sci., , 36 : 958 – 972 .
- Perry , K. D. , Cliff , S. S. and Jimenez-Cruz , M. P. 2004 . Evidence for Hygroscopic Mineral Dust Particles from the Intercontinental Transport and Chemical Transformation Experiment . J. Geophys. Res., , 109 : D23S28
- Petit , R. H. , Legrand , M. , Jankowiak , I. , Molinié , J. , Asselin de Beauville , C. Marion , G. 2005 . Transport of Saharan Dust over the Caribbean Islands: Study of an Event . J. Geophys. Res., , 110 : D18S09
- Prospero , J. M. 1999 . Long-Range Transport of Mineral Dust in the Global Atmosphere: Impact of African Dust on the Environment of the Southeastern United States . Proc. Natl. Acad. Sci. U. S. A., , 96 : 3396 – 3403 .
- Salam , A. , Lohmann , U. , Crenna , B. , Lesins , G. , Klages , P. Rogers , D. 2006 . Ice Nucleation Studies of Mineral Dust Particles with a New Continuous Flow Diffusion Chamber . Aerosol Sci. Technol., , 40 : 134 – 143 .
- Sassen , K. 2002 . Indirect Climate Forcing over the Western US from Asian Dust Storms . Geophys. Res. Lett., , 29 ( 10 ) : 1465
- Schuttlefield , J. D. , Cox , D. and Grassian , V. H. 2007 . An Investigation of Water Uptake on Clays Minerals Using ATR-FTIR Spectroscopy Coupled with Quartz Crystal Microbalance Measurements . J. Geophys. Res. D: Atmos., , 112 : D21303
- Seisel , S. , Pashkova , A. , Lian , Y. and Zellner , R. 2005 . Water Uptake on Mineral Dust and Soot: A Fundamental View of the Hydrophilicity of Atmospheric Particles . Faraday Discuss., , 130 : 437 – 451 .
- Smith , J. D. and Atkinson , D. B. 2001 . A Portable Pulsed Cavity Ring-Down Transmissometer for Measurement of the Optical Extinction of the Atmospheric Aerosol . Analyst, , 126 : 1216 – 1220 .
- Sokolik , I. N. and Toon , O. B. 1996 . Direct Radiative Forcing by Anthropogenic Airborne Mineral Aerosols . Nature, , 381 : 681 – 683 .
- Takeshi , H. 1978 . “ Smectites ” . In Developments in Sedimentology Edited by: Toshio , S. and Susumu , S. 221 – 242 . Elsevier
- Tegen , I. and Fung , I. 1994 . Modeling of Mineral Dust in the Atmosphere: Sources, Transport, and Optical Thickness . J. Geophys. Res. , 99 : 22897 – 22914 .
- Uematsu , M. , Wang , Z. and Uno , I. 2003 . Atmospheric Input of Mineral Dust to the Western North Pacific Region Based on Direct Measurements and a Regional Chemical Transport Model . Geophys. Res. Lett., , 30 : 1342
- Usher , C. R. , Michel , A. E. and Grassian , V. H. 2003 . Reactions on Mineral Dust . Chem. Rev., , 103 : 4883 – 4940 .
- Vlasenko , A. , Sjogren , S. , Weingartner , E. , Gaggeler , H. W. and Ammann , M. 2005 . Generation of Submicron Arizona Test Dust Aerosol: Chemical and Hygroscopic Properties . Aerosol Sci. Technol. , 39 : 452 – 460 .
- Vlasenko , A. , Sjogren , S. , Weingartner , E. , Stemmler , K. , Gaggeler , H. W. and Ammann , M. 2006 . Effect of Humidity on Nitric Acid Uptake to Mineral Dust Aerosol Particles . Atmos. Chem. Phys. , 6 : 2147 – 2160 .
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range . J. Aerosol Sci. , 19 : 387 – 389 .
- Xue , H. , Khalizov , A. F. , Wang , L. , Zheng , J. and Zhang , R. 2009 . Effects of Dicarboxylic Acid Coating on the Optical Properties of Soot . Phys. Chem. Chem. Phys. , 11 : 7869 – 7875 .
- Zender , C. S. , Miller , R. L. and Tegen , I. 2004 . Quantifying Mineral Dust Mass Budgets: Terminology, Constraints, and Current Estimates . Eos. Trans. , : 85 AGU