Abstract
The 2 chromium oxidation states found in ambient atmospheric particulate matter (PM) are trivalent [Cr(III)] and hexavalent [Cr(VI)] chromium. Cr(III) is a trace element essential for the proper function of living organisms. However, Cr(VI) is toxic and exposure to Cr(VI) may lead to cancer, nasal damage, asthma, bronchitis, pneumonitis, inflammation, dermatitis, and skin allergies. Therefore, it is important to accurately discriminate between these 2 species in atmospheric PM samples. This work focuses on the effect of deliquescence and pH on chromium speciation in filter samples of airborne PM collected in the northeastern USA. The deliquescence relative humidity (DRH) and liquid water mass content determined for the ambient particle samples are in good agreement with previously reported values for ammonium sulfate and ammonium nitrate suggesting that these 2 salts control the hygroscopic properties of the ambient particles in the northeastern USA. The loss of Cr(VI) increases significantly up to 85% at acidic pH as Cr(III) becomes more stable. Under basic pH conditions, deliquescence increases the loss of Cr(VI) such that up to 33% reduction was observed at 96% relative humidity (RH). No statistically significant difference was observed for Cr(VI) and Cr(III) interconversion over a range of ambient PM mass. Because of the effect of deliquescence on chromium speciation at basic pH, a new design criterion for Cr(VI) samplers can be defined to preserve the collected Cr(VI). DRH of sodium bicarbonate, K2Cr2O7, and Cr(NO3)3 was found to be 91%, 94%, and 52%, respectively.
Copyright 2012 American Association for Aerosol Research
INTRODUCTION
Chromium primarily exists as trivalent [Cr(III)] and hexavalent [Cr(VI)] states in ambient atmospheric particulate matter (PM; Rahman et al. Citation2005). Cr(III) is a trace element essential for the proper function of living organisms. In contrast, Cr(VI) is toxic and exposure to Cr(VI) may lead to cancer, nasal damage, asthma, bronchitis, pneumonitis, inflammation, dermatitis, and skin allergies. Therefore, it is important to be able to accurately discriminate between these 2 species in atmospheric samples.
In aqueous systems, Cr(III) can be found as Cr3+, Cr(OH)2+, Cr(OH)+ 2, and Cr(OH)− 4 (Guertin et al. Citation2005). Cr(III) precipitates at pH values between 6 and 12 as Cr(OH)3 (Rai et al. Citation1987). At very low pH values around 1, H2CrO4 becomes the predominant species of Cr(VI). Only CrO2− 4 exists at pH values above 6 (Davis and Olsen Citation1995). For the pH range of atmospheric aerosols and droplets, HCrO− 4 is the dominant species (Seigneur and Constantino Citation1995). In the oxidation of Cr(III) to Cr(VI), it was found that Mn(III) and Mn(IV) are important intermediates in the oxidation mechanisms (Seigneur and Constantino Citation1995; Nico and Zasoski Citation2000).
Several pathways have been reported for the reduction of Cr(VI) to Cr(III). Iron(II) (Pettine et al. Citation1998), reduced sulfur species such as S, S2−, H2S, and S2O3, (Schroeder and Lee Citation1975), humic and fulvic acids (Palmer and Plus Citation1994), and Cu(I) especially at low pH (Abu-Saba et al. Citation2000) can reduce Cr(VI) to Cr(III). The efficiency of cations and anions on the conversion of Cr(VI) to Cr(III) was reported to be in the order of Cu2+ > Fe3+> Ni2+ and NO− 3> Cl−> SO2− 4, respectively (Goshu et al. Citation2007).
There is a lack of experimental data for Cr(VI) and Cr(III) reduction and oxidation under atmospheric conditions. Grohse et al. (Citation1988) studied the conversion of Cr(VI) in the presence of mixtures of reactants including HNO3, HCHO, O3, NO2, SO2, m-xylene, benzene, Fe2+, and V2+ in a laboratory reaction chamber under typical atmospheric conditions. The conversion of Cr(VI) to Cr(III) after 24 h ranged from <5% to 99%. This large variation in conversion rates was attributed to the variations in the concentrations of the species that react with Cr(VI). They also estimated an average half-life of 16 h for the Cr(VI) in the atmosphere.
Seigneur and Constantino (Citation1995) simulated the solution chemistry of chromium at atmospheric pH and concentration conditions using kinetic data for the oxidation of Cr(III) and reduction of Cr(VI) available for aqueous media. They found that the overall trend is toward the reduction of Cr(VI) to Cr(III) with half-lives ranging from a few seconds to several minutes. Their modeling did not consider the reactions of Cr(VI) with atmospheric organic compounds or gaseous reactants.
Werner et al. (Citation2006) and Nico et al. (Citation2009) simulated the atmospheric aging of chromium through the reaction of ozone and reactive oxygen species (ROS) with chromium ultrafine particles. ROS was generated through the reaction of ozone (∼1 ppmv) and sunlight in the presence of water vapor (70% RH). In their studies, chromium particles were generated by combustion, and consisted of pure Cr, Cr-Fe or Cr-Fe-Mn matrices. Werner et al. (Citation2006) showed that ROS played an important role in the reduction of pure Cr particles under simulated atmospheric aging. Ozone (in the presence of water vapor) did not significantly change the Cr(VI)/Cr(total) ratio. In addition, Nico et al. (Citation2009) found that reaction of the ozone with the Cr-Mn-Fe matrix in the presence of light and water vapor caused reduction followed by the oxidation of chromium during atmospheric aging. However, in the absence of ozone, chromium reduction was observed, while the reduction pathway resulted from either the reaction of volatile organic compounds or, more likely, the transfer of an electron from water to the metal because of irradiation. Since Werner et al. (Citation2006) and Nico et al. (Citation2009) did not utilize an ambient particle matrix in their studies, the simultaneous influences of the ambient matrix and the RH on the ambient chromium speciation is still unknown.
Interconversion between chromium oxidation states can occur in solution. One of the phenomena that can supply the water to facilitate chromium reactions is deliquescence. When the activity of water in the air is high enough, soluble salt particles absorb water and form a liquid phase on the particle surface. Koloutso-Vakakis and Rood (Citation1994) reported deliquescence relative humidity (DRH) values between 74% and 79% for PM samples from Riverside, California. Semeniuk et al. (Citation2007) studied the DRH of different particles generated from eighty individual aerosol particles collected from young smoke of flaming and smoldering fires. They found that soot tar balls did not deliquesce. Hameri et al. (Citation2002) investigated the DRH for internally mixed ammonium sulfate and carboxylic acid and also ammonium sulfate and phthalic acid particles. The same approach was used to investigate the effect of the organic compounds on the hygroscopicity of NaCl particles by Hansson et al. (Citation1998). Both groups found that the impact of the organic compounds on the DRH of inorganic–organic particles was negligible.
Various studies (Saxena et al. Citation1995; Cruz and Pandis Citation2000; Speer et al. Citation2003) have shown that oxidized organic materials such as organic carbon or pinonic and glutaric acids add to the hygroscopicity of ambient aerosol. Xu et al. (Citation2003) reported a DRH of NaCl–formic acid of about 68.5% while the DRH of pure NaCl was observed to be 75%. Semeniuk et al. (Citation2007) studied the effect of different organic compounds mixed with NaCl on water uptake of particles including a sulfate-coated NaCl/silicate aggregate particle, a sulfate coated sea-salt particle, and a Mg-rich, chloride-coated sea-salt particle. These samples showed that initial water uptake began between 50% and 60% RH with the first major morphological changes occurring at 70% RH.
Current sampling methods do not control the RH of the sampled airstream such that collected PM is subjected to a range of RH values over the course of the sampling interval. The aim of this work is to investigate the impact of deliquescence and pH on chromium speciation in ambient PM samples. This information is important for understanding chromium chemistry on air filters and is needed to provide a basis for designing better Cr(VI) air samplers.
EXPERIMENTAL METHODS
Eighty cellulose filters were leached overnight in 10% nitric acid solutions to remove any chromium contamination (initial values of 0.3 to 0.7 μg Cr/g filter). The filters were washed with ultrapure water and dried overnight in a clean bench. After drying, forty of the filters were soaked again in a fresh 10% nitric acid solution overnight and then dried in a clean bench. The pH of the filters was measured using pH indicator paper and found to be between 1 and 2. To prepare basic cellulose filters, the other acid-washed filters were impregnated with 500 mL of a 0.12 M sodium bicarbonate solution. The filters were dried in a clean bench producing filters with pH values between 9 and 10.
To provide samples with similar mass and components for subsequent chromium stability studies, 4 FRM samplers (Rupprecht and Patachnick Model 2000H) equipped with TSP inlets were operated simultaneously with 4 basic or acidic cellulose filters prepared each sampling day, from November 2010 to February 2011. Sampling was performed with flow rates of 16.7 L min−1 on the roof of an academic building on the Clarkson University campus in Potsdam, New York. Daily samples provided different PM masses on each set of 4 filters. After sampling, the filters were transferred to petri dishes and stored in a freezer at −20°C until needed. To measure the DRH of ambient PM, the 4 FRM samplers equipped with PM2.5 inlets and Teflon filters (Whatman) were operated for 72 h with flow rates of 16.7 L min−1 in Potsdam.
To study the influence of ambient PM mass on chromium speciation, sampling was performed using 4 FRM samplers for 14, 24, 48, and 72 h with flow rates of 27 L min−1 from March to May 2011. Three samplers with basic cellulose filters and 1 sampler with a baked quartz filter were utilized. The quartz filters were used for the determination of organic and elemental carbons as well as metal concentrations. A punch was taken from the filter and analyzed for OC and EC using the NIOSH protocol (Birch and Cary Citation1996). The remainder of the filter was acid digested with nitric acid using a CEM MARS5. The digested samples were filtered and analyzed using a Thermo X-series ICP/MS. The experiments were repeated for 8 quartz filters. Part of the extract was analyzed for ferrous iron concentrations using the method developed by Gendel and Lahav (Citation2008).
Solutions of Cr(III) (Cr(NO3)3) and Cr(VI) (K2Cr2O7) isotopically enriched standards were purchased from Applied Isotope Technology (Pittsburgh, Pennsylvania, USA) and refrigerated at 4°C. 53Cr(VI) and 50Cr(III) isotopes were separately spiked on the basic and acidic filters such that spiked filters contained isotopically enriched Cr(VI) and Cr(III) (). All of the spiked filters were dried in vacuum desiccators after spiking and stored in a freezer at −20°C until use. All of the experiments were repeated a minimum of 4 times.
TABLE 1 Average mass of sampled PM and spiked chromium isotopes in this work
A 20 cm × 10 cm × 10 cm plastic glove chamber was used to conduct experiments in which the basic and acidic cellulose filters and Teflon filters were exposed to a well-defined RH. The chamber was kept in the dark. The RH in the chamber was adjusted using saturated salt solutions at 25°C (Lide 2004). Relative humidity and temperature were measured using an RH meter and thermometer (Rotronic Model HygroPalm 3). The experiments for Cr(VI) were repeated 5 times for sampled basic filters and 6 times for sampled acidic filters. After exposure to the specific humidity, the samples were sonicated for 1 h in 20 mM sodium bicarbonate solution at a pH of approximately 9 before analysis.
To investigate the deliquescence points of K2Cr2O7, Cr(NO3)3, and sodium bicarbonate, saturated salt solutions of each compound at 25°C were made. Each solution was placed in the glove chamber separately for 24 h to maintain RH inside the chamber. The final RH and temperature for each saturated salt solution was determined as their DRH. All experiments were replicated 3 times. shows the measured DRH of K2Cr2O7, Cr(NO3)3, and sodium bicarbonate.
TABLE 2 Deliquescence RH values for K2Cr2O7, Cr(NO3)3, and sodium bicarbonate in this work
Determination of Cr(VI) was performed using HPLC coupled with an ICP-MS (Thermo X-series, MA). The analysis system consisted of HPLC Spectrosystem, peristaltic pump, and a quartz spray chamber with a Conikal concentric glass nebulizer. Collision cell technology (CCT) was optimized to reduce polyatomic interferences such as 52ArC+ and 53ClO+. The instrument was optimized daily using a Thermo A25 Tune solution. External calibration curves were generated using a blank and Cr(VI) standards of 0.2, 0.5, 1, 2, 5, and 10 ng/mL. The method detection limit (MDL; as defined in Appendix B to Part 136 of U.S. Environmental Protection Agency's Code of Federal Regulation No. 40) was determined as 0.15 ng/mL. The mean of the blank filter extract was 0.004 ng/mL (n = 4), which was far below the MDL. The spike recovery of 8 ng Cr(VI) was 92 ± 13% (n = 10). Relevant HPLC parameters and analytical conditions for HPLC-ICP-MS are given in .
TABLE 3 HPLC-ICP-MS operating conditions
The conventional approach to measure the conversion of Cr oxidation states uses isotopically enriched Cr species (Meng et al. Citation2011). Conversion of Cr(VI) to Cr(III) is defined as the ratio of the 53Cr(III) mass determined in the sample extract to the spiked 53Cr(VI) amount. In the present study, the ratio of the difference in spiked 53Cr(VI) and the measured 53Cr(VI) mass in the sample extract divided by the spiked 53Cr(VI) mass was defined as “loss” of 53Cr(VI). This definition is more reliable because it eliminates the influence of the precipitation of Cr(III), which can occur during atmospheric Cr(VI) sampling and analysis under basic conditions (James et al. Citation1995; Vitale et al. Citation1997; Huo et al. Citation1998). The percent conversion of 50Cr(III) to 50Cr(VI) was defined as the measured mass of 50Cr(VI) in the sample extract divided by the spiked 50Cr(III) mass.
RESULTS AND DISCUSSIONS
The average (n = 8) composition of the ambient PM at the sampling site in Potsdam was determined (). The total chromium concentration was determined to be 21.7 ng/m3, which is in good agreement with values reported for other cities: 27 ng/m3 in Isfahan, Iran (Talebi Citation2003), 25 ng/m3 in Radom, Poland (Swietlik et al. Citation2011), 42–79 ng/m3 in Flanders, Belgium (Tirez et al. Citation2011), and 16.3 ng/m3 in Frankfurt, Germany (Zereini et al. Citation2005). Mn (11.96 ng/m3) and Cr concentrations have the same order of magnitude, similar to previous observations by Herner et al. (Citation2006) and Lough et al. (Citation2005). shows that the total Fe concentration is about an order of magnitude higher than Cr and Mn, which is similar to other reported values (Herner et al. Citation2006).
TABLE 4 Elemental and organic carbon concentrations in ambient PM
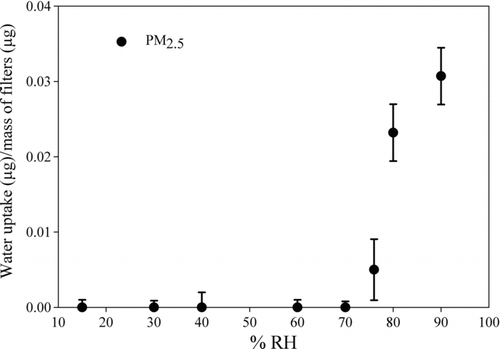
To study the effect of deliquescence on the interconversion of Cr(VI) and Cr(III), the DRH of the PM samples was determined by measuring water uptake by PM2.5 sampled on Teflon filters at different relative humidities (). At an RH of ∼76% ± 2%, the ambient PM began to deliquesce. This DRH is in agreement with Koloutso-Vakakis and Rood (Citation1994). Since ammonium nitrate and ammonium sulfate comprise the majority of the water-soluble mass of ambient PM in this region (Sunder Raman et al. Citation2008), these salts dominate the ambient PM hygroscopicity. The deliquescence point shifts between the DRH of ammonium sulfate (80% at 23.1°C; Lee and Hsu Citation1998) and ammonium nitrate (68% at 21.3°C; Lee and Hsu Citation2000). The amount of the water absorbed at the DRH is approximately 0.4 times the particle mass. The liquid water mass for ammonium sulfate is between 0.34 and 1 particle mass at 79% and 80% RH, respectively (Lee and Hsu Citation1998, Citation2000).
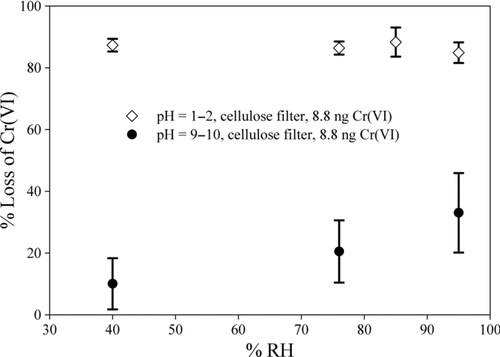
For strongly acidic conditions, the pH controls chromium speciation in contrast to basic conditions where deliquescence is dominant. Over a wide range of RH values in acidic media, over 85% of the Cr(VI) loss occurs (). It is expected that Cr(III) accounts for most of the chromium in liquid coated particles with acidic pHs, for example in many cloud and fog droplets. This result is in agreement with previous measurements of ambient chromium that found that Cr(VI) comprises up to 25% of the Cr present in particles (Talebi Citation2003; Swietlik et al. Citation2011; Tirez et al. Citation2011).
Organic compounds play an important role in reduction of atmospheric hexavalent chromium. Reduction of Cr(VI) to Cr(III) can be driven by different organic carbon compounds such as humic and fulvic acids (Palmer and Plus Citation1994) as well as oxalate, malonate, lactate, ascorbate, cysteine, methionine, and various thiols (Khan and Hashmi Citation1998). The rate of the reduction increases (Palmer and Plus Citation1994) and half-life of Cr(VI) decreases (Eckert et al. Citation1990) with decreasing pH (Palmer and Plus Citation1994). Werner et al. (Citation2006) and Nico et al. (Citation2009) also reported reduction of Cr(VI) occurring in the presence of organic carbon at pH values around 5. Ferrous ions also add considerably to the Cr(VI) reduction at pH 2 while the rate of Cr(VI) reduction increases as pH increases (Fendorf et al. Citation2000). Therefore, the reduction of Cr(VI) at acidic pH in this study could occur because of the reaction of Cr(VI) with the organic carbon that comprises a major part of the ambient PM mass as well as ferrous ions ().
Increasing the RH from 40% to 96% for basic pH values increased Cr(VI) loss from 10 ± 8.3% to 33 ± 10% (). This difference in the mean values is statistically significant between the converted fractions at 40% and 96% RH (t = −3.360, p = 0.010). Above the DRH, the deliquesced particles have an aqueous phase allowing the chromium to react with other constituents. At 96% RH that is very close to the deliquescence point of the potassium dichromate and sodium bicarbonate, the water activity reach to the point that dry Cr(VI) and sodium bicarbonate crystals deliquesce. Below the deliquescence point, Cr(VI) losses of ∼10% were observed. Some losses may be the result of limited deliquescence below 76%. The loss of Cr(VI) reached a maximum value of 33% at 96% RH. Fe(II) is the major reactant that reduces Cr(VI) to Cr(III). Laboratory studies showed that this reaction is complete in less than 5 min (Eary and Rai Citation1988). Fe(II) seems to control the reduction of Cr(VI) in aqueous anoxic systems under natural to alkaline conditions (Pettine et al. Citation1998). The results presented by Sedlak and Chan (Citation1997) showed that the reaction rate constant for the reduction of Cr(VI) by Fe(II) increases up to 2 order of magnitude from acidic to basic pH. It was shown that the rate of reduction of Cr(VI) by Fe(II) in NaCl solution increases in the pH range of 5.0 to 8.7 (Pettine et al. Citation1998).
Cr-Fe spinel comprises the majority of the chromium particles in the ambient PM matrix (Huggins et al. Citation2000; Werner et al. Citation2007; Tirez et al. Citation2011), and coal and biomass combustion products (Stam et al. Citation2011). Chromium in Cr-Fe spinel appears to be Cr(III) in a chromite (FeCr2O4) structure (Huggins et al. Citation2000; Werner et al. Citation2006, Citation2007). In atmospheric PM, the dominant speciation of Fe has been found to be Fe(III) (Huggins et al. Citation2000; Tong et al. Citation2001; Qi et al. Citation2003; Majestic et al. Citation2007). However, the soluble fraction of iron is Fe(II) (Majestic et al. Citation2007). demonstrates the total Fe concentration in the ambient PM measured in this study was found to be an order of magnitude higher than total chromium concentration. The fraction of water soluble Fe(II) to total water soluble Fe in the ambient PM in this study was found to be 0.54. Also, Cr(III) represents the majority of the total chromium in ambient PM in agreement with prior studies (Bell and Hipfner Citation1997 [80%]; Talebi Citation2003 [75%]; Tirez et al. Citation2011 [more than 96%]). Therefore, sufficient mass of Fe(II) is available in the ambient PM matrix for the reduction of Cr(VI) to Cr(III). This reaction is facilitated at the basic pH as shown in . Manganese also contributes in the Cr-Fe matrix (Nico et al. Citation2009) with similar concentrations as chromium, but much lower than iron as shown in . In addition to Fe(II), organic carbon reduces Cr(VI) to Cr(III). However, the rate of this type of reaction decreases at basic pH compared to acidic pH (Palmer and Plus Citation1994).
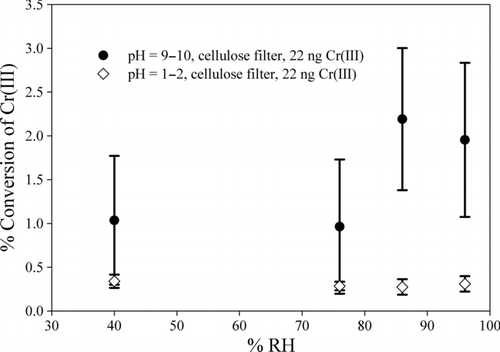
The conversion of Cr(III) to Cr(VI) is very low for acidic pH values and less than 4% under basic conditions both below and above the DRH () indicating that Cr(III) is very stable under most atmospheric conditions. This result is in agreement with that indicates the instability of Cr(VI) under acidic conditions. At basic pH values, the conversion of Cr(III) is very low although there was a sufficient mass of Mn available in the ambient PM matrix for oxidation of Cr(III). The low conversion rate is likely because of the essentially complete precipitation of the Cr(III) (James et al. Citation1995; Vitale et al. Citation1997; Huo et al. Citation1998). The precipitated Cr(III) cannot be oxidized to Cr(VI) under the current experiment conditions (Meng et al. Citation2011). However, at 86% and 96% RH values, statistically significant different conversion rates (p = 0.003 and p < 0.001, respectively) were observed between the basic and acidic conditions as shown in .
Impregnating the filters with acidic or basic solution strongly controls the local pH of the deliquesced particles. Considerable mass of the nitric acid or sodium bicarbonate compared to mass of particles is deposited inside the filters, and, therefore, upon formation of a liquid phase, the local pH is as basic or acidic. Furthermore, the basic or acidic impregnated filters can absorb acidic or basic gases, respectively, during sampling, and also in the reaction chamber. The amount of such gas in the current experimental chamber is very low. Thus, this phenomenon is negligible. However, to ensure that gas absorption during sampling does not change the pH of the filters, rough measurements of the pH of the moistened PM samples at different locations on the filter were made using a pH meter. No change in the pH of the filters was observed. In addition, this observation confirms that the local pH of the deliquesced particles is similar to initial pH of the filter. This method was applied by Werner et al. (Citation2006) to roughly estimate the local pH of the PM samples surrounded by a liquid layer.
IMPACT OF PM MASS ON CHROMIUM SPECIATION
The reactions of Cr(III) and Cr(VI) at 4 different levels of PM masses under basic pH and 3 levels of RH values (83%, 93%, and 99%) were studied in this investigation. Different amounts of PM mass resulted in different concentrations of possible oxidants and reductants in the PM samples. However, no statistically significant difference was observed for the conversion of Cr(III) to Cr(VI) and loss of Cr(VI) with respect to 4 different levels of PM masses at each RH value. For example, at 83% RH, the mean of Cr(VI) losses for 4 different levels of PM masses (14 h, 24 h, 48 h, and 72 h sampling) was found to be 28.1%, 29.0%, 34.5%, and 28.7%, respectively. The masses of iron and organic carbon are in excess compared to the mass of spiked Cr(VI). Thus, the reaction rate of Cr(VI) with ambient PM is independent of ambient PM mass, and expected to be constant.
PRACTICAL IMPLICATIONS
Current practice for sampling atmospheric Cr(VI) in the National Air Toxics Network (NATTS) is for collection over 24 h from midnight to midnight using an ERG sampler (ERG Citation2006, Citation2009) that collects total suspended particles (TSP). ERG (Citation2006) has shown that when the filters are left in the NATTS sampler for longer than 12–24 h, a loss of Cr(VI) occurs. When filters are spiked with Cr(VI) solution and left in the field for 33–105 h, the negative bias in the Cr(VI) values ranges from 30% to 58%. Meng et al. (Citation2011) found the average loss of Cr(VI) to be 43% for 24-h samples in Paterson, NJ, and Chester, NJ, using filters spiked prior to sampling, and 33% with post-sampling spiked filters. Their results suggest that ∼10% of the Cr(VI) was lost during sampling. Based on the results presented here for basic pH of the Cr(VI) sampling filters, the loss of Cr(VI) during sampling could be a result of deliquescence of the collected PM. As the RH increases to over 95%, significant loss of Cr(VI) occurred. The 33% Cr(VI) loss after 24 h, reported in this work at high relative humidities, is in good agreement with the 30% loss of Cr(VI) reported by ERG (Citation2006) and 43% loss of Cr(VI) found by Meng et al. (Citation2011). However, the reported values in this investigation as well as those in the studies of ERG (Citation2006) and Meng et al. (Citation2011), include loss of Cr(VI) because of filter storage, extraction, and sample analysis.
Under real atmospheric conditions, the RH varies over the 24-h sampling period. It may exceed the DRH at least 2 times during the 24-h sampling periods commonly employed: after sunset and early in the morning. Once the RH reaches or exceeds the DRH, loss of Cr(VI) can occur even if the RH subsequently drops below the DRH. Typically soluble ambient particles show a hysteresis behavior between the crystallization and DRH values.
The crystallization RH is less than the DRH (Semeniuk et al. Citation2007). Once the RH decreases below the crystallization RH, the water content of the soluble particles markedly decreases such that almost dry particles are obtained. The crystallization RH of ammonium nitrate and ammonium sulfate is 32% (Lee and Hsu Citation2000) and 35% (Lee and Hsu Citation1998), respectively. If the particles deliquesce, then the water will remain longer than just at the point at which the RH drops below the deliquescence point due to hysteresis. Therefore, after sunrise, when RH is lower than the DRH but higher than the crystallization point, Cr (VI) loss can still occur in the residual aqueous phase. Thus, it is important to keep the RH below the DRH throughout the sampling period by diluting the ambient air with clean, dry air.
Chromium speciation changes during sampling can be attributed to the reactions of soluble chromium species with ambient PM and dissolved gaseous compounds. Also, gas–solid phase reactions encompassing reactions of insoluble chromium species with gaseous reactants may become important. However, the contribution of each type of reactions in interconversion of Cr(III) and Cr(VI) during sampling has not been investigated.
For basic pH values, deliquescence is important for the loss of Cr(VI), while precipitation controls the solution chemistry of Cr(III). Under highly acidic conditions, the pH becomes very important for the interconversion of Cr(VI) and Cr(III). However, deliquescence showed no effect on Cr(VI) and Cr(III) chemistry for acidic conditions. Thus, control of humidity to avoid deliquescence should be a design criterion for Cr(VI) samplers to minimize the loss of Cr(VI) during sampling as has been observed in previous works.
Acknowledgments
This work was supported by the U.S. Environmental Protection Agency through grant number XA-97247301–0. Although the research described in this article has been funded wholly or in part by the U.S. Environmental Protection Agency, it has not been subjected to the Agency's peer and policy review and therefore does not necessarily reflect the views of the Agency, and hence no official endorsement should be inferred.
REFERENCES
- Abu-Saba , K. E. , Sedlak , D. L. and Flegal , A. R. 2000 . Indirect Reduction of Hexavalent Chromium by Copper in the Presence of Superoxide . Mar. Chem. , 69 : 33 – 41 .
- Bell , R. W. and Hipfner , J. C. 1997 . Airborne Hexavalent Chromium in Southwestern Ontario . J. Air Waste Manage. , 47 : 905 – 910 .
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . Aerosol Sci. Technol , 25 : 221 – 241 .
- Cruz , C. N. and Pandis , S. N. 2000 . Deliquescence and Hygroscopic Growth of Mixed Inorganic−Organic Atmospheric Aerosol . Environ. Sci. Technol. , 34 : 4313 – 4319 .
- Davis , A. and Olsen , R. L. 1995 . The Geochemistry of Chromium Migration and Remediation in the Surface . Ground Water , 33: : 759 – 768 .
- Eary , L. E. and Rai , D. 1988 . Chromate Removal from Aqueous Wastes by Reduction with Ferrous Iron . Environ. Sci. Technol. , 22: : 972 – 977 .
- Eckert , J. M. , Stewart , J. J. , Waite , T. D. , Szymezek , R. and Williams , K. L. 1990 . The Reduction of Chromium(VI) at sub-μgL−1 Levels by Fulvic Acid . Anal. Chim. Acta , 236 : 357 – 362 .
- ERG . 2006 . Collection and Analysis of Hexavalent Chromium in Ambient Air , NC. : Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, Research Triangle Park .
- ERG . 2009 . Standard Operating Procedure for the Determination of Hexavalent Chromium in Ambient Air Analyzed By Ion Chromatography (IC) , NC : Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, Research Triangle Park . ERG No. MOR-063
- Fendorf , S. E. , Wielenga , B. W. and Hansel , C. M. 2000 . Chromium Transformations in Natural Environments: The Role of Biological Processes in Chromium(VI) Reduction . Int. Geol. Rev. , 42 : 691 – 701 .
- Gendel , Y. and Lahav , O. 2008 . Accurate Determination of Fe(II) Concentrations in the Presence of a Very High Soluble Fe(III) Background . Appl. Geochem. , 23 : 2123 – 2129 .
- Goshu , I. V. , Tsarev , V. and Kostrov , V. V. 2007 . Study of Chromium(VI) Reduction in the Presence of Metal Salt Additives . Inorg. Syn. Ind. Inorg. Chem. , 80 : 1946 – 1949 .
- Grohse , P. M. , Gutkenecht , W. F. , Hodson , L. and Wilson , B. M. 1988 . “ The Fate of Hexavalent Chromium in the Atmosphere ” . In California Air Resources Board Sacramento , NC : Research Triangle Institute, Research Triangle Park . CARB Contract No. A6–096-32.
- Guertin , J. , Jacobs , J. A. and Avakian , C. A. 2005 . Chromium (VI) Handbook , Boca Raton, FL : CRC Press .
- Hameri , K. , Charlson , R. and Hansson , H. C. 2002 . Hygroscopic Properties of Mixed Ammonium Sulfate and Carboxylic Acids Particles . AIChE , 48 : 1309 – 1316 .
- Hansson , H. C. , Rood , M. J. , Koloutsou-Vakakis , S. , Hämeri , K. , Orsini , D. and Wiedensohler , A. 1998 . NaCl Aerosol Particle Hygroscopicity Dependence on Mixing with Organic Compounds . J. Atmos. Chem. , 31 : 321 – 346 .
- Herner , J. D. , Green , P. G. and Kleeman , M. J. 2006 . Measuring the Trace Elemental Composition of Size-Resolved Airborne Particles . Environ. Sci. Technol. , 40 : 1925 – 1933 .
- Huggins , F. E. , Huffman , G. P. and Robertson , J. D. 2000 . Speciation of Elements in NIST Particulate Matter SRMs 1648 and 1650 . J. Hazard. Mater. , 74 : 1 – 23 .
- Huo , D. W. , Lu , Y. S. and Kingston , H. M. 1998 . Determination and Correction of Analytical Biases and Study on Chemical Mechanisms in the Analysis of Cr(VI) in Soil Samples Using EPA Protocols . Environ. Sci. Technol. , 32 : 3418 – 3423 .
- James , B. R. , Petura , J. C. , Vitale , R. J. and Mussoline , G. R. 1995 . Hexavalent Chromium Extraction from Soils: A Comparison of 5 Methods . Environ. Sci. Technol. , 29 : 2377 – 2381 .
- Khan , Z. and Hashmi , A. A. 1998 . Reduction of Chromium(VI) by Phosphonic Acid . Transit. Metal Chem. , 23 : 147 – 150 .
- Koloutso-Vakakis , S. and Rood , M. J. 1994 . The (NH4)2SO4-Na2SO4-H2O System: Comparison of Deliquescence Humidities Measured in the Field and Estimated from Laboratory Measurements and Thermodynamic Modeling . Tellus , 46B : 1 – 15 .
- Lee , C. T. and Hsu , W. C. 1998 . A Novel Method to Measure Aerosol Water Mass . J. Aerosol Sci. , 29 : 827 – 837 .
- Lee , C. T. and Hsu , W. C. 2000 . The Measurement of Liquid Water Mass Associated with Collected Hygroscopic Particles . J. Aerosol Sci. , 31 : 189 – 197 .
- Lide , D. R. 2004 . Handbook of Chemistry and Physics, , 85th ed. , Boca Raton, FL : CRC Press .
- Lough , G. C. , Schauer , J. J. , Park , J. S. , Shafer , M. M. , DeMinter , J. T. and Weinstein , J. P. 2005 . Emissions of Metals Associated with Motor Vehicle Roadways . Environ. Sci. Technol. , 39 : 826 – 836 .
- Majestic , B. J. , Schauer , J. J. and Shafer , M. M. 2007 . Application of Synchroton Radiation for Measurement of Iron Red-Ox Speciation in Atmospherically Processed Aerosols . Atmos. Chem. Phys. , 7 : 2475 – 2487 .
- Meng , Q. , Fan , Z. , Buckley , B. , Lin , L. , Huang , L. Yu , C. 2011 . Development and Evaluation of a Method for Hexavalent Chromium in Ambient Air Using IC-ICP-MS . Atmos. Environ. , 45 : 2021 – 2027 .
- Nico , P. S. , Kumfer , B. M. , Kennedy , I. M. and Anastasio , C. 2009 . Redox Dynamics of Mixed Metal (Mn, Cr, and Fe) . Aerosol Sci. Technol. , 43 : 60 – 70 .
- Nico , P. S. and Zasoski , R. J. 2000 . Importance of Mn(II) Availability of Cr(III) Oxidation on Birnessite . Environ. Sci. Technol. , 38 : 5253 – 5260 .
- Palmer , C. D. and Plus , R. W. 1994 . Natural Attenuation of Hexavalent Chromium in Ground Water and Soils , Ada, OK : Superfund Technology Support Center for Ground Water, Robert S. Kerr Environmental Research Laboratory . Ground Water EPA/ 540/S-94/505
- Pettine , M. , D’Ottone , L. , Campanella , L. , Millero , F. J. and Passino , R. 1998 . The Reduction of Chromium(VI) by Iron(II) in Aqueous Solutions . Geochin. Cosmochim. Acta , 62 : 1509 – 1519 .
- Qi , J. H. , Zhang , M. P. , Feng , L. J. , Li , X. G. , Xie , Z. Sun , Z. H. 2003 . An EXAFS Study on the Local Structure Around Iron in Atmospheric Aerosols Collected in the Qingdao Area . Molecules , 8 : 31 – 39 .
- Rahman , G. M. , Kingston , H. M. , Towns , T. G. , Vitale , R. J. and Clay , K. R. 2005 . Determination of Hexavalent Chromium by Using Speciated Isotope-Dilution Mass Spectrometry After Microwave Speciated Extraction of Environmental and Other Solid Materials . Anal. Bioanal. Chem. , 382 : 1111 – 1120 .
- Rai , D. , Sass , B. M. and Moore , D. A. 1987 . Chromium(III) Hydrolysis Constants and Solubility of Chromium(III) Hydroxide . Inorg. Chem. , 26 : 345 – 349 .
- Saxena , P. , Hildemann , L. M. , Mcmurry , P. H. and Seinfeld , J. H. 1995 . Organics Alter Hygroscopic Behavior of Atmospheric Particles . J. Geophys. Res. , 100 ( D9 ) : 18755 – 18770 .
- Sedlak , D. L. and Chan , P. G. 1997 . Reduction of Hexavalent Chromium [Cr(VI)] by Ferrous Iron [Fe(II)] . Geochim. Cosmochim. Acta , 61 : 2185 – 2192 .
- Seigneur , C. and Constantino , E. 1995 . Chemical Kinetic Mechanism for Atmospheric Chromium . Environ. Sci. Technol. , 29 : 222 – 231 .
- Semeniuk , T. A. , Wise , M. E. , Martin , S. T. , Russell , L. M. and Buseck , P. R. 2007 . Water Uptake Characteristics of Individual Atmospheric Particles Having Coatings . Atmos. Environ. , 41 : 6225 – 6235 .
- Schroeder , D. C. and Lee , G. F. 1975 . Potential Transformation of Chromium in Natural Waters . Water Air Soil Poll. , 4 : 355 – 365 .
- Speer , R. E. , Edney , E. O. and Kleindeinst , T. E. 2003 . Impact of Organic Compounds on the Concentrations of Liquid Water in Ambient PM2.5 . Aerosol Sci. , 34 : 63 – 77 .
- Stam , A. F. , Meij , R. , Winkel , H. , Eijk , R. J. , Huggins , F. E. and Brem , G. 2011 . Chromium Speciation in Coal and Biomass Co-Combustion Products . Environ. Sci. Technol. , 45 : 2450 – 2456 .
- Sunder Raman , R. , Hopke , P. K. and Holsen , T. M. 2008 . Characterization of Fine Aerosol and Its Inorganic Components at Two Rural Locations in New York State . Environ. Monit. Assess. , 144 : 351 – 366 .
- Swietlik , R. , Molik , A. , Molenda , M. , Trojanowska , M. and Siwiec , J. 2011 . Chromium(III/VI) Speciation in Urban Aerosol . Atmos. Environ. , 44 : 1364 – 1368 .
- Talebi , S. M. 2003 . Determination of Total and Hexavalent Chromium Concentrations in the Atmosphere of the City of Isfahan . Environ. Res. , 92 : 54 – 56 .
- Tirez , K. , Silversmit , G. , Bleux , N. , Adriaensens , E. , Roekens , E. Servaes , K. 2011 . Determination of Hexavalent Chromium in Ambient Air: A Story of Method Induced Cr(III) Oxidation . Atmos. Environ. , 45 : 5332 – 5341 .
- Tong , Y. , Li , A. , Cai , Y. , Ni , X. , Zhang , Y. Wang , J. 2001 . Mossbauer Study of Atmospheric Aerosols of Shanghai . Environ. Sci. Technol. , 35 : 1432 – 1436 .
- Vitale , R. J. , Mussoline , G. R. , Petura , J. C. and James , B. R. 1997 . Cr(VI) Soil Analytical Method: A Reliable Analytical Method for Extracting and Quantifying Cr(VI) in Soils . Soil Contam. , 6 ( 6 ) : 581 – 593 .
- Werner , M. , Nico , P. , Guo , B. , Kennedy , I. and Anastasio , C. 2006 . Laboratory Study of Simulated Atmospheric Transformations of Chromium in Ultrafine Combustion Aerosol Particles . Aerosol Sci. Technol. , 40 : 545 – 556 .
- Werner , M. L. , Nico , P. S. , Marcus , M. A. and Anastasio , C. 2007 . Use of Micro-XANES to Speciate Chromium in Airborne Fine Particles in the Sacramento Valley . Environ. Sci. Technol. , 41 : 4919 – 4924 .
- Xu , Q. , Dewitte , M. and Sloan , J. J. 2003 . The Effect of Formic Acid on the Deliquescence of Model Sea-Salt Aerosol Particles . Atmos. Environ. , 37 : 911 – 919 .
- Zereini , F. , Alt , F. , Messerschmidt , J. , Wiseman , C. , Feldmann , I. Bohlen , A. 2005 . Concentration and Distribution of Heavy Metals in Urban Airborne Particulate Matter in Frankfurt am Main, Germany . Environ. Sci. Technol. , 39 : 2983 – 2989 .