Abstract
The displacement of ammonium by triethylammonium (TEAH) in aerosol particles of about 15–35 μm in diameter was investigated using an electrodynamic balance (EDB) coupled with in situ Raman spectroscopy. The phase state of particles played a crucial role in the extent of triethylamine (TEA) uptake. At 50 or 75% relative humidity (RH), the heterogenous uptake of about 40-ppm TEA by aqueous ammonium salts of sulfate [(NH4)2SO4], bisulfate (NH4HSO4), nitrate (NH4NO3), chloride (NH4Cl), and oxalate [(NH4)2C2O4] led to increases in particle mass of over 90%. A complete displacement of ammonium by TEAH was confirmed by direct EDB mass measurements and the Raman spectra obtained. TEAH sulfate was formed during the exposure of aqueous droplets of (NH4)2SO4 and NH4HSO4 to TEA vapor at 50% RH; but a fraction of it decomposed to TEAH bisulfate when the TEA supply was removed. Crystalline solid particles of (NH4)2SO4 and (NH4)2C2O4 experienced small mass increases of <5%, both of which were attributed to the hindered mass transfer of TEA in crystalline solids. However, TEA reacted with the amorphous solid NH4NO3 particle at <3% RH as effectively as if it was in the aqueous NH4NO3 droplet (50% RH) and formed TEAH nitrate. On the other hand, the amorphous NH4HSO4 solid particle reacted with TEA at <3% RH to form crystalline (NH4)2SO4 and liquid TEAH bisulfate and sulfate. The formation of rather inert crystalline (NH4)2SO4 suppressed the ammonium exchange.
Copyright 2012 American Association for Aerosol Research
INTRODUCTION
Short-chain aliphatic amines are emitted from a wide range of sources such as animal husbandry (Schade and Crutzen Citation1995), vehicle exhausts (Cadle and Mulawa Citation1980), waste incinerators and sewage treatment plants (Leach et al. Citation1999). In intense agricultural regions, ambient levels of amines could reach hundreds of parts per billion (ppb) (Rabaud et al. Citation2003), but they are generally lower in other areas. The typical concentrations of amines are <1–100 parts per trillion (ppt) (Gronberg et al. Citation1992), 1–3 orders of magnitude lower than those of ammonia (Reis et al. Citation2009). Particulate amines have been detected in fine aerosols (Zhang et al. Citation2002; Facchini et al. Citation2008; Sorooshian et al. Citation2008; Pratt et al. Citation2009), aqueous fog and rain drops (Collett et al. Citation2008; Rehbein et al. Citation2011); and their abundance has been found to be size dependent (VandenBoer et al. Citation2011). Elevated levels of particle-phase amines were observed during periods of acidic cloud/fog processing (Rehbein et al. Citation2011), indicating the possible importance of aqueous-phase chemistry and aerosol acidity in particulate amine formation.
Similar to other reactive organic vapors (Kroll and Seinfeld Citation2008; Hallquist et al. Citation2009; Li et al. Citation2011), amines may undergo rapid oxidation with ozone, hydroxyl and nitrate radicals (Angelino et al. Citation2001; Murphy et al. Citation2007; Silva et al. Citation2008; Zahardis et al. Citation2008) to produce secondary organic aerosols. Moreover, as one of the few atmospheric alkaline vapors other than ammonia (Swartz et al. Citation1999), amines exhibit acid–base chemistry which is of concern in aerosol science (Ge et al. Citation2011). Theoretical calculations (Barsanti et al. Citation2009) and field measurements (Makela et al. Citation2001; Smith et al. Citation2010; Wang et al. Citation2010b) consistently showed that amines formed aminium salts with sulfuric acid, nitric acid, and organic acids, and that they comprised a significant fraction of ambient aerosols, particularly in nucleation mode. The molar ratio of aminium to ammonium in fine aerosol has been reported to range from 0.005 to 0.2 (VandenBoer et al. Citation2011). Aminium salt formation leads to tremendous decreases in the vapor pressures of the involved species (amines and acids), which may assist nucleation by counteracting the Kelvin curvature effect (Zhang and Wexler Citation2002; Barsanti et al. Citation2009). There is evidence that amines enhanced nucleation in sulfuric acid-water system more effectively than ammonia (Kurten et al. Citation2008). Bzdek, Johnston, and coworkers conducted an extensive investigation on the uptake of amines by small clusters (1–2 nm) of ammonium sulfate, bisulfate, and nitrate using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) (Bzdek et al. Citation2010a; Bzdek et al. Citation2010b). Complete displacement of ammonium by aminium was observed, suggesting that the sub-3-nm clusters were likely to be aminium salts.
Using reaction flow tubes, the uptake coefficients of low molecular-weight alkylamines (Wang et al. Citation2010a; Yin et al. Citation2011) residing on highly acidic sulfuric acid surfaces were measured at various temperatures and sulfuric acid concentrations. Irreversible reactive uptakes were observed. To explore the displacement of ammonium by aminium ion, Qiu et al. (Citation2011) investigated the heterogenous interaction between amines and ammonium sulfate or bisulfate using a coated-wall flow reactor coupled to an ion-drift chemical ionization mass spectrometer (ID-CIMS). Simultaneous loss of amines and generation of ammonia in the gas phase were observed and the results suggested that amines were important contributors to the growth of ambient aerosols. Using a flow tube coupled with ion trap time-of-flight (IT-TOF) aerosol mass spectrometer, Lloyd et al. (Citation2009) investigated the uptake of trimethylamine (TMA) by ammonium nitrate and showed the displacement of NH+ 4 ions by aminium ions.
In this paper, we systematically investigated the heterogenous uptake of triethylamine (TEA) by common inorganic and organic particles. Five salts of ammonium and two salts of sodium in both droplet and solid (crystalline and amorphous) forms under <3, 50, or 75% relative humidity (RH) were studied. An electrodynamic balance (EDB) coupled with Raman spectroscopy (Lee et al. Citation2008) was employed for simultaneous measurements of the temporal changes in mass, chemical composition, and post-reaction hygroscopicity of levitated droplets and solid particles. TEA [(C2H5)3N] uptake can be tremendously enhanced in an aqueous acidic medium. Protonation of TEA leads to the formation of triethylammonium ions ((C2H5)3NH+, abbreviated as TEAH ions hereafter), and shifts the equilibrium to the aqueous phase and further promotes TEA uptake (Ge et al. Citation2011):
This acidic condition facilitated TEA uptake. In this study, all ammonium-containing aqueous droplets had overwhelming TEA uptake. Particle mass increases were attributed to the formation of TEAH. Complete displacement of ammonium by TEAH was observed for aqueous droplets of ammonium salts and reactions were confirmed directly by matching the product spectra with the Raman spectra of standard compounds collected using the EDB/Raman system.
Aqueous ammonia [NH3(aq)] was formed after reaction (Equation (3)) and partitioned into the gas phase in the ammonia-free EDB chamber:
EXPERIMENTAL SETUP
1. Single Particle Generation
Ammonium sulfate (>99.0%, Sigma-Aldrich), ammonium bisulfate (>99.5%, Fluka), ammonium oxalatemonohydrate (>99.5%, Fluka), ammonium nitrate (99.999%, Aldrich), ammonium chloride (99.99%, Aldrich), sodium sulfate (>99.0%, Sigma-Aldrich), sodium chloride (>99.5%, Sigma-Aldrich), and the mixture of sulfuric acid (95–98 wt%, Mallinckrodt Chemicals) and TEA (>99%, Aldrich) were dissolved in high-purity Milli-Q water (18.2 Ω) in 10-ml beakers. A small amount of the aqueous solution was introduced to a piezoelectric particle generator (MicroFab Tech., Inc.) and a single droplet of 15–35 μm in diameter (after solvent evaporation) was produced by applying an electric pulse to the generator. The droplet was charged as it passed through the induction plate mounted at the top entrance of the EDB.
2. EDB Equilibrium Mass and Hygroscopic Measurements
EDB has been used extensively in direct measurements of particle mass changes (Davis Citation1997; Chan et al. Citation2010). A study on the reactive uptake of nonanal by sulfuric acid/organic mixture has been reported (Chan and Chan Citation2011). In brief, a charged particle of inorganic or organic salt was trapped and levitated at the null point of the EDB by setting up a combination of AC and DC electric fields around the particle. Assuming that there was no loss of charge, the weight of the single particle would be proportional to the balancing DC voltage. The relative mass change of a particle during TEA vapor uptake can be determined by monitoring the change in the balancing DC voltage. The uptake data obtained was used to calculate the mass ratio, defined as mt /mo , and the percentage mass increase, defined as (1 – mt /mo ) × 100%, where mt is the mass of the particle after TEA uptake and mo is the initial mass of the particle (reference state). For hygroscopic measurements, the mass ratio was defined as the ratio of mass at an elevated RH to the mass at <3% RH.
3. Generation and Detection of TEA Vapor
TEA vapor was generated by evaporating liquid TEA using a syringe pump (kdScientific, KDS-100). The gas-phase TEA concentration was controlled by diluting the TEA vapor-laden air with a stream of purified air at controlled RH using mass flow controllers (Smart-Trak Series 100). The diluted TEA vapor was split into two streams: one for EDB experiments, which was fixed at ∼180 cm3 min−1, and the other was for TEA monitoring with a portable volatile organic compound (VOC) detector (Ion-science Inc., PhoCheck 5000+) and for RH monitoring with a dew point monitor (EdgeTech Inc., Dew Prime I). For all uptake experiments, the EDB stream was allowed to flow once the monitoring stream had become stable and the TEA vapor concentration had a fluctuation of approximately ±5%. Calibration of the VOC detector was done by evaporating known amounts of TEA at controlled flow rates prior to EDB measurements.
4. Raman Spectroscopy of Single Levitated Particles
To probe the temporal chemical changes of levitated particles, a Raman spectroscopy system similar to that of Lee et al. (Citation2008) was employed. The system consisted of a 150-mW diode-pumped solid-state laser (Spectra-Physics Excelsior, 532-150-SLM-CDRH) and a 0.5-m monochromator (Acton SpectraPro 500) attached to a charge-coupled device (CCD) detector (Andor Technology DV420-OE), which was integrated into the EDB system. The 532.2-nm line of the laser was used as the source of excitation. A pair of lenses, which matched the f/7 optics of the monochromator, was used to focus the 90° scattering of the levitated droplet in the EDB onto the slit of the monochromator. A Raman notch filter was placed between the two lenses to remove the strong Rayleigh scattering. A 300 g mm–1 grating was selected for the monochromator. The integration time of each spectrum was 10 s (10 frames, each with an accumulation time of 1 s). The resolution of the spectra obtained was about 6 cm−1. The assignments of Raman peaks were based on those used by Dawson et al. (1986), Lin-Vien et al. (Citation1991), and Dong et al. (2007).
RESULTS AND DISCUSSION
1. Enhanced TEA Uptake of Ammonium Salts
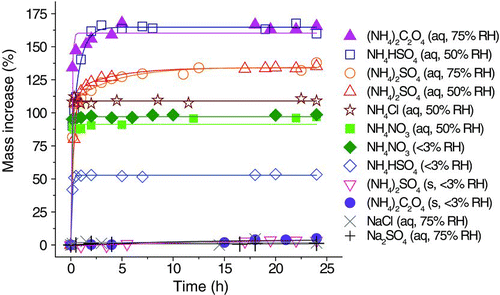
Levitated droplets and solid particles of the chosen inorganic and organic salts were exposed to 40-ppm TEA in an EDB for 24 h at controlled RHs (<3, 50, or 75%). Their uptake kinetics are shown in .
The droplets of neutral salts, such as sodium chloride and sodium sulfate (NaCl and Na2SO4, 75% RH), had negligible mass changes during the course of TEA exposure. Using the Henry's Law constant of TEA in water (6.6 M atm–1), the estimated mass uptake of the aqueous droplets equilibrated at 40-ppm TEA vapor was <0.1%, consistent with the observed constant NaCl and Na2SO4 masses.
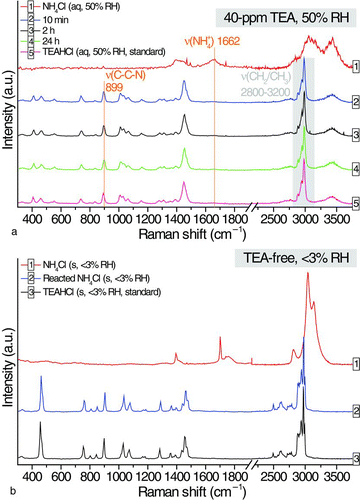
FIG. 2 (a) Temporal changes in Raman signals of an aqueous ammonium chloride droplet at 40-ppm TEA and (b) comparison of the Raman spectra of a solid particle of ammonium chloride, the dried particle of the reacted ammonium chloride droplet in TEA-free EDB chamber, and a solid particle of TEAHCl. (Color figure available online.)
TEA uptake can be dramatically enhanced in an aqueous acidic medium. Protonation of TEA leads to the formation of TEAH ions and shifts the equilibrium to the aqueous phase [Equations (1) and (2)]. Ammonium salts are mildly acidic [Equation (3)] and their aqueous solutions facilitated TEA uptake. In this study, all ammonium-containing droplets had overwhelming TEA uptake. Particle mass increases were attributed to the formation of TEAH and will be discussed in the detailed Raman analysis later. At 50 or 75% RH, aqueous droplets of ammonium oxalate [(NH4)2C2O4], ammonium bisulfate (NH4HSO4), ammonium sulfate [(NH4)2SO4], ammonium chloride (NH4Cl), and ammonium nitrate (NH4NO3) had mass increases of more than 90% after 1 h of exposure to 40-ppm TEA. However, the reactions of TEA with ammonium crystalline solids were inefficient. Crystalline solid particles of (NH4)2SO4 and (NH4)2C2O4 had mass increases of less than 5% after 24 h of exposure. The phase states of particles played an important role in TEA uptake. To elucidate the changes in particle mass, detailed chemical analysis was carried using Raman spectroscopy. The analysis of aqueous ammonium salts is discussed first and then followed by the discussion of crystalline and amorphous solid ammonium salts.
TABLE 1 Summary of the reaction conditions, the particle mass changes on a dry basis and the theoretical mass ratios
2. Raman Analysis of NH4 + Displacement and TEAH Formation in Aqueous NH4 + Salts
Temporal chemical changes of an aqueous NH4Cl droplet when reacting with 40-ppm TEA at 50% RH were monitored by Raman spectroscopy (). The spectrum of the aqueous NH4Cl droplet at 50% is shown as a control.
At 10 min, the totally symmetric stretching mode of NH4 + at 1662 cm−1 vanished. On the other hand, the symmetric stretching mode of carbon—carbon–nitrogen (C–C–N) at 899 cm−1 and the unresolved multiple peaks of combined CH2/CH3 stretching at 2800–3200 cm−1 emerged due to the formation of TEAH ions. The substantial formation of TEAH suggested that protonation of TEA was effective, resulting in a huge mass increase (110%, ). Concurrently, the removal of proton by TEA promoted the dissociation of ammonium into aqueous ammonia (Equation (3)). Aqueous ammonia eventually partitioned into the gas phase in the ammonia-free EDB chamber (Equation (4)), causing the ammonium signals to disappear. The EDB chamber was connected to a continuous flow system and the ammonia generated by the single levitated particle was not expected to accumulate throughout the experiment. Overall, ammonium ions were displaced by TEAH ions in the aqueous NH4Cl droplet. After the initial 10 min, the Raman features of the reacted NH4Cl droplet remained constant, consistent with the observation of a stable particle mass (). The ultimate spectrum of the reacted NH4Cl droplet at 24 h was similar to the standard spectrum of triethylammonium chloride (TEAHCl) droplet, confirming the displacement of ammonium by TEAH. The reacted NH4Cl droplet was dried at <3% RH in the absence of TEA vapor and its spectrum is compared with the spectra of unexposed pure NH4Cl particle and pure TEAHCl particle collected using the EDB/Raman system (). Again, the spectra of the reacted NH4Cl particle matched most of the TEAHCl Raman features.
lists the mass ratios of the reacted particles (<3, 50, or 70% RH; 40-ppm TEA) subsequently dried (<3% RH) in the TEA-free EDB chamber to the corresponding initial particles and the theoretical mass ratios (see also the footnotes of ).
The EDB-measured dry mass ratio of the reacted particle to the initial NH4Cl solid particle was very close to the molecular weight ratio of TEAHCl to NH4Cl (theoretical mass ratio), validating the use of the coupled EDB/Raman system in ammonium/triethylammonium exchange studies. Similar to the NH4Cl droplet, the NH4NO3 droplet reacted with TEA to form TEAH nitrate at 50% RH (Supp. Info., Figure S1a). The ammonium displacement was confirmed by matching the Raman signals of the exposed NH4NO3 droplet with the signals of nitrate and TEAH from NH4NO3 and TEAHCl droplets, respectively. The generalized overall reaction of aqueous (mono-) ammonium salts was:
3. Decomposition of TEAH Sulfate into TEAH Bisulfate
As will be shown later, reactions of TEA with aqueous (50 or 75% RH) (NH4)2SO4 and NH4HSO4 particles form TEAH sulfate. However, TEAH sulfate can convert to TEAH bisulfate (and vice versa) and hence a discussion of the conversion is given here before the data analysis of the uptake by aqueous (NH4)2SO4 and NH4HSO4 droplets. We examined the uptake of TEA by TEAH bisulfate (TEAHBS) droplet, which was synthesized by mixing TEA with diluted aqueous sulfuric acid solution in a 1:1 molar ratio, for Raman characterization. From the observed Mie scattering patterns, TEAHCl particles crystallized at <3% RH while the mixed particle of TEA and sulfuric acid (i.e., TEAHBS) did not and remained in droplet form. Liquid TEAHBS is commonly referred to as a protic ionic liquid (Drummond and Greaves Citation2008) and has a reported melting point of 84.2°C (Belieres and Angell Citation2007). Interestingly, the single levitated TEAHBS droplet remained as a supercooled liquid (Debenedetti and Stillinger Citation2001; Zobrist et al. Citation2008) and did not crystallize at room temperature (∼23°C), about 61°C below its melting point. Suppressed crystallization has often been observed in hygroscopic studies involving an EDB (Peng et al. Citation2001). To show the presence of TEAH ions in the synthesized TEAHBS, the Raman spectrum of the synthesized droplet was compared with the spectra of NH4HSO4 and TEAHCl particles at <3% RH (Supp. Info., Figure S2a). TEAH ions were identified in the synthesized TEAHBS particles, as confirmed by the presence of characteristic CH3 deformation at 1450 cm−1 (shaded), unresolved multiple peaks of CH2/CH3 stretching at 2800–3200 cm−1 and C–C–N stretching at ∼899 cm−1. Sulfate ions (SO4 2−) are characterized by peaks at 452, 613, 973, and 1080 cm−1 from (NH4)2SO4 while bisulfate ions (HSO4 −) are characterized by peaks at 411, 428, 583, 600, 870, and 1013 cm−1 from NH4HSO4. All bisulfate Raman features were identified in the spectrum of TEAHBS particle but sulfate peaks were absent. Overall, the Raman features of the TEAHBS particle were the superposition of the peaks of TEAH and bisulfate ions, confirming that the mixing of TEA and sulfuric acidin a 1:1 molar ratio produced TEAHBS. In aqueous state (50% RH, Supp. Info., Figure S2b), NH4HSO4 dissociated into sulfate and bisulfate ions (Clegg et al. Citation1998). Again, the Raman features of aqueous TEAHBS droplet were the superposition of the TEAH and bisulfate features of TEAHCl and NH4HSO4 droplets, respectively. No sulfate peaks were observed in the spectrum of TEAHBS droplet.
Triethylammonium sulfate (TEAH sulfate) was produced when particles of TEAHBS were exposed to the 40-ppm TEA vapor. The uptake was studied at 50 and <3% RH. A high RH favored the formation of TEAH sulfate. At 50% RH (), the TEAHBS particle had a mass increase of about 80% while the reaction at <3% RH yielded a 10% mass increase only. Most of the uptake occurred in the first 10 min of the reaction.
FIG. 3 Kinetics of the uptake of 40-ppm TEA by TEAHBS droplets at 50 and <3% RH. (Color figure available online.)
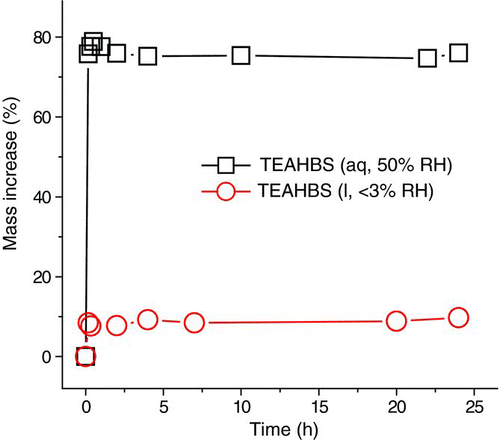
FIG. 4 Temporal changes in (a) Raman signals of the aqueous TEAHBS droplet at 40-ppm TEA (50% RH); (b) Raman signals of the aqueous TEAH sulfate droplet during decomposition in the TEA-free EDB chamber (50% RH); (c) Raman signals of the partially decomposed TEAH sulfate particle during decomposition at <3% RH and its Raman spectrum after re-humidification. (Color figure available online.)
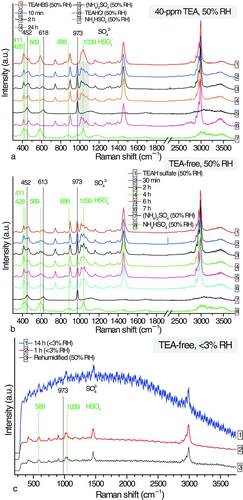
The Raman features of the TEAHBS droplet reacted at 50% RH are shown in . At 10 min, the characteristic peaks of bisulfate (411, 428, 589, 886, and 1039 cm−1) reduced significantly and the peaks of sulfates (452, 618, and 973 cm−1) emerged while the TEAH Raman features persisted, confirming that TEAH sulfate was indeed formed. Except for the potential overlap with the bisulfate peak at 1039 cm−1, the characteristic peaks of TEAH ions in TEAH sulfate at 1000–1100 cm−1 and the three peaks of TEAHCl at the same Raman shift were the same. No spectroscopic change was observed beyond 10 min, which was consistent with the observed stable mass of reacting TEAHBS droplet during the same period ().
TEAH sulfate was stable in the presence of gaseous TEA. After 24 h of exposure, TEA supply was cut off and replaced by TEA-free air at the same 50% RH. In , the temporal spectral changes of the TEAH sulfate droplet in the absence of TEA vapor are shown. Sulfate signals weakened whilst bisulfate signals strengthened, indicating that TEAH sulfate decomposed into aqueous TEAH and TEAHBS, albeit at a much lower rate than the initial TEA uptake. A mass decrease of about 25% was measured with the EDB 7 h after the TEA supply was cut off, suggesting that a significant amount of particle-phase free-TEA, from both decomposition and absorption, escaped into the gas phase. Since the RH was kept constant at 50% during the decomposition, the contribution of water evaporation to the mass decrease was expected to be less significant compared with the liberation of free-TEA. Afterwards, the particle was left in the EDB chamber for another 14 h for drying at <3% RH. In the first hour, the sulfate peak at 973 cm−1 was significantly weaker while the bisulfate peak at 1039 cm−1 was stronger than those before the particle was dried (but after it was exposed to TEA-free air at 50% RH) (, <3% RH).
The particle then became highly fluorescent after being dried for 14 h, masking the Raman features. The same particle was then rehumidified at 50% RH and the Raman features of sulfate and bisulfate were found to be similar to those of the particle dried for 1 h only. This suggested that the conversion of sulfate to bisulfate may have ceased after 1 h at <3% RH. It is noted that from our later experiments, trace amounts of gaseous TEA may have remained in the EDB chamber which might have then suppressed further decomposition of TEAH sulfate to liberate TEA. The percentage of TEAH sulfate remained after drying was estimated using the EDB-measured dry mass of the TEA-exposed particle at 14 h and that of the initial TEAHBS. The molar ratio of TEAHBS to TEAH sulfate was determined to be approximately 88:12. This ratio was used as the ratio of stable TEAH sulfate to TEAHBS after drying at <3% RH in a later analysis of uptake experiments of aqueous (NH4)2SO4 and NH4HSO4 droplets.
In additional experiments that the TEAHBS droplet reacted with TEA vapor at <3% RH, the sulfate signal at 973 cm−1 increased while the bisulfate signal decreased but did not vanish (Figure s3a, Supp. Info). This was different from the reaction at 50% RH at which the bisulfate signal had almost disappeared and the conversion to TEAH sulfate was complete (). In the TEA uptake experiment at <3% RH, only a portion of TEAHBS was converted to TEAH sulfate, leaving a mixture of TEAH sulfate and TEAHBS at 24 h. As shown in the uptake kinetics (), the mass increase of the TEAHBS particle reacted at <3% RH was 8 times smaller than that reacted at 50% RH. We speculate that TEAH sulfate may not be stable in highly concentrated droplet at <3% RH. Twenty hours after cutting off the TEA supply (Supp. Info., Figure S3b), TEAH sulfate decomposed and the mixed TEAH sulfate/bisulfate particle (formed at <3% RH, 40-ppm TEA) transformed into a TEAHBS particle with Raman features resembling those of the initial TEAHBS particle.
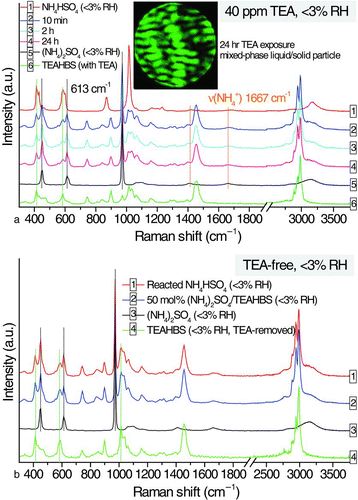
FIG. 5 (a) Temporal changes in the Raman signals of an amorphous ammonium bisulfate solid particle at 40-ppm TEA (<3% RH) and (b) comparison of the Raman spectra of the dried particle of the reacted ammonium bisulfate in TEA-free EDB chamber, a 50 mol% ammonium sulfate/TEAHBS mixed particle, a crystalline ammonium sulfate solid particle, and a TEAHBS droplet, all at <3% RH. (Color figure available online.)
4. Reactions of Aqueous NH4HSO4 and (NH4)2SO4 Droplets with TEA
As indicated from the changes of Raman spectra, both droplets of NH4HSO4 and (NH4)2SO4 reacted with TEA to form the same reaction product, TEAH sulfate, at 50 or 75% RH. Ammonium ions were replaced by TEAH ions as reflected by the loss of NH4 + peaks and the advent of C–C–N stretching due to TEAH (Supp. Info., Figures S4a–c). Unlike aqueous (NH4)2SO4 droplets and other aqueous droplets of ammonium salts used in this study (NH4Cl, NH4NO3),aqueous NH4HSO4 droplets provided extra protons from the proton dissociation of bisulfate ions for the neutralization reaction with TEA to form TEAH. Both neutralization and ammonium displacement were responsible for the mass and chemical changes of the aqueous NH4HSO4 droplet. The overall reaction of aqueous NH4HSO4 droplet with TEA led to the removal of one mole of ammonium per mole of NH4HSO4. Stoichiometrically, it can be represented by:
On the other hand, aqueous (NH4)2SO4 (at both 50 and 75% RH) droplets reacted with TEA and two moles of ammonium were removed per mole of (NH4)2SO4:
The TEA uptake and ammonium displacement were effective in aqueous droplets. The mass increase of aqueous NH4HSO4 droplet after 40-ppm TEA uptake at 50% RH was about 160% (with reference to aqueous NH4HSO4 at 50% RH). (NH4)2SO4 droplets reacted at 50 and 75% RH had mass increases of about 130%. One extra ammonium was removed from the (NH4)2SO4-TEA reaction compared with the NH4HSO4-TEA reaction but the same reaction product, TEAH sulfate, was formed. Hence, the mass increases of (NH4)2SO4 droplets after a 24-h exposure were less than that of the NH4HSO4 droplet in aqueous state. It is noted that the mass increases during TEA exposure () were complicated by the difference in hygroscopicity between parent [(NH4)2SO4 or NH4HSO4] and reacted (TEAH sulfate) droplets, and the potential mass contribution by the physical partitioning of TEA vapor. Nevertheless, the EDB-measured dry mass ratios of exposed to initial particles of NH4HSO4 and (NH4)2SO4 were consistent with the theoretical mass (). A mass ratio of TEAHBS to TEAH sulfate of 88:12 was assumed after decomposing the reacted NH4HSO4 and (NH4)2SO4 droplets (i.e., TEAH sulfate) at <3% RH in the TEA-free EDB chamber, as discussed in Section 3.
5. Enhanced TEA Uptake by Amorphous Solids over Crystalline Solids
Crystalline solid particles of ammonium oxalate [(NH4)2C2O4] and (NH4)2SO4 (<3% RH) reacted slowly with TEA, presumably due to the diffusion limitation of the TEA in the crystalline solids. Weak signals of combined CH2/CH3 stretching vibrations appeared at 2800–3200 cm−1 in the Raman spectrum of reacted (NH4)2C2O4 and (NH4)2SO4 particles (Supp. Info., Figure S5), accompanied by a small mass increase of less than 5% after 24 h of reactions ().
Unlike (NH4)2SO4 and (NH4)2C2O4 which formed crystalline solids at <3% RH, NH4NO3 particle did not effloresce and may form amorphous solid (Martin Citation2000). The formation of amorphous NH4NO3 solid particle was inferred from the observed light scattering pattern in our experiments that resembled patterns closer to those of droplets than the irregular pattern of crystalline solids. The TEA uptake by amorphous NH4NO3 solid particle was as effective as that of the aqueous NH4NO3 droplet. In , both the NH4NO3 droplet (50% RH) and the NH4NO3 amorphous solid (<3% RH) had similar equilibrium mass increases of about 97–99% at 24 h. Martin (Citation2000) reported that NH4NO3 did not effloresce even under extremely dry condition (∼0% RH) and particle-phase water might have remained in the amorphous solids of NH4NO3 at <3% RH. The presence of residual water may have facilitated the ammonium displacement of NH4NO3 during reaction with TEA at <3% RH. It is noted that the spectrum of NH4NO3 particle that reacted at <3% RH had a doublet at about 450 cm−1 (Supp. Info., Figure S1b) which belonged to neither nitrate nor TEAH. The doublet was also observed in the spectrum when the reacted NH4NO3 droplet (50% RH) was dried at <3% RH (Supp. Info., Figure S1c). The origin of the doublet is not known at this point.
Amorphous solid NH4HSO4 (Martin Citation2000; Mikhailov et al. Citation2009) at <3% RH responded to TEA vapor swiftly and attained a mass increase of about 50% at equilibrium (). At 10 min (), the Raman spectrum of reacted NH4HSO4 particle contained all features of (NH4)2SO4, including both sulfate and ammonium, and the Raman signals of TEAHBS and TEAH sulfate. Hence, the reaction products were ionic mixtures of TEAH, ammonium, bisulfate, and sulfate.
The physical state (e.g., crystalline or liquid) of reaction products can be identified by analyzing the full width at half maximum (FWHM) (Ling and Chan Citation2008), also known as peak broadness, of the peaks of interest. In general, Raman peaks of crystalline compounds had smaller FWHM values (or were sharper) compared with their liquid (or aqueous) counterparts. The sulfate peak at 613 cm−1 was investigated. The FWHM(613) of liquid TEAH sulfate was 39.3 cm−1, almost double that of crystalline (NH4)2SO4 (20.8 cm−1). The FWHM(613) of reacted NH4HSO4 was 21.2 cm−1, suggesting that the sulfate of reacted NH4HSO4 particle was in crystalline state. As deduced from the list of product ions mentioned above, the crystalline phase is likely (NH4)2SO4. It should be noted that TEAHBS partially reacted with TEA at <3% RH, forming a fraction of TEAH sulfate. Hence, in addition to that of crystalline (NH4)2SO4, mixed signals of TEAH sulfate and TEAHBS are expected if TEAHBS is produced from NH4HSO4-TEA reactions. In our experiments at<3% RH, TEAHBS dominated over TEAH sulfate. When Raman signals of TEAHBS were subtracted from the spectrum of reacted NH4HSO4 particle, the resultant spectrum matched most features of crystalline (NH4)2SO4 particle, except for the potential contribution of sulfate signals from liquid TEAH sulfate. The reacted NH4HSO4 particle showed the Mie scattering pattern of a mixed-phase liquid/solid particle (), consistent with the deduction of crystalline (NH4)2SO4 mixed in liquid TEAHBS and TEAH sulfate mixtures. Overall, the reaction of NH4HSO4 at <3% RH is proposed to be:
This is in contrast to the reaction of aqueous NH4HSO4 droplet with TEA at 50% RH (Equation (6)), which liberated ammonia. It is because of the crystallization of (NH4)2SO4 at <3% RH, which suppressed the displacement of ammonium. When the TEA supply was removed after 24 h, TEAH sulfate decomposed into TEAHBS. The spectrum of TEA-removed reacted NH4HSO4 matched the spectrum of the synthesized 50 mol% (NH4)2SO4/TEAHBS mixture at <3% RH (). The EDB-measured mass ratio of the reacted NH4HSO4 particle to the initial NH4HSO4 is also consistent with the mass ratio of the sum of (NH4)2SO4 and TEAHBS to NH4HSO4 at the stoichiometric ratio determined using Equation (Equation8) (). Unlike TEA reactions of aqueous ammonium salts (e.g., NH4Cl), the TEA reaction of NH4HSO4 under dry condition (<3% RH) did not result in the displacement of ammonium (1667 cm−1). The mass increase of amorphous solid NH4HSO4 was attributed to the acid-base neutralization reactions (Qiu et al. Citation2011) of acidic bisulfate and alkaline TEA (Equation (Equation8)). From the results above, an aqueous phase plays a crucial role in effective uptake of TEA by ammonium salts. Fully deliquesced droplets had much higher TEA uptake compared to those of solid particles. Owing to less severe mass transfer limitation, the amorphous solids tended to have enhanced uptake over those of the crystalline solids.
6. Uptake at Low TEA Concentrations
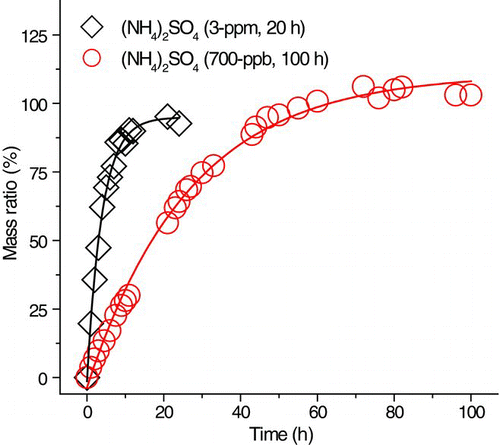
Low TEA concentration (3-ppm or 700-ppb) uptake experiments were conducted for aqueous (NH4)2SO4 droplets at 50% RH and the kinetics are shown in . As in the TEA reaction of (NH4)2SO4 droplet at 40-ppm (50% and 75% RH), TEAH sulfate was formed when the aqueous (NH4)2SO4 droplet (50% RH) was exposed to 700-ppb TEA for 100 h (Supp. Info., Figure S6). The particle that reacted at 700 ppb remained as a droplet and became fluorescent when dried at <3% RH.
For the 20-h 3-ppm TEA uptake experiment using (NH4)2SO4 droplet (50% RH), the displacement of ammonium by TEAH was not fully complete. Hence, the reacted particle was an aqueous mixture of ammonium, TEAH, bisulfate, and sulfate. From the FWHM analysis and the Mie scattering pattern (similar to the analysis of amorphous solid NH4HSO4 reaction), crystallization of the unreacted (NH4)2SO4 occurred when the 3-ppm TEA-exposed (NH4)2SO4 particle was dried at <3% RH while the reaction product, TEAHBS, remained as a liquid at <3% RH in the absence of TEA. To determine the ratio of (NH4)2SO4 to TEAHBS, a calibration based on the collected Raman spectra of synthesized mixtures of (NH4)2SO4 and TEAHBS with 11, 20, 43, 69, and 87 mol% (NH4)2SO4 was established (Supp. Info., Figure S7). The normalized sulfate peak area ratio (Asulfate), defined as the area of sulfate (613 cm−1) over the sum of the areas of sulfate (613 cm−1) and bisulfate (583 cm−1), was plotted against the mol% of (NH4)2SO4 ().
FIG. 7 Normalized sulfate peak area ratio against mol% ammonium sulfate. (Color figure available online.)
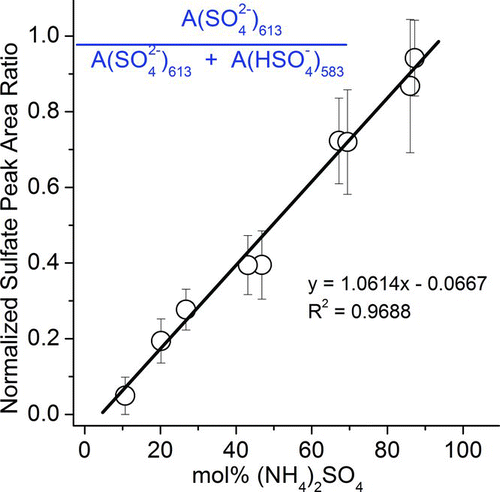
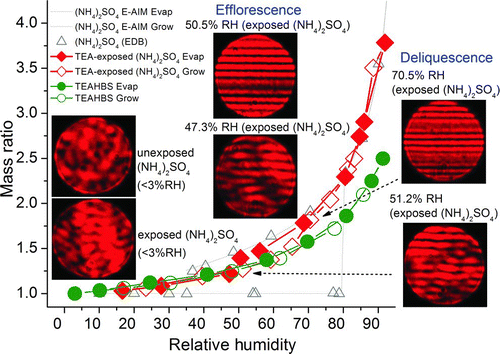
FIG. 8 Hygroscopicities of E-AIM predictions of ammonium sulfate and EDB-measurements of ammonium sulfate particles, the 3-ppm TEA-exposed (20 h, 50% RH) ammonium sulfate particle and a TEAHBS particle, and Mie scattering patterns of the 3-ppm TEA-exposed ammonium sulfate particle. (Color figure available online.)
The Asulfate of 3-ppm TEA-exposed (NH4)2SO4 was 0.365. From , the corresponding mol% of (NH4)2SO4 was 41% (and the mol% of TEAHBS was 59%). The mass ratio of exposed particle to initial (NH4)2SO4 (on a dry basis) particle, calculated using the mole ratio of (NH4)2SO4 to TEAHBS, was 1.30, consistent with the EDB-measured mass ratio of 1.29.
The hygroscopicity of (NH4)2SO4 particle reacted at 50% RH (in aqueous state) with 3-ppm TEA for 20 h was measured and compared with those of unexposed (NH4)2SO4 particle, TEAHBS particle and E-AIM predictions of (NH4)2SO4 ().The EDB-measured hygroscopicity of unexposed (NH4)2SO4 particle matched the E-AIM predictions well (Clegg et al. Citation1998) and (NH4)2SO4 was observed to deliquesce at about 80% RH. Each TEAHBS ion had 3 extra hydrophobic ethyl groups, making the TEAHBS particle less hygroscopic than (NH4)2SO4. The hygroscopicity of 3-ppm TEA-exposed (NH4)2SO4 particle (determined as a mixture of ∼40 mol% (NH4)2SO4 and ∼60 mol% TEAHBS) was between those of unexposed (NH4)2SO4 particle and TEAHBS particle and showed partial deliquescence and efflorescence properties. The TEA-exposed (NH4)2SO4 particle completely deliquesced at about 70.5% RH (open diamonds), as reflected by the formation of regular fringes. The hygroscopicity of the completely deliquesced droplet was similar to that of unexposed (NH4)2SO4 particle. The (NH4)2SO4 portion of TEA-exposed (NH4)2SO4 crystalized at 47–51% RH (closed diamonds), as supported by the FWHM analysis.
As shown in , at <3% RH, the crystalline (NH4)2SO4 particle (unexposed) exhibited an irregular scattering pattern while the TEA-exposed (NH4)2SO4 particle (reacted at 50% RH but dried at <3% RH subsequently) exhibited a pattern of distorted fringes owing to the mixing of liquid TEAHBS and crystalline (NH4)2SO4.
Using a smog chamber, Murphy et al. (2007) studied the uptake of several short-chain amines by submicron (NH4)2SO4 particles at 10% RH. The particle composition was measured simultaneously by an Aerodyne Time of Flight Aerosol Mass Spectrometer (cToF-AMS) and a Particle-Into-Liquid Sampler coupled to Ion Chromatography (PILS-IC). They found a sudden increase in AMS-measured sulfate concentration when the uptake of amines started. However, the sulfate concentration measured by IC remained constant. They speculated that the amine reactions of (NH4)2SO4 may have turned the particle more liquid-like and increased the collection efficiency of the AMS. From this study, we showed the formation of liquid (or supercooled liquid) aminium sulfates (e.g., TEAHBS) as a product of TEA and (NH4)2SO4 reaction. The formation of liquid aminium sulfates may help explain the increase in particle collection efficiency in the AMS/chamber experiments of amine uptake by (NH4)2SO4. The reaction was more favorable in aqueous condition; yet, we still observed a <5% mass increase by crystalline (NH4)2SO4 under<3% RH condition. The mass increase at 10% RH is expected to be larger since TEAHBS uptakes water continuously, according to our measurements. Furthermore, the submicron particles used in Murphy et al. (Citation2007) are significantly smaller than the particles we used here and hence they are expected to have a larger mass transfer rate.
CONCLUSIONS AND ATMOSPHERIC IMPLICATIONS
Using a coupled EDB/Raman system, the heterogenous uptakes of TEA vapor by aqueous and solid (crystalline or amorphous) salts of common inorganic aerosol surrogates were investigated. Although we used particles much larger than atmospheric particles, the effects of the phase state of the particles on uptake of TEA were clearly observed. Aqueous droplets of (NH4)2SO4 and NH4HSO4 (50 or 75%) underwent an overwhelming equilibrium mass increase of >90% after exposure to 40-ppm TEA and a complete displacement of ammonium by TEAH was observed. However, crystalline solid (NH4)2SO4 and (NH4)2C2O4 were comparatively inert (<5% mass increase) to TEA uptake, presumably due to the diffusion limitation in these solids. Aqueous-phase chemistry played a significant role in ammonium displacement. Unlike the crystalline solids, amorphous NH4NO3 had a mass increase of >90% after an exposure to 40-ppm TEA at <3% RH. Moreover, amorphous NH4HSO4 (<3% RH) was also effectively neutralized by TEA, resulting in an equilibrium mass increase of about 50%. Enhanced TEA uptake by amorphous solid over that of crystalline solid in these experiments was attributed to the reduced mass transfer limitation. Amorphous and liquid-like particles have been postulated in the atmosphere (Mikhailov et al. Citation2009). Enhanced amine uptake by amorphous particles over those of crystalline particles in the atmosphere is expected. Qiu et al. (Citation2011) studied the reaction of alkylamines with NH4HSO4 and proposed the formation of alkylaminium ammonium sulfate based on vapor phase measurements by ID-CIMS. They suggested that NH4HSO4 was neutralized by vapors of alkylamines but did not address the bulk composition and the physical states of products. In this study, using Raman spectroscopy, we showed that the reaction of TEA and NH4HSO4 at <3% RH produced solid (NH4)2SO4 and liquid TEAH bisulfate/sulfate. We also examined for the first time to our knowledge the inter-conversion between TEAH bisulfate and sulfate.
Recent field observations have suggested that cloud and fog processing (Rehbein et al. Citation2011) enhanced the gas-to-particle partitioning of trimethylamine into sulfate-containing particles. Ambient acidic sulfate aerosols tend to have a higher fraction of TMA. According to the results of the current study, acidic NH4HSO4 can contribute to particulate TEAH formation through both neutralization reaction (bisulfate and TEA) and ammonium displacement. However, for (NH4)2SO4, TEAH formation can only be effective through ammonium displacement in the aqueous phase. Acidic NH4HSO4 had a higher potential to react with TEA through neutralization. This may help explain the increased particulate fractions of amines in acidic aerosol. In ambient environments, low RH conditions may induce potential crystallization of (NH4)2SO4 or ammoniated sulfates (Clegg et al. Citation1998), which in turn would suppress further aqueous ammonium displacement. Fog events and high humidity may favor the formation of particle-phase amines. We noted that the ambient concentration of ammonia is 1–3 orders higher than those of amines. Experiments involving the co-absorption of different amines, and amines/ammonia mixtures are warranted for a more complete understanding of amine uptake.
uast_a_618815_sup_21383194.zip
Download Zip (1.7 MB)Acknowledgments
This work was supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (GRF project nos. 600208 and 610909).
[Supplementary materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Angelino , S. , Suess , D. T. and Prather , K. A. 2001 . Formation of Aerosol Particles from Reactions of Secondary and Tertiary Alkylamines: Characterization by Aerosol Time-of-Flight Mass Spectrometry . Environ. Sci. Technol. , 35 ( 15 ) : 3130 – 3138 .
- Barsanti , K. C. , McMurry , P. H. and Smith , J. N. 2009 . The Potential Contribution of Organic Salts to New Particle Growth . Atmos. Chem. Phys. , 9 ( 9 ) : 2949 – 2957 .
- Belieres , J. P. and Angell , C. A. 2007 . Protic Ionic Liquids: Preparation, Characterization, and Proton Free Energy Level Representation . J. Phys. Chem. B , 111 ( 18 ) : 4926 – 4937 .
- Bzdek , B. R. , Ridge , D. P. and Johnston , M. V. 2010a . Amine Exchange into Ammonium Bisulfate and Ammonium Nitrate Nuclei . Atmos. Chem. Phys , 10 ( 8 ) : 3495 – 3503 .
- Bzdek , B. R. , Ridge , D. P. and Johnston , M. V. 2010b . Size-dependent Reactions of Ammonium Bisulfate Clusters with Dimethylamine . J. Phys. Chem. A , 114 ( 43 ) : 11638 – 11644 .
- Cadle , S. H. and Mulawa , P. A. 1980 . Low-molecular Weight Aliphatic-amines in Exhaust from Catalyst-equipped Cars . Environ. Sci. Technol. , 14 ( 6 ) : 718 – 723 .
- Chan , L. P. and Chan , C. K. 2011 . Enhanced Reactive Uptake of Nonanal by Acidic Aerosols in the Presence of Particle-Phase Organics . Aerosol Sci. Technol. , 45 ( 7 ) : 872 – 883 .
- Chan , L. P. , Lee , A. K. Y. and Chan , C. K. 2010 . Gas-Particle Partitioning of Alcohol Vapors on Organic Aerosols . Environ. Sci. Technol. , 44 ( 1 ) : 257 – 262 .
- Clegg , S. L. , Brimblecombe , P. and Wexler , A. S. 1998 . Thermodynamic Model of the System H+–NH4 +–SO4 2–NO3–H2O at Tropospheric Temperatures . J. Phys. Chem. A , 102 ( 12 ) : 2137 – 2154 .
- Collett , J. L. , Herckes , P. , Youngster , S. and Lee , T. 2008 . Processing of Atmospheric Organic Matter by California Radiation Fogs . Atmos. Res. , 87 ( 3–4 ) : 232 – 241 .
- Davis , E. J. 1997 . A History of Single Aerosol Particle Levitation . Aerosol Sci. Technol. , 26 ( 3 ) : 212 – 254 .
- Dawson , B. S. W. , Irish , D. E. and Toogood , G. E. 1986 . Vibrational Spectral Studies of Solutions at Elevated-Temperatures and Pressures 8. A Raman Spectral Study of Ammonium Hydrogen Sulfate-Solutions and the HSO4–SO4 2− Equilibrium . J. Phys. Chem. , 90 ( 2 ) : 334 – 341 .
- Debenedetti , P. G. and Stillinger , F. H. 2001 . Supercooled Liquids and the Glass Transition . Nature , 410 ( 6825 ) : 259 – 267 .
- Dong , J. L. , Li , X. H. , Zhao , L. J. , Xiao , H. S. , Wang , F. and Guo , X. 2007 . Raman Observation of the Interactions Between NH4 +, SO4 2-, and H2O in Supersaturated (NH4)2SO4 Droplets . J. Phys. Chem. B , 111 ( 42 ) : 12170 – 12176 .
- Drummond , C. J. and Greaves , T. L. 2008 . Protic Ionic Liquids: Properties and Applications . Chem. Rev. , 108 ( 1 ) : 206 – 237 .
- Facchini , M. C. , Decesari , S. , Rinaldi , M. , Carbone , C. , Finessi , E. Mircea , M. 2008 . Important Source of Marine Secondary Organic Aerosol from Biogenic Amines . Environ. Sci. Technol. , 42 ( 24 ) : 9116 – 9121 .
- Ge , X. L. , Wexler , A. S. and Clegg , S. L. 2011 . Atmospheric Amines—Part II: Thermodynamic Properties and Gas/Particle Partitioning . Atmos. Environ. , 45 ( 3 ) : 561 – 577 .
- Gronberg , L. , Lovkvist , P. and Jonsson , J. A. 1992 . Measurement of Aliphatic-Amines in Ambient Air and Rainwater . Chemosphere , 24 ( 10 ) : 1533 – 1540 .
- Hallquist , M. , Wenger , J. C. , Baltensperger , U. , Rudich , Y. , Simpson , D. and Claeys , M. et al. 2009 . The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues . Atmos. Chem. Phys , 9 ( 14 ) : 5155 – 5236 .
- Kroll , J. H. and Seinfeld , J. H. 2008 . Chemistry of Secondary Organic Aerosol: Formation and Evolution of Low-Volatility Organics in the Atmosphere . Atmos. Environ. , 42 ( 16 ) : 3593 – 3624 .
- Kurten , T. , Loukonen , V. , Vehkamaki , H. and Kulmala , M. 2008 . Amines are Likely to Enhance Neutral and Ion-Induced Sulfuric Acid-Water Nucleation in the Atmosphere more Effectively than Ammonia . Atmos. Chem. Phys , 8 ( 14 ) : 4095 – 4103 .
- Leach , J. , Blanch , A. and Bianchi , A. C. 1999 . Volatile Organic Compounds in an Urban Airborne Environment Adjacent to a Municipal Incinerator, Waste Collection Centre and Sewage Treatment Plant . Atmos. Environ. , 33 ( 26 ) : 4309 – 4325 .
- Lee , A. K. Y. , Ling , T. Y. and Chan , C. K. 2008 . Understanding Hygroscopic Growth and Phase Transformation of Aerosols Using Single Particle Raman Spectroscopy in an Electrodynamic Balance . Faraday Discuss. , 137 : 245 – 263 .
- Li , Y. J. , Chen , Q. , Guzman , M. I. , Chan , C. K. and Martin , S. T. 2011 . Second-generation Products Contribute Substantially to the Particle-phase Organic Material Produced by Beta-Caryophyllene Ozonolysis . Atmos. Chem. Phys , 11 ( 1 ) : 121 – 132 .
- Lin-Vien , D. , Colthup , N. B. , Fateley , W. G. and Grasselli , J. G. 1991 . The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules , Boston : Academic Press .
- Ling , T. Y. and Chan , C. K. 2008 . Partial Crystallization and Deliquescence of Particles Containing Ammonium Sulfate and Dicarboxylic Acids . J. Geophys. Res-Atmos. , 113 ( D14 ) : D14205
- Lloyd , J. A. , Heaton , K. J. and Johnston , M. V. 2009 . Reactive Uptake of Trimethylamine into Ammonium Nitrate Particles . J. Phys. Chem. A , 113 ( 17 ) : 4840 – 4843 .
- Makela , J. M. , Yli-Koivisto , S. , Hiltunen , V. , Seidl , W. , Swietlicki , E. Teinila , K. 2001 . Chemical Composition of Aerosol During Particle Formation Events in Boreal Forest . Tellus B , 53 ( 4 ) : 380 – 393 .
- Martin , S. T. 2000 . Phase Transitions of Aqueous Atmospheric Particles . Chem. Rev. , 100 ( 9 ) : 3403 – 3453 .
- Mikhailov , E. , Vlasenko , S. , Martin , S. T. , Koop , T. and Poschl , U. 2009 . Amorphous and Crystalline Aerosol Particles Interacting with Water Vapor: Conceptual Framework and Experimental Evidence for Restructuring, Phase Transitions and Kinetic Limitations . Atmos. Chem. Phys , 9 ( 24 ) : 9491 – 9522 .
- Murphy , S. M. , Sorooshian , A. , Kroll , J. H. , Ng , N. L. , Chhabra , P. Tong , C. 2007 . Secondary Aerosol Formation from Atmospheric Reactions of Aliphatic Amines . Atmos. Chem. Phys , 7 ( 9 ) : 2313 – 2337 .
- Peng , C. , Chan , M. N. and Chan , C. K. 2001 . The Hygroscopic Properties of Dicarboxylic and Multifunctional Acids: Measurements and UNIFAC Predictions . Environ. Sci. Technol. , 35 ( 22 ) : 4495 – 4501 .
- Pratt , K. A. , Hatch , L. E. and Prather , K. A. 2009 . Seasonal Volatility Dependence of Ambient Particle Phase Amines . Environ. Sci. Technol. , 43 ( 14 ) : 5276 – 5281 .
- Qiu , C. , Wang , L. , Lal , V. , Khalizov , A. F. and Zhang , R. Y. 2011 . Heterogeneous Reactions of Alkylamines with Ammonium Sulfate and Ammonium Bisulfate . Environ. Sci. Technol. , 45 ( 11 ) : 4748 – 4755 .
- Rabaud , N. E. , Ebeler , S. E. , Ashbaugh , L. L. and Flocchini , R. G. 2003 . Characterization and Quantification of Odorous and Non-Odorous Volatile Organic Compounds near a Commercial Dairy in California . Atmos. Environ. , 37 ( 7 ) : 933 – 940 .
- Rehbein , P. J. G. , Jeong , C. H. , McGuire , M. L. , Yao , X. H. , Corbin , J. C., and Evans , G. J. 2011 . Cloud and Fog Processing Enhanced Gas-to-Particle Partitioning of Trimethylamine . Environ. Sci. Technol. , 45 ( 10 ) : 4346 – 4352 .
- Reis , S. , Pinder , R. W. , Zhang , M. , Lijie , G. and Sutton , M. A. 2009 . Reactive Nitrogen in Atmospheric Emission Inventories . Atmos. Chem. Phys , 9 ( 19 ) : 7657 – 7677 .
- Schade , G. W. and Crutzen , P. J. 1995 . Emission of Aliphatic-Amines from Animal Husbandry and Their Reactions—Potential Source of N2O and HCN . J. Atmos. Chem. , 22 ( 3 ) : 319 – 346 .
- Silva , P. J. , Erupe , M. E. , Price , D. , Elias , J. , Malloy , Q. G. J. Li , Q. 2008 . Trimethylamine as Precursor to Secondary Organic Aerosol Formation via Nitrate Radical Reaction in the Atmosphere . Environ. Sci. Technol. , 42 ( 13 ) : 4689 – 4696 .
- Smith , J. N. , Barsanti , K. C. , Friedli , H. R. , Ehn , M. , Kulmala , M. Collins , D. R. 2010 . Observations of Aminium Salts in Atmospheric Nanoparticles and Possible Climatic Implications . Proc. Nat. Acad. Sci. U.S.A. , 107 ( 15 ) : 6634 – 6639 .
- Sorooshian , A. , Murphy , S. N. , Hersey , S. , Gates , H. , Padro , L. T. Nenes , A. 2008 . Comprehensive Airborne Characterization of Aerosol from a Major Bovine Source . Atmos. Chem. Phys , 8 ( 17 ) : 5489 – 5520 .
- Swartz , E. , Shi , Q. , Davidovits , P. , Jayne , J. T. , Worsnop , D. R. and Kolb , C. E. 1999 . Uptake of Gas-phase Ammonia. 2. Uptake by Sulfuric Acid Surfaces . J. Phys. Chem. A , 103 ( 44 ) : 8824 – 8833 .
- VandenBoer , T. C. , Petroff , A. , Markovic , M. Z. and Murphy , J. G. 2011 . Size Distribution of Alkyl Amines in Continental Particulate Matter and Their Online Detection in the Gas and Particle Phase . Atmos. Chem. Phys , 11 ( 9 ) : 4319 – 4332 .
- Wang , L. , Lal , V. , Khalizov , A. F. and Zhang , R. Y. 2010a . Heterogeneous Chemistry of Alkylamines with Sulfuric Acid: Implications for Atmospheric Formation of Alkylaminium Sulfates . Environ. Sci. Technol. , 44 ( 7 ) : 2461 – 2465 .
- Wang , X. F. , Gao , S. , Yang , X. , Chen , H. , Chen , J. M. Zhuang , G. S. 2010b . Evidence for High Molecular Weight Nitrogen-Containing Organic Salts in Urban Aerosols . Environ. Sci. Technol. , 44 ( 12 ) : 4441 – 4446 .
- Yin , S. , Ge , M. F. , Wang , W. G. , Liu , Z. and Wang , D. X. 2011 . Uptake of Gas-phase Alkylamines by Sulfuric Acid . Chinese Science Bulletin , 56 ( 12 ) : 1241 – 1245 .
- Zahardis , J. , Geddes , S. and Petrucci , G. A. 2008 . The ozonolysis of Primary Aliphatic Amines in Fine Particles . Atmos. Chem. Phys , 8 ( 5 ) : 1181 – 1194 .
- Zhang , K. M. and Wexler , A. S. 2002 . A Hypothesis for Growth of Fresh Atmospheric Nuclei . J. Geophys. Res-Atmos. , 107 ( D21 ) : 4577
- Zhang , Q. , Anastasio , C. and Jimemez-Cruz , M. 2002 . Water-soluble Organic Nitrogen in Atmospheric Fine Particles (PM2.5) from Northern California . J. Geophys. Res-Atmos. , 107 ( D11 ) : 4112
- Zobrist , B. , Marcolli , C. , Pedernera , D. A. and Koop , T. 2008 . Do Atmospheric Aerosols form Glasses? . Atmos. Chem. Phys , 8 ( 17 ) : 5221 – 5244 .