Abstract
Reactive oxygen species (ROS) have been related to adverse health effects in recent years. Previous studies have reported ROS concentrations in mainstream smoke, but the reports have shown considerable variability and conclusions. There have been no prior measurements on sidestream smoke. In this study, the amounts of gas-phase and particle-bound ROS in tobacco smoke were determined using 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) as the fluorescent probe with hydrogen peroxide as the standard. Both research and commercially available cigarettes were tested using mainstream and sidestream smoke generated by a Single Cigarette Smoking Machine. For mainstream smoke from regular and light cigarettes, the total quantities of ROS were 120–150 nmol and 90–110 nmol, respectively. For sidestream smoke, the values were 60–90 nmol and 30–70 nmol for regular and light cigarettes, respectively. The effects of the cigarette filter on the emissions were to reduce the particle mass and particle-phase ROS in the mainstream smoke.
Copyright 2012 American Association for Aerosol Research
1. INTRODUCTION
Reactive oxygen species (ROS) are chemical constituents with oxygen atoms that are highly reactive in the ambient air, can cause respiratory problems, and produce adverse health effects in humans. It has been defined to include oxygen-centered or related free radicals such as hydroxyl (·OH), hydroperoxyl (HOO·), alkoxyl (RO·), and organic peroxy radicals (ROO·); ions such as superoxide (O2 −), hypochlorite (ClO−), and peroxynitrite (ONOO−); and molecules such as hydroperoxide (ROOH) and organic peroxide (ROOR′). It is a new and promising research field in atmospheric science.
ROS are generated during normal cell metabolism in human body and are essential to life. These ROS are involved in many kinds of biological functions, e.g., vascular smooth muscle function. ROS can also be harmful (Gomes et al. Citation2005). In the ambient air, both gas-phase and particle-bound ROS can be generated through combustion and other atmospheric chemical processes. Particle-bound ROS inside the human body can be classified as endogenous that is generated by the inhaled particulate matter (PM) in vivo and exogenous that is transported into the lungs on respirable particles.
Although ROS could produce adverse health effects, these species represent a broad suite of constituents that are difficult to individually quantify and limited work has been undertaken to date to characterize them (Pavlovic and Hopke Citation2011). However, ROS can be considered collective rather than individual species. Since the delivery of oxidants into the lungs will have relatively similar health effects, this approach focuses on the shared oxidative characteristics and, therefore, allows a general analytical procedure to be applied. At present, the most common ROS measurement approach is a manual method involving a fluogenic probe that responds to the oxidants (Hung and Wang Citation2001; Mudway et al. Citation2004; Huang et al. Citation2005; Venkatachari et al. Citation2005a, Citation2005b). This approach uses sample collection for a period of time and subsequent analysis (Venkatachari and Hopke Citation2008).
Different fluorescent probes have been used to detect ROS. A good probe should have the ability to respond to most oxidative functional groups including the major ones in the atmosphere and not react with non-ROS. Moreover, the probe should be stable over time. Common probes include 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA), dihydrorhodamine 123 (DHR-123), dihydrorhodamine 6G (DHR 6G), 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, (2S, 3S)-1, 4-bis-sulfanylbutane-2,3-diol (DTT), and p-hydroxyphenylacetic acid (POHPAA). These oxidant-sensitive fluorescent probes can be oxidized from their reduced (nonfluorescent) form to an oxidized (fluorescent) form. Although conditions vary, DCFH has been chosen in many cases and provided reproducible results (Hung and Wang Citation2001; Huang et al. Citation2005; Venkatachari et al. Citation2005a, Citation2007; See et al. Citation2007; Venkatachari and Hopke Citation2008). This reagent was used in this study based on its measured broad response to potential oxidants (Venkatachari and Hopke Citation2008).
Combustion and photochemical reactions are two main sources for environmental ROS (Huang et al. Citation2005). Hasson and Paulson (Citation2003) detected hydroperoxide concentrations in both the gaseous phase and particulate phase (May–August 2001) at 0.5–3.5 ppbv and 0–13 ng/m3, respectively. Kang et al. (Citation2002) reported 0.104 ± 0.068 ppbv of gas-phase hydrogen peroxide in the summer in downtown Seoul, Korea. In the past research, people have studied various combustion sources, e.g., vehicles (Hung and Wang Citation2001), incense burning (Kao and Wang Citation2002), and cigarettes (Huang et al. Citation2005; Ou and Huang Citation2006). Both gas phase and PM (mainly tar) are generated in cigarette smoke and both contain radical species (Pryor Citation1997). In addition, both phases may be harmful to humans because of their strong oxidizing properties and the ability to induce oxidative stress in an organism (Church and Pryor Citation1992; Penn and Snyder Citation1993).
2. MATERIALS AND METHODS
2.1. Preparation of Fluorescent Probes and Standards
The fluorescent probe used in this study was DCFH. A 1 mM stock solution was prepared by dissolving 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Calbiochem, CA, USA) into ethyl alcohol (ACS grade, Pharmo, CT, USA). A 10 mL solution was mixed with 40 mL 0.01 M sodium hydroxide (NaOH) and left in a dark at room temperature for 30 min to hydrolyze. Then 200 mL of phosphate buffer produced by mixing sodium phosphate dibasic (Na2HPO4, Sigma Aldrich, MO, USA) with sodium dihydrogen phosphate anhydrous (NaH2PO4, Fluka, Germany) to achieve a pH of 7.2 was added to the solution. Horseradish peroxidase (HRP, Sigma Aldrich, MO, USA) was used as the catalyst with a concentration of 0.5 units/mL. The final DCFH concentration of this working solution was 5 μM.
Equivalent H2O2 concentration was used to express the ROS concentrations by converting fluorescence intensity using a standard H2O2 calibration curve. Four H2O2 standards with the concentrations of 1.0, 2.0, 3.0, and 4.0 × 10−7 nmol were prepared by mixing 0.1 mL hydrogen peroxide (ACS grade, Sigma Aldrich, MO, USA) with 3 mL DCFH–HRP working solution. Standard blanks were obtained by mixing 0.1 mL deionized Milli-Q water (resistivity >18.2 MΩ) with probe. The standards were placed in cuvettes and incubated at 37°C in a water bath. Formation of 2,7-dichlorofluorescein was monitored by measuring fluorescence (excitation wavelength: 490 nm; emission wavelength: 515 nm) using a Turner QuantechTM Digital Filter Fluorimeter (model # FM109535, Barnstead Thermolyne, IA, USA).
2.2. Sampling System
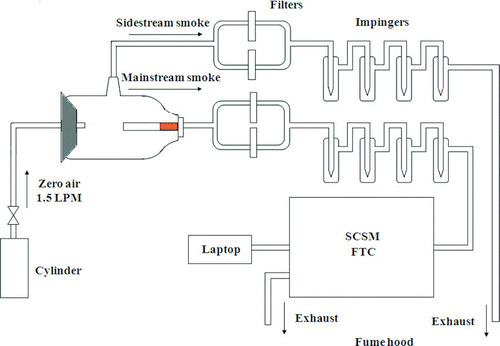
A computer-controlled Single Cigarette Smoking Machine (SCSM, CH Technologies) was used to generate mainstream cigarette smoke under standard smoking conditions (cigarettes burn for 8–9 min with a 2-s, 35-mL puff every minute) according to the Federal Trade Commission (FTC) protocol (Bradford et al. Citation1936; Ogg Citation1964; International Standards Organization [ISO] 2000). A custom-made glass collection system was used for sidestream with the cigarette burning inside and connected to the SCSM. A flow of 1.5 LPM commercial zero air containing less than 0.1 parts per million of total hydrocarbons was introduced to the system to support the cigarette burning and dilute the sidestream smoke. Four impingers were filled with 10 mL DCFH–HRP solution and used to collect gas-phase ROS for mainstream and sidestream, respectively. The experimental system is shown schematically in .
Research cigarettes 3R4F and 1R5F from the University of Kentucky and commercial brands including Marlboro (red), Marlboro (gold), and Camel were tested. As suggested by the Kentucky Tobacco Research & Development Center (KTRDC), all of the cigarettes were kept sealed and stored in a refrigerator (4°C, 50%–60% relative humidity) before use. Prior to the experiments, the cigarettes were taken out and left at room temperature (24°C, 60% relative humidity) for 15 min and then immediately burned. Among the commercial cigarette brands, Marlboro (red) and Camel are considered to be regular cigarettes, while Marlboro (gold) is classified as light cigarettes. For the reference cigarettes obtained from the University of Kentucky, 1R5F research cigarette is described as containing ultra-low nicotine. 3R4F was made to replace 2R4F that is now out of stock as a low-nicotine type (Mottier and Jeanneret Citation2011). Moreover, Marlboro (red) cigarettes were used to determine filter effect by measuring the ROS produced by cigarettes after the filters were removed.
Mainstream, particle-bound ROS was collected on two preweighed PTFE (polytetrafluoroethylene) filters (Whatman, Piscataway, NJ, PTFE 46.2 mm, 2 μm pore size). Particle-phase ROS in the sidestream smoke was sampled with two preweighed quartz filters (Pall, Port Washington, NY, Tissuquartz 47 mm, 2 μm pore size) because of the relatively large amount of PM mass in the sidestream smoke tended to clog PTFE filters. Similarly, mainstream smoke from the cigarettes without the filters also clogged the PTFE filters and quartz filters were used for these experiments.
2.3. Sample Analysis
Subsequent to sampling, 3 mL of the reagent solution was removed from each impinger (each contains 10 mL DCFH working solution), placed into a cuvette, and incubated for 15 min at 37°C in water bath. Generally, the fluorescence intensities of the solutions in impingers were within the range of standards. After using the volume of the solution to get the amount of ROS in each impinger, the contents of all four impingers were combined. The filters were weighed and then placed face down into beakers and filled with fluorescent probe solution. A Branson B5510DTH Ultrasonic bath (Branson, Danbury, CT) was set to 37°C and was used to sonicate and incubate the particle sample for 15 min. An aliquot of the solution was taken from each beaker and the fluorescence intensity was measured. Sampling blanks were obtained by operating the smoking system without any cigarette burning and analyzed in the same way. Sampling blank values were subtracted from sample results. If the values were found to exceed the calibration range, both the samples and the sampling blank were diluted by the same factor and measured again.
3. RESULTS
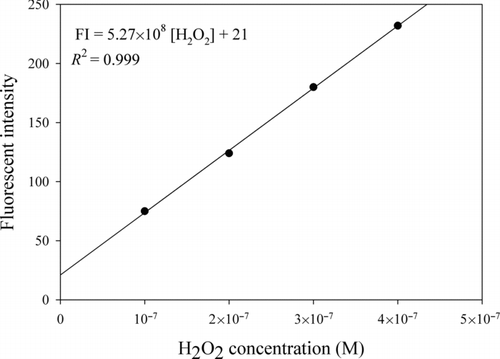
3.1. H2O2 Calibration Assay
shows the calibration relationship between the fluorescent intensity and standard H2O2 concentration (R 2 = 0.999). The reactivity of ROS in the samples was expressed as an equivalent H2O2 concentration.
3.2. ROS in Mainstream and Sidestream Cigarette Smoke
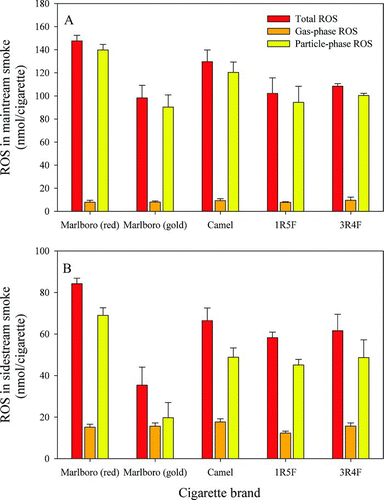
The concentrations of ROS detected in both mainstream and sidestream smoke in nanomoles of H2O2 are presented in . At least five replicate measurements were made for each brand reported. The ROS detected in the mainstream smoke of regular and light cigarettes were 120–150 nmol and 90–110 nmol, respectively. For sidestream smoke, the ROS values were 60–90 nmol and 30–70 nmol, respectively, for regular and light cigarettes. Thus, more ROS was detected in mainstream smoke than in sidestream smoke irrespective of brand as shown in and b.
TABLE 1 ROS detected in mainstream and sidestream smoke
FIG. 3 ROS detected in (a) mainstream smoke and (b) sidestream smoke. (Color figure available online.)
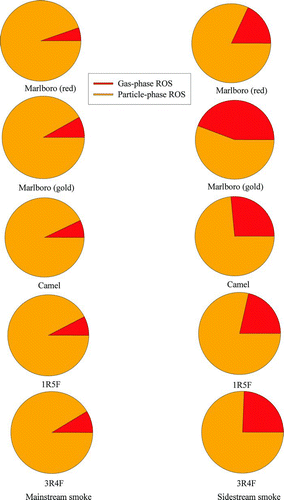
The “light” cigarettes had lower ROS concentrations. For the commercial light cigarettes (Marlboro [gold]), the particulate ROS values in both mainstream and sidestream smoke were substantially lower. For the reference cigarettes, lower particle-phase ROS values were measured in the mainstream smoke. However, the light cigarette sidestream smoke ROS values were similar to Camels.
The ROS concentrations in the particle phase were much greater than that in gas phase for both mainstream and sidestream smoke. The gas-phase/particle-phase ROS partitioning ratios for mainstream smoke in regular and light cigarettes () were ∼0.06–0.08 and 0.22–0.36, respectively. The corresponding values for sidestream smoke were 0.08–0.1 and 0.27–0.79 for regular and light cigarettes, respectively. Thus, the particle phase contained the major fraction of the generated ROS.
In general, the ROS amounts in different brands were in relative narrow ranges. Given the control over the combustion conditions by the smoking machine, it may not be surprising that the resulting ROS values were quite similar.
3.3. ROS Concentration in PM Mass
ROS concentrations in PM mass were calculated using the measured particle-phase ROS and PM mass (). For mainstream smoke, approximately 10 mg of PM mass was collected per cigarette. There were no significant differences in mass concentrations between regular brands (10.7 mg) and light brands (10.9 mg). The ROS concentrations were in the range of 10.3–11.1 nmol/mg. Thus, the mass concentrations were very similar. Sidestream smoke was more variable in ROS mass concentration. Only 0.8 nmol of H2O2 equiv./mg was found for Marlboro (gold) as a representative light cigarette. This concentration was ∼25% of the 3.1 nmol/mg in Marlboro (red). Thus, light cigarettes produce 1/4 to 1/3 of the ROS concentration in their sidestream smoke relative to regular brands.
By comparing the ROS mass concentrations from mainstream and sidestream smoke, it can be seen that despite twice as much PM mass being collected in sidestream smoke, the ROS concentrations were much less than from mainstream smoke.
3.4. Effect of Cigarette Filters
To determine the effect of the cigarette filter on the removal of PM mass and/or ROS in mainstream smoke, the filters of Marlboro (red) cigarettes were removed. These unfiltered cigarettes were then tested in the same manner as the filtered cigarettes. As shown in , the filters eliminated 52.0% PM mass and 17.1% particle-phase ROS in mainstream smoke (data from a minimum of five repeats). Since most of the generated ROS was found to be in particle phase, the cigarette filters reduced the inhaled PM mass and ROS. However, the filters only produced a small reduction in the particle-phase ROS.
These results show that the filter reduced the gas-phase ROS by 26.5%. However, given that gas-phase ROS represented a relatively small fraction of the total ROS, this reduction is relatively negligible compared with the total aerosol ROS. Also, the observed reduction could be measurement variability because the unfiltered gas-phase ROS amount was small. Any variations in the measurements might lead to large apparent differences.
Since the fraction of particle-phase ROS removed by the filters was less than the PM mass removed, the concentrations of ROS in PM mass increased with the use of the filter.
4. DISCUSSION
Huang et al. (Citation2005) measured the amount ROS in mainstream smoke using 2,7-dichlorofluorescin as the fluorescent probe as was done in this study. They detected gas-phase ROS with impingers, collected total suspended particles with 37 mm polycarbonate filters, and sampled PM2.5 with a PEM PM2.5 sampler (Personal Environmental Monitor PM2.5, SKC, PA, USA). Using three commercial brand cigarettes, they found mainstream smoke contained 18.64–54.81 nmol H2O2/L in which gas-phase ROS represented 71.21%–85.99% with the concentration of 14.32–39.03 nmol H2O2/L. They also concluded that cigarette filters are not effective in removing ROS, while this study shows that filtration is effective to reduce certain amounts. They detected more ROS in gas phase. Thus, it was understandable that filters were not very effective to remove ROS in this phase. However, depending on the pore size of the polycarbonate filters used in their study, there could be substantial penetration of the smoke particles through the filter pores (Spurny et al. Citation1969a, Citation1969b) resulting in an underestimation of the particle-bound ROS. In the present study, the particulate phase was the major source of ROS and it was reasonable that filters could eliminate particle-bound ROS and PM mass. By detecting ROS concentration in tobacco leaves and cigarette ash, Huang et al. (Citation2005) concluded that mainstream ROS was primarily generated by the combustion process.
Miljevic et al. (Citation2010) measured the combined mainstream and sidestream smoke together using a novel profluorescent nitroxide probe, 9-(1,1,3,3-tetramethylisoindolin-2-yloxyl-5-ethynyl)-10-(phenylethynyl) anthracene (BPEAnit). The cigarettes they used were Kentucky 3R4F research cigarettes. They reported 101 ± 29 nmol ROS/cigarette as the total concentration in which 41 ± 7 nmol ROS/cigarette for gas phase and 61 ± 30 nmol ROS/cigarette for particle phase. A sidestream smoke concentration of 50 ± 2 nmol per mg of PM was provided that they believed to be decreased with the aging of the PM. The mainstream smoke results were comparable to ours but with a different gas/particle partitioning ratio. However, the BPEAnit probe mainly traps radicals such as carbon-centered radicals, peroxyl radicals, and hydroxyl radicals. It could also oxidize transition metals such as Cu+ and Fe2+. Carbon-centered radicals could react with oxygen to produce ROO and Fenton reactions have contribution to ROS generation, but the efficiency of these reactions in ambient air is not clear. As it describes in Bernhard et al. (Citation2005), transition metals such as Cu exist in cigarette smoke so the BPEAnit probe may also react with such metals other than ROS. As a result, since the probe reacts with carbon-centered radicals and transition metals directly, it may lead to overestimation by including non-ROS or underestimation by missing actual ROS in the reported ROS concentrations. Moreover, the probe may also miss ionic and molecule ROS.
TABLE 2 ROS concentration in PM mass
TABLE 3 Cigarette filter effect
TABLE 4 Particle-bound ROS concentrations measured in previous studies
In their sidestream smoke experiments, they lit a cigarette and let it smolder in a chamber that meant the cigarette did not burn because of a flow through it. Since there was no mainstream smoke, the sample they collected cannot be simply defined as sidestream smoke since puffing a cigarette could affect both the amount and nature of the smoke produced.
Ou and Huang (Citation2006) detected a concentration of 391 nmol of ROS/cigarette in mainstream using 2R4F research cigarette under FTC protocol. They used DHR 6G as the fluorescent probe which was found to be reactive with radicals (e.g., R·, RO·, ROO·) but not H2O2 (Flicker and Green Citation2001). They also report burley tobacco cigarette has 10 times higher ROS content than bright tobacco cigarette.
Flicker and Green (Citation2001) used a nitroxide trap, 2-amino-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (3AP), to trap gas-phase carbon-centered radicals (R·). On the basis of their experiments, they found 54 ± 2 nmol/cigarette from Marlboro (red). However, they did not follow the FTC protocol as was done in the present study. Bartalis et al. (2007, 2009) also focused on radicals in cigarette smoke using a method similar to that of Flicker and Green (Citation2001). They identified 7 acyl and 11 alkylaminocarbonyl radicals and reported ∼225 nmol (1.4 × 1017 radicals) from the 2R4F and 168–245 from single tobacco type cigarettes using the FTC protocol. Since these studies mainly detected ROS-related radicals but not all of the possible ROS, their results cannot be directly compared with the values reported here.
summarized the particle-bound ROS concentration measured in previous ambient aerosol studies (Hung and Wang Citation2001; Venkatachari et al. Citation2005a, Citation2005b, Citation2007; See et al. Citation2007; Wang et al. in press) in units of equivalent nmol H2O2 per cubic meter air. According to respiratory study, normal human breathing rate is ∼12 breaths per minute with approximately half a liter air inhaled at once (Wilkerson Citation2001). Thus, the ROS concentrations could be converted to the ROS amount inhaled by human breath per hour (also shown in the table). Combined with the data from this study, we can estimate the length of human exposure to ambient air that is equivalent to smoking or being exposed to the sidestream smoke of a Marlboro (red) cigarette. Thus, if one were to continuously breathe ambient air for a total of 2–3 days, the resulting ROS exposure would be equivalent to smoking one Marlboro (red) cigarette. Air heavily impacted by traffic is more polluted (See et al. Citation2007), and one day of continuous ambient air exposure is equivalent to smoking one cigarette.
Acknowledgments
The authors want to thank Dr. Oberdorster, Bob Gelein, Pamela Wade-Mercer, and Dr. Rahman from the University of Rochester for their help on the preliminary work in this study. This work was supported by US Environmental Protection Agency's Science to Achieve Results (STAR) Program through a subcontract from the University of Rochester PM and Health Center grant RD832415. Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency, it has not been subjected to the Agency's required peer and policy review and, therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
REFERENCES
- Bartalis , J. , Chan , W. G. and Wooten , J. B. 2007 . A New Look at Radicals in Cigarette Smoke . Anal. Chem. , 79 : 5103 – 5106 .
- Bartalis , J. , Zhao , Y.-L. , Flora , J. W. , Paine , J. B. and Wooten , J. B. 2009 . Carbon-Centered Radicals in Cigarette Smoke: Acyl and Alkylaminocarbonyl Radicals . Anal. Chem. , 81 : 631 – 641 .
- Bernhard , D. , Rossmann , A. and Wick , G. 2005 . Metals in Cigarette Smoke . IUBMB Life , 57 : 805 – 809 .
- Bradford , J. A. , Harlan , W. R. and Hanmer , H. R. 1936 . Nature of Cigarette Smoke. Technique of Experimental Smoking . Ind. Eng. Chem. , 28 : 836 – 839 .
- Church , D. F. and Pryor , W. A. 1992 . “ The Oxidative Stress Placed on the Lung by Cigarette Smoke ” . In Lung Injury , Edited by: Crystal , R. G. and West , J. B. 215 – 219 . New York : Raven Press .
- Flicker , T. M. and Green , S. A. 2001 . Comparison of Gas-Phase Free-Radical Populations in Tobacco Smoke and Model Systems by HPLC . Environ. Health Perspect. , 109 : 765 – 771 .
- Gomes , A. , Fernands , E. and Lima , J. L. F. C. 2005 . Fluorescence Probes Used for Detection of Reactive Oxygen Species . Biochem. Biophys. Methods , 65 : 45 – 80 .
- Hasson , A. S. and Paulson , S. E. 2003 . An Investigation of the Relationship Between Gas-Phase and Aerosol-Borne Hydroperoxides in Urban Air . J. Aerosol Sci. , 34 : 459 – 468 .
- Huang , M. F. , Lin , W. L. and Ma , Y. C. 2005 . A Study of Reactive Oxygen Species in Mainstream of Cigarette . Indoor Air , 15 : 135 – 140 .
- Hung , H. F. and Wang , C. S. 2001 . Experimental Determination of Reactive Oxygen Species in Taipei Aerosols . J. Aerosol. Sci. , 32 : 1201 – 1211 .
- International Standards Organization (ISO) . 2000 . Routine Analytical Cigarette-Smoking Machine—Definitions and Standard Conditions ISO 3308, ISO, Case postale 56, CH-1211 Geneva 20 http://www.iso.ch
- Kang , C.-M. , Han , J.-S. and Sunwoo , Y. 2002 . Hydrogen Peroxide Concentrations in the Ambient Air of Seoul, Korea . Atmos. Environ. , 36 : 5509 – 5516 .
- Kao , M. C. and Wang , C. S. 2002 . Reactive Oxygen Species in Incense Smoke . Aerosol Air Qual. Res. , 2 : 61 – 69 .
- Miljevic , B. , Fairfull-Smith , K. E. , Bottle , S. E. and Ristovski , Z. D. 2010 . The Application of Profluorescent Nitroxides to Detect Reactive Oxygen Species Derived from Combustion-Generated Particulate Matter: Cigarette Smoke—A Case Study . Atmos. Environ. , 44 : 2224 – 2230 .
- Mottier , N. and Jeanneret , F. 2011 . Evaluation of Two Derivatization Reagents for the Determination by LC-MS/MS of Ammonia in Cigarette Mainstream Smoke . J. Agric. Food Chem. , 59 : 92 – 97 .
- Mudway , I. S. , Stenfors , N. , Duggan , S. T. , Roxborough , H. , Zielinski , H. Marklund , S. L. 2004 . An In Vitro and In Vivo Investigation of the Effects of Diesel Exhaust on Human Airway Lining Fluid Antioxidants. . Arch. Biochem. Biophys , 423 : 200 – 212 .
- Ogg , C. L. 1964 . Determination of Particulate Matter and Alkaloids (as Nicotine) in Cigarette Smoke . J. Assoc. Off. Agric. Chem. , 47 : 356 – 362 .
- Ou , B. and Huang , D. 2006 . Fluorescent Approach to Quantitation of Reactive Oxygen Species in Mainstream Cigarette Smoke . Anal. Chem , 78 : 3097 – 3103 .
- Pavlovic , J. and Hopke , P. K. 2011 . Detection of Radical Species Formed by the Ozonolysis of 01-pinene . J. Atmospheric Chem. , DOI: 10.1007/s10874-011-9197-y
- Penn , A. and Snyder , C. A. 1993 . Inhalation of Sidestream Cigarette Smoke Accelerates Development of Arteriosclerotic Plaques . Circulation , 88 : 1820 – 1825 .
- Pryor , W. A. 1997 . Cigarette Smoke Radicals and the Role of Free Radicals in Chemical Carcinogenicity . Environ. Health Perspect. , 105 : 875 – 882 . Suppl. 4
- See , S. W. , Wang , Y. H. and Balasubramanian , R. 2007 . Contrasting Reactive Oxygen Species and Transition Metal Concentrations in Combustion Aerosols . Environ. Res. , 103 : 317 – 324 .
- Spurny , K. R. , Lodge , J. P. Jr. , Frank , E. R. and Sheesley , D. C. 1969a . Aerosol Filtration by Means of Nuclepore Filters: Structural and Filtration Properties . Environ. Sci. Technol. , 3 : 453 – 464 .
- Spurny , K. R. , Lodge , J. P. Jr. , Frank , E. R. and Sheesley , D. C. 1969b . Aerosol Filtration by Means of Nuclepore Filters: Aerosol Sampling and Measurement . Environ. Sci. Technol. , 3 : 464 – 468 .
- Venkatachari , P. and Hopke , P. K. 2008 . Development and Laboratory Testing of an Automated Monitor for the Measurement of Atmospheric Particle-Bound Reactive Oxygen Species (ROS) . Aerosol Sci. Technol. , 42 : 629 – 635 .
- Venkatachari , P. , Hopke , P. K. , Brune , W. H. , Ren , X. , Lesher , R. Mao , J. 2007 . Characterization of Wintertime Reactive Oxygen Species Concentrations in Flushing, New York . Aerosol Sci. Technol. , 41 : 97 – 111 .
- Venkatachari , P. , Hopke , P. K. , Grover , B. D. and Eatough , D. J. 2005a . Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols . J. Atmos. Chem. , 50 : 49 – 58 .
- Venkatachari , P. , Hopke , P. K. , Grover , B. D. and Eatough , D. J. 2005b . Erratum: Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols . J. Atmos. Chem. , 52 : 325 – 326 .
- Wang , Y. , Hopke , P. K. , Sun , L. , Chalupa , D. C. and Utell , M. J. in press . Laboratory and Field Testing of an Automated Atmospheric Particle-Bound Reactive Oxygen Species Sampling-Analysis System . J. Toxicol. , 2011 Article ID 419476, 9 pages doi: 10.1155/2011/419476
- Wilkerson , J. A. 2001 . Medicine for Mountaineering & Other Wilderness Activities , Seattle : The Mountaineers Books .