Abstract
As organic pigments are produced mainly in wet chemical processes, a novel gas to particle process for generating organic nanoscale pigments is introduced. This process is based on the expansion of a nearly saturated gas flow from an overpressure regime to ambient pressure conditions.
The expansion is achieved in a Laval nozzle and subsequently continues as an expanding free jet in an expansion chamber. This expansion chamber is equipped with an adjustable cooling system. Copper phthalocyanine and Paliogen Red, two organic pigments, were used as test materials. By controlling the temperature in the expansion chamber, particularly in the free jet, nanoscale pigments with high (needle shape) to low (grainy shape) aspect ratios are generated. Particle size distribution measurements as well as scanning electron microscopy and transmission electron microscopy analyses show that newly generated particles differ significantly in size and shape from the cubic-shaped bulk material. Particles with low aspect ratio are below 100 nm in all dimensions. Hence, specific control of temperatures in the expanding free jet offers a tool to produce nanoscale organic pigments with defined particle sizes.
INTRODUCTION
In recent years, the scientific community and industry have explored the potential of nanoparticles for new applications and developments. These nanoscale materials possess to some extent distinctly different properties compared to bulk materials. Especially organic materials in nanoscale size represent a unique combination of properties that make them ideal candidates for new nanotechnological applications in materials sciences or industrial, pharmaceutical, and biomedical applications.
Such organic particles can be produced in wet chemical or gas-phase processes but the majority, in particular for pharmaceutical and chemical applications, are still produced in wet chemical processes. Contaminations of solid product with substances from the liquid phase, complex production steps, as well as high liquid volumes created the desire for alternative production processes. Gas to particle conversion processes offer a high purity of the generated particles with almost no contamination and thus provide incentives for the development of new and innovative processes.
In this work, a gas to particle conversion process for generating organic nanoscale pigments out of a supersaturated gas flow is introduced. By sublimation of bulk material, a nearly saturated gas flow is reached and subsequently super saturation arises from adiabatic expansion of a high-pressure gas flow in a Laval nozzle and an attached expansion chamber. Thus, high and fast temperature drops are realized. In the last decades, particle formation in nozzles of materials with small molecule size (mostly inorganic materials) or metal atoms has been the subject of many studies (McBride et al. Citation1971; Saito et al. Citation1983; Liu et al. Citation1995; Bayazitoglu et al. Citation1996; Friedlander Citation1998). Only in the recent years, the generation of higher molecular materials in nozzles, in particular organic materials, draws the attention to scientific research and the complex formation mechanisms are far from being fully understood. One of those new processes for producing organic particles in expanding fluid flows is the rapid expansion of supercritical solutions (RESS). This is a novel process where water-insoluble drugs can be generated by rapid expansion through capillary nozzles (Helfgen et al. Citation2000; Türk Citation2001; Türk et al. Citation2002; Sane et al. Citation2003). Other researchers investigated, for example, the particle formation of succinic acid in expanding gas jets (Kodde Citation1996; Wagner Citation1999; Wagner and Mewes Citation1999)
In this work, copper phthalocyanine and Paliogen Red L4120 were applied as organic test materials. These substances are traditionally used as color pigments but have also been tested for alternative applications. The most promising new application fields for both materials are the information and communication technology, for example, in semiconductors and organic light emitting diodes (Hao and Iqbal Citation1997; Roberts Citation1999; Alfredssona et al. Citation2005; Hertel et al. Citation2005). Moreover, new fields of application are reported including photovoltaics and cancer therapy (Mather Citation1999; El-Nahass et al. Citation2002; Michaelis Citation2003). This broad application spectrum where high purity of the materials is essential was one of the main technology drivers to choose both the expansion process via Laval nozzle and these two organic pigments as test materials.
The motivation for conducting this work is to apply the relatively widespread process of particle formation in expanding gas flows for higher molecular weight organic materials. Originating from these reasons, a pilot plant was designed and built to investigate the particle formation process of organic nanoparticles.
This pilot plant was developed to investigate different techniques for the generation, modification, and conditioning of organic particles. The presented study focuses on:
| • | the generation of organic particles due to rapid expansion of gas flows in Laval nozzles and in the subsequent free jet, | ||||
| • | steering of particle size and shape by controlling temperatures in the expanding free jet. | ||||
The generated particles were characterized by using particle size distribution measurements (scanning mobility particle sizer [SMPS] and fast mobility particle sizer [FMPS]) and electron microscopy (transmission electron microscopy [TEM] and scanning electron microscopy [SEM] in combination with energy dispersive X-ray spectroscopy [EDX]). Computational fluid dynamics (CFD) simulations were used to understand and visualize the flow phenomena which appear in the expansion chamber. First results of the experimental work are presented and a short introduction of the formation mechanism is given.
FUNDAMENTALS OF GAS TO PARTICLE CONVERSION
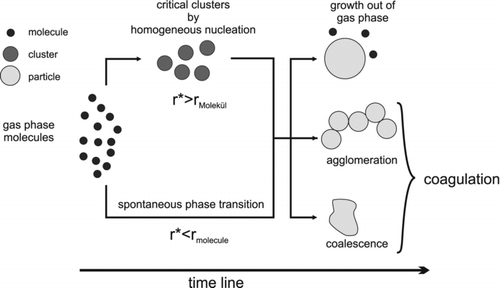
The theoretical description of the gas to particle conversion process is diverse. shows a scheme of the chronological procedure during the gas to particle conversion process. In this approach, it is assumed that generated primary particles have a spherical shape. Referring to our organic pigments, this is a very simplistic assumption, as will be shown in the Results and Discussion section.
Starting with a supersaturated vapor phase, single molecules begin to attach to each other to form molecule clusters. After these clusters reach a critical cluster size r*, they become stable and continue to exist as a new phase. If the saturation in the gas phase is relatively low and the requisite critical cluster size is bigger than a single molecule size, molecule clusters have to overcome that critical size. In other words, they have to surpass an energetic barrier by forming particles which are thermodynamically stable.
A widely used theoretical approach to describe this homogeneous nucleation of sublimated material out of the gas phase is the classical nucleation theory (CNT) (Volmer and Weber Citation1926;Becker and Döring Citation1935; Lothe and Pound Citation1962). On the other hand, if it is found that this critical cluster size is of the order of the molecule size or smaller, no energetic barrier has to be surpassed and stable clusters are formed instantaneously.
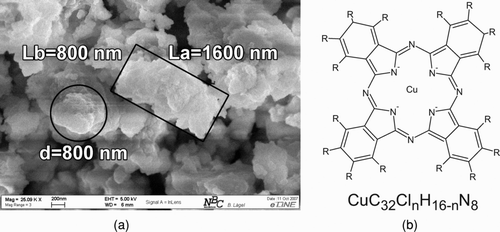
FIG. 2 Shape of the bulk material and structural formula of copper phthalocyanine. (a) Cubic shape of CuPc bulk material. (b) Structural formula of α-crystal modification CuPc with R as the replacement position for chlorine atoms.
The subsequent particle growth happens simultaneously by three different processes. Particle growth out of the gas phase via molecule transport onto the particle surface is dominated by the coupled heat and mass transport and limited by the molecule reservoir in the gas phase. Agglomeration and coalescence are two processes where temperature and material properties are important parameters for the formation of hard and soft agglomerates or new particles. The particle number distribution will change for the latter processes whereas for the particle growth out of the gas phase it remains constant. Additionally, operating parameters such as temperature and residence time determine which process will take place preferentially. A good overview about these processes can be found in Pratsinis and Vemury (Citation1996) andFriedlander (Citation2000).
As mentioned earlier, a spherical model is not realistic for organic pigments as used in this study. We will see in the Results and Discussion section that generated organic pigments show an oriented growth with needlelike and grainylike shapes. The observed needlelike or two-dimensional nucleation and growth is relatively complex and not yet sufficiently investigated in the scientific community. Many researchers report on oriented growth in supersatured liquid systems (Banfield et al. Citation2000; Cölfen and Mann Citation2003) or oriented growth via vapor-phase transport (VPT) which is a very slow formation process compared to the gas to particle conversion due to expansion of gas flows in a nozzle (Xu et al. Citation2004). Oriented growth of high molecular weight organic crystals in expanding gas flows has been less investigated.
Crystals can be grown out of the gas phase when the vapor molecules attach themselves to a surface and move into the crystal arrangement. In analogy to the CNT, for crystals to grow, the gas–solid system has to be in a steady nonequilibrium state such that more molecules are undergoing desublimation into solid state than sublimation from solid into gas phase. The main difference to the assumption of spherical shape and isomeric growth is the oriented growth of crystals.
Crystals, like the test materials, typically own a preferred growth face. Molecules from the same species will attach preferably on this crystal face (Vere Citation1987; Xu et al. Citation2004). However, these crystal growth faces are responsible for the two-dimensional growth due to the fact that the larger vapor gradient over the sharp growth face serves as a sink of gas molecules.
A measure for growth in general and crystal growth in particular is the mass flux I of molecules out of the gas phase and onto the surface of the crystal. The physical and mathematical description of the mass flux is distinguished in different growth regimes. These regimes are described by the dimensionless Knudsen number (Jennings Citation1988):
The regime for bigger particle sizes and therefore small Knudsen numbers Kn ≥ 0 corresponds to diffusion-limited growth, in which the mass flux is based on Fick's law (Zhang and Harrington Citation2010). The surrounding medium of the particles behaves, in contradiction to the free molecular regime, like a continuum fluid and consequently this regime is named as the continuum regime. The mass flux IC of molecules onto the particle surface is expressed by the Stefan flux and the mathematical expressions can be found in (Kulmala and Vesala Citation1991).
Note that the mathematical expressions for mass fluxes which are given in the indicated literature are valid for spherical particles. However, some numerical models for two-dimensional growth of ice crystals have been developed in the recent years with focus on atmospheric cloud modeling (Wood et al. Citation2001; Zhang and Harrington Citation2010). Only with a good data base of material properties and thus for simple systems like ice and water vapor can these models be used for the prediction of crystal growth.
EXPERIMENTAL SETUP AND FLUID DYNAMIC SIMULATIONS
Particle Characterization
As already mentioned, copper phthalocyanine (CuPc) and Paliogen Red L4120 were applied as organic test materials in the experiments. The bulk material was provided by BASF S.E. and shows the most relevant physical properties.
TABLE 1 Physical properties of copper phthalocyanine and Paliogen Red
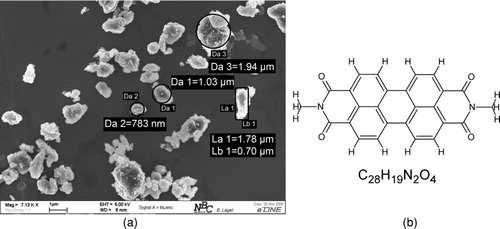
FIG. 3 Shape of the bulk material and structural formula of Paliogen Red L4120. (a) Cubic shape of Paliogen Red bulk material. (b) Structural formula of Paliogen Red L4120.
CuPc has a relatively planar molecule structure and hydrogen atoms can be substituted by chlorine atoms as indicated in the structural formula (). Copper phthalocyanine can exist as α-, β-, γ- or x-polymorphic crystal modification (Prabakaran et al. Citation2002). Those crystal modifications are differentiated against each other by their arrangement of molecules. This arrangement of molecules which is also called stacks is the main reason that the crystal modifications differ in their electrical and chromophoric properties. The most frequent ones are α- and β-crystal modification.
CuPc as α-crystal modification can transform, if not stabilized, at temperature above 200°C into the β-crystal modification (Sharp and Abkowitz Citation1973; Mohammed and Collins Citation1986).
The substitution of hydrogen by chlorine causes the unstable α-crystal modification become thermally and electrically stable. The copper phthalocyanine that is used here is a stabilized α-crystal modification with the trivial name trichlorine copper phthalocyanine. The stabilization is reached by a partial replacement of three hydrogen atoms by chlorine.
shows that the bulk material of CuPc has an amorphous geometric shape and a broad size distribution where most particles are about 1μm in size.
The second pigment which was investigated is Paliogen Red L4120. This pigment belongs to the perylenebisimide family. The crystal structure () exhibits a planar geometry of perylenebisimides molecules. Both pigments are traditionally synthesized in wet chemical processes which results mostly in a cubic microscale shape as is shown in and .
Pilot Plant Setup
Expansion of gas flows can be realized in two ways, either from ambient pressure to vacuum or from overpressure to ambient pressure. Scalability and a better process control are the main advantages of the latter way and thus our pilot plant was designed for the expansion from overpressure (2.5 bara) to ambient pressure (1.1 bara).
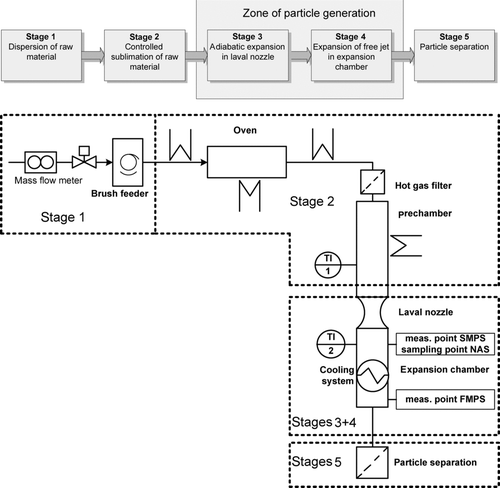
Viewed as a block scheme, the presented process can be divided into five stages. shows schematically these process stages with the corresponding P&ID diagram. In the first stage, a brush feeder disperses the organic bulk material into a carrier gas stream. The feeding speed was set in such a way that the mass fraction of organic pigment in the gas flow was constant at 0.4–0.5 g/kg all the time. Higher mass fractions caused that the material was not sublimed entirely and then blocked the filter downstream. Subsequently, the organic matter has to be transferred into the gas phase in a manner that decomposition is minimized. Decomposition effects at high temperatures are typical for organic compounds and should be minimized in order to minimize material losses and reach a high purity of the generated particles. Optimized residence time and temperatures in the sublimation zone as well as nitrogen (99.8%) as the inert carrier gas are essentially to minimize those decomposition effects of the organic matter.
A temperature-adjustable sublimation zone with an oven, heated piping, and filter ensure that only sublimated organic matter will arrive at the prechamber.
This chamber ensures that laminar flow conditions are reached before the gas flow as the sublimated organic material enters the nozzle. Temperatures of 500–510°C for CuPc and 450–460°C for Paliogen Red are adjusted to be slightly above the sublimation temperature so as to ensure that no material will desublime in the prechamber. The temperatures are measured in the chamber center at TI1. In the Laval nozzle, the gas expands adiabatically with an expansion rate of P = 105/s and forms a free jet in the adjacent expansion chamber. The chamber walls are equipped with a cooling system, which is continuously adjustable and therefore different temperatures of the chamber walls can be set. Without cooling, the expansion chamber temperatures of approximately 290°C were measured at TI2 in the free jet. The temperature sensor is placed 30 mm downstream of the nozzle outlet and in the center of the expanding free jet. Using the cooling system, temperatures could be decreased stepwise down to 160°C. Assuming a constant operational pressure and temperature in the prechamber, nozzle design leads to a constant flow rate of 2 Nm3/h. In the last stage, the generated particles are separated by a filter.
Particle size distributions (PSD) were measured online downstream of the Laval nozzle using SMPS. The SMPS-system (TSI Modell 3936) includes an electrostatic classifier (TSI model 3081) and a condensation particle counter (CPC) (TSI model 3775) with a measuring range from 13.8 to 835.4 nm. As particle number concentration of the generated particles was higher than detection limits of the CPC (N = 107/cm3), a dilutor was used (VKL; Palas, Germany). Additionally, an FMPS (TSI model 3091) was applied to measure PSD at very small time intervals. Within 1 s, a full particle spectum from 5.6 to 560 nm is recorded. For the analysis with SEM/EDX and TEM, generated particles were separated via nanometer aerosol sampler (NAS Modell 3089, TSI Inc., Shoreview, MN, USA).
Computational Fluid Dynamics
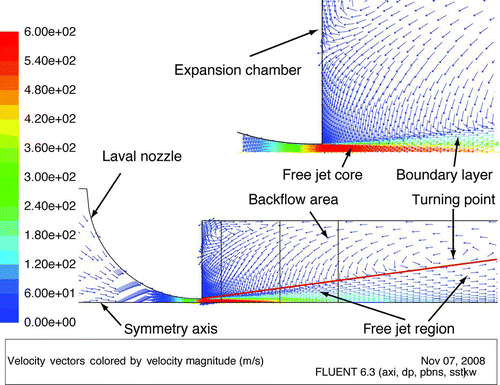
Simulations with CFD (ANSYS FLUENT® 6.3) are used as a visualization tool for the expansion process in nozzle and expansion chamber. Both flow and heat transfer equations were solved. The widely used shear stress transport (SST) k–ω turbulence model with compressible flow turned out to be the most adequate numerical flow model. shows the different flow patterns which appear in the expansion chamber. The expanding free jet can be divided into a core with a constant velocity profile and a boundary layer with a decreasing velocity profile from the center outwards. The boundary layer is expanding with increasing distance from the nozzle until the core disappears completely and a fully developed free jet remains. Due to viscous forces between the expanding and surrounding fluids, a backflow (or eddy) is established in a manner that gas from the backflow re-enters the free jet (see zoom view in ). As can be seen later in the Results and Discussion section, cooling of the expansion chamber walls will significantly impact the temperature in the backflow area and thus the temperature profile in the boundary layer. To adjust our simulation, temperatures of the expansion chamber wall for different settings of the cooling system were measured and integrated in the model. Thereafter, to verify the model predictions, we compared measured temperatures at TI2 with calculated temperatures at the same distance from the nozzle outlet and found a good match of the values.
lists the most important and relevant parameters for the design of nozzle and expansion chamber.
TABLE 2 Relevant design and operating parameter
RESULTS AND DISCUSSION
Particle Generation Process
As expansion of the gas flow with the sublimated material is happening very fast, particle generation takes place in Laval nozzle and expansion chamber. Starting with CuPc, the experiments were carried out first without cooling the chamber walls. shows the typical needlelike growth of CuPc particles. As described before, this pigment has a preferred growth face where molecules out of the gas phase will accumulate. The observed particle sizes are in their lateral dimension (length) in the order of >500 nm and in their longitudinal dimension (wideness) <100 nm.
FIG. 6 Comparison of shape and size of generated copper phthalocyanine particles on the influence of cooled backflow. (a) Needle-shaped CuPc particles without cooling system. (b) SEM picture of grainy-shaped CuPc particles (nanorods) with cooling system. (c) TEM picture of grainy-shaped CuPc particles with cooling system.
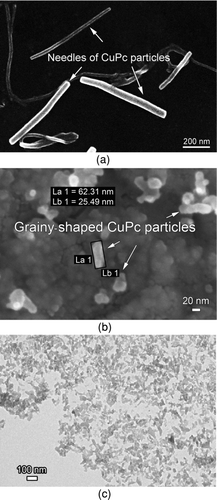
In the second step, we applied the cooling system of expansion chamber walls. Cooling the walls means cooling the backflow (see CFD results of temperature profiles in and ), which enters as a chilled gas flow the boundary layer of the expanding free jet and leads to temperature drops in the free jet down to 160°C. These measured temperatures match very good with the values of the CFD simulation. The thereby generated particles are significantly smaller and particle structure changes from needle to grainy shape ( and ) and particle sizes are below 100 nm in all dimensions.
The cooled backflow inhibits the crystal growth process and shifts the particle size distribution to a nanoscale region (<100 nm). We run numerical flow simulations to see the influence of temperature reduction on the saturation profile in the freejet ( and ). Material properties of CuPc were used to calculate the saturation profile in the expansion chamber. Temperature and thus saturation in the free jet core remains constant but as the temperature in the boundary layer of the free jet is reduced, due to the cooled backflow saturation increases. Still high computational efforts are required to combine particle formation with fluid dynamics in one numerical simulation. Therefore, to keep our model simple and the computational effort moderate, we assumed that no nucleation occurs downstream of the nozzle. So, neither decrease of saturation due to particle formation or latent heat is considered. What we can deduce from simulation results is, in consequence of the radial and axial distribution of the saturation ratio, and the particle formation process will take place preferably in the boundary layer of the free jet. Furthermore, the saturation in the cooled system is significantly higher compared to the noncooled system, especially in the boundary layer. In reality, this leads to higher nucleation rates and therefore to more and smaller clusters which degrade the saturation faster. This leads to smaller particle sizes because the vapor molecule reservoir of the sublimated species is exhausted quicker than it would be for lower saturations. The high values for the saturation profile in the free jet, particularly in the boundary layer, are attributed to the steep incline of the vapor pressure curve of copper phthalocyanine. Vapor pressure for temperatures above sublimation point is relatively high compared to temperatures below sublimation point, where vapor pressure is negligibly small.
Of course, results of the saturation distribution will change significantly if we consider nucleation and latent heat. In a perfect manner, one should consider both homogeneous and heterogeneous nucleation since already generated clusters can be transported back into the free jet by the backflow and serve as nucleation seeds.
The same behavior was observed for Paliogen Red pigments. The needlelike growth if backflow is not cooled is even more distinct as it is for CuPc. In , examples of particles shapes for noncooled and cooled backflow are shown.
FIG. 7 Comparison of temperature and saturation profiles in the expansion chamber based on CuPc material properties. (a) Temperature profile of a noncooled system. (b) Temperature profile of a cooled system. (c) Saturation profile of a noncooled system. (d) Saturation profile of a cooled system.
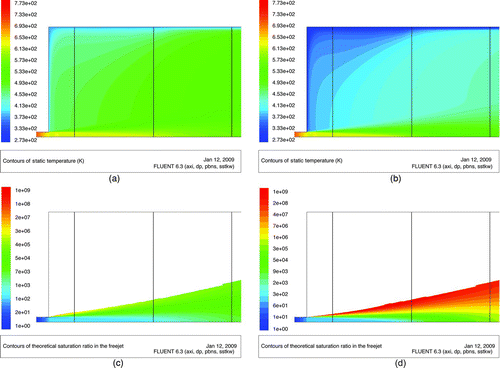
FIG. 8 Comparison of shape and size of generated Paliogen Red particles on the influence of cooled backflow. (a) Long needles of Paliogen Red without cooled backflow. (b) Grainy-shaped Paliogen Red particles with cooled backflow.
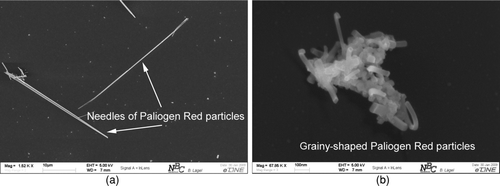
Another difference between generated particles of both pigments is that particle sizes, in particular the longitudinal dimension for Paliogen Red are generally bigger, whether cooling is applied or not independently. A possible explanation for differences in size, assuming constant and comparable operation conditions, is a lower nucleation rate for Paliogen Red compared to CuPc. The nucleation rate J for the critical cluster size r *, i.e., smallest cluster size which is stable after phase transition, can be derived from kinetic gas theory (Becker and Döring Citation1935):
FIG. 9 Free energy of copper phthalocyanine and Paliogen Red. (a) Free energy depending on molecule number in a cluster; peak of graph marks the critical free energy. (b) Critical free energy of both pigments depending on the theoretical saturation.
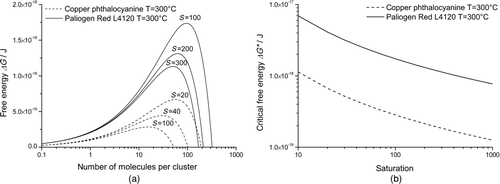
Furthermore, we believe that the subsequent dominant growth process here is particle growth out of the gas phase. Tests with particle agglomerates like that in or showed that agglomerates could be dispersed easily in alcoholic/aqueous solutions.
Particle Size Distribution
As the size and shape of the generated particles depends on temperatures in the free jet, the cooling system of the chamber walls can be used as a control element for manufacturing particles. shows SMPS number size distribution measurements from the generated copper phthalocyanine particles while the temperature in the expansion chamber was decreased stepwise due to the cooling of the backflow area. The reference temperature of the free jet in was measured at TI2. Mode diameters vary from 50 to 100 nm depending on the adjusted temperature in the gas flow and the measurements were repeatable with only slightly differences between the size distributions.
FIG. 10 Particle size distribution measured with SMPS for different temperatures in the free jet at TI2.
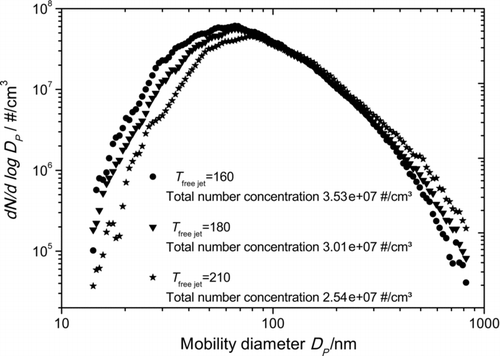
The shift of the PSD emphasizes the assumption of the numerical simulation that lower temperatures in the free jet lead to a higher saturation and therefore to more primary particles whereas a higher number concentration of growable primary particles degrades the saturation in the free jet faster, which results in smaller particle sizes and higher particle number concentrations.
With an FMPS, we were able to demonstrate the influence of a cooled backflow on the PSD. shows the measured size distribution for CuPc of an experimental test run where at the beginning the free jet was not cooled. Approximately, after 75 s, the chamber cooling system was applied and thus temperatures in the free jet start to drop due to the cooled backflow which re-enters the free jet. Starting with a noncooled free jet, a broad particle size distribution develops, which is an indicator for needlelike growth. The point when temperatures begin to decrease, i.e., the cooled gas flow re-enters the boundary layer of the free jet, can be seen clearly. From this moment, the particle size distribution becomes narrower and mode diameters smaller.
FIG. 11 Particle size distributions measured with FMPS are showing the effect of a cooled backflow on the particle size.
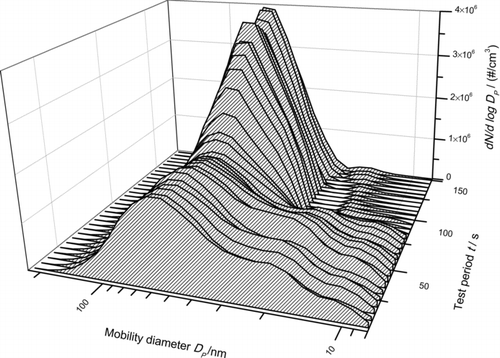
Mobility measurement for particles with shapes very different from spherical shape (aspect ratios » 1) should be interpreted with care. The peak at 20 nm during the period when the free jet is not cooled is caused due to unwanted orientation effects of needle-shaped particles in the internal flow field of the FMPS. The width is then measured instead of length. If backflow is cooled and the particle shape changes toward grainylike (aspect ratios 1), this peak diminishes because these grainy nanoparticles do not orient themeselves in the flow field of the FMPS.
The more pronounced needlelike growth of Paliogen Red in combination with the orientation of particles in the internal flow field of mobility analyzers reinforces the effect of apparently smaller particles with higher temperatures in the free jet. The measured size distributions conflict with electron microscopy pictures and therefore no meaningful PSD of Paliogen Red could be recorded. A different measurement approach has to be considered for future experiments to measure the particle size distribution and avoid such effects.
EDX Analysis of Generated Particles
From the microscopy analysis and PSD measurements, we saw that starting from cubic-shaped raw material, nanoscale particles are generated by gas to particle formation. As mentioned before, those materials are sensitive to decomposition processes. High temperatures coupled with long residence time could damage the material. To verify that the generated particles did not decompose during the phase transition, we used EDX which allows the elemental composition and distribution of materials to be measured.
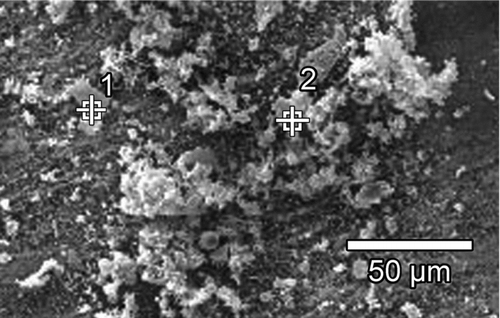
Notwithstanding the fact that detection of hydrogen with EDX is nearly impossible and for lighter elements (e.g., carbon and oxygen) is quantitatively not very reliable, EDX offers a possibility to indicate if decomposition takes place. shows the SEM picture of an analyzed CuPc particle agglomerate and the corresponding element distribution. The numbers in the SEM picture indicate where the X-ray beam of the EDX was focused.
If decomposition is occurring, a good indicator that those decomposition effects are little is the ratio of measured chlorine since the binding forces of chlorine are the weakest and would decompose first. Carbon has the highest mass specific ratio followed by nitrogen, chlorine, and copper. Considering the measurement uncertainty of EDX and the structural formula of , the measured element distribution meets qualitatively the ratio of elements in a copper phthalocyanine-chlorinated molecule. Besides the detection of elements which appear in the molecule, a relatively high ratio of other elements was measured. These other components are impurities due to unavoidable short exposures to ambient conditions and the coated filter material where the sample was placed (e.g., O2; F; Si).
TABLE 3 EDX element distribution of generated CuPc particle agglomerates
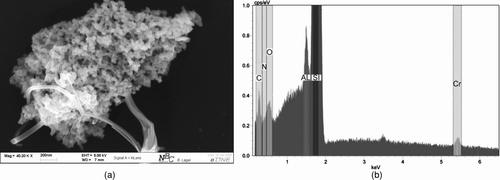
In the same manner, Paliogen Red particle agglomerate () was also analyzed by EDX. Due to different SEM-EDX systems, the element distribution in is expressed in cps (or counts per second). Again, all elements (except hydrogen) could qualitatively be measured. Thereby, carbon exhibits the highest peak followed by oxygen and nitrogen according to the structural and chemical formulas (). However, silicon and traces of other metals are measured since the sample was placed here for analysis on a silicon wafer.
FIG. 13 EDX mapping of Paliogen Red. (a) SEM picture of generated and analyzed Paliogen Red particle agglomerate. (b) EDX Element distribution from Paliogen Red.
A secondary indicator for both pigments is that visual testing of the pigment color with the naked eye results in the same color for bulk material and generated particles. Targeted tests for generated particles which underwent a high degree of decomposition showed that those pigments own a visible grayish colorshade. We are persuaded that decomposition effects during the sublimation and desublimation processes of copper phthalocyanine and Paliogen Red are minimal even though it could not be fully excluded. For further investigations, other analysis techniques (e.g., X-ray diffraction [XRD] or Fourier transform infrared spectroscoy [FTIR]) have to be considered to substantiate the point that decomposition is little.
SUMMARY AND CONCLUSION
A novel gas to particle process for generating organic nanoscale particles was presented. Two organic pigments, copper phthalocyanine chlorinated and Paliogen Red L4120 have been investigated. The particle generation takes place due to adiabatic expansion in a Laval nozzle and the subsequent expansion of the free jet in an expansion chamber. This chamber is equipped with a cooling system to control temperatures in the free jet. We could demonstrate by means of particle size measurements and electron microscopy that without cooling both pigments exhibit a needlelike crystal growth. Those particles are not nanoscale in their longitudinal length. Cooling the backflow of the free jet suppresses crystal growth because more growable clusters are formed, which decrease the saturation in the system faster. Particles become smaller and in contrast to the needle-shaped particles, these grainy-shaped particles are nanoscale in all dimensions. Electron microscopy and EDX were used to characterize shape and material composition of generated organic particles.
Generally, the generated particles show a different shape and size compared to their bulk material whether the cooling system is used or not independently. A uniform particle shape whether needlelike or grainy nanoparticles could be obtained due to the controlled crystal growth during the gas to particle conversion. Controlling temperatures in the free jet, in particular temperatures in the boundary layer represent a parameter for controlling particle sizes with the smart advantage of a cooling process without diluting the gas–particle system. Different techniques for future PSD measurements have to be tested to avoid unwanted effects of oriented flow of particles with high aspect ratios in mobility analyzers.
REFERENCES
- Alfredssona , Y. , Åhlunda , J. , Nilsona , K. , Kjeldgaardb , L. , O’Sheac , J. , Theobaldc , J. , Baoa , Z. , Mårtenssona , N. , Sandella , A. , Puglia , C. and Siegbahn , H. 2005, December . Phase and Molecular Orientation in Metal-Free Phthalocyanine Films on Conducting Glass: Characterization of Two Deposition Methods . Thin Solid Films , 493 ( 1–2 ) : 13 – 19 .
- Banfield , J. F. , Welch , S. A. , Zhang , H. , Ebert , T. T. and Penn , R. L. 2000 . Aggregation-Based Crystal Growth and Microstructure Development in Natural Iron Oxyhydroxide Biomineralization Products . Science , 4 : 751 – 754 .
- Barrett , J. C. and Clement , C. F. 1988 . Growth Rates for Liquid Drops . J. Aerosol Sci. , 19 ( 2 ) : 224 – 242 .
- Bayazitoglu , Y. , Brotzen , F. R. and Zhang , Y. 1996 . Metal Vapor Condensation in a Converging Nozzle . Nanostruct. Mater. , 7 ( 7 ) : 789 – 803 .
- Becker , R. and Döring , W. 1935 . Kinetische Behandlung der Keimbildung in Übersättigten Dämpfen . Ann. Phys. , 416 ( 8 ) : 719 – 752 .
- Cölfen , H. and Mann , S. 2003 . “ Higher-Order Organization by Mesoscale Self-Assembly and Transformation of Hybrid Manostructures ” . In Angewandte Chemie International Edition Vol. 42 , 2350 – 2365 .
- Dettmer , R. 1997 . Thermodynamische Betrachtungen zur Kondensation , Fachbereich Chemie : Philipps-Universität Marburg/Lahn, Marburg, Germany . Ph.D. thesis
- El-Nahass , M. M. , Bahabri , F. S. , Ghamdi , A. A. A. and Al-Harbi , S. R. 2002 . Structural and Transport Properties of Copper Phthalocyanine (CuPc) Thin Films . Egypt. J. Sol. , 25 ( 2 ) : 307 – 321 .
- Fladerer , A. 2002 . Keimbildung und Tröpfchenwachstum in Übersättigtem Argon-Dampf , Universität Köln, Colone, Germany . Ph. D. thesis
- Friedlander , S. K. 1998 . “ Synthesis of Nanoparticles and Their Agglomerates: Aerosol Reactors ” . In R&D Status and Trends in Nanoparticles, Nanostructured Materials, and Nanodevices in the United States , International Technology Research Institute, Los Angeles, CA, USA .
- Friedlander , S. 2000 . Smoke, Dust and Haze , 2nd ed. , New York : Oxford University Press .
- Hao , Z. and Iqbal , A. 1997 . Some Aspects of Organic Pigments . Chem. Soc. Rev. , 26 : 203 – 213 .
- Helfgen , B. , Türk , M. and Schaber , K. 2000 . Theoretical and Experimental Investigations of the Micronization of Organic Solutes by Rapid Expansion of Supercritical Solutions . J. Powder Technol. , 110 : 22 – 28 .
- Hertel , D. , Müller , C. D. and Meerholz , K. 2005 . Bilderzeugung Organische Leuchtdioden . Chem. Unserer Zeit , 39 : 336 – 347 .
- Jennings , S. G. 1988 . The Mean Free Path in Air . J. Aerosol Sci. , 19 ( 2 ) : 159 – 166 .
- Kodde , M. 1996 . Partikelbildung durch Desublimation infolge direkter Kühlung , Institut für Verfahrenstechnik, Universität Hannover, Hannover, Germany . Ph.D. thesis
- Kulmala , M. and Vesala , T. 1991 . Condensation in the Continuum Regime . J. Aerosol Sci. , 22 ( 3 ) : 337 – 346 .
- Liu , P. , Ziemann , P. , Kittelson , D. B. and McMurry , P. H. 1995 . Generating Particle Beams of Controlled Dimensions and Divergence: II. Experimental Evaluation of Particle Motion in Aerodynamic Lenses and Nozzles Expansions . Aerosol Sci. Technol. , 22 : 314 – 324 .
- Lothe , J. and Pound , G. M. 1962 . Reconsiderations of Nucleation Theory . J. Chem. Phys. , 36 ( 8 ) : 2080 – 2085 .
- Mather , R. R. 1999 . The Effect of Crystal Properties on the Manufacture and Application Performance of Copper Phthalocyanine Pigments . J. Porphyrins Phthalocyanines , 3 : 643 – 646 .
- McBride , D. D. , Sherman , P. M. and Pierce , T. H. 1971 . Condensation of Zinc Vapor in a Supersonic Carrier Gas Dynamics . Appl. Sci. Res. , 25 : 83 – 96 .
- Michaelis , W. 2003 . Einfluß der Wachstumsbedingungen auf Morphologie,Struktur und Elektrische Eigenschaften von Organischen Halbleiterfilmen , FachbereichBiologie/Chemie, Universität Bremen, Bremen, Germany . Ph.D. thesis
- Mohammed , K. A. and Collins , R. A. 1986 . Phase Behaviour of Copper and Zinc Phthalocyanines . Thermochim. Acta , 104 : 377 – 381 .
- Prabakaran , R. , Kesavamoorthy , R. , Reddy , G. L. N. and Xavier , F. P. 2002 . Structural Investigation of Copper Phthalocyanine Thin Films Using X-Ray Diffraction, Raman Scattering and Optical Absorption Measurements . Phys. Status Solidi b , 229 ( 3 ) : 1175 – 1186 .
- Pratsinis , S. E., and Vemury , S. 1996 . Particle Formation in Gases: A Review . Powder Technol , 88 : 267 – 273 .
- Roberts , L. C. , Lewis , E. R. , Wilson , S. A. and Losee , D. L. 1999, February . Forming Pigment Color Filter Arrays . U.S Patent Document: 5874188
- Saito , Y. , Mihama , K. and Noda , T. 1983, November . Formation of Lead Clusters in Supersonic Nozzle Expansion: Effect of Nozzle Geometry . Jpn. J. Appl. Phys. , 22 ( 11 ) : 715 – 717 .
- Sane , A. , Taylor , S. , Sun , Y.-P. and Thies , M. C. 2003 . RESS for the Preparation of Fluorinated Porphyrin Nanoparticles . Chem. Commun. (Camb.) , 7 : 2720 – 2721 .
- Sharp , J. H. and Abkowitz , M. 1973 . Dimeric Structure of a Copper Phthalocyanine Polymorph . J. Phys. Chem. , 77 ( 4 ) : 477 – 481 .
- Türk , M. 2001 . Erzeugung von organischen Nanopartikeln mit überkritischen Fluiden , Habilitationsschrift : Universität Karlsruhe (TH), Karlsruhe, Germany .
- Türk , M. , Hils , P. , Helfgen , B. , Schaber , K. , Martin , H.-J. and Wahl , M. 2002 . Micronization of Pharmaceutical Substances by Rapid Expansion of Supercritical Solutions (RESS): A Promising Method to Improve the Bioavailability of Poorly Soluble Pharmaceutical Agents . J. Supercrit. Fluids , 22 ( 1 ) : 75 – 84 .
- Vere , A. 1987 . Crystal Growth: Principles and Progress , New York : Plenum Press .
- Voigt , T. 2010 . Maßnahmen zur kontrollierten Generierung von organischen Nanopartikeln in einer übersättigten Gasströmung , University of Kaiserslautern, Kaiserslautern, Germany . Ph.D. thesis
- Volmer , M. and Weber , A. 1926 . Keimbildung in Übersättigten Gebilden . Z. Phys. Chem. , 119 : 277 – 301 .
- Wagner , A. 1999 . Partikelbildung durch Desublimation in einer adiabaten expandierenden Gasströmung. Ph.D. thesis , Institut für Verfahrenstechnik, Universität Hannover, Hannover, Germany .
- Wagner , A. and Mewes , D. 1999 . Erzeugen feinster fester Partikel durch Desublimation in einer adiabaten expandierenden Gasströmung . Chemie Ingenieur Technik , 71 ( 6 ) : 598 – 601 .
- Wood , S. , Baker , M. and Calhoun , D. 2001 . New Model for the Vapor Growth of Hexagonal Ice Crystals in the Atmosphere . J. Geophys. Res. , 106 : 4845 – 4870 .
- Xu , C. , Sun , X. , Dong , Z. , Yu , M. , My , T. , Zhang , X. , Chua , J. and White , T. 2004 . Zinc Oxide Nanowires and Nanorods Fabricated by Vapour-Phase Transport at low Temperature . Nanotechnology , 15 : 839 – 842 .
- Zhang , C. and Harrington , J. Y. 2010 . A Unified Theory for Including Surface Kinetic Effects on Ice Vapor Growth in Cloud Models . Am. Meteorol. Soc.—Extended Abstracts ,