Abstract
Pairs of Institute of Occupational Medicine (IOM) and 37 mm closed face cassette samplers (CFC) were deployed where occupational exposures to lead-containing dusts were known to occur. Discrete particle analyses of wall and filter deposits were performed by Scanning Electron Microscopy—Energy Dispersive X-ray Spectrometry (SEM-EDX). From the elemental composition and projected area diameter of each particle a density, volume, and mass were calculated, and a mass-weighted size distribution for each filter and corresponding wall deposit determined. Comparison of pairs of wall and filter mass-weighted size distributions by Mann–Whitney statistical analysis shows that in only 3 of 72 examples from either sampler were the distributions significantly different that suggests that the mechanisms of particle deposition on the sampler walls for particles in this size range (0.5 μm through 20 μm) do not differ for the different samplers. Furthermore, in only 4 of 33 sampler pairs did the IOM and CFC results differ. Although these results originate from several distinct processes characterized by different chemical and physical dust generation mechanisms, they suggest that in these environments the measurement of “total dust” by the CFC and inhalable dust by the IOM will be very similar when both samplers are processed the same way with respect to the including or excluding wall deposits with the filter catch. However, these results may not be applicable to environments where larger particles exist.
INTRODUCTION
Various apparatus have been applied to measure lead dust exposures, but questions about the appropriate methodology remain (Harper et al. Citation2004; Harper and Pacolay Citation2006; Harper et al. Citation2006; Lee et al. Citation2009). Current occupational hygiene practice for assessing exposure to lead dusts is based on the concept that all particles that can be inhaled will contribute to the body burden. Hence, the historic aim of the procedure has been to sample total dust and then analyze for the lead content. In the 1940s, a holder was developed that could allow a vacuum to be applied to one side of a circular filter. Ambient air was pulled through from the other side of the filter causing entrained particles to be trapped on the filter for subsequent recovery and analysis. Because of the common assumption that all particles of interest would be collected this way the sample became colloquially known as “total dust”. Having a filter open to accidental or deliberate abuse was a problem, so a more enclosed holder known as the “Millipore Monitor” was developed in the 1950s, and by the 1960s had become the standard sampler for dust in the United States and elsewhere. The Millipore Monitor was also manufactured by other companies and so became known generically as the closed face cassette (CFC). It is typically 37 mm (1.5 inches) diameter, but 25 mm diameter cassettes are also popular, especially in Europe.
In the 1980s, the occupational hygiene community felt that a sampler should assess potential inhaled dose as closely as possible to mimic the human nose and mouth that do not inhale all particles sizes equally. An “inhalable” convention was agreed upon, and samplers were developed whose aspiration matched this convention. A sampler was developed to meet this convention at the Institute of Occupational Medicine (IOM) in Scotland. When this IOM sampler was tested against the CFC, it was found to collect considerably more mass of particulate from the air than the CFC, especially as the particle size increased (Werner et al. Citation1996). However, one problem with these tests is that they did not compare “apples to apples.” In the early development of the IOM sampler, the developers noted that a significant fraction of the dust aspirating into the sampler deposited on the internal surfaces of the sampler as well as on the filter (Mark Citation1990). These deposits were considered an important and integral part of the sample and were thus included in the analysis. However, this same consideration was not properly given to the CFC sampler.
More recently, it has been shown that including similar internal surface deposits in the analysis of CFC samples provides results much closer to those of collocated IOM samplers (while, conversely, analyzing only the filter catch of both samplers typically provides results that are not easily distinguishable) (Harper and Demange Citation2007). It is clear that analysis of only the filter catch of either sampler does not provide an accurate assessment of the inhaled potential dose (unless it can be demonstrated the nonfilter portion is insignificant—which can only be done by testing each sampler). Therefore, the Occupational Safety and Health Administration (OSHA) has clarified its position that the entire sample entering the sampler orifice is relevant. This has always been the situation for gravimetric sampling by OSHA method PV2121 (U.S. Department of Labor 2003), since this method requires that an internal capsule that contains both filter and wall deposits be weighed. The procedures in the OSHA methods for metals now include a wipe of the internal surfaces of the CFC as a part of the analysis. While this is not also the case for National Institute for Occupational Safety and Health (NIOSH) methods, there is a discussion of the issue in part 7 of the preamble to Chapter O: “Factors Affecting Aerosol Sampling” (NIOSH Manual of Analytical Methods) that suggests that it should be done.
It has been reported in a previous study (Lee et al. Citation2009) that the number—weighted particle size distribution of filter and internal surface deposits of lead sulfide and lead oxide aerosols up to 20 μm aerodynamic equivalent diameter (AED) generated and sampled by CFC and IOM samplers under laboratory conditions are indistinguishable. However, large particles could not be generated in that study and the strong contribution that larger particles could make to the overall measured mass concentrations and to differences between samplers is recognized. The primary goal of the present research is to extend that study to real workplaces where lead is the primary metal of concern and assess and compare the mass-weighted size distributions of aerosols captured on the filter and internal surfaces of the CFC and IOM samplers in environments where larger particles may occur. However, few such particles were actually observed, and certainly insufficient to allow statistical comparison of their size distribution.
In field studies conducted in France by the INRS (Demange et al. Citation2002) wall deposits added to the filter catch in CFC samplers resulted in mass concentrations of lead not considerably different from those found from collocated IOM samplers where both filters and walls were also accounted for. Similarly, the filter deposits of collocated IOM and CFC samplers in studies in the USA by Harper and Pacolay (Citation2006) also contained similar quantities of lead. In this work, samples were obtained and analyzed from field situations representing a range of metal-working environments where different particle size distributions were expected to exist. However, the results of this investigation are in line with previous field investigations that suggest larger particles are rare and even when present apparently contribute little to sampler collection differences in these environments.
METHODS
Sites Selected
The 5 field sites chosen for this study, a bronze foundry, a solder manufacturer, a lead ore concentrate mill, a lead-acid battery recycling facility, and a copper smelter, have already been studied for lead dust exposures (Harper et al. Citation2004; Harper et al. Citation2005; Harper and Pacolay Citation2006; Harper et al. Citation2007) and detailed descriptions of the facilities are given in the references. These sites were chosen because they present lead-containing aerosol exposures and include a wide variety of chemical and physical forms. The previous studies at these sites compared lead mass deposits on filters and in a few cases on walls, but did not compare the size distributions of particles. A summary description of sampling the 5 sites follows.
The bronze foundry was sampled at some locations where lead-containing aerosols were generated by condensation of metal vapor or processing of liquid metal, and at other locations where aerosols were generated by hammering the moulds to remove them from the solid cast metal (“shakeout”). The lead solder manufacturer was sampled at locations where aerosols were generated by condensation of metal vapor or by processing of liquid metal. The lead ore concentrate mill was sampled at locations where finely ground ore was processed in froth tanks, the aerosol exposure being generated by bursting bubbles on the surface of the froth, and also at two points earlier in the process where coarse ore was crushed, ground, and shaken. At the lead-acid battery recycling facility the sampled aerosols were generated from crushing old batteries made of lead and lead sulfate and by vapor phase condensation above smelters. At the copper smelter, aerosol samples were acquired near the kettles where metal vapors form and where liquid metals are processed, and at crushing operations, where aerosols were generated from the solid phase. Thus, these samples represent a wide range of particle generation processes where large differences in size distributions of the aerosols might be anticipated. However, this has not been checked by direct measurement of size distributions.
Sample Collection
From the sites described above area samples were obtained simultaneously from collocated pairs of samplers, one metal IOM and one plastic CFC. The samples were acquired according to the standard methods wherein the IOM sampler is oriented with its opening facing horizontally, and the CFC is oriented with its opening facing approximately 45o downwards (Buchan et al. Citation1986) and air drawn through them by calibrated pumps operating at a nominal 2 L/min. However, no special care was taken to ensure that all the samples were taken in this orientation, and some of the CFC samples may have been acquired with the opening facing horizontally. Standard 0.8 micron mixed cellulose ester (MCE) filters were used in the samplers. Back-up pads supported the filters in the CFC samplers. As all samplers were deployed as area samples, they may not have sampled the very largest particles to which a worker may be exposed. If they were acquired slightly more distant from the point where a worker stands then large particles may have had time to settle out of the collection zone. On the other hand, for many exposures the source of an aerosol exposure may not be a single point immediately adjacent to a worker, and therefore, an area sample may be equivalent to a personal sample. Area samplers also have the advantage over personal samplers in paired operation in that a single worker wearing a pair of samplers usually has them placed one to the left and one to the right. The worker's handedness can bias the sampling if he works with one side closer to the aerosol source, or if there is ambient air movement that favors one side or the other (Vaughan et al. Citation1990). In the lead ore concentrate mill, some of the samplers were located a few inches above the froth tanks and any particles small enough to be launched by bursting bubbles would enter the capture zone of the samplers. The total time that these samplers were accumulating aerosol varied from approximately 1 h to a full 8 h shift. Dustier locations were sampled for shorter periods of time to prevent overloading the filters. In addition to the paired IOM and CFC samplers, one set of IOM-only area samples was taken for 2 min exposures at several locations in the lead ore concentrate mill. These were taken for direct on-filter analysis to check for particle agglomeration. If agglomerates were present, it is possible that the sample preparation method used in this study might disrupt the agglomeration, thus altering the measured particle size in comparison to the airborne size distribution.
After pairs of samples were taken the IOM interior cassette components were removed and sealed in holders according to manufacturers’ instructions and the CFC samplers were sealed by plugging the inlet and outlet ports according to manufacturers’ instructions. If the flow rate through a sampler deviated from the NIOSH Method limits (i.e., from a nominal 2 L/min), the sample was discarded. To minimize the possibility of rough treatment that could dislodge dust from the filters and on to the walls or vice versa, all samples were hand carried from the sampled locations to the laboratory. The samples were then stored in a laboratory cabinet where the possibility of excessive movement or handling was minimized.
Analysis by SEM-EDX
Scanning Electron Microscopy—Energy Dispersive X-ray Spectrometry (SEM-EDX) was the method chosen to characterize the particle size distributions of the wall and filter deposits of the samplers and has been described previously (Lee et al. Citation2009). Particles were ultrasonically removed from filters or wall wipes into an iso-propanol suspension and aliquots taken by pipet and re-deposited with vacuum filtration onto 13 mm diameter, 0.2 μm pore size polycarbonate filters. The filters were attached to SEM stubs by conductive carbon tape and then carbon coated.
Stubs that were to be compared were interrogated with 20 keV incident beam energy and with approximately equal incident electron beam currents, between 1 and 5 nA, with most experiments performed between 1.9 and 2.4 nA. Excitation currents in this range were found to excite an X-ray count rate from sub-micron particles of low atomic number sufficient to generate a usable spectrum in 5 s, but did not cause an excessive detector dead time when large particles of high atomic number were interrogated. The X-ray spectrum of each individual particle was recorded from 0 to 20 keV and stored along with its projected area. The number of particles detected and characterized for each stub varied between 500 and 2200, but in most cases 700–1500 particles were characterized. These numbers do not include particles whose spectrum was very weak, that is, from filter artifacts or very small particles. In all, more than 130,000 particles were analyzed for this study.
As noted above, one separate set of sampling and SEM-EDX experiments was performed to validate the size distribution behavior of the redeposition method using the one set of IOM-only, 2 min personal samples captured on 0.2 μm pore size polycarbonate filters. From each filter 2 samples were prepared for SEM analysis. The filter was cut along a diameter and one half was attached to a 25 mm SEM stub by double stick carbon tape and carbon coated. The other half was treated as described above, using the entire 5 mL aliquot. The net result is that from one homogeneous sample 2 separate stubs were made, one containing one half of the original filter with all the dust still on it as it originally was captured, the other stub containing the re-deposited dust from the second half of the filter. As the sample filters were exposed to dust only for 2 min, the dust loading on the filter was light enough that overlapping particles were not an issue. Nevertheless, by using the entire aliquot from the dust suspended in alcohol there was enough dust recovered to render the SEM-EDX experiment practical.
Analysis by ICP-MS
The efficiency of lead recovery by the ultrasonic extraction and redeposition process of representative filter samples was quantified for a few random filters. The filter samples included an IOM filter from the solder manufacturer, an IOM and a CFC filter from the copper smelter, an IOM and 2 CFC filters from the lead battery recycler, and a CFC filter from the lead ore processor. The 7 filters were each split into 2 identical pieces. One piece was subjected to the redeposition procedure and the filter with redeposited dust was dissolved in nitric acid (5 mL each of Fisher Scientific Ultima and 18.2 M-Ω-cm water) and analyzed by inductively coupled plasma mass spectrometery (ICP-MS, Perkin Elmer Nexion 300D). The other half was dissolved in nitric acid and analyzed with no other treatment. Dissolutions were performed in a microwave reactor (CEM MARS) in PTFE (polytetrafluoroethylene) sample tubes at a maximum temperature of 200°C. After cooling, the solutions were quantitatively transferred and diluted to volume with 10% nitric acid. Immediately prior to analysis by the Perkin–Elmer Nexion ICP-MS, they were filtered through PTFE filters. Assuming the 2 pieces of each filter contained identical masses of lead, the recovery efficiency is the ratio of the mass of recovered lead to the mass of lead on the original half filter. Mass balance was checked by analyzing the remnant lead on the ultrasonically treated filter pieces in the same way.
Data Analysis
The image analyses and X-ray integrations were performed by PGT IMIX software, version 9, as described previously (Lee et al. Citation2009). The X-ray intensities were imported into a Microsoft Excel spreadsheet for all subsequent steps of the data analysis. For each individual particle the elemental X-ray intensities weighted by Cliff–Lorimer factors (Reed Citation1993) were used to calculate its elemental composition, omitting elements lighter than sodium. If the total integrated X-ray intensities from elements of atomic number 11 and larger were below a threshold of hundred X-ray counts per nano-amp excitation beam current, the particle was ignored in subsequent data analysis. The threshold was established by inspection of typical particle spectra and typical background spectra. The purpose of dropping these data from the analysis is to exclude features in the polycarbonate filter from being counted as particles, which can happen if the bremsstrahlung background is sufficiently intense. The volume of a single particle was estimated from its two-dimensional image by using the measured projected area equivalent diameter as the diameter of a sphere. Multiplying that sphere's volume by the density of the particle yields the particle's mass. There was no attempt to include a shape factor correction, so the result of the calculation is an approximation to aerodynamic diameter. The approximate mass-weighted size distributions of particles on filters and walls of the different samplers can be compared because they would have similar shapes, even though the calculated size distributions may not be accurately representative of the actual aerodynamic sizes of the particles. The density of the particle was estimated from its elemental composition as derived from the X-ray spectrum. Elements of atomic number 11 through 20 (sodium through calcium) were assumed to be present as minerals with a density of 2500 kg/m3, which is a reasonable approximation for common oxides, silicates, and carbonates. Transition metals were assumed to have their macroscopic elemental densities. The density of lead was assumed to be that of the sulfide in samples from locations where the sulfide is known to be predominant, and the density assumed to be that of the element in cases where the lead is known to be aerosolized in elemental or alloy form. The density of tin was assumed to be that of the element. Finally, this approximate “aerodynamic equivalent diameter” of a particle was calculated by multiplying the particle volume by the square root of its specific gravity (Hinds Citation1999).
TABLE 1 Results of comparisons between particle mass-weighted size distributions of filter and wall deposit pairs and IOM and CFC filter pairs
After the particle masses for a given sample were calculated, they were grouped into fifty-two intervals between 0.0749 pg and 4070.5 pg. (These mass intervals correspond to aerodynamic equivalent diameters of spheres ranging from 0.523 to 19.81 μm, size intervals used by the TSI Aerodynamic Particle Sizer described and used previously (Lee et al. Citation2009)). Although this method will not yield absolute values of particle mass-weighted size distributions, it will generate relative approximations that can be usefully compared, which is what is required here. By the calculation used here, the vast majority of particles in the field samples were found to be smaller than 20 μm “AED,” which allows us to compare the results directly to those obtained in the laboratory study (Lee et al. Citation2009).
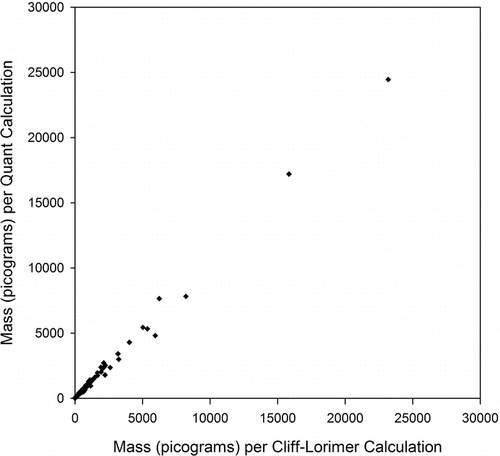
A check on the validity of using Cliff–Lorimer analysis to calculate composition from an X-ray spectrum was performed by analyzing 1 set of 988 particles from a sampler used at the lead ore processing facility, calculating the masses with both the PGT IMIX Quant software package and the Cliff–Lorimer method (Reed Citation1993) and comparing the results. The Quant software package calculates elemental composition by accounting for absorption and fluorescence and is, in fact a more accurate way to analyze a particle, but it is not practical to use it to determine the composition of the more than 130,000 particles studied here. In calculating individual particle mass, the two methods were found to be linearly related with a slope near unity as shown in .
FIG. 1 Comparison of individual particle masses of 988 particles from the lead ore processing facility calculated by the IMIX Quant and the Cliff–Lorimer methods. The vast majority of particles have a mass less than 1000 pg.
SEM data (as pairs of cumulative mass-weighted size distributions) were compared using the Mann–Whitney U Test (Ramsey and Schafer Citation2002). While generally distributions are compared using the Anderson–Darling or Kolmogorov–Smirnov tests, those both rely on specific distributions; however, these data were not assumed to come from any specific probability distribution and the Mann–Whitney has been used as a nonparametric distribution comparison test.
RESULTS
The results of all 5 sampling campaigns are listed in . The entries in the column listing the number of job locations denote how many distinct exposures were sampled by a pair of IOM and CFC samplers. For example, in the bronze foundry, there were 2 locations of shakeout that were sampled, but they would be expected to generate indistinguishable exposures and so they are counted as only 1 job location. Entries in the (second) column listing number of particles denote the total number of particles each of which generated enough X-ray intensity in elements of atomic number larger than 10 to be identified as a metal-containing particle. The total includes the wall and filter samples from both IOM and CFC samplers. Entries in the wall versus filter differences (third) column denote the number of paired mass-weighted size distributions that were statistically significantly different compared to the total number of paired distributions. For example, in analyses of paired wall and filter deposits from fourteen samplers from the bronze foundry, only one pair had different mass distributions. The fifth column of lists the results of comparing the CFC and IOM filter deposits.
The first sampling campaign at the bronze foundry provided a total of 7 pairs of sampler analyses spanning 4 job exposures in the foundry including pouring, molding, shakeout, and sandblasting. There were a total of 41,527 particles identified and analyzed. Statistical analysis of the mass-weighted size distributions as a function of “AED” (as estimated here without shape factor) indicated that for only one sampler, positioned near a metal pouring location, did the filter deposit size distribution differ significantly from the wall deposit size distribution. In none of the 7 pairs of samplers did the IOM and CFC filter sample size distributions significantly differ (p < .05). The data sets for different locations of the same job having been combined, for example, all the shakeout data having been combined, all the sandblasting data having been combined, and all the mould data having been combined, it was still observed that there were no significant differences between the filters and walls and between the IOM and the CFC filters.
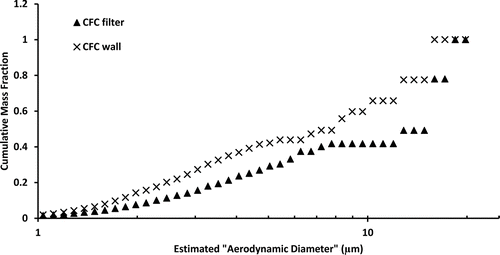
An example of the (normalized) mass-weighted size distribution curves from the filters of a CFC and IOM pair that were not statistically different is shown in . As an example of distributions that are statistically different, shows the normalized distributions of the wall and filter pair of a CFC sampler used at a metal pouring location.
FIG. 2 Example of normalized mass distributions of an IOM and CFC filter pair that were not significantly different (according to Mann–Whitney statistical analysis.)
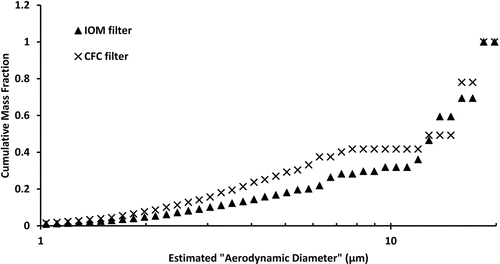
FIG. 3 Example of normalized mass distributions of a CFC wall and filter pair that were significantly different (according to Mann–Whitney statistical analysis.)
The solder manufacturing plant provided a total of 5 pairs of IOM and CFC sampler analyses taken from 3 job locations in the facility. There were a total of 21,601 particles imaged and analyzed. Statistical analysis of their mass-weighted size distributions indicated that only 1 filter sample differed significantly from its corresponding wall deposit sample, and only 1 IOM filter sample differed significantly from its corresponding CFC filter sample.
There were 2 sampling campaigns executed at the lead ore concentrate mill, the first was for the same purposes as the other campaigns and which provided a total of eight IOM and CFC filter pairs. One each of an additional IOM and CFC sample were taken but not as a simultaneous collocated pair. There were a total of 36,951 particles analyzed. Statistical analyses of the normalized distributions show that no filter sample differed significantly from its corresponding wall deposit sample, and only one IOM filter sample differed significantly from its corresponding CFC filter sample.
The second sampling campaign at the lead ore concentrate mill involved only IOM filters that were exposed for a very brief time to aerosol. As described earlier, the purpose of these IOM only samples was to validate the method by which the samples were treated to make SEM stubs. The method was validated for homogenous laboratory samples previously (Lee et al. Citation2009), but it was necessary to re-validate the method for heterogeneous field samples. The measurements of size distributions of these pairs, re-deposited versus direct-on-filter, were treated statistically as described above. A total of fourteen IOM sample filters from the lead ore concentrate mill were collected, split, and analyzed. In all cases, the direct-on-filter measured particle mass-weighted size distributions did not differ from their corresponding measurements made by redeposition, thereby validating the method for field samples.
The lead acid battery recycler sampling campaign provided 6 pairs of IOM and CFC samplers and an additional 3 unpaired samplers. These were located at 5 distinct job locations in the facility, and 24,295 particles were imaged and analyzed. There was 1 example of a filter and corresponding wall distribution that differed and there was 1 example where the distributions of corresponding IOM and CFC filter deposits differed.
The copper smelter sampling campaign provided a total of 7 IOM and CFC filter pair analyses and 1 unpaired sampler stationed at a total of 5 distinct job locations in the smelter. There were a total of 19,050 particles identified. In none of the 15 wall and filter pairs was there a difference in mass-weighted size distributions, and in only one of the seven pairs of IOM and CFC filters was there a difference.
The seven measurements by ICP-MS of the efficiency of lead recovery averaged 50% and ranged from 18 to 102%, where efficiency is defined as redeposited lead mass divided by lead mass on the original half filter. Overall mass balances averaged 80% and ranged from 43 to 133%, where overall mass is defined as the sum of redeposited lead mass and remnant (on the ultrasonically treated half filter) lead mass, divided by the lead mass on the original half filter. For 2 filters the overall mass balance was near unity and the ultrasonic recovery was a reasonable value near 70%. The cause of the variability in lead measurements in the other 5 samples is unknown.
DISCUSSION
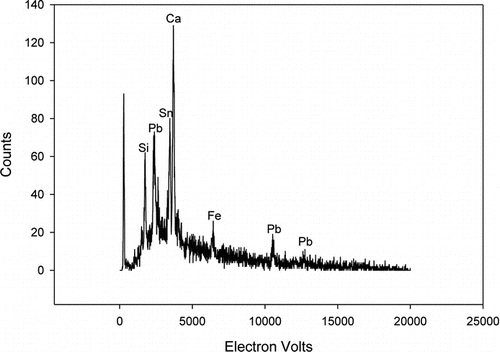
An example of an SEM-back-scattered-electron (BSE) image of a single field of particles from the wall of a CFC sampler is shown in , the sample having been taken where a 38% tin and 60% lead alloy was cast. The majority of the particles in this field have area equivalent diameters around 1 μm. By way of illustrating the methods used here, the large particle circled in the field image is discussed in some detail. It has an area equivalent diameter of 2.1 μm. The X-ray spectrum from that particle is shown in , where strong lines from lead, tin, calcium, sulfur, and silicon are readily apparent. There are also weaker lines from iron, potassium, and chlorine. The density of the particle was calculated to be 5400 kg/m3, intermediate between the assumed value of 2500 kg/m3 for calcium and silicate minerals and the elemental values of 7300 and 11,300 kg/m3 for tin and lead, respectively. Its “approximate aerodynamic equivalent diameter,” calculated from its density and its area equivalent diameter (by the method described above), is found to be 4.9 μm and its mass calculated to be 27 pg.
FIG. 4 Typical field of particles on a polycarbonate filter, imaged by backscattered electrons at a magnification of 1500 X. The circled particle has an “estimated aerodynamic equivalent diameter” of 2.1 μm and is composed of a mixture of mostly calcium, tin, silicon, and lead, with traces of iron, potassium, and chlorine.
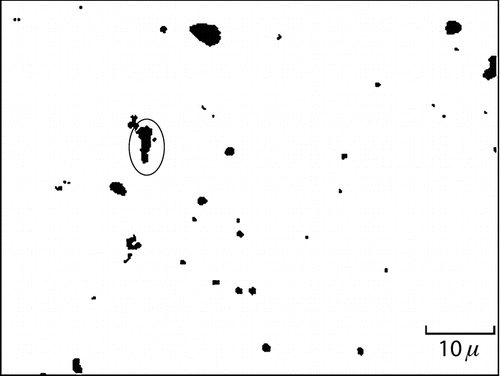
FIG. 5 X-ray spectrum of particle shown in . Five seconds of data acquisition excited by 20 keV electrons at 2.1 nA current. Two weaker lines not labeled in this spectrum are from potassium and chlorine.
Size-related deposition differences in the IOM sampler have previously been noted (Mark Citation1990; Aitken and Donaldson Citation1996; Kenney et al. 1997) in laboratory studies where particle sizes were used that were much larger than those found in these representative workplaces. Given that the wall and filter deposits of both the CFC samplers and IOM samplers are very similar in terms of the mass weighted size distribution for all of the exposures studied here, it is apparent that the mechanisms of wall deposition for particles smaller than approximately 20 μm AED are not size selective. We observed very few particles above 20 μm, as estimated here without shape factor. Prior explanations of wall deposits postulated a number of size selective mechanisms, including electrostatic deposition, gravitational settling, particle bounce, turbulence, and inertial impaction (NIOSH Citation2010). Many of these processes are dependent on particle size. As size bias between walls and filters were not observed, it may rule out the operation of some processes in the size range encountered. Thus, gravitational settling is unlikely to be a dominant process. Particle bounce arises from particle inertia and therefore also favors larger particles and hence it too cannot be a dominant mechanism here (Li et al. Citation2000). The effects of turbulence on deposition are complicated, but in general larger particles deposit faster (Guha Citation2008) suggesting that turbulence is not a dominant mechanism either. Furthermore, if turbulence were a dominant mechanism there would likely be a large difference in the IOM and CFC results reflecting the large difference in entrance air velocity between the two samplers (approximately a factor of 14). Inertial projection of large particles at an angle through the orifice may also cause particles to impact on and adhere to the walls, but again, that process would affect only particles massive enough to maintain a continuous trajectory over many centimeters of travel and in that case the wall deposit would comprise more large particles. Thus, wall deposits appear to result from some combination of electrostatic, inertial, gravitational, turbulence, and diffusion mechanisms that are not size selective. Finally, because of the special diligence exercised here it is certain that the act of transporting samplers from the field to the laboratory cannot explain the presence of wall deposits as simply dust that was dislodged by rough handling from the filter surface and fell onto the interior walls.
CONCLUSIONS AND RECOMMENDATION
The first conclusion to be drawn from these results is that when lead-containing aerosols of a wide variety of composition, size, and mode of generation are sampled in these metal-working environments by IOM and CFC samplers as area samplers, the size distribution of the material deposited on the walls of the samplers is generally indistinguishable from the filter deposits since the sampled aerosol generally contains only particles smaller than 20 μm AED. As the wall deposits do not differ in aerodynamic size from the filter deposits, they should be considered as biologically relevant whenever they comprise a substantial fraction of the total catch, which is often the case. Previous work has reported that the CFC and IOM median wall fraction of lead in deposits from 4 of these same 5 sites ranged from 0 to 30%, (Harper et al. Citation2007). Therefore, it is recommended that the proper metric of any “total dust” or inhalable aerosol sampling with CFC and IOM samplers should include wall deposits with the filter catch. Unfortunately, this suggests that the large body of historical exposure data gathered from CFC and IOM filter-only analyses under-reports total exposures, and this also suggests a large uncertainty in those exposure measurements. Moreover, the findings reported here are consistent with those reported earlier for the example of lead oxide and lead sulfide dusts generated in a laboratory where the parent aerosol populations also contained very few particles larger than 20 μm AED. The IOM and CFC filter deposits were the same, and for both samplers in all cases the wall deposits and filter deposits were the same (Lee et al. Citation2009).
The second conclusion is that under circumstances where it is likely that only particles of AED less than 20 μm are present, as appears to be the case for area samples at the range of metal-processing establishments studied in this work, the CFC and IOM filter and wall deposit results are very similar in terms of particle mass-weighted collection. This observation should be useful in comparing historical exposures taken with CFC samplers to exposures taken with the IOM.
Acknowledgments
This article not subject to United States Copyright law. Thanks are due to Mr. Patrick J. Hintz (NIOSH, Spokane Research Laboratory, Spokane, Washington, now with the USFDA in MD) and Mr. Bruce Pacolay (NIOSH, Health Effects Laboratory Division, Morgantown, WV, now with RJ Group, Pittsburgh, PA) for obtaining many of the samples used in this work. Thanks are also due to the referees whose time and efforts contributed significantly to the final form of this paper. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- Aitken , R. J. and Donaldson , R. 1996 . Large Particle and Wall Deposition Effects in Inhalable Samplers , Sudbury, Suffolk, UK : HSE Contract Research Report No. 117/1996. Health and Safety Executive .
- Buchan , R. M. , Soderholm , S. C. and Tillery , M. I. 1986 . Aerosol Sampling Efficiency of 37 mm Filter Cassettes . Am. Ind. Hyg. Assoc. J. , 47 : 825 – 831 .
- Demange , M. , Gorner , P. , Elcabache , J. M. and Wrobel , R. 2002 . Field Comparison of 37-mm Closed-Face Cassettes and IOM Samplers . Appl. Occup. Environ. Hyg. , 17 : 200 – 208 .
- Guha , A. 2008 . Transport and Deposition of Particles in Turbulent and Laminar Flow . Annu. Rev. Fluid Mech. , 40 : 311 – 41 .
- Harper , M. and Demange , M. 2007 . Analytical Performance Criteria—Concerning Sampler Wall Deposits in the Chemical Analysisi of Airborne Metals . J. Occup. Env. Hyg. , 4 : D81 – D86 .
- Harper , M. , Hallmark , T. S. , Andrew , M. E. and Bird , A. J. 2004 . A Comparison of X-ray Fluorescence and Wet Chemical Analysis of Air Filter Samples from a Scrap Lead Smelting Operation . J. Environ. Monitor. , 6 : 819 – 826 .
- Harper , M. and Pacolay , B. 2006 . A Comparison of X-ray Fluorescence and Wet Chemical Analysis for Lead on Air Filters from Different Personal Samplers Used in a Secondary Lead Smelter/Solder Manufacturer . J. Environ. Monitor. , 8 : 140 – 146 .
- Harper , M. , Pacolay , B. and Andrew , M. E. 2005 . A Comparison of X-ray Fluorescence and Wet Chemical Analysis for Lead on Air Filters from Different Personal Samplers Used in a Bronze Foundry . J. Environ. Monitor. , 7 : 592 – 597 .
- Harper , M. , Pacolay , B. , Hintz , P. and Andrew , M. E. 2006 . A Comparison of Portable XRF and ICP-OES Analysis for Lead on Air Filter Samples from a Lead Ore Concentrator Mill and a Lead-Acid Battery Recycler . J. Environ. Monitor. , 8 : 384 – 392 .
- Harper , M. , Pacolay , B. , Hintz , P. , Bartly , D. L. , Slaven , J. E. and Andrew , M. E. 2007 . Portable XRF Analysis of Occupational Air Filter Samples from Different Workplaces Using Different Sampler: Final Results, Summary and Conclusion . J. Environ. Monitor. , 9 : 1263 – 1270 .
- Hinds , W. C. 1999 . Aerosol Technology , New York : John Wiley & Sons Inc .
- Kenny , L. C. , Aitken , R. , Chalmers , C. , Fabriès , J. F. , Gonzalez-Fernandez , E. Kromhout , H. 1997 . A Collaborative European Study of Personal inhalable Sampler Performance . Ann. Occup. Hyg. , 41 : 135 – 153 .
- Lee , T. , Chisholm , W. P. , Slaven , J. E. and Harper , M. 2009 . Size Distributions of 0.5 to 20 μm Aerodynamic Diameter Lead-Containing Particles from Aerosol Sampler Walls and Filters . Aerosol. Sci. Tech. , 43 : 1042 – 1050 .
- Li , S. N. , Lundgren , D. A. and Rovell-Rixx , D. 2000 . Evaluation of Six Inhalable Aerosol Samplers . Amer. Ind. Hyg. Assoc. J. , 61 ( 4 ) : 506 – 516 .
- Mark , D. 1990 . The Use of Dust-Collecting Cassettes in Dust Samplers . Ann. Occup. Hyg. , 34 : 281 – 291 .
- National Institute for Occupational Safety and Health (NIOSH) . 2010 . NIOSH Manual of Analytical Methods, NIOSH Publications . Center for Disease Control and Prevention , http://www.cdc.gov/niosh/docs/2003–154/pdfs/chapter-o.pdf. (Accessed 12/10/2010)
- Ramsey , F. and Schafer , D. 2002 . The Statistical Sleuth, A Course in Methods of Data Analysis, Second Edition , Duxbury Resource Center, Pacific Grove, California, USA. .
- Reed , S. J. B. 1993 . Electron Microprobe Analysis, Second Edition , Cambridge : Cambridge University Press .
- U.S. Department of Labor . 2003 . U.S. Department of Labor Occupational Safety and Health Administration: OSHA Analytical Method PV2121, Gravimetric Determination. . OSHA, 2003, Sampling and Analysis Methods. Sandy, UT: OSHA, Salt Lake Technical Center, v 2003
- Vaughan , N. P. , Chalmers , C. P. and Botham , R. A. 1990 . Field Comparison of Personal Samplers for Inhalable Dust . Ann. Occup. Hyg. , 34 : 553 – 573 .
- Werner , M. A. , Spear , T. M. and Vincent , J. H. 1996 . Investigation Into the Impact of Introducing Workplace Aerosol Standards Based on the Inhalable Fraction . Analyst , 121 : 1207 – 1214 .