Abstract
The use of cavity ring down spectroscopy to retrieve aerosol complex refractive index from optical property measurements has seen increasing popularity over the past few years. However, few studies have looked at the limit which sources of error and uncertainty inherent in the cavity ring down method place on the accuracy with which the refractive index can be retrieved. In this paper, we consider both experimental sources of error and those which compromise the theoretical models against which measurements are compared, both reviewing previously published work and presenting new data. Our results show that for absolute measurements made using single-cavity instruments, factors such as uncertainty in the length of the ring down cavity occupied by aerosol and the counting efficiency of the CPC can introduce an error of ∼2.5% into the real part of the refractive index retrieved from experiment. This is significantly higher than the typical 1% error quoted in previously published work. We note that due to the dependence of particle extinction efficiency on diameter, the effect of a given error on measurements for different particle sizes is not constant.
1. INTRODUCTION
The optical properties of aerosol particles, dependent on factors such as size, shape, and complex refractive index, affect the radiative balance of the atmosphere directly through the ability of particles to both absorb and scatter solar and terrestrial radiation. The sum of scattering and absorption by aerosol is termed extinction and the relative extents to which the two processes occur, described by the single scattering albedo, govern whether a particle contributes to the warming or cooling of the atmosphere (Seinfeld and Pandis Citation1998). The scattering and absorption properties of a particle are determined by the real and imaginary parts of its complex refractive index, respectively. The magnitude of the direct radiative forcing effect of atmospheric aerosol has been identified as one of the major uncertainties in predicting future climate change (Forster et al. Citation2007).
During the past decade, the number of studies employing aerosol cavity ring down spectroscopy (A-CRDS) to measure optical properties has grown rapidly due to its high sensitivity and ability to measure the absolute extinction of an aerosol sample without instrument calibration. A-CRDS compares the temporal decay of a light pulse trapped within an optical resonator both with and without aerosol present through measurement of the ring down time of the cavity. This is defined as the time taken for the light intensity exiting the cavity to fall to 1/e of its original value. For an empty cavity, the ring down time, τ0, depends only on the cavity length, L, and the reflectivity of the mirrors. The introduction of aerosol into the cavity leads to a reduction in the cavity ring down time to a value τ, caused by additional losses from scattering and absorption by the particles.
The difference in the decay rates for the empty and filled cavity is dependent on the number density, N, extinction efficiency, Q ext, and geometric cross-section, σgeom, of the aerosol particles being probed:
By measuring the extinction coefficient b ext as a function of particle number density N for size-selected particles of known diameter, the size-dependent values of the particle extinction efficiency can be extracted from the experimental data. Measuring the value of Q ext at a range of particle radii, r, or size parameter (x = 2πr/λ) allows the complex refractive index of the aerosol to be determined through comparison with theoretical Q ext values calculated from Mie theory (Riziq et al. Citation2007; Dinar et al. Citation2008; Riziq et al. Citation2008; Butler et al. Citation2009; Freedman et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010a, Citation2010b; Adler et al. Citation2010; Hasenkopf et al. Citation2010; Miles et al. Citation2010a, Citation2010b). This approach relies critically on knowing the size distribution of particles within the cavity, their morphology, and their number density.
A-CRDS is now routinely used in laboratory (Sappey et al. Citation1998; Vander Wal and Ticich Citation1999; Smith and Atkinson Citation2001; Bulatov et al. Citation2002, Citation2003, Citation2006; Strawa et al. Citation2003; Pettersson et al. Citation2004; Baynard et al. Citation2006; Garland et al. Citation2007; Riziq et al. Citation2007; Rudić et al. Citation2007; Dinar et al. Citation2008; Riziq et al. Citation2008; Butler et al. Citation2009; Freedman et al. Citation2009; Khalizov et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010a, Citation2010b; Radney et al. Citation2009; Xue et al. Citation2009; Adler et al. Citation2010; Hasenkopf et al. Citation2010; Miles et al. Citation2010a, Citation2010b) and field studies (Thompson et al. Citation2002, Citation2003; Strawa et al. Citation2006; Garland et al. Citation2008; Nakayama et al. Citation2010) to measure extinction by both aerosol ensembles (Vander Wal and Ticich Citation1999; Thompson et al. Citation2002, Citation2003, Citation2008; Strawa et al. Citation2003; Pettersson et al. Citation2004; Baynard et al. Citation2006, Citation2007; Garland et al. Citation2007; Riziq et al. Citation2007; Beaver et al. Citation2008; Dinar et al. Citation2008; Riziq et al. Citation2008; Butler et al. Citation2009; Freedman et al. Citation2009; Khalizov et al. Citation2009; Lack et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010a, Citation2010b; Radney et al. Citation2009; Xue et al. Citation2009; Adler et al. Citation2010; Beaver et al. Citation2010; Hasenkopf et al. Citation2010; Nakayama et al. Citation2010) and single particles (Butler et al. Citation2007; Miller and Orr-Ewing Citation2007; Sanford et al. Citation2008). Rudić and co-workers (Citation2007) studied the effect of aerosol mixing state on optical properties, comparing experimentally measured extinctions from homogeneous and core-shell particles with predictions from different theoretical mixing rules and Mie simulations of homogeneous and layered spheres (Riziq et al. Citation2007; Dinar et al. Citation2008; Riziq et al. Citation2008; Lang-Yona et al. Citation2010a). Cavity ring down has been used in series with nephelometry to measure the single scattering albedo of single component and external mixtures of inorganic salts and organic dyes (Radney et al. Citation2009), as well as the change in the optical properties of freshly generated soot particles caused by introduction and removal of coatings of sulphuric acid (Khalizov et al. Citation2009) and dicarboxylic acids (Xue et al. Citation2009) to mimic atmospheric processing. Thompson et al. (Citation2008) used a ring down cavity (RDC) situated within an integrating sphere nephelometer to determine the single scattering albedo of polystyrene spheres, india ink, ammonium sulphate and aerosol generated by pine needle combustion. In addition, the enhancements in extinction and absorption when absorbing polystyrene beads were coated with varying-layer thicknesses of nonabsorbing oleic acid were measured by Lack et al. (Citation2009) using a combination of A-CRDS and photoacoustic spectroscopy. Several groups have studied the impact of particle organic mass fraction on light extinction for internal mixtures of inorganic salts and organic compounds (Freedman et al. Citation2009), as well as the effect of relative humidity (Baynard et al. Citation2006; Garland et al. Citation2007; Beaver et al. Citation2008, Citation2010). Studies have also reported the complex refractive index of organic carbon extracted from freshly generated diesel soot (Adler et al. Citation2010), fresh biogenic secondary organic aerosol (SOA) formed from the Holm Oak volatile organic carbon (VOC) emissions (Lang-Yona et al. Citation2010b) and laboratory-generated aerosol thought to be representative of those found in the atmosphere of Titan and the early Earth (Hasenkopf et al. Citation2010).
In contrast with other optical measurement techniques such as nephelometry (Anderson et al. Citation1996; Varma et al. Citation2003; Abu-Rahmah et al. Citation2006; Bond et al. Citation2009; Massoli et al. Citation2009) aethalometry (Hansen et al. Citation1984; Weingartner et al. Citation2003; Fialho et al. Citation2006; Lack et al. Citation2008) and photoacoustic spectroscopy (Arnott et al. Citation1999, Citation2003; Raspet et al. Citation2003), there have been few studies of the instrumental limitations that can compromise the accuracy of aerosol optical properties retrieved by A-CRDS. Baynard and co-workers (Citation2007) determined an absolute uncertainty in the measured aerosol extinction coefficient of <1% when including factors such as aerosol transmission efficiency, gas-phase interference, evaporation of volatile compounds and determination of aerosol sample volume. In addition, Pettersson et al. (Citation2004) found that extinction measurements at low particle number densities were limited by statistical fluctuations in the number of particles within the measurement volume. The authors also considered the effect on the total error in the extinction measurement caused by multiply charged particles within the cavity (∼1%) and uncertainty in the length of the cavity occupied by aerosol (<0.5%). To our knowledge, there has so far been no study which explicitly considers the effect of these and other instrumental limitations, such as the particle counting efficiency, on the accuracy with which complex refractive index values can be retrieved. Where uncertainties in experimentally retrieved complex refractive indices have been given, these are generally on the order of ±1% for the real part (Dinar et al. Citation2008; Freedman et al. Citation2009; Lack et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010b; Adler et al. Citation2010; Hasenkopf et al. Citation2010) and range from a few to several hundred percent for the imaginary term (Dinar et al. Citation2008; Lack et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010b; Adler et al. Citation2010; Hasenkopf et al. Citation2010).
In this paper, we present a comprehensive review of all the sources of error and uncertainty in the A-CRDS technique, paying specific attention to their effect on retrieval of the real part of the refractive index for accumulation-mode aerosol. Using a combination of experimental data and theoretical modeling, we consider factors which affect both experimental determination of the extinction efficiency and calculation of the theoretical values against which these are compared to determine refractive index. Compared with the generally accepted uncertainty of ±1% in the real part of the refractive index retrieved from A-CRDS measurements, this assessment suggests that the uncertainties reported to date in the literature may be conservative if the sources of error we identify have failed to be carefully addressed.
2. EXPERIMENTAL
The experimental system used in this work consists of the same components as the majority of those to be found in the literature (Bulatov et al. Citation2002, Citation2006; Riziq et al. Citation2007; Beaver et al. Citation2008, Citation2010; Dinar et al. Citation2008; Freedman et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010a; Radney et al. Citation2009; Adler et al. Citation2010; Nakayama et al. Citation2010) and has been described in detail in previous publications (Miles et al. Citation2010a, Citation2010b). Briefly, particles were generated from an aqueous suspension/solution of the aerosol medium (polystyrene beads or sodium chloride) using a constant output atomizer (TSI Model 3076), before being passed through a Nafion dryer (Perma Pure PD-100T-12SS, http://www.permapure.com/) to remove residual water. Particle number density was varied via addition of a flow of clean, dry nitrogen to the aerosol flow, followed by equilibration inside a conditioning volume. A differential mobility analyzer (DMA; GRIMM Aerosol Technik, Vienna/Reischl type, L-DMA), complete with variable impactor, was used to pass particles of one electrical mobility into the sealed RDC, which was formed between two highly reflective plano-convex mirrors (Layertec, R = 99.98% at λ = 560 nm, diameter = 12.7 mm, radius of curvature = 1 m) separated by a distance of 90 cm. Aerosol was introduced and removed from the cavity via two 4.3-mm-diameter ports perpendicular to the cavity axis and separated by 79 cm. The flow rate through the cavity was 0.3 l/min, with a condensation particle counter (CPC; GRIMM Aerosol Technik, Model 5.403) used to record the number density of particles exiting the cavity. The inside of the RDC was lined with silicon conductive tubing to reduce particle wall losses to below 4%.
Pulsed probe laser light of wavelength 560 nm was generated at a repetition rate of 10 Hz by an optical parametric oscillator (OPO; Continuum, Panther, CA, USA) pumped by the 355-nm third harmonic of a Nd:YAG laser (Continuum, Surelight SL-II-10). The pulse duration was 5 ns, with a bandwidth less than 6 cm−1. The visible beam passed through a horizontal polarizer and optical broadband adjustable isolator (OFR, IO-5-VIS-HP), before being coupled into the RDC using an f = 50 cm coupling lens placed ∼30 cm from the input high reflectivity mirror. The linearly polarized laser pulses (at 45°) had a power of approximately 5 mW at λ = 560 nm as measured in between the coupling lens and the cavity.
The exponentially decaying light intensity leaking from the cavity was loosely focused by a lens on to a photomultiplier tube (PMT; Hamamatsu, Japan) and the intensity sampled by an 8-bit and 350-MHz bandwidth digital oscilloscope (Wavesurfer 434, 2 GS/s; LeCroy, New York). Further analysis of the data was performed on a computer. For most of the measurements, a 200-MS/s sampling rate was used. To protect the PMT from background light interference and ensure that the laser light intensity was uniformly distributed across the photosensitive element, a diffuser followed by a 515-nm-long pass filter was placed in front of the detector.
3. RESULTS
In this paper, we have divided the different sources of error into two groups: firstly, those resulting from the optical measurement itself and affecting the experimentally determined extinction coefficient, b ext; secondly, those associated with extraction of extinction efficiency values from the experimental data and calculation of theoretical results against which these are compared to retrieve a value for the refractive index. These two classes of error are addressed in turn in the following discussion, with attempts made to quantify the impact of each variable on the accuracy with which refractive index values can be retrieved.
3.1. Sources of Error in the Measurement of b ext
The extinction coefficient, b ext, is determined from measurements of the empty and filled cavity ring down times, τ0 and τ, respectively, using EquationEquation (2). As the length of the cavity, L, can be measured accurately and the value of c is well know, it can be assumed that only the variables τ, τ0, and l, could introduce an uncertainty into the value of b ext:
3.1.1. Single Exponential Character of the Ring Down Decay Signal
The values of τ0 and τ are determined from the rate of decay in the intensity of the laser pulse within the RDC. To ensure a single exponential decay of the light intensity, it is desirable to excite only the central transverse electromagnetic (TEM00) mode of the cavity. Different cavity modes have different ring down times and thus the presence of multiple, faster-decaying exponential decays in the ring down trace from higher-order modes will affect the accuracy of the values of τ and τ0 retrieved (Kogelnik and Li Citation1966; Wheeler et al. Citation1998; Berden et al. Citation2000; Baynard et al. Citation2007; Riziq et al. Citation2007). Several techniques, such as the placing of a coupling lens before the first cavity mirror and the use of narrow bandwidth laser sources, can be used to promote coupling into the TEM00 mode.
To investigate the possible contribution of higher-order cavity modes to the ring down time, the region over which the exponential decay was sampled was varied and the differences in the values of τ and τ0 were observed. The natural logarithm of the sum of three successive empty cavity ring down traces for the experimental system described in this paper is shown in the inset in . The ring down time was determined by performing a weighted least squares linear fit to the data within a user-specified time gate, the width and position of which on the trace could be varied. Although the signal-to-noise ratio decreases with increasing time, the decay appears to remain linear, suggesting a good approximation to a single exponential decay. The main body of shows the variation observed in the measured empty cavity ring down time when the starting point of a 15-μs-long time gate on the ring down trace was altered. Each data point shows the average and standard deviation of the ring down time when over a 1000 consecutive ring down events were recorded and fitted individually. It was found that ignoring the tail end of the ring down decay where data collection was limited by bit noise (∼20 μs onwards), variation in the width and position of the time gate gave a systematic error in both τ and τ0 of at worst ∼2%. This is shown by the grey-shaded region in . The 2% precision in τ and τ0 could be improved by using a digitizer with greater resolution and increased data averaging.
FIG. 1 The variation in the empty cavity ring down time with the starting position on the ring down trace of the 15-μs time gate, over which the experimental data are fitted. The average and standard deviation from fitting over a 1000 consecutive ring down events is shown. Inset: Natural logarithm of a typical empty cavity ring down trace, summed over three events. The linearity shows the single exponential nature of the ring down decay over ∼2.5 ring down times.
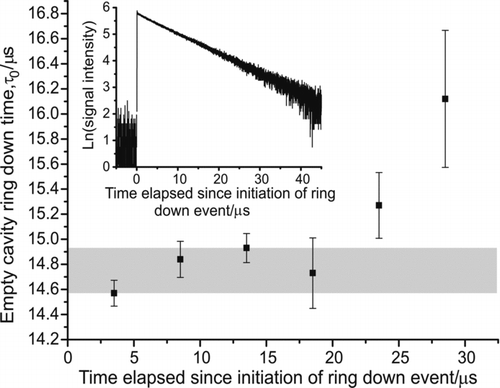
Simulations using ring down times measured during an experiment with 404-nm diameter polystyrene beads were used to examine the effect of a ±2% error in the values of τ and τ0 on the extracted value of Q ext. The systematic nature of the error introduced by gate placement means the sign of the error would be expected to be the same for both τ and τ0. Two extreme situations were considered, one where τ and τ0 were both increased by 2%, and another where they were both decreased by the same amount. In each instance, the extinction coefficient was recalculated for the modified ring down times using EquationEquation (2) and the new value of Q ext was determined assuming all other experimental parameters were accurate. For the 404-nm particles, the changes in the experimentally measured Q ext values were −1.6% and +2.4% for the increase and the decrease in the ring down time, respectively. This variation was observed to be less than the difference in extinction efficiency values obtained from repeated experimental runs on the same-diameter particle (∼5.5%). Thus, for single exponential traces such as that shown in , errors in the determination of the ring down time are not thought to be significant for determining Q ext, provided care is taken to avoid the regions of low signal-to-noise ratio in the ring down trace.
3.1.2. Changes in τ0 During the Measurement Period
As the A-CRDS technique is based on a comparison between the empty-cavity ring down time and the ring down time with particles present inside the cavity, if τ0 were to change during the measurement period, the reference point against which the values of the ring down time were compared would no longer be valid, introducing an error into the calculation of the extinction coefficient. The empty-cavity ring down time depends on the length of the cavity, L, and the mirror reflectivity, R, according to EquationEquation (3):
Any change in mirror reflectivity, for example, due to deposition of particles on the mirror surface, will lead to a change in the empty-cavity ring down time. It is therefore good experimental practice to measure the empty-cavity ring down time at regular intervals during a measurement period to identify and correct for any drift in the value of τ0 (Strawa et al. Citation2003). This applies even if a mirror purge flow is used to protect the mirrors.
In our studies, we found that the drift in the value of the empty-cavity ring down time was only 0.3% after a period of 8 h of continuous operation (Miles et al. Citation2010b). This indicates that the aerosol in this instance, polystyrene beads, was not adhering to the mirror surfaces at the concentrations used, despite the lack of a mirror purge flow. However, this behaviour may not be repeated for different aerosol media, particularly liquid droplets, or at higher particle concentrations.
3.1.3. Intra-Cavity Absorption by Nebulization Solvent
In laboratory-based A-CRDS studies, often also used for the initial characterization of field instruments, aerosol particles are commonly generated by atomizing a solution of the aerosol medium, which is then subjected to a drying process to remove the solvent both from the particle surface and the gas flow. The extinction measured when the aerosol sample is passed into the cavity is then assumed to be solely due to scattering and absorption by the dry particles, with no contribution from gas-phase absorption by any remaining solvent. The effect of a residual solvent coating on the particles is addressed in a later section, and here we consider only the consequences of gas-phase absorption, which if present could lead to an overestimation in particle extinction.
The degree to which the solvent is removed from the gas flow depends on the efficiency of the drying process. Several different methods of drying have been reported in the cavity ring down literature to date, including mixing of the aerosol flow with large volumes of dry air (Radney et al. Citation2009), use of Nafion dryers (Butler et al. Citation2009; Miles et al. Citation2010a, Citation2010b) or those containing molecular sieves, activated alumina or silica gel crystals (Baynard et al. Citation2006; Bulatov et al. Citation2006; Garland et al. Citation2007; Riziq et al. Citation2007; Spindler et al. Citation2007; Riziq et al. Citation2008; Thompson et al. Citation2008; Adler et al. Citation2010), or a combination of several of the above (Freedman et al. Citation2009; Khalizov et al. Citation2009; Xue et al. Citation2009; Beaver et al. Citation2010). The absorption cross-section of water at λ = 560 nm is very low (∼5 × 10−27 cm2 molec−1 (HITRAN)); thus, the effect of any residual solvent in our study is likely to be minimal; for τ0 = 14 μs, a relative humidity of 50% at 20°C would be required to reduce the cavity ring down time by ∼0.01 μs.
The efficiency of the Nafion drying process was confirmed by nebulizing three polystyrene bead suspensions with a solvent composed of different ratios of water and ethanol. The higher volatility of the alcohol makes it easier for it to be removed by the Nafion dryer. If removal of water by the Nafion dryer were inefficient, resulting in residual solvent in the flow, the airflows from the different water/ethanol solutions would be dried to different extents. The DMA was used to send a particle free airflow, generated from atomization of each water/ethanol solution, into the cavity and the cavity ring down time was measured. All three nebulization solutions tested gave ring down times within 0.01 μs (0.07%) of each other, indicating either that there was no appreciable difference in the volume of water left in the gas flow, or that absorption by the water at the wavelength of the measurement was small enough that its presence in the cavity did not matter. This conclusion was confirmed by a second experiment, in which increasing volumes of dilution air were added to the flow generated from atomization of a purely aqueous polystyrene bead solution to dilute down any residual solvent. Again, no difference in the ring down times was seen as the degree of dilution was varied, supporting our previous measurements and theoretical calculations. Although these measurements confirm that residual water was not affecting the cavity ring down time with the drying system used, care should be taken when generalizing this finding to other A-CRDS instruments given the large variety of drying methods reported in the literature and wavelengths used.
3.1.4. Uncertainty in the Length of the Aerosol Sample Within the Cavity
In order to calculate the value of the extinction coefficient, b ext, the fraction of the total cavity length physically occupied by aerosol must be known. It is assumed that within this region, aerosol particle number density is uniform both longitudinally and radially. The design of the RDC used in this work, analogous to the majority of those described to date (Bulatov et al. Citation2002, Citation2006; Garland et al. Citation2007; Riziq et al. Citation2007; Spindler et al. Citation2007; Beaver et al. Citation2008, Citation2010; Dinar et al. Citation2008; Riziq et al. Citation2008; Freedman et al. Citation2009; Khalizov et al. Citation2009; Lang- Yona et al. Citation2009; Radney et al. Citation2009; Xue et al. Citation2009; Adler et al. Citation2010), poses a challenge in determining the exact cavity length occupied by aerosol. To prevent mirror contamination, the aerosol flow cannot be introduced into or removed from the RDC directly over the mirrors, and consequently, the distance between the aerosol inlet and outlet ports is less than the total cavity length. This leads to an ambiguity in the true length of the cavity occupied by aerosol, as in principle, the particles are free to diffuse throughout the entire volume as well as following the stream of the air flow.
To better constrain the length of the cavity occupied by aerosol, a number of instruments use apertures to separate the mirrors from the rest of the RDC, creating a small volume into which a purge flow of particle-free gas is introduced (Thompson et al. Citation2003, Citation2008; Pettersson et al. Citation2004; Moosmuller et al. Citation2005; Baynard et al. Citation2007; Radney et al. Citation2009; Nakayama et al. Citation2010). The aerosol sample length is then assumed to be given by the distance between the apertures. Several authors also report the use of mirror purge flows without apertures to try to restrict the length of the cavity occupied by aerosol, assuming that the aerosol sample length is then equal to the inter-port distance (Pettersson et al. Citation2004; Riziq et al. Citation2007, Citation2008; Dinar et al. Citation2008; Lang-Yona et al. Citation2009). However, it is often unclear in both these situations whether the extent of diffusion, either by the mirror purge flow back into the body of the cavity or by the aerosol sample flow towards the mirrors, has been confirmed experimentally. This will impact on the accuracy of the stated aerosol sample length.
In the instrument described in this paper, the distance between the cavity mirrors was 90 cm, whereas the inlet and outlet ports for the aerosol were separated by 79 cm. No mirror purge flow was used, and as such, an aerosol length of 90 cm was routinely assumed in order to represent the upper limit on the length of the cavity occupied by aerosol (Miles et al. Citation2010a, Citation2010b). We identified in our previous publications that the value of the extinction efficiency extracted from the experimental data showed a significant dependence on the value chosen for the length of the cavity occupied by aerosol. This is because a value of l is required to convert the experimentally measured empty- and filled-cavity ring down times into values of the extinction coefficient b ext (EquationEquation (2)).
If the length of the cavity occupied by aerosol was assumed to be 79 cm, there is a 14.5% increase in the experimentally retrieved value of the extinction efficiency for 404-nm-diameter particles compared with when a value of 90 cm was assumed (Q ext = 3.616 for l = 79 cm compared with Q ext = 3.158 for l = 90 cm). To show the effect that using an aerosol sample length of 79 cm instead of 90 cm would have on the retrieval of the particle refractive index, we performed the same correction (changing l = 90 cm to l = 79 cm) on our previously published Q ext values for polystyrene bead diameters of 240 nm, 299 nm, 349 nm, 404 nm, 453 nm, 596 nm, and 707 nm (Miles et al. Citation2010a). These data were then fitted using a procedure that minimized the error between experimental and theoretical Q ext values to determine the refractive index (Miles et al. Citation2010a, Citation2010b). The real part of the refractive index returned by the program for the corrected data was 1.668, an increase of 2.5% on the value of 1.627 calculated if aerosol was assumed to fill the whole 90-cm length of the cavity. In both calculations, the imaginary part of the refractive index was taken as 5 × 10−4 (Ma et al. Citation2003). The potential systematic error introduced by considering the two extremes in the value of l is significantly higher than the 1% precision typically quoted in A-CRDS publications.
To state categorically what fraction of the total cavity length is occupied by aerosol, the fluid mechanics of the aerosol flow inside the cavity must be well characterized. This highlights an important issue often neglected in publication of A-CRDS data, where the length of the cavity physically occupied by aerosol is frequently either omitted or stated without any reference as to whether it has been confirmed experimentally (Bulatov et al. Citation2006; Garland et al. Citation2007; Beaver et al. Citation2008, Citation2010; Thompson et al. Citation2008; Freedman et al. Citation2009; Baynard et al. Citation2007; Riziq et al. Citation2007; Spindler et al. Citation2007; Dinar et al. Citation2008; Riziq et al. Citation2008; Khalizov et al. Citation2009; Radney et al. Citation2009; Xue et al. Citation2009; Nakayama et al. Citation2010). As the above discussion clearly shows, any uncertainty in the length of the cavity occupied by aerosol has a large impact on the values of the extinction efficiency retrieved by experiment and consequently on the refractive index returned by the fitting process. As the ratios of cavity length to aerosol sample length published in the literature to date vary from 1.13 (Lang-Yona et al. Citation2009; Hasenkopf et al. Citation2010) to 1.67 (Khalizov et al. Citation2009; Xue et al. Citation2009), the uncertainty introduced by this parameter will vary between instruments.
3.2. Sources of Error in the Extraction of Particle Extinction Efficiency, Q ext
The extinction efficiency, Q ext, is extracted from the optical measurements using EquationEquation (4), requiring the number density measured by the CPC, N, as well as an assumption about the geometrical cross-section of the particles, σgeom, within the RDC:
An error in particle diameter affects not only the experimentally determined Q ext value retrieved from the values of b ext measured, but also the theoretical extinction efficiency against which the experimental results are compared through the diameter dependence of the size parameter. This has been discussed at length in a previous publication, where it was shown that the uncertainty in the diameter of polystyrene beads (typically ±6 nm) could lead to errors in the retrieved refractive index of up to 2.9% (Miles et al. Citation2010a). Notably, this will be important to account for not only when polystyrene beads are used to calibrate/validate the optical measurement in an A-CRDS instrument, but also if they are used to calibrate the voltage-to-diameter conversion of the DMA. The following discussion will focus solely on errors introduced into the measurements by uncertainties in the number density measured by the CPC, uncertainty in particle morphology (influencing the theoretical value of Q ext calculated for comparison with the measurements), and the effect of multiply charged particles passed into the cavity by the DMA (impacting on the geometric size used to retrieve the extinction efficiency).
3.2.1. Absolute Value of the Particle Number Density Measured by the CPC
As the value of the extinction efficiency is calculated from the dependence of the extinction coefficient on particle concentration, the accuracy of the A-CRDS technique depends critically on the ability to measure the number density of particles within the RDC. In the majority of papers published to date, as in the work presented here, particle number density is measured using a CPC (Strawa et al. Citation2003; Pettersson et al. Citation2004; Baynard et al. Citation2006, Citation2007; Garland et al. Citation2007; Riziq et al. Citation2007, Citation2008; Beaver et al. Citation2008, Citation2010; Dinar et al. Citation2008; Freedman et al. Citation2009; Khalizov et al. Citation2009; Lang-Yona et al. Citation2009, Citation2010a, Citation2010b; Radney et al. Citation2009; Xue et al. Citation2009; Adler et al. Citation2010; Hasenkopf et al. Citation2010; Nakayama et al. Citation2010). This device uses light scattering to count the number of particles in a controlled gas flow that pass through a known volume in a given time interval. A consistent over- or undercounting of the particle number density by the CPC would result in a systematic error in the value of the extinction efficiency retrieved from experimental data, which in turn would feed through into determination of particle refractive index. Short-term fluctuations in particle number or random noise in the counting efficiency would affect the precision with which the refractive index can be retrieved (Pettersson et al. Citation2004; Bulatov et al. Citation2006).
CPCs are commonly calibrated using a mobility-classified aerosol sample and an aerosol electrometer, or using a secondary CPC which has previously undergone calibration with an electrometer (Liu and Pui Citation1974). An electrometer measures the electrical current generated by a flow of charged particles collected on an electrically isolated filter and converts this into a number concentration assuming singly charged aerosol particles. Due to the wide range of commercial particle counting instruments available, intercomparisons between instruments are often performed to highlight differences in the size-dependent particle counting efficiencies or recorded number concentrations (Wiedensohler et al. Citation1997; Ankilov et al. Citation2002a, Citation2002b; Mordas et al. Citation2008). Several studies have reported systematic discrepancies between the number densities measured by different CPCs and a suitably chosen reference count, with the degree of consistent under- or overcounting ranging between +10% and –10% (Ankilov et al. Citation2002a; Mordas et al. Citation2008).
Calibrating a CPC using a reference electrometer at the low number densities (typically <2000 cm−3 (Strawa et al. Citation2003; Riziq et al. Citation2007; Freedman et al. Citation2009; Lang-Yona et al. Citation2009; Nakayama et al. Citation2010)) used for A-CRDS studies is problematic because of the increased uncertainty in the electrometer count at such low concentrations. This was demonstrated by Fletcher et al. (Citation2009), who used electrostatically classified 80-nm-diameter polystyrene beads to calibrate a TSI 3760A CPC against an electrometer using the traceable methods by National Institute of Standards and Technology (NIST). The electrometer itself was calibrated by a fA current source and had a minimum detection limit of 129 cm−3, below which the signal from the aerosol could not be distinguished above the background noise.
FIG. 2 (a) Uncertainty in the concentration measured by an aerosol electrometer (AE) as a function of particle number density. Data taken from of Fletcher et al. (Citation2009). The solid line is the 1:1 relationship, with the dashed lines marking the bounds of the uncertainty. (b) Particle number density measured by the Grimm CPC (black squares) relative to that recorded by the calibrated TSI 3775 for the 156-nm-diameter soot particles. The black line shows the 1:1 line based on the TSI count. The dashed line is a linear fit to the Grimm CPC data with a gradient of 0.897. (Color figure available online.)
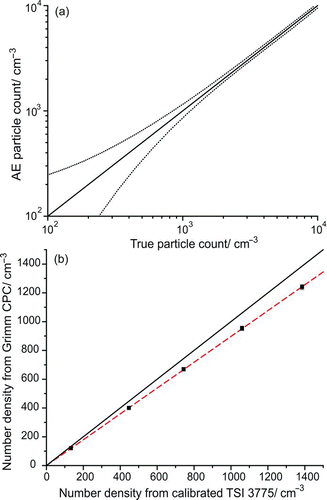
The uncertainty in the electrometer concentration was calculated from the uncertainties in measurement of the current and the aerosol flow rate through the instrument. As shown in , plotted using data taken from in Fletcher et al. (Citation2009), the error in the electrometer count was found to be very significant for the lower concentrations relevant to A-CRDS. This demonstrates that a CPC must be calibrated at high concentrations and a linear extrapolation must be assumed for its behaviour at lower number densities. After accounting for coincidence errors (Gebhart Citation2005) and the presence of multiply charged particles in the monomobile aerosol fraction, Fletcher et al. (Citation2009) observed that the TSI 3760A consistently undercounted with respect to the electrometer by around 5%.
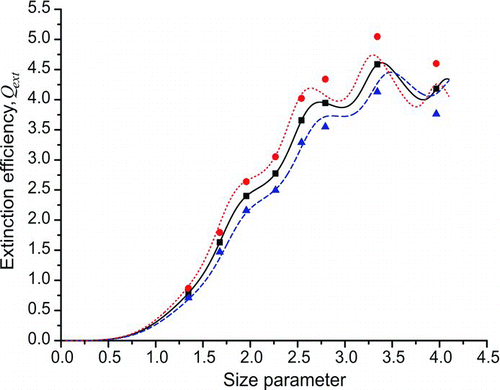
The Grimm CPC used in this study is calibrated by the manufacturers either with an aerosol electrometer or a standardized CPC, which has itself been calibrated against an electrometer. The manufacturer's specified accuracy in the count is ±10% (Spielvogel 2009). For particle diameters greater than ∼100 nm, no size dependence in the counting efficiency is expected (GRIMM Aerosol Technik GmbH Citation2005). Mie theory was used to calculate the theoretical value of the extinction efficiency for seven polystyrene bead diameters in the range 240–596 nm, assuming a polystyrene refractive index at λ = 560 nm of 1.5947 + 5 × 10−4 i (Matheson and Saunderson Citation1952; Ma et al. Citation2003). These are shown in by the black squares, with the solid black line denoting the Q ext curve resulting from a refractive index fit to the data. The effect that a ±10% uncertainty in the CPC count would have on the extinction efficiency values retrieved from a cavity ring down experiment was simulated by multiplying the theoretically calculated Q ext values, deemed the “true” values, by 0.9 (to simulate a 10% overcount by the CPC) and 1.1 (to simulate a 10% undercount by the CPC). The results for each particle diameter are shown in as blue triangles (CPC overcount) and red circles (CPC undercount).
Each set of “corrected” extinction efficiency values was used to retrieve the apparent refractive index using the fitting program already described (Miles et al. Citation2010a, Citation2010b). An imaginary part of 5 × 10−4 was assumed in all calculations. The real part of the refractive index returned for the data when a 10% undercount by the CPC was assumed was 1.636 ± 0.021, with that obtained assuming a CPC overcount of 10% was 1.555 ± 0.014. These are both an error of ∼2.5% from the value of 1.5947 used to generate the original Q ext values and span a larger refractive index range than values reported in the literature to date for polystyrene at λ = 560 nm (Miles et al. Citation2010a). Once again, this uncertainty range is significantly larger than the value of 1% typically quoted in A-CRDS papers. The dashed blue and dotted red lines in show how markedly different the variation in the extinction efficiency with size parameter is for the two extremes in the refractive index.
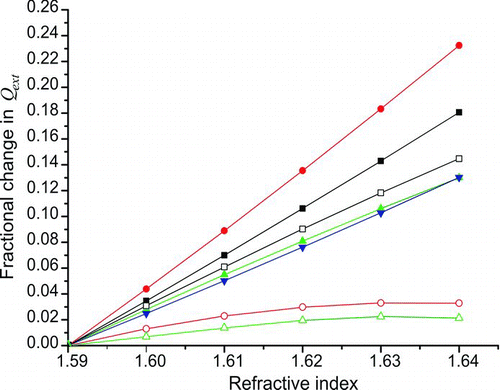
The standard deviation in the refractive index values retrieved in the two limiting cases considered above arises from the fact that because of the structure in the Q ext curve, a consistent error in the experimentally retrieved Q ext value, for example, caused by an error in the CPC count, affects each particle diameter differently. This is demonstrated in , which shows the fractional change in the theoretical extinction efficiency relative to that at a refractive index of 1.59 + 5 × 10−4 i, as the real part of the refractive index is increased for particle diameters in the range 240–700 nm. From the figure, it can be seen that a 2% change in the Q ext value caused by experimental error (i.e., CPC counting efficiency) would lead to only a 0.005 error in the retrieved refractive index for the 300-nm beads but a 0.02 error in the retrieved refractive index for the 600-nm beads. Where experimental Q ext values for different particle diameters are fitted simultaneously to retrieve refractive index, this phenomenon could lead to a bias in the retrieved refractive index, as data for each particle diameter will be given a different natural weighting in the fitting process arising from the local topography of the Q ext curve. To avoid such complications in our work, refractive index fitting was performed on each experimental Q ext value individually and then an average was taken for all particle diameters, ensuring all data were given equal weighting in the fit and allowing systematic discrepancies with size to become apparent.
FIG. 3 Simulation showing the effect of a ±10% CPC counting error on the extinction efficiencies measured in an A-CRDS experiment. The squares are theoretical Q ext values calculated using Mie theory for 240-nm, 299-nm, 349-nm, 404-nm, 453-nm, 498-nm, 596-nm, and 707-nm-diameter particles for a refractive index of n = 1.5947 + 5 × 10−4 i at λ = 560 nm (Ma et al. Citation2003; Matheson and Saunderson Citation1952). The circles show the extinction efficiencies that would be measured by experiment if these values were subject to a CPC undercount of 10%, with the triangles showing the Q ext values expected if the CPC were overcounting by 10%. The lines show the variation in extinction efficiency with size parameter for the refractive indices retrieved from fits to each of the data sets: solid line (nr = 1.5947), dotted line (nr = 1.636), and dashed line (nr = 1.555). The imaginary part of the refractive index was fixed at 5 × 10−4 i. (Color figure available online.)
The Grimm CPC used in this work and that previously published (Miles et al. Citation2010a, Citation2010b) was compared against an electrometer-calibrated TSI 3775 CPC owned by the National Physical Laboratory, UK. When repeatedly tested with different particle media in the diameter range 156–498 nm and at number densities spanning ∼10 cm−3 to 15,500 cm−3, the Grimm instrument was observed to undercount consistently with respect to the calibrated TSI by, on average, 9.3 ± 0.7%. An example of the Grimm CPC count compared with the calibrated TSI 3775 count for 156-nm soot particles is shown in to illustrate the linearity of the instrument response. Correcting our previously published extinction efficiency data for polystyrene beads (Miles et al. Citation2010a) to take into account the undercounting by the Grimm CPC and refitting the data for the refractive index gives a value of the real part of the refractive index of polystyrene of 1.585 ± 0.031, very close to the value measured by Ma et al. (Citation2003) of 1.584. This is a reduction of 2.6% compared with the originally determined value of 1.627 ± 0.027. All calculations assumed an imaginary part of the refractive index of 5 × 10−4. This large decrease in the value of the refractive index retrieved from the measurements when the CPC counting efficiency correction is made, together with the simulation shown in , provides a striking example of the importance of accurately calibrating a CPC prior to undertaking A-CRDS measurements. In the literature to date, we could only find one example in which the counting efficiency of the CPC was stated (±5%) (Lack et al. Citation2009). It must also be remembered that the accuracy of the CPC count at the concentrations of typical A-CRDS measurements is most often reliant on an extrapolation of a calibration performed with an aerosol electrometer at higher concentrations to avoid the significant increase in error at low concentrations apparent in .
FIG. 4 The fractional change in the theoretical extinction efficiency relative to that at a refractive index of n = 1.59 + 5 × 10−4 i as the real part of the refractive index increases for particle diameters of 240 nm (filled squares), 299 nm (filled circles), 349 nm (filled triangles), 404 nm (filled inverted triangles), 453 nm (empty squares), 596 nm (empty circles), and 707 nm (empty triangles). The imaginary part of the refractive index was kept constant at 5 × 10−4 i and a wavelength of 560 nm was used to calculate the size parameter. (Color figure available online.)
3.2.2. Layered Spheres – Surfactant and Adsorbed Water
In addition to size and refractive index, particle morphology affects aerosol optical properties and thus it is important to know the morphology of the particles inside the RDC (Riziq et al. Citation2008; Lang-Yona et al. Citation2010b). In laboratory-based A-CRDS studies, aerosol particles are commonly generated from a suspension or solution of the aerosol medium. If not all solvent is removed from the particles in the drying process, for example, if the aerosol forms a kinetically arrested glassy state or a thin layer of solvent remains on the particle surface following the drying process, the aerosol optical properties will be different from those expected for a homogeneous particle of uniform refractive index. Under such circumstances, reproducible drying conditions are essential if repeated measurements on particles of varying size are to be made.
For the polystyrene beads used in our study, a several-nanometre-thick layer of residual water or surfactant used to stabilize the particles in solution is likely to be left on the particle surface in the drying process, increasing the geometrical cross-section and affecting optical properties (Hassan 2008). Evidence for this was observed by Pettersson et al. (Citation2004) in their measurements on polystyrene beads. The resulting aerosol can be modeled as a core-shell particle composed of a homogenous polystyrene core surrounded by a uniformly thick, homogeneous surface layer, with the refractive index within the shell and the core assumed to be independent of the radial position because of the homogeneity of the individual media (Aden and Kerker Citation1951). A program for calculating the extinction efficiency of core-shell particles was used to simulate the effect of the presence of such a layer on the extinction efficiency for polystyrene cores with diameters up to 750 nm, as a function of both the layer thickness and its refractive index.
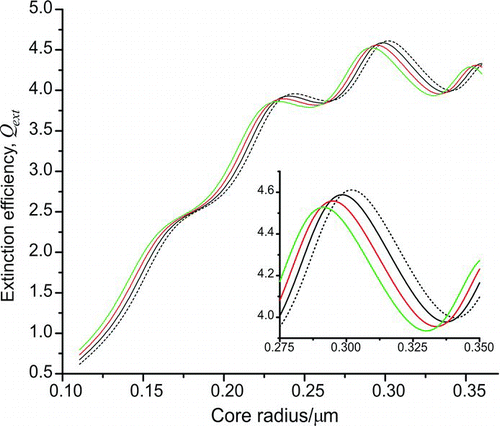
The identity of the surfactant used by bead manufacturer Thermo Scientific is proprietary (Hassan 2008); thus, a range of refractive index values from 1.42 to 1.48 was used to model the surfactant, corresponding to several C16–C18 organic fatty acids approximated as being likely representatives of the surfactant (Lide Citation2010). A refractive index range of 1.33–1.35 was used to simulate the effect of a water layer on the particle, allowing for the possibility of solvation of the surfactant (Hale and Querry Citation1973). The real refractive index of the polystyrene core was assumed to be 1.5947, as recommended by the bead manufacturer (Matheson and Saunderson Citation1952; Thermo Scientific 1996). Calculations were performed for layer thicknesses in the range 1–15 nm. shows the shift in the extinction efficiency curve when the thickness of a surfactant layer with refractive index 1.45 was increased from 0 nm to 15 nm. The presence of the shell is seen to shift the curve to lower size parameter, with the effect more pronounced for thicker shells. For a given layer thickness of surfactant, increasing the refractive index of the shell shifts the extinction efficiency curve to smaller size parameter.
FIG. 5 Shift in the Q ext curve with increasing layer thickness calculated using MieLayer, assuming a surfactant shell of refractive index 1.45 on a polystyrene core of refractive index 1.5947. The inset shows an enlarged view of the Q ext curves at the larger core radii. The black dashed line depicts the result for an uncoated particle, with the remaining lines having shell thicknesses of 5 nm, 10 nm, and 15 nm, showing increasing shift from the homogeneous predictions in the inset. (Color figure available online.)
The effect of core-shell polystyrene-surfactant particles on the value of the extinction efficiency which would be retrieved experimentally in any A-CRDS instrument validation measurement was modeled and the ultimate effect on retrieval of the particle refractive index was investigated. For seven polystyrene core diameters in the range 240–707 nm, the value of the extinction efficiency was calculated for particles covered by surfactant layers 1–5-nm thick, assuming a surfactant refractive index of 1.45. These Q ext values were multiplied by the geometrical cross-section of the core-shell particle to give a theoretical value of the extinction cross-section. This was assumed to be representative of what would be measured in a cavity ring down experiment with core-shell polystyrene-surfactant particles in the cavity. The theoretical extinction cross-section was processed in the same way as experimental data would be, dividing it by the “known” (assumed) geometrical cross-section of the polystyrene core only. Coating of the polystyrene beads is not routinely considered in such measurements and the DMA does not have the resolution to reliably detect nanometre increases in particle radius due to layer formation (Lack et al. Citation2009).
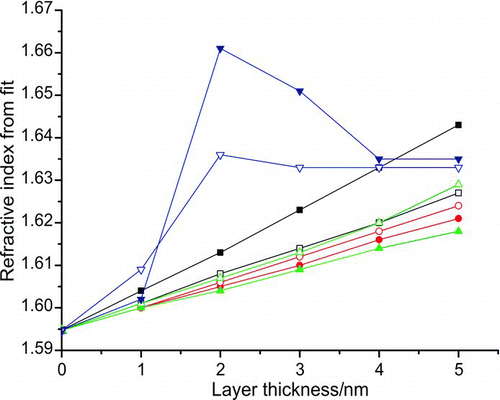
The resulting extinction efficiency values for each core diameter and layer thickness were then fitted for the refractive index assuming a homogeneous sphere with a diameter equal to the polystyrene core only. The results are shown in . As before, the effect of neglecting the presence of a surfactant layer on the polystyrene beads is not the same for all particle diameters due to the structure in the Q ext curve. The figure shows that only a 2-nm-thick layer of surfactant on each polystyrene bead core, a realistic assumption given the mass fraction of surfactant in the solution as stated by the manufacturer (Hassan 2008), leads to retrieved refractive indices for different particle diameters spanning the range 1.60 to 1.66. This is an increase of between 0.3% and 4.1% on the value of 1.5947 used for the polystyrene refractive index in the simulation. This clearly illustrates the importance of a correct assessment of the morphology of the particles being studied, whether in validation measurements using polystyrene beads or in measurements on real aerosol for which the morphology may be more ambiguous.
FIG. 6 The real part of the refractive index that would be retrieved from experimentally measured extinction efficiency values as a function of core diameter and layer thickness if polystyrene-surfactant core-shell particles were present in the ring down cavity but not accounted for in the data processing. The data points correspond to polystyrene core diameters of 240 nm (filled black squares), 299 nm (empty black squares), 349 nm (filled circles), 404 nm (empty circles), 453 nm (filled triangles), 498 nm (empty triangles), 596 nm (filled inverted triangles), and 707 nm (empty inverted triangles). A wavelength of 560 nm was assumed in all calculations and the imaginary part of the refractive index was kept constant at 5 × 10−4 i. (Color figure available online.)
3.2.3. Multiple Particle Aggregates
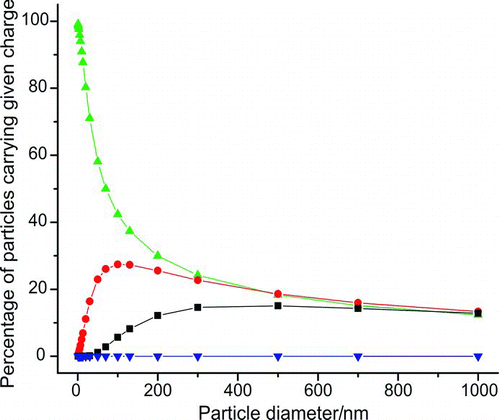
Knowledge of the diameter of particles within the RDC is commonly achieved through the use of a differential mobility analyzer which passes only aerosol particles of a given electrical mobility, or diameter-to-charge ratio (Hewitt Citation1957; Whitby and Clark Citation1966). However, it is important to recognize that this electrostatically selected, monomobile aerosol fraction can contain particles of different diameters carrying a range of charges. Prior to electrical classification, aerosol entering the DMA is charge neutralized, generating a Boltzmann distribution of both positive and negative particle charges centred around zero. shows the size-dependent charging efficiency for neutral and negatively charged particles (carrying up to three charges) in a fully neutralized aerosol sample as calculated by Wiedensohler (Citation1988). The figure shows that even at particle diameters as small as 100 nm, there are a significant number of particles which carry more than a single charge.
FIG. 7 The percentages of neutral, singly, doubly, and triply negatively charged particles as a function of particle diameter. Calculations were performed using equations and data contained in Wiedensohler (Citation1988). Triangles = neutral particles, circles = single negative charge, squares = double negative charge, inverted triangles = triple negative charge. (Color figure available online.)
It is important to take multiply charged particles into account when selecting monomobile aerosol from a polydispersed distribution, as the presence of larger particles with greater extinction cross-section inside the RDC will impact on the measurement of the aerosol optical properties (Baynard et al. Citation2006; Garland et al. Citation2007; Beaver et al. Citation2008, Citation2010; Freedman et al. Citation2009; Khalizov et al. Citation2009; Xue et al. Citation2009; Hasenkopf et al. Citation2010). Such larger particles would be detected in the ring down measurement but would not be discriminated against in the particle count, as the CPC outputs raw counts with no sizing information.
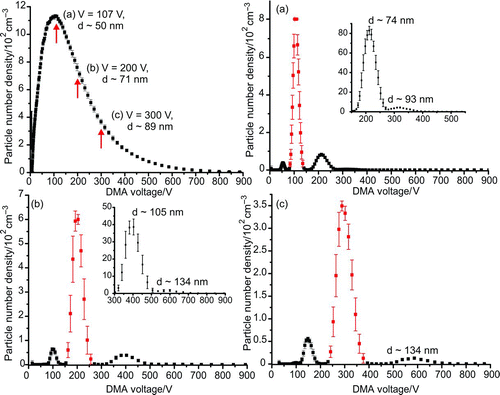
FIG. 8 Number density of crystalline NaCl aerosol generated from atomization of a 0.02 wt% aqueous solution, plotted as a function of DMA voltage. The diameter conversion of the three marked voltages is shown. (a) The size distribution measured by the second DMA when the primary DMA was set to a voltage of 107 V. The target peak is the most intense, with smaller peaks due to particles which passed in the original monomobile fraction with higher charges clearly seen. Panels (b) and (c) show the size distribution measured by the second DMA when the primary DMA was set at voltages of 200 V and 300 V, respectively. (Color figure available online.)
Several A-CRDS studies to date have sought to account for the presence of multiply charged particles. In their measurements of optical growth factor, Baynard et al. (Citation2006), Beaver et al. (Citation2008), and Garland et al. (Citation2007) measured the extinction coefficient as a function of particle number density to calculate the extinction cross-section of aerosol passed by the DMA at a given voltage. This was then compared with theoretical values of the extinction cross-section calculated using Mie theory to determine an optical effective diameter. For ammonium sulfate aerosol size selected by the DMA with nominal diameters in the range 100–450 nm, the corresponding optical effective diameters were found to be in the range 150–501 nm (Beaver et al. Citation2008). Khalizov et al. (Citation2009) and Xue et al. (Citation2009) used a tandem DMA to reduce the number of multiply charged particles passed to the RDC. Particles passed through the first DMA at a voltage corresponding to diameter D p were reneutralized and sent to a second DMA, selecting at a voltage corresponding to 1.5 D p. The authors found that this reduced in number but did not eliminate multiply charged particles, with experimentally measured scattering cross-sections for ammonium sulfate being 17–47% higher than those predicted by Mie theory, and those for polystyrene beads being 2–12% higher (Khalizov et al. Citation2009). Worse agreement was observed at smaller particle diameters. Hasenkopf and co-workers (Citation2010) explicitly included the effect of multiply charged particles in their optical measurements by determining for a given DMA voltage which particle diameters would be passed with a double charge. The number density of these particles carrying a single charge was determined from the size distribution, with the formalism of Wiedensohler (Citation1988) then used to convert this into the number carrying a double charge. Agreement to within 5% was seen between the predicted interference from multiply charged particles and that measured experimentally (Hasenkopf et al. Citation2010). Nakayama et al. (Citation2010) used a DMA in series with a particle mass analyzer to avoid possible interference from multiply charged particles.
When selecting particles from a polydispersed distribution, the ratio of singly to multiply charged particles passed in the monomobile aerosol fraction depends on both the diameter-dependent charging efficiency and the concentration ratio of the larger particles to the target diameter in the size distribution. To minimize the effect of the latter, size distributions can be generated whose modal diameter is significantly smaller than the diameter at which particle size selection is to occur (Pettersson et al. Citation2004; Freedman et al. Citation2009; Hasenkopf et al. Citation2010). However, the concentration at the measurement diameter must still be large enough to perform ring down measurements without the reduction in sensitivity seen at lower particle number densities (Pettersson et al. Citation2004). Impactors of varying cutoff diameter can also be used to try to limit the contribution from multiply charged larger particles.
shows the polydispersed, crystalline NaCl aerosol distribution generated from atomization and drying of an ∼0.02 wt% aqueous salt solution. To determine the proportion of multiply charged particles passed in the monomobile aerosol fraction as a function of classification voltage, a tandem DMA was used. Three voltages were chosen for the primary DMA: 107 V, 200 V, and 300 V, corresponding to particle diameters of ∼50 nm, 71 nm, and 89 nm, respectively. The output from the primary DMA was reneutralized and passed to a second identical instrument, which was used to scan across the entire voltage range. As the aerosol is reneutralized before passing through the second classifier, peaks due to the singly charged species of any larger particles that passed through the first DMA with multiple charges should be seen. The results of these scans are shown in the three panels of the figure.
Panels (a) and (b) in , corresponding to selection voltages of 107 V and 200 V, both show doubly and triply charged particles of a larger diameter that were passed in the monomobile aerosol fraction selected by the primary DMA. This is to be expected given the proximity of the selection voltages to the modal value for the size distribution. For a higher initial selection voltage of 300 V, shown in panel (c), doubly charged particles were still passed in the monomobile fraction, although there is no evidence of triply charged particles. A decrease in the relative concentrations of the singly:doubly:triply charged particles is seen as the primary DMA voltage moves further away from the modal value for the size distribution. These findings support the practice of selecting particle diameters only from the tail end of a polydispersed distribution to minimize the contribution from multiply charged particles (Freedman et al. Citation2009; Hasenkopf et al. Citation2010).
The transmission of multiply charged particles into the RDC was also investigated using polystyrene beads. Even though the beads are monodisperse, multiple particle aggregates are able to form during the nebulization and drying of the particle suspension, which then behave as larger particles. These can be in the form of a cluster or a chain, with the aggregate formed from two particles called a doublet, and that from three particles called a triplet. The proportion of multiple particle aggregates produced in the aerosol flow depends on the concentration of the polystyrene bead solution and the size of the droplets produced by the atomizer, as aggregate formation requires more than one polymer bead to be present in an atomized droplet (Hinds Citation1982).
FIG. 9 (a) DMA voltage scan for the 299-nm-diameter beads, indentifying peaks due to singlet (m), doublet (2m), and triplet (3m) particles, with both single (e–) and double (2e–) charges. (b) The peaks seen in the voltage scan of the second DMA after reneutralization of the monomobile aerosol fraction passed by the primary DMA for the 299-nm-diameter beads. Strong peaks attributed to singlet particles with various charges were seen, as well as triplet particles with a single charge. (Color figure available online.)
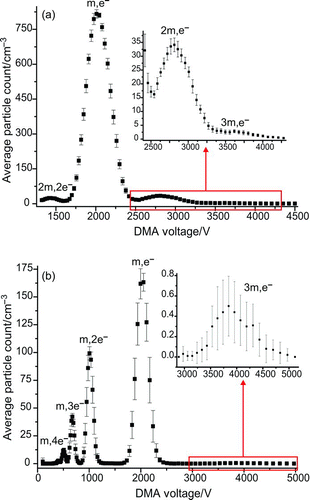
As a result of their increased diameter and irregular shape, doublet and triplet particles experience different drag forces inside the DMA than those experienced by singlet particles. The differences can be represented in terms of a dynamic shape factor, χ, defined as the ratio of the drag force experienced by the particle aggregate to that experienced by a sphere of the same volume-equivalent diameter (Zelenyuk et al. Citation2006). The value of χ depends not only on the number of particles in the aggregate, but also on the ambient gas pressure, the size of the primary particles, and the orientation of the aggregate in the electric field within the DMA. Zelenyuk and co-workers (Citation2006) found that for aggregates formed from 302-nm polystyrene beads passed through a DMA, a doublet had a χ value of 1.01, while a compact triplet had a χ value of 1.09.
TABLE 1 A summary of the sources of error and uncertainty in A-CRDS experiments and their relative importance when considering the retrieval of particle refractive index
Scans across the voltage range of the Grimm DMA showed that doublet and triplet particle aggregates were able to pass through the impactor even when its cutoff diameter was reduced to be close to that of the primary bead diameter. shows the peaks in the DMA scan around the singly charged singlet peak of 299-nm diameter polystyrene beads. The singly charged doublet and triplet peaks are clearly visible. The voltage of the singly charged triplet particles was such that the doubly charged triplet particles would be expected at a voltage equivalent to that of the singly charged singlet beads. The primary DMA was set to the voltage of the apex of the singly charged singlet polystyrene bead peak and the output was scanned with the second DMA. Multiple peaks due to singlet particles carrying varying charges were observed in the mobility spectrum of the second DMA, as shown in . Examination of the baseline also showed a weak peak corresponding to singly charged triplet particles.
Measurements were repeated for eight bead sizes in the range 240–707 nm. For the smaller particle diameters, clear peaks corresponding to singly charged doublet and triplet particles were seen due to the more concentrated nebulization solutions needed for optical measurements with these bead sizes. For the larger bead diameters, which were generated from more dilute solutions, no evidence of doublet or triplet particles was seen. These outcomes support the finding of Khalizov et al. (Citation2009), who saw greater disagreement between experimental measurements and theoretical predictions caused by multiple charge effects for smaller particle diameters.
The investigations with both NaCl aerosol and polystyrene beads clearly show that particles of varying diameter carrying different charges are passed in the monomobile aerosol fraction selected by the DMA. Increased optical extinction by the larger-diameter particles within the RDC must be explicitly considered, particularly when size selecting aerosol from a polydispersed distribution, if an accurate value of the refractive index is to be retrieved. Panel (c) in demonstrates this, as the extinction cross-section of the 134-nm diameter particles is over 11 times larger than that of the 89-nm diameter particles (assuming n = 1.544 (Lide Citation2010)). Tailoring of the modal value of the aerosol size distribution, particle selection from the tail of the distribution and the use of impactors can minimize the effect of multiply charged particles, but it will not eliminate them completely.
4. CONCLUSION
We have presented a detailed and systematic review of potential sources of error and uncertainty in the measurement of aerosol optical properties using cavity ring down spectroscopy, considering both experimental and theoretical factors. Our results indicate that the typical uncertainty of ∼1% quoted in the literature for the real part of the refractive index retrieved by single-cavity A-CRDS studies may be conservative if the uncertainties we identify have not been carefully considered. We summarize these key uncertainties and their influence in . When performing comparative measurements using two or more ring down cavities at multiple wavelengths or different RHs, the relative nature of the measurements may reduce the impact of some of these sources of error.
The length of the cavity physically occupied by the aerosol particles and the counting efficiency of the CPC were identified as key areas in which uncertainty can be introduced during the retrieval of particle refractive index. For measurements on polystyrene beads, an increase of 2.5% in the real part of the refractive index returned by the fitting program was observed when the length of the cavity occupied by aerosol was reduced from 90 cm to 79 cm, corresponding to a move from the inter-mirror distance to the inter-port distance in our experimental apparatus. The aerosol length in the cavity is often stated without any reference as to whether it has been experimentally determined, despite its significance in the measurement of the extinction efficiency and thus retrieval of complex refractive index. Modeling the effect of a manufacturer-specified error in the CPC count of ±10% on the optical properties that would be determined for polystyrene beads gave retrieved refractive index values in the range of 1.636 to 1.555. This range is broader than the range of values published in the literature to date at the wavelength of interest. After calibrating the Grimm CPC used in this work against an aerosol electrometer-calibrated CPC and correcting for the observed systematic 9.3% undercounting, the value of the polystyrene refractive index determined from our measurements decreased from 1.627 to 1.585, a fall of 2.6%. Both results demonstrate very clearly the crucial dependence of the refractive index retrieved from the A-CRDS technique on an accurate determination of particle number density. This can only be ensured by calibrating the count recorded by the CPC against a suitable standard, such as an aerosol electrometer. Even then, this often requires extrapolation of calibrations performed at higher particle concentrations than are typical in A-CRDS measurements, as errors associated with aerosol electrometer counts at number densities below 1000 cm−3 can approach 100% by a number concentration of 100 cm−3. Attempts to calibrate the aerosol sample length and CPC counting efficiency of an A-CRDS instrument using polymer bead standards of known size must also consider the errors inherent in such measurements, such as the uncertainty in bead diameter and the morphology of the polymer bead standards after drying.
As observed in previous publications, the concentration of multiply charged particles in the monomobile fraction passed by the DMA was shown to be significant when sampling from a polydisperse distribution close to the modal diameter. Such particles will have an increased extinction cross-section and must be accounted for in the optical measurements if accurate values of the complex refractive index are to be retrieved. Uncertainties in particle morphology and the counting efficiency of the CPC were also demonstrated to have a different significance depending on particle diameter, potentially compromising global fitting of experimental extinction efficiency data.
In summary, we recommend that A-CRDS measurements fully report methods of determining the length of the cavity occupied by the aerosol and the calibration of the CPC. Ambiguity in these two key experimental parameters can lead to systematic errors in estimates of the refractive index that far outweigh the currently reported random errors from variations between repeated measurements.
Acknowledgments
This study was funded by the NERC through grant NE/C512537/1. Rachael E. H. Miles is grateful to the EPSRC for studentship and postdoctoral funding, and Svemir Rudić acknowledges the NERC for postdoctoral support. Andrew J. Orr-Ewing thanks the Royal Society and Wolfson Foundation for a Research Merit Award, and Jonathan P. Reid acknowledges the EPSRC for Leadership Fellowship funding. Drs Richard Gilham, Marc Coleman, Fabrizio Innocenti, and Tom Gardiner (National Physical Laboratory), and Dr Jin Kim (University of Bristol) are thanked for their assistance in measuring the counting efficiency of the Grimm CPC.
REFERENCES
- Abu-Rahmah , A. , Arnott , W. P. and Moosmuller , H. 2006 . Integrating Nephelometer with a Low Truncation Angle and an Extended Calibration Scheme . Meas. Sci. Tecnol. , 17 : 1723 – 1732 .
- Aden , A. L. and Kerker , M. 1951 . Scattering of Electromagnetic Waves from Two Concentric Spheres . J. Appl. Phys. , 22 : 1242 – 1246 .
- Adler , G. , Riziq , A. A. , Erlick , C. and Rudich , Y. 2010 . Effect of Intrinsic Organic Carbon on the Optical Properties of Fresh Diesel Soot . Proc. Natl. Acad. Sci. USA , 107 : 6699 – 6704 .
- Anderson , T. L. , Covert , D. S. , Marshall , S. F. , Laucks , M. L. , Charlson , R. J. Waggoner , A. P. 1996 . Performance Characteristics of a High-Sensitivity, Three-Wavelength, Total Scatter/Backscatter Nephelometer . J. Atmos. Ocean Tech. , 13 : 967 – 986 .
- Ankilov , A. , Baklanov , A. , Colhoun , M. , Enderle , K.-H. , Gras , J. Julanov , Y. 2002a . Intercomparison of Number Concentration Measurements by Various Aerosol Particle Counters . Atmos. Res. , 62 : 177 – 207 .
- Ankilov , A. , Baklanov , A. , Colhoun , M. , Enderle , K.-H. , Gras , J. Julanov , Y. 2002b . Particle Size Dependent Response of Aerosol Counters . Atmos. Res. , 62 : 209 – 237 .
- Arnott , W. P. , Moosmuller , H. , Rogers , C. F. , Jin , T. and Bruch , R. 1999 . Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description . Atmos. Environ. , 33 : 2845 – 2852 .
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. Slaton , W. V. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity . J. Geophys. Res. , 108 doi: 10.1029/2002JD002165
- Baynard , T. , Garland , R. M. , Ravishankara , A. R. , Tolbert , M. A. and Lovejoy , E. R. 2006 . Key Factors Influencing the Relative Humidity Dependence of Aerosol Light Scattering . Geophys. Res. Lett. , 33 : L06813
- Baynard , T. , Lovejoy , E. R. , Pettersson , A. , Brown , S. S. , Lack , D. Osthoff , H. 2007 . Design and Application of a Pulsed Cavity Ring-Down Aerosol Extinction Spectrometer for Field Measurements . Aerosol. Sci. Tech. , 41 : 447
- Beaver , M. R. , Freedman , M. A. , Hasenkopf , C. A. and Tolbert , M. A. 2010 . Cooling Enhancement of Aerosol Particles Due to Surfactant Precipitation . J. Phys. Chem. A , 114 : 7070 – 7076 .
- Beaver , M. R. , Garland , R. M. , Hasenkopf , C. A. , Baynard , T. , Ravishankara , A. R. and Tolbert , M. A. 2008 . A Laboratory Investigation of the Relative Humidity Dependence of Light Extinction by Organic Compounds from Lignin Combustion . Environ. Res. Lett. , 3 : 1 – 8 .
- Berden , G. , Peeters , R. and Meijer , G. 2000 . Cavity Ring-Down Spectroscopy: Experimental Schemes and Applications . Int. Rev. Phys. Chem. , 19 : 565
- Bond , T. C. , Covert , D. S. and Muller , T. 2009 . Truncation and Angular-Scattering Corrections for Absorbing Aerosol in the TSI 3563 Nephelometer . Aerosol. Sci. Tech. , 43 : 866 – 871 .
- Bulatov , V. , Chen , Y. , Khalmanov , A. and Schechter , I. 2006 . Absorption and Scattering Characterization of Airborne Microparticulates by a Cavity Ringdown Technique . Anal. Bioanal. Chem. , 384 : 155 – 160 .
- Bulatov , V. , Fisher , M. and Schechter , I. 2002 . Aerosol Analysis by Cavity-Ring-Down Laser Spectroscopy . Anal. Chim. Acta. , 466 : 1 – 9 .
- Bulatov , V. , Khalmanov , A. and Schechter , I. 2003 . Study of the Morphology of a Laser-Produced Aerosol Plume by Cavity Ringdown Laser Absorption Spectroscopy . Anal. Bioanal. Chem. , 375 : 1282 – 1286 .
- Butler , T. J. A. , Mellon , D. , Kim , J. , Litman , J. and Orr-Ewing , A. J. 2009 . Optical-Feedback Cavity Ring-Down Spectroscopy Measurements of Extinction by Aerosol Particles . J. Phys. Chem. A , 113 : 3963 – 3972 .
- Butler , T. J. A. , Miller , J. L. and Orr-Ewing , A. J. 2007 . Cavity Ring-Down Spectroscopy Measurements of Single Aerosol Particle Extinction. I: The Effect of Position of a Particle within the Laser Beam on Extinction . J. Chem. Phys. , 126 : 174302 – 174307 .
- Dinar , E. , Riziq , A. A. , Spindler , C. , Erlick , C. , Kiss , G. and Rudich , Y. 2008 . The Complex Refractive Index of Atmospheric and Model Humic-Like Substances (HULIS) Retrieved by a Cavity Ring Down Aerosol Spectrometer (CRD-AS) . Discuss. Neurosci. , 137 : 279 – 295 .
- Fialho , P. , Freitas , M. C. , Barata , F. , Vieira , B. , Hansen , A. D. A. and Honrath , R. E. 2006 . The Aethalometer Calibration and Determination of Iron Concentration in Dust Aerosols . Aerosol. Sci. , 37 : 1497 – 1506 .
- Fletcher , R. A. , Mulholland , G. W. , Winchester , M. R. , King , R. L. and Klinedinst , D. B. 2009 . Calibration of a Condensation Particle Counter Using a NIST Traceable Method . Aerosol. Sci. Tech. , 43 : 425 – 441 .
- Forster , P. , Ramaswamy , V. , Artaxo , P. , Berntsen , T. , Betts , R. Fahey , D. W. 2007 . Changes in Atmospheric Constituents and in Radiative Forcing, in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. and Averyt , K. B. Cambridge : Cambridge University Press . et al., eds., pp. 131–234.
- Freedman , M. A. , Hasenkopf , C. A. , Beaver , M. R. and Tolbert , M. A. 2009 . Optical Properties of Internally Mixed Aerosol Particles Composed of Dicarboxylic Acids and Ammonium Sulfate . J. Phys. Chem. A , 113 : 13584 – 13592 .
- Garland , R. M. , Ravishankara , A. R. , Lovejoy , E. R. , Tolbert , M. A. and Baynard , T. 2007 . Parameterization for the Relative Humidity Dependence of Light Extinction: Organic-Ammonium Sulfate Aerosol . J. Geophys. Res. , 112 doi: 10.1029/2006JD008179
- Garland , R. M. , Yang , H. , Schmid , O. , Rose , D. , Nowak , A. Achtert , P. 2008 . Aerosol Optical Properties in a Rural Environment Near the Mega-City Guangzhou, China: Implications for Regional Air Pollution, Radiative Forcing and Remote Sensing . Atmos. Chem. Phys. , 8 : 5161 – 5186 .
- Gebhart , J. 2005 . “ Optical Direct-Reading Techniques: Light Intensity Systems ” . In Aerosol Measurement: Principles, Techniques and Applications , 2nd ed. , Edited by: Baron , P. A. and Willeke , K. New York, NY : Wiley-InterScience . pp. 419–454
- GRIMM Aerosol Technik GmbH. 2005 . SMPS (Sequential Mobility Particle Sizer) Series 5.400 and 5.500 , Ainring, , Germany : GRIMM Aerosol Technik GmbH .
- Hale , G. M. and Querry , M. R. 1973 . Optical Constants of Water in the 200 nm to 200 μm Wavelength Region . Appl. Optics. , 12 : 555 – 563 .
- Hansen , A. D. A. , Rosen , H. and Novakov , T. 1984 . The Aethalometer – An Instrument for the Real-Time Measurement of Optical Absorption by Aerosol Particles . Sci. Total Environ. , 36 : 191 – 196 .
- Hasenkopf , C. A. , Beaver , M. R. , Trainer , M. G. , Dewitt , H. L. , Freedman , M. A. Toon , O. B. 2010 . Optical Properties of Titan and Early Earth Haze Laboratory Analogs in the Mid-Visible . Icarus , 207 : 903 – 913 .
- Hassan , Z. 2008 . Personal Communication. Thermo Scientific .
- Hewitt , G. W. 1957 . The Charging of Small Particles for Electrostatic Precipitation . Trans. Am. Inst. Elec. Engrs. , 76 : 300 – 306 .
- Hinds , W. C. 1982 . Aerosol Technology: Properties, Behaviour, and Measurement of Airborne Particles , New York, NY : John Wiley & Sons .
- Khalizov , A. F. , Xue , H. , Wang , L. , Zheng , J. and Zhang , R. 2009 . Enhanced Light Absorption and Scattering by Carbon Soot Aerosol Internally Mixed with Sulfuric Acid . J. Phys. Chem. A , 113 : 1066 – 1074 .
- Kogelnik , H. and Li , T. 1966 . Laser Beams and Resonators . Appl. Optics. , 5 : 1550 – 1567 .
- Lack , D. A. , Cappa , C. D. , Covert , D. S. , Baynard , T. , Massoli , P. Sierau , B. 2008 . Bias in Filter-Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Ambient Measurements . Aerosol. Sci. Tech. , 42 : 1033 – 1041 .
- Lack , D. A. , Cappa , C. D. , Cross , E. S. , Massoli , P. , Ahern , A. T. Davidovits , P. 2009 . Absorption Enhancement of Coated Absorbing Aerosols: Validation of the Photo-Acoustic Technique for Measuring the Enhancement . Aerosol. Sci. Tech. , 43 : 1006 – 1012 .
- Lang-Yona , N. , Riziq , A. A. , Erlick , C. , Segre , E. , Trainic , M. and Rudich , Y. 2010a . Interaction of Internally Mixed Aerosols with Light . Phys. Chem. Chem. Phys. , 12 : 21 – 31 .
- Lang-Yona , N. , Rudich , Y. , Mentel , T. F. , Bohne , A. , Buchholz , A. Kiendler-Scharr , A. 2010b . The Chemical and Microphysical Properties of Secondary Organic Aerosols from Holm Oak Emissions . Atmos. Chem. Phys. , 10 : 7253 – 7265 .
- Lang-Yona , N. , Rudich , Y. , Segre , E. , Dinar , E. and Abo-Riziq , A. 2009 . Complex Refractive Indices of Aerosols Retrieved by Continuous Wave-Cavity Ring Down Aerosol Spectrometer . Anal. Chem. , 81 : 1762 – 1769 .
- Lide , D. R. 2010 . CRC Handbook of Chemistry and Physics , 91st ed. , Boca Raton, FL : CRC Press .
- Liu , B. Y. H. and Pui , D. Y. H. 1974 . A Submicron Aerosol Standard and Primary, Absolute Calibration of the Condensation Nuclei Counter . J. Colloid. Interf. Sci. , 47 : 155 – 171 .
- Ma , X. , Lu , J. Q. , Brock , R. S. , Jacobs , K. M. , Yang , P. and Hu , X.-H. 2003 . Determination of Complex Refractive Index of Polystyrene Microspheres from 370 to 1610 nm . Phys. Med. Biol. , 48 : 4165 – 4172 .
- Massoli , P. , Murphy , D. M. , Lack , D. A. , Baynard , T. , Brock , C. A. and Lovejoy , E. R. 2009 . Uncertainty in Light Scattering Measurements by TSI Nephelometer: Results from Laboratory Studies and Implications for Ambient Measurements . Aerosol. Sci. Tech. , 43 : 1064 – 1074 .
- Matheson , L. A. and Saunderson , J. L. 1952 . “ Optical and Electrical Properties of Polystyrene ” . In Styrene: Its Polymers, Copolymers and Derivatives , Edited by: Boundy , R. H. and Boyer , R. F. 517 – 546 . New York, NY : Reinhold Publishing Corporation .
- Miles , R. E. H. , Rudić , S. , Orr-Ewing , A. J. and Reid , J. P. 2010a . Influence of Uncertainties in the Diameter and Refractive Index of Calibration Polystyrene Beads on the Retrieval of Aerosol Optical Properties Using Cavity Ring Down Spectroscopy . J. Phys. Chem. A , 114 : 7077 – 7084 .
- Miles , R. E. H. , Rudić , S. , Orr-Ewing , A. J. and Reid , J. P. 2010b . Measurements of the Wavelength Dependent Extinction of Aerosols by Cavity Ringdown Spectroscopy . Phys. Chem. Chem. Phys. , 12 : 3914 – 3920 .
- Miller , J. L. and Orr-Ewing , A. J. 2007 . Cavity Ring-Down Spectroscopy Measurement of Single Aerosol Particle Extinction. II: Extinction of Light by an Aerosol Particle in an Optical Cavity Excited by a CW Laser . J. Chem. Phys. , 126 : 174303 – 174307 .
- Moosmuller , H. , Varma , R. and Arnott , W. P. 2005 . Cavity Ring-Down and Cavity-Enhanced Detection Techniques for the Measurement of Aerosol Extinction . Aerosol. Sci. Tech. , 39 : 30
- Mordas , G. , Manninen , H. E. , Petaja , T. , Aalto , P. P. , Hameri , K. and Kulmala , M. 2008 . On Operation of the Ultra-Fine Water-Based CPC TSI 3786 and Comparison with Other TSI Models (TSI 3776, TSI 3772, TSI 3025, TSI 3010, TSI 3007) . Aerosol. Sci. Tech. , 42 : 152 – 158 .
- Nakayama , T. , Hagino , R. , Matsumi , Y. , Sakamoto , Y. , Kawasaki , M. Yamazaki , A. 2010 . Measurements of Aerosol Optical Properties in Central Tokyo During Summertime Using Cavity Ring-Down Spectroscopy: Comparison with Conventional Techniques . Atmos. Environ. , 44 : 3034 – 3042 .
- Pettersson , A. , Lovejoy , E. R. , Brock , C. A. , Brown , S. S. and Ravishankara , A. R. 2004 . Measurement of Aerosol Optical Extinction at 532 nm with Pulsed Cavity Ring Down Spectroscopy . J. Aerosol. Sci. , 35 : 995 – 1011 .
- Radney , J. G. , Bazargan , M. H. , Wright , M. E. and Atkinson , D. B. 2009 . Laboratory Validation of Aerosol Extinction Coefficient Measurements by a Field-Deployable Pulsed Cavity Ring-Down Transmissometer . Aerosol. Sci. Tech. , 43 : 71
- Raspet , R. , Slaton , W. V. , Arnott , W. P. and Moosmuller , H. 2003 . Evaporation-Condensation Effects on Resonant Photoacoustics of Volatile Aerosols . J. Atmos. Ocean Tech. , 20 : 685 – 695 .
- Riziq , A. A. , Erlick , C. , Dinar , E. and Rudich , Y. 2007 . Optical Properties of Absorbing and Non-Absorbing Aerosols Retrieved by Cavity Ring Down (CRD) Spectroscopy . Atmos. Chem. Phys. , 7 : 1523 – 1536 .
- Riziq , A. A. , Trainic , M. , Erlick , C. , Segre , E. and Rudich , Y. 2008 . Extinction Efficiencies of Coated Absorbing Aerosols Measured by Cavity Ring Down Aerosol Spectrometry . Atmos. Chem. Phys. , 8 : 1823 – 1833 .
- Rudić , S. , Miles , R. E. H. , Orr-Ewing , A. J. and Reid , J. P. 2007 . Optical Properties of Micrometer Size Water Droplets Studied by Cavity Ringdown Spectroscopy . Appl. Optics. , 46 : 6142 – 6150 .
- Sanford , T. J. , Murphy , D. M. , Thomson , D. S. and Fox , R. W. 2008 . Albedo Measurements and Optical Sizing of Single Aerosol Particles . Aerosol. Sci. Tech. , 42 : 958 – 969 .
- Sappey , A. D. , Hill , E. S. , Settersten , T. and Linne , M. A. 1998 . Fixed-Frequency Cavity Ringdown Diagnostic for Atmospheric Particulate Matter . Opt. Lett. , 23 : 954 – 956 .
- Seinfeld , J. H. and Pandis , S. N. 1998 . Atmospheric Chemistry and Physics: From Air Pollution to Climate Change New York, NY : John Wiley & Sons, .
- Smith , J. D. and Atkinson , D. B. 2001 . A Portable Pulsed Cavity Ring-Down Transmissometer for Measurement of the Optical Extinction of the Atmospheric Aerosol . Analyst , 126 : 1216 – 1220 .
- Spielvogel , J. 2009 . Private Communication. GRIMM Aerosol Technik GmbH .
- Spindler , C. , Riziq , A. A. and Rudich , Y. 2007 . Retrieval of Aerosol Complex Refractive Index by Combining Cavity Ring Down Aerosol Spectrometer Measurements with Full Size Distribution Information . Aerosol. Sci. Tech. , 41 : 1011 – 1017 .
- Strawa , A. W. , Castaneda , R. , Owano , T. , Baer , D. S. and Paldus , B. A. 2003 . The Measurement of Aerosol Optical Properties using Continuous Wave Cavity Ring-Down Techniques . J. Atmos. Ocean Tech. , 20 : 454 – 465 .
- Strawa , A. W. , Elleman , R. , Hallar , A. G. , Covert , D. , Ricci , K. Provencal , R. 2006 . Comparison of in Situ Aerosol Extinction and Scattering Coefficient Measurements Made during the Aerosol Intensive Operating Period . J. Geophys. Res. - Atmos. , 111 : D05S03
- Thermo Scientific . 1996 . Technical Note TN-007.02A: Index of Refraction , Waltham, MA : Thermo Scientific .
- Thompson , J. E. , Barta , N. , Policarpio , D. and DuVall , R. 2008 . A Fixed Frequency Aerosol Albedometer . Opt. Express , 16 : 2191 – 2205 .
- Thompson , J. E. , Nasajpour , H. D. , Smith , B. W. and Winefordner , J. D. 2003 . Atmospheric Aerosol Measurements by Cavity Ringdown Turbidimetry . Aerosol. Sci. Tech. , 37 : 221
- Thompson , J. E. , Smith , B. W. and Winefordner , J. D. 2002 . Monitoring Atmospheric Particulate Matter through Cavity Ring-Down Spectroscopy . Anal. Chem. , 74 : 1962 – 1967 .
- Vander Wal , R. L. and Ticich , T. M. 1999 . Cavity Ringdown and Laser-Induced Incandescence Measurements of Soot . Appl. Optic. , 38 : 1444 – 1451 .
- Varma , R. , Moosmuller , H. and Arnott , W. P. 2003 . Towards an Ideal Integrating Nephelometer . Opt. Lett. , 28 : 1007 – 1009 .
- Weingartner , E. , Saathoff , H. , Schnaiter , M. , Streit , N. , Bitnar , B. and Baltensperger , U. 2003 . Absorption of Light by Soot Particles: Determination of the Absorption Coefficient by Means of Aethalometers . J. Aerosol. Sci. , 34 : 1445 – 1463 .
- Wheeler , M. D. , Newman , S. M. , Orr-Ewing , A. J. and Ashfold , M. N. R. 1998 . Cavity Ring-Down Spectroscopy . J. Chem. Soc. Faraday Trans. , 94 : 337 – 351 .
- Whitby , K. T. and Clark , W. E. 1966 . Electrical Aerosol Particle Counting and Size Distribution Measuring Measuring System for the 0.015 to 1 μ Size Range . Tellus , 18 : 573 – 586 .
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range . J. Aerosol. Sci. , 19 : 387 – 389 .
- Wiedensohler , A. , Orsini , D. , Covert , D. S. , Coffmann , D. , Cantrell , W. Havlicek , M. 1997 . Intercomparison Study of the Size-Dependent Counting Efficiency of 26 Condensation Particle Counters . Aerosol. Sci. Tech. , 27 : 224
- Xue , H. , Khalizov , A. F. , Wang , L. , Zheng , J. and Zhang , R. 2009 . Effects of Dicarboxylic Acid Coating on the Optical Properties of Soot . Phys. Chem. Chem. Phys. , 11 : 7869 – 7875 .
- Zelenyuk , A. , Cai , Y. and Imre , D. 2006 . From Agglomerates of Spheres to Irregularly Shaped Particles: Determination of Dynamic Shape Factors from Measurements of Mobility and Vacuum Aerodynamic Diameters . Aerosol. Sci. Tech. , 40 : 197