Abstract
Although tobacco smoke is well known for its adverse health effects, the hygroscopicity and droplet growth properties of the aerosol have not been thoroughly explored. In this study, cigarette smoke is further characterized and several state-of-art analysis techniques are applied to understand the effects of particle chemistry and hygroscopicity for enhanced condensational growth (ECG) by water vapor and wet particle deposition. Low nicotine (LN) and ultra-low nicotine (ULN) research cigarettes are tested with a Walton Smoking Machine (WSM); mainstream and sidestream environmental tobacco smoke (ETS) are produced. Online and offline analysis are combined to analyze the smoke. More than 99% of the mainstream and sidestream ETS mass is semivolatile aerosol and nonelemental carbon, of which more than 95% is organic. The water-soluble organic comprises 30-85% of the aerosol mass fraction and has no effect on surface tension when dissolved in water. The oxygen-to-carbon ratio (O/C) and nitrogen-to-carbon ratio (N/C) from High Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS or HR-AMS) data show that more oxidized components are present in mainstream smoke. Differences in the bulk aerosol composition have little effect on the overall water uptake. The two types of cigarettes produce aerosols of similar hygroscopicity (with single hygroscopicity parameter, κ ∼0.15 or less) in mainstream and sidestream smoke. Droplets grow at the same rate within the instrument. However, ULN reference cigarettes that produce dry particles at larger sizes are more likely to experience ECG.
Copyright 2012 American Association for Aerosol Research
Abbreviations
| CN | = |
Condensation Nuclei |
| CCN | = |
Cloud Condensation Nuclei |
| CPC | = |
Condensational Particle Counter |
| EC | = |
Elemental Carbon |
| ECG | = |
Enhanced Condensational Growth |
| ETS | = |
Environmental Tobacco Smoke |
| HR-ToF-AMS or HR-AMS | = |
High Resolution Time-of-Flight Aerosol Mass Spectrometer |
| ISO | = |
International Organization for Standardization |
| LN | = |
Low Nicotine |
| N/C | = |
Nitrogen-to-Carbon ratio |
| O/C | = |
Oxygen-to-Carbon ratio |
| OM/OC | = |
Organic-Mass-to-Organic-Carbon ratio |
| RH | = |
Relative Humidity |
| SMPS | = |
Scanning Mobility Particle Sizer |
| ULN | = |
Ultra-Low Nicotine |
| UMR | = |
Unit Mass Resolution |
| WSM | = |
Walton Smoking Machine |
| WSOC | = |
Water-Soluble Organic Carbon |
| WSOM | = |
Water-Soluble Organic Mass |
INTRODUCTION
Adverse health effects from particulate matter (PM) are caused by the deposition of particles in the respiratory tract during inhalation. The water vapor uptake by hygroscopic components can alter dry particle size and deposition efficiencies (Blanchard and Willeke Citation1984; Broday and Georgopoulos Citation2001; Schroeter et al. Citation2001; Londahl et al. Citation2009; Varghese and Gangamma Citation2009; Kane et al. Citation2010; Phalen et al. Citation2010). Therefore, the dose and deposition site of these aerosols are influenced by the growth of the particles. Relative humidity (RH) in the lung is estimated at 99.5% RH (Anselm et al. Citation1990), and more recent work has calculated instantaneous values in the supersaturated range (up to 104% RH; Longest and Xi Citation2008; Varghese and Gangamma Citation2009; Longest et al. Citation2010) in the upper airways. At large RH, enhanced condensational growth (ECG) may occur, droplets may form (Longest et al. Citation2010) and dry particles will effectively behave as cloud condensation nuclei (CCN) within the respiratory system. Longest and Xi (Citation2008) suggests that condensational growth may play a significant role in the deposition of cigarette smoke particles in the upper airways. Both size and composition influence the ability of particles to act as CCN (Andreae and Rosenfeld Citation2008). The following study explores the aerosol composition of inhaled cigarette smoke in relation to ECG via the deposition of water vapor and CCN properties.
Cigarette smoke is an aerosol of liquid droplets (i.e., particulate phase) suspended in a mixture of gases and semivolatile compounds (Ingebrethsen Citation1986). More than 4000 components have been identified in tobacco smoke (Thielen et al. Citation2008), of which at least 50 are carcinogenic (Roberts Citation1988; Hoffmann and Hoffmann Citation1993). A number of potent lung carcinogens for humans, such as the polycyclic aromatic hydrocarbons (PAHs), tobacco-specific nitrosamines (TSNAs), phytosterols, and the metals, are found only in the particulate phase (Hoffmann et al. Citation1996). The dry size of these particles in fresh inhaled smoke ranges from 0.1 to 1 μm in diameter and may reach the deep lung where the gas exchange takes place. Secondhand or environmental tobacco smoke (ETS) is aged and the combination of diluted “sidestream” smoke (released between puffs from the lit end of the cigarette) and “mainstream” smoke (exhaled by a smoker). Here, we note that the mainstream smoke is not humidified in the set-up; we characterize the water-uptake of the dry particle formed by the mainstream process. The mixture between sidestream and exhaled mainstream smoke in the environment after dilution and aging is often referred to as ETS and is a significant source of human exposure to fine particles (Klepeis et al. Citation2003).
TABLE 1 Aerosol Composition Characteristics of Cigarette Smoke: The aerosol phase is composed of semivolatile organics, and EC makes up <1% of the total organic carbon (TOC). O/C ratio of mainstream ETS is larger than sidestream smoke
The source and transformation during transport before inhalation are important for aerosol chemistry. Yadav et al. (Citation2004) used a single particle time-of-flight mass spectrometer to show that the size and composition of fresh and aged smoke significantly differ. Sleiman et al. (Citation2010) showed that additional products are formed during the photo-oxidative aging of ETS and suggested the water-soluble organic aerosol content is enhanced in the presence of water vapor. Aerosol components that are water-soluble or surface active may affect the droplet growth rates.
The aged aerosol contributions to droplet growth from the respective mainstream and sidestream types of ETS are explored. Understanding the inhalation of smoke, specifically the links between the physicochemical properties and wet deposition rates are important. The goal of this study is to further characterize cigarette smoke, with an emphasis on wet ECG, represented by CCN activity and droplet formation properties. Changes in particle size, chemistry, and volatility are correlated to changes in droplet behavior from offline and online chemistry measurements.
EXPERIMENTAL METHODS
A Walton Smoking Machine (WSM) produced mainstream and sidestream ETS. The operation and characterization of the instrument has been previously reported (Guerin et al. Citation1979; Chen et al. Citation1990). Two types of reference cigarettes, 1R5F and 3R4F (College of Agriculture, University of Kentucky), were tested with the WSM. The 3R4F is a low nicotine (LN) research cigarette and the 1R5F is an ultra-low nicotine (ULN) research cigarette. shows manufacturer specifications of both cigarettes. The ULN 1R5F reference cigarette produces significantly (∼5 to 7 times) less particle, tar, and nicotine mass per cigarette than the LN 3R4F (, http://www.ca.uky.edu/refcig/). Hence, measurements in this study are normalized by total number concentration for direct comparison of cigarette composition and contribution to water uptake ability. Two cigarettes of the same type were simultaneously smoked at the WSM. Testing followed the International Organization for Standardization (ISO) smoking protocol for smoking puff volume, duration, and frequency. The puff air volume was 35 cm3 per puff; the duration was 2 s per puff and the frequency was 1 cycle per 60 s. As per ISO protocol, the vent blocking was 0% for WSM measurements. Mainstream smoke via the vent hole fitting of the WSM was collected in a 27 L stainless steel reservoir chamber. The chamber provided constant sample flow and consistent aerosol phase mixing conditions for subsequent particle sizing and hygroscopicity measurements. Hence, the smoke produced and sampled by this method is representative of aged, nonhumidified mainstream ETS. Sidestream ETS was funneled and diluted with compressed air by an ejector pump to simulate dilution conditioned secondhand smoke, which then was also collected in the stainless steel reservoir chamber.
Mainstream and sidestream ETS were size-selected with a scanning mobility particle sizer (SMPS, TSI 3080) and a condensation particle counter (CPC, TSI 3772). A Droplet Measurement Technologies Continuous-Flow Streamwise Thermal Gradient CCN Counter (CCNC) was operated in parallel with the CPC (Roberts and Nenes Citation2005); CCNC counted the CCN concentration of size-selected aerosol while the CPC counted the condensation nuclei (CN) concentration. The CCNC instrument was calibrated with (NH4)2SO4 aerosol. The critical diameter where CCN/CN = 0.5, d p50, of the (NH4)2SO4 aerosol at the custom-set supersaturation (e.g., 0.6% and 1.0%) determined the varying instrument supersaturation s (e.g., 0.54% and 0.85%). s = S (saturation ratio) – 1 and is usually expressed as a percentage. The sample flow rates of CPC and CCNC were 1 and 0.5 L min−1, respectively. The sheath-to-aerosol ratio of both SMPS and CCNC instruments was 10:1. Previous work of Robinson and Yu (Citation1998) suggest that s < 0.5% is not large enough to activate cigarette smoke aerosol. In this study, the CCNC s was set to be 0.54% and 0.85%, much smaller values and more likely to be instantaneous high RH in airways. At a constant CCNC supersaturation, s, the activated fraction (CCN/CN) of the dry particle mobility size distribution was obtained every minute (∼one cycle of the WSM). d p50 was calculated using Scanning Mobility CCN Analysis (SMCA) (Moore et al. Citation2010). By applying the κ-Köhler theory proposed by Petters and Kreidenweis (Citation2007), CCN activity of the smoke was represented using a single hygroscopicity parameter κ, which gathers all the composition-dependent variables and can be determined experimentally from CCNC data (s and d p50). κ can be used to describe hygroscopic particle growth at both subsaturated and supersaturated RH regimes (Petters and Kreidenweis Citation2007).
In addition to dry d p50 and hygroscopicity information, the CCNC can also measures droplet growth size at the exit of the instrument column. The instrument optical particle counter provides size resolved droplet concentrations. The droplet growth information was assessed using the method of Threshold Droplet Growth Analysis (TDGA, e.g., but not limited to, Asa-Awuku et al. Citation2010; Padro et al. Citation2010). TDGA can directly assess the impact of organics on CCN growth kinetics by comparing the droplet sizes of activated aerosol particles with (NH4)2SO4 particles at identical instrument s. Wet diameters of particles with dry diameters equal to critical diameters (d p50) were selected in this study.
The aerosol composition was characterized with online and offline methods in separate experiments. A catalytic stripper (CS) removed volatile components from smoke samples (Abdul-Khalek and Kittelson Citation1995; Stenitzer Citation2003; Kittelson et al. Citation2005; Zheng et al. Citation2010). Two different catalysts, Oxicat® and S-trap, remove hydrocarbon and sulfur components from the aerosol at 300°C. Afterwards, a coil cools the sample to ambient temperatures. The flow rate through the CS is 1 L min−1 for this study.
Smoke was sampled by an Aerodyne High Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS or HR-AMS) (Jimenez et al. Citation2003; DeCarlo et al. Citation2006) at a rate of 1.3 cm3 s−1. Unit mass resolution (UMR) data discerns differences in mass to charge (m/z) ion fragmentation. The elemental composition and oxidation state was examined with high resolution (HR) data analysis (Aiken et al. Citation2007; Aiken et al. Citation2008).
Offline filter measurements characterize the surface tension, water-soluble organic carbon (WSOC) and elemental carbon (EC) fraction of the organic aerosol. Filters were preweighed to estimate the total aerosol and organic mass (OM). Teflon Filters (PALL® Life Sciences, 47 mm) were extracted in 18.2 MΩ of ultra pure water (Milli-Q Advantage A10 Ultrapure Water Purification System). Large insoluble particles were filtered from the solution using Whatman® 25 mm GD/X syringe filters, and then analyzed by a Sievers 900 portable Total Organic Carbon (TOC) Analyzer (GE) for dissolved organic carbon concentration. The water-soluble organic mass fraction, f WSOM, is inferred by multiplying the dissolved organic carbon concentration from TOC measurement by the organic mass to organic carbon (OM/OC) ratio defined by HR-AMS. The EC/OC content was obtained by analyzing loaded quartz filter with a Sunset Laboratory (Forest Grove) Thermal/Optical Carbon Aerosol Analyzer. Surface tension values of the water extracted filter samples were measured with an Attension T-200 Pendant Drop Tensiometer.
RESULTS AND DISCUSSION
Size Distribution, Volatility, and Organic Chemical Composition
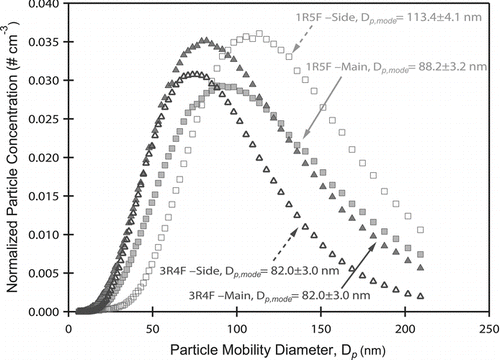
Previous studies report particle sizes of cigarette smoke ranging from 0.1 to 1.5 μm, using various cigarette types, dilution ratios and measurement techniques (Chen et al. Citation1990; Bernstein Citation2004). In comparison, we use the current, state-of-the-art real time particle sizing instrument, SMPS, which selects particles with size ∼6–210 nm. Furthermore, the aerosol are from two low-nicotine research cigarettes. As a result, differences in cigarette smoke particle sizes and composition are expected. Results from the WSM are consistent with Chen et al. (Citation1990) and show that after one puff, the composition and number of particles remains consistent for a given cigarette type. Both LN and ULN smoke are formed with the aforementioned experimental set-up. Mainstream and sidestream ETS produce well mixed and stable particle size distributions (). The particle number concentration was normalized by total concentration of each sample. For a given cigarette type, the gas to condensed phase partitioning from mainstream and sidestream ETS forms similar aerosol distributions. LN 3R4F ETS forms smaller particles than ULN 1R5F (D p,mode ∼88 nm, in mainstream and sidestream ETS). 1R5F ETS forms aerosol distributions with larger modes, D p,mode = 113 ± 4.1 nm and D p,mode = 88.2 ± 3.2 nm in sidestream and mainstream modes, respectively. ULN cigarettes produce aerosol at larger sizes (). Differences in dry aerosol size distribution are due to variations in partitioning and condensation of volatiles into particle phase.
FIG. 1 Particle Size distributions from mainstream and sidestream ETS. 1R5F cigarettes form particles of larger modes in both mainstream and sidestream ETS. Because the activation diameters for both cigarette types produce aerosol of similar hygroscopicity (κ ∼ 0.15), ULN 1R5F cigarettes will produce more aerosol that activate and form droplets.
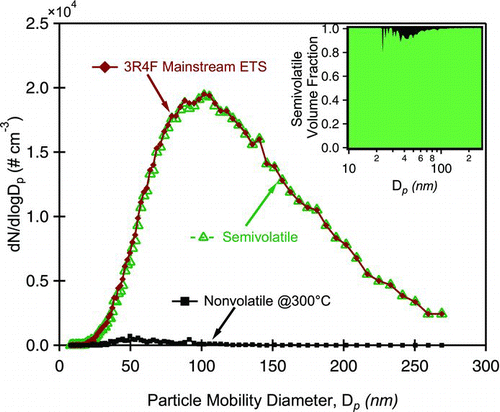
The majority of mainstream and sidestream ETS are organic and semi-volatile (, and ). The EC/OC fraction constitutes less than 1% of the aerosol mass fraction. CS data in supports EC/OC measurements and shows that ∼1% (by volume) of the material is nonvolatile (evaporates at >300°C) between 60 nm to 100 nm.
FIG. 2 Exemplary volatility data from Catalytic Stripper (CS) measurements. Log normal particle distribution of ETS and nonvolatile aerosol are measured. The semivolatile distribution is inferred. Inset graph shows the semivolatile fraction as a function of particle mobility diameter. The majority of 3R4F aerosol is semi-volatile and evaporates at temperatures below 300°C. (Color figure available online.)
HR-AMS results compare the nonrefractory and semivolatile particulate composition formed by the two cigarette types in mainstream and sidestream ETS. Organics comprise more than ∼95% of the total nonrefractory and semivolatile aerosol mass. K+ is the most prevalent inorganic ion mass fragment (<3%) in both cigarettes. The OM/OC, O/C, and N/C ratios in mainstream smoke are larger than those in sidestream (); indicative of more oxidative materials present in the particulate phase of the main stream smoke due to higher combustion temperature. The properties of LN and ULN ETS are similar; however, ULN mainstream aerosol contains slightly more nitrogen containing compounds than LN mainstream ETS in the particulate phase (). The inferred f WSOM ranges from 32% to 85%. 3R4F contains similar amounts of WSOC in side-stream and mainstream ETS. 1R5F ETS contains more soluble mass; f WSOM = 45% and 85% in mainstream and sidestream ETS, respectively (). For samples with 10.6 ppm WSOC, the surface tension was 71.5 ± 0.7 mN/m and similar to that of water at room temperature (72 mN/m). At higher concentrations (∼39 ppm), the dissolved material only slightly depress surface tension (<5%). Hence, water-soluble components of both main and sidestream ULN and LN smoke are non-surface active at ECG activation concentrations.
All experiments show abundance in the UMR m/z 43 fragmentation ion ( and ). We normalized the signal intensity of all fragment ions by that of m/z 43. The fraction of m/z 43 ion in the total organic signal, f43 , equals 8.5 ± 0.1% in both LN and ULN mainstream ETS. HR analysis indicates that both oxidized (C2H3O+) and non-oxidized (C3H7 +) species exist in the m/z 43 ion fragment. Significant differences are observed in the UMR and HR-AMS data. For example, the ratio (R x) of LN to ULN mass spectra mainstream ETS at m/z 29 ion, R 29 = 1.6 (); 3R4F forms 60% more aerosol fragments at m/z 29 (Formyl radical, CHO). However, the majority of differences, R x < 0.95 and >1.10, are at m/z ions >43 (). LN aerosol forms larger molecular weight fragments than ULN. Prevalent R x differs by 14 ions (e.g., R 57, R 71, R 85); consistent with changes in CH2 + and N+ fragments.
FIG. 3 Exemplary m/z 43 normalized UMR mass spectra of LN 3R4F (darker color) to ULN 1R5F (lighter color) mainstream ETS. Prominent peaks where the ratio LN to ULN mass spectra, R, is (a) <0.95 and (b) >1.10 are shown. (Color figure available online.)
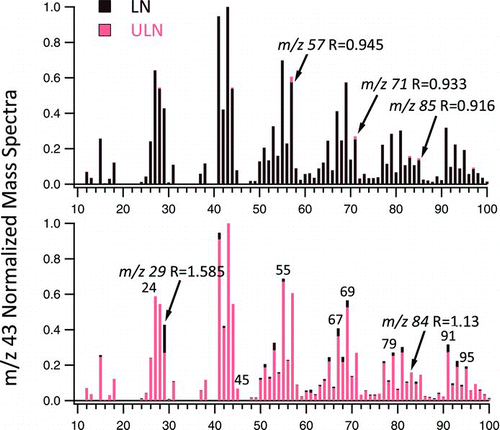
FIG. 4 Exemplary m/z 43 normalized UMR mass spectra of ULN 3R4F main (darker color) to ULN 3R4F (lighter color) sidestream ETS. The m/z 43 is the most abundant fragment in both cigarette types but is formed less in Main (8.5%) versus Side (9.8%) stream smoke. (Color figure available online.)
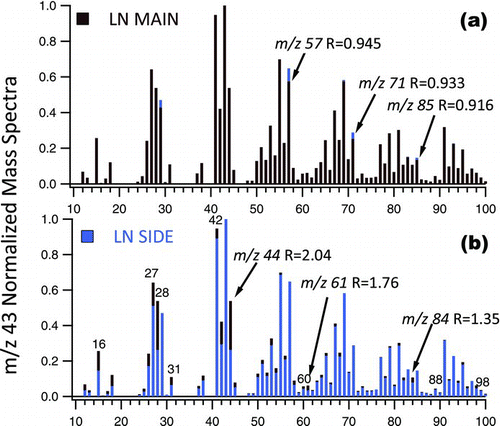
A comparison of LN 3R4F ETS shows f 44 ion in mainstream smoke is twice that in sidestream (). HR data shows key fragments have significantly more oxidized components. In LN mainstream and sidestream ETS, the oxidized contribution to UMR m/z 44 differs; the relative contribution of CO2 + ions is more in mainstream (0.89 ± 0.08) versus sidestream (0.66 ± 0.03) LN smoke. In ULN sidestream m/z 44 ion fragment contains less oxidized CO2 + (0.53 ± 0.02).
FIG. 5 Activated fraction (CCN/CN) versus particle mobility diameter of mainstream ETS for s c at 0.85% (closed) and 0.54% (open). (NH4)2SO4 calibration data (circles) is highly soluble in water and shown for comparison. LN 3R4F (triangles) have slightly smaller activation diameters, d p50, compared to ULN IR5F (squares) aerosol. κ (∼0.15) of both cigarette types are consistent with partially soluble organic aerosol components.
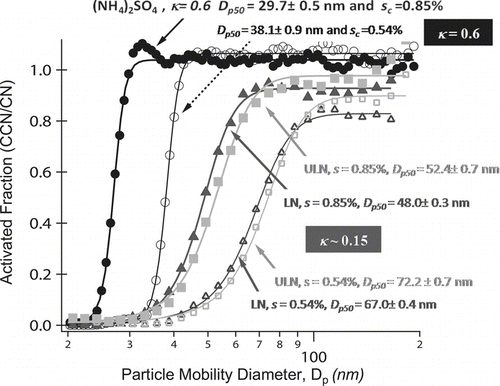
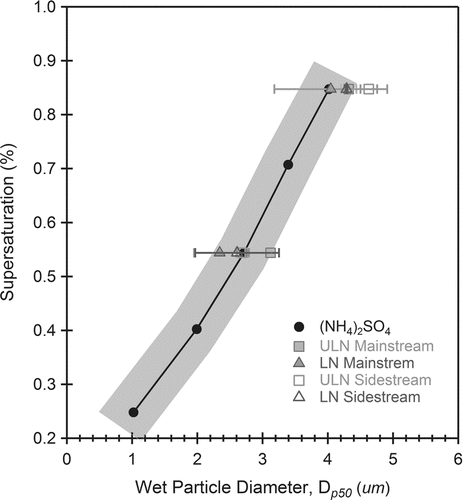
FIG. 6 Droplet growth of LN and ULN ETS. Both LN 3R4F (triangles) and ULN IR5F (squares) aerosol grow to similar sizes as (NH4)2SO4 (circles) at activation in the CCNC instrument. This indicated similar growth kinetics. Despite being less hygroscopic (κ < 0.6, Table 2), organic ETS have the same droplet growth rates as soluble (NH4)2SO4 particles. The shaded area is the region of measurement uncertainty ±0.5 μm.
CCN Activity and Droplet Formation
The ability of ETS to form droplets is less than that of inorganic salts (, ). At s = 0.85% and 0.54%, (NH4)2SO4 aerosol will form droplets at 30 nm and 38 nm respectively. These values respond to κ ∼ 0.6. Calculation of κ assumes no surface tension depression and complete solubility in the droplet, in which case κ is inversely proportional to activation diameter (d p50). Mainstream LN and ULN aerosol will form droplets at larger dry sizes (∼50 nm and 70 nm) and are less hygroscopic than inorganic salts. The calculated κ is consistent with values of organic and semivolatile origin (Duplissy et al. Citation2008; Poulain et al. Citation2010). The smoke formed in mainstream and sidestream smoke of LN cigarettes are of similar distribution and hygrosocopicity (κ ∼ 0.15) (; ). The change in oxygenation (OM/OC or O/C) of the bulk aerosol composition has little or no effect on bulk aerosol hygrosocpicity. shows that the droplets formed at activation from 3R4F and 1R5F smoke grow to sizes, statistically similar to (NH4)2SO4, and indicates similar growth kinetics. Despite being less hygroscopic (κ < 0.6, ), organic ETS has the same droplet growth rate as soluble (NH4)2SO4 particles.
The particle size distribution of the aerosol formed will determine the number of particles that experience ECG. Because the activation diameters for both cigarette types produce aerosol of similar hygroscopicity (κ ∼ 0.15), ULN 1R5F cigarettes will produce more aerosol that activate and form droplets. At 0.54% and 0.85% s, 58% and 79% of the 3R4F mainstream and 68% and 86% of the 1R5F mainstream respectively experience ECG and will form droplets at larger μm sizes. The presented values are percentages of the total aerosol distribution; as LN particles can produce 5 times more PM than ULN cigarette types the absolute number concentrations must be accounted for ().
TABLE 2 Aerosol activation and average single-parameter hygroscopicity data
SUMMARY AND IMPLICATIONS
ETS aerosol is organic and is comprised of slightly soluble compounds. Particles exhaled through mainstream breath produce more oxidized aerosol. The single hygroscopicity parameter, κ ∼ 0.15 is consistent for partially soluble organic aerosol and suggests that the smallest particles are unlikely to form droplets, and experience ECG by water vapor. With this single hygroscopicity parameter, the water uptake of the aerosol can be estimated at sub and supersaturated RH levels. The aerosol that experience ECG in supersaturated regimes will grow to similar droplet sizes as soluble inorganic aerosol. The droplet growth kinetics of ETS is not dependent on solute composition for cigarette smoke particles tested in this study. Depression of surface tension is minor (<5%) for mainstream and sidestream smokes of both cigarette types. Slight changes in bulk aerosol composition do not significantly affect overall CCN activity; hence, the wet deposition of mainstream and sidestream ETS in supersaturated regimes is expected to be similar. Our study shows that reference cigarette ETS are hygroscopic and will have similar lung deposition rates despite source. Additional study may be required for the diverse varieties of commercially available cigarettes. Significant changes in aerosol composition (beyond differences in nicotine content) may alter ETS number, size, and composition. The fraction of particles that experience ECG must be treated differently in deposition models from those that do not. Particle size distribution and hygroscopicity must be considered to model inhalation, dry, and wet deposition rates of ETS. The single parameter, κ ∼ 0.15, reported here can be used to approximate the importance of ECG for ETS.
Acknowledgments
The authors would like to thank Michael Kleeman for use of the Walton Smoking Machine and Daniel Short for his contribution to EC/OC measurements. Xiaochen Tang and Akua Asa-Awuku would like to thank the National Science Foundation Proposal 1032388 for funding and support. Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect the views of the NSF.
REFERENCES
- Abdul-Khalek , I. S. and Kittelson , D. B. 1995 . Real Time Measurement of Volatile and Solid Exhaust Particles Using a Catalytic Stripper . Society of Automotive Engineers Technical Paper Series. Paper no. 950236 ,
- Aiken , A. C. , DeCarlo , P. F. and Jimenez , J. L. 2007 . Elemental Analysis of Organic Species with Electron Ionization High-Resolution Mass Spectrometry . Anal. Chem., , 79 : 8350 – 8358 .
- Aiken , A. C. , Decarlo , P. F. , Kroll , J. H. , Worsnop , D. R. , Huffman , J. A. Docherty , K. S. 2008 . O/C and Om/Oc Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-Resolution Time-of-Flight Aerosol Mass Spectrometry . Environ. Sci. Technol., , 42 : 4478 – 4485 .
- Andreae , M. O. and Rosenfeld , D. 2008 . Aerosol-Cloud-Precipitation Interactions. Part 1. The Nature and Sources of Cloud-Active Aerosols . Earth-Sci. Rev. , 89 : 13 – 41 .
- Anselm , A. , Heibel , T. , Gebhart , J. and Ferron , G. 1990 . Invivo Studies of Growth-Factors of Sodium-Chloride Particles in the Human Respiratory-Tract . J. Aerosol Sci. , 21 : S427 – S430 .
- Asa-Awuku , A. , Nenes , A. , Gao , S. , Flagan , R. C. and Seinfeld , J. H. 2010 . Water-Soluble Soa from Alkene Ozonolysis: Composition and Droplet Activation Kinetics Inferences from Analysis of Ccn Activity . Atmos. Chem. Phys., , 10 : 1585 – 1597 .
- Bernstein , D. M. 2004 . A Review of the Influence of Particle Size, Puff Volume, and Inhalation Pattern on the Deposition of Cigarette Smoke Particles in the Respiratory Tract . Inhal. Toxicol., , 16 : 675 – 689 .
- Blanchard , J. D. and Willeke , K. 1984 . Total Deposition of Ultrafine Sodium-Chloride Particles in Human Lungs . J. Appl. Physiol., , 57 : 1850 – 1856 .
- Broday , D. M. and Georgopoulos , P. G. 2001 . Growth and Deposition of Hygroscopic Particulate Matter in the Human Lungs . Aerosol Sci. Tech., , 34 : 144 – 159 .
- Chen , B. T. , Namenyi , J. , Yeh , H. C. , Mauderly , J. L. and Cuddihy , R. G. 1990 . Physical Characterization of Cigarette-Smoke Aerosol Generated from a Walton Smoke Machine . Aerosol Sci. Tech., , 12 : 364 – 375 .
- DeCarlo , P. F. , Kimmel , J. R. , Trimborn , A. , Northway , M. J. , Jayne , J. T. Aiken , A. C. 2006 . Field-Deployable, High-Resolution, Time-of-Flight Aerosol Mass Spectrometer . Anal. Chem., , 78 : 8281 – 8289 .
- Duplissy , J. , Gysel , M. , Alfarra , M. R. , Dommen , J. , Metzger , A. Prevot , A. S. H. 2008 . Cloud Forming Potential of Secondary Organic Aerosol under near Atmospheric Conditions . Geophys. Res. Lett., , 35:L03818
- Guerin , M. R. , Stokely , J. R. , Higgins , C. E. , Moneyhun , J. H. and Holmberg , R. W. 1979 . Inhalation Bioassay Chemistry – Walton Horizontal Smoking Machine for Inhalation Exposure of Rodents to Cigarette-Smoke . J. Natl. Cancer. I, , 63 : 441 – 448 .
- Hoffmann , D. and Hoffmann , I. 1993 . “ Tobacco Smoke as a Respiratory Carcinogen ” . In Lung Biology in Health and Disease; Prevention of Respiratory Diseases , Edited by: Hirsch , A. , Goldberg , M. , Martin , J. P. and Masse , R. 497 – 532 . Marcel Dekker, Inc., Basel, Switzerland .
- Hoffmann , D. , Rivenson , A. , and Hecht , S. S. 1996 . The Biological Significance of Tobacco-Specific N-Nitrosamines: Smoking and Adenocarcinoma of the Lung . Crit. Rev. Toxicol., , 26 : 199 – 211 .
- Ingebrethsen , B. J. 1986 . Evolution of the Particle-Size Distribution of Mainstream Cigarette-Smoke During a Puff . Aerosol Sci. Tech., , 5 : 423 – 433 .
- Jimenez , J. L. , Jayne , J. T. , Shi , Q. , Kolb , C. E. , Worsnop , D. R. Yourshaw , I. 2003 . Ambient Aerosol Sampling Using the Aerodyne Aerosol Mass Spectrometer . J. Geophys. Res.-Atmos., , 108(D7)
- Kane , D. B. , Asgharian , B. , Price , O. T. , Rostami , A. and Oldham , M. J. 2010 . Effect of Smoking Parameters on the Particle Size Distribution and Predicted Airway Deposition of Mainstream Cigarette Smoke . Inhal. Toxicol., , 22 : 199 – 209 .
- Kittelson , D. B. , Watts , W. F. , Savstrom , J. C. and Johnson , J. P. 2005 . Influence of a Catalytic Stripper on the Response of Real Time Aerosol Instruments to Diesel Exhaust Aerosol . J. Aerosol Sci., , 36 : 1089 – 1107 .
- Klepeis , N. E. , Apte , M. G. , Gundel , L. A. , Sextro , R. G. and Nazaroff , W. W. 2003 . Determining Size-Specific Emission Factors for Environmental Tobacco Smoke Particles . Aerosol Sci. Tech., , 37 : 780 – 790 .
- Londahl , J. , Massling , A. , Swietlicki , E. , Brauner , E. V. , Ketzel , M. Pagels , J. 2009 . Experimentally etermined Human Respiratory Tract Deposition of Airborne Particles at a Busy Street . Environ. Sci. Technol., , 43 : 4659 – 4664 .
- Longest , P. W. , McLeskey , J. T. and Hindle , M. 2010 . Characterization of Nanoaerosol Size Change During Enhanced Condensational Growth . Aerosol Sci. Tech., , 44 : 473 – 483 .
- Longest , P. W., and Xi , J. X. 2008 . Condensational Growth May Contribute to the Enhanced Deposition of Cigarette Smoke Particles in the Upper Respiratory Tract . Aerosol Sci. Tech., , 42 : 579 – 602 .
- Moore , R. H. , Nenes , A. and Medina , J. 2010 . Scanning Mobility Ccn Analysis-a Method for Fast Measurements of Size-Resolved Ccn Distributions and Activation Kinetics . Aerosol Sci. Tech., , 44 : 861 – 871 .
- Padro , L. T. , Tkacik , D. , Lathem , T. , Hennigan , C. J. , Sullivan , A. P. Weber , R. J. 2010 . Investigation of Cloud Condensation Nuclei Properties and Droplet Growth Kinetics of the Water-Soluble Aerosol Fraction in Mexico City . J. Geophys. Res.-Atmos., , 115 : D09204
- Petters , M. D. and Kreidenweis , S. M. 2007 . A Single Parameter Representation of Hygroscopic Growth and Cloud Condensation Nucleus Activity . Atmos. Chem. Phys., , 7 : 1961 – 1971 .
- Phalen , R. F. , Mendez , L. B. and Oldham , M. J. 2010 . New Developments in Aerosol Dosimetry . Inhal. Toxicol., , 22 : 6 – 14 .
- Poulain , L. , Wu , Z. , Petters , M. D. , Wex , H. , Hallbauer , E. Wehner , B. 2010 . Towards Closing the Gap between Hygroscopic Growth and Ccn Activation for Secondary Organic Aerosols - Part 3: Influence of the Chemical Composition on the Hygroscopic Properties and Volatile Fractions of Aerosols . Atmos. Chem. Phys., , 10 : 3775 – 3785 .
- Roberts , D. L. 1988 . Natural Tobacco Flavor . Recent Adv. Tob. Sci., , 14 : 49 – 81 .
- Roberts , G. C. and Nenes , A. 2005 . A Continuous-Flow Streamwise Thermal-Gradient Ccn Chamber for Atmospheric Measurements . Aerosol Sci. Tech., , 39 : 206 – 221 .
- Robinson , R. J. and Yu , C. P. 1998 . Theoretical Analysis of Hygroscopic Growth Rate of Mainstream and Sidestream Cigarette Smoke Particles in the Human Respiratory Tract . Aerosol Sci. Tech. , 28 : 21 – 32 .
- Schroeter , J. D. , Musante , C. J. , Hwang , D. M. , Burton , R. , Guilmette , R. and Martonen , T. B. 2001 . Hygroscopic Growth and Deposition of Inhaled Secondary Cigarette Smoke in Human Nasal Pathways . Aerosol Sci. Tech. , 34 : 137 – 143 .
- Sleiman , M. , Destaillats , H. , Smith , J. D. , Liu , C. L. , Ahmed , M. Wilson , K. R. 2010 . Secondary Organic Aerosol Formation from Ozone-Initiated Reactions with Nicotine and Secondhand Tobacco Smoke . Atmos. Environ., , 44 : 4191 – 4198 .
- Stenitzer , M. 2003 . Nano Particle Formation in the Exhaust of Internal Combustion Engines . Diplom-Ingenieurs, Technischen Universität Wien, Fakultät für Maschinenbau (in English) , Diploma thesis
- Thielen , A. , Klus , H. and Muller , L. 2008 . Tobacco Smoke: Unraveling a Controversial Subject . Exp. Toxicol. Pathol., , 60 : 141 – 156 .
- Varghese , S. K. and Gangamma , S. 2009 . Particle Deposition in Human Respiratory System: Deposition of Concentrated Hygroscopic Aerosols . Inhal. Toxicol., , 21 : 619 – 630 .
- Yadav , R. , Saoud , K. , Rasouli , F. , Hajaligol , M. and Fenner , R. 2004 . Study of Cigarette Smoke Aerosol Using Time of Flight Mass Spectrometry . J. Anal. Appl. Pyrol., , 72 : 17 – 25 .
- Zheng , Z. Q. , Tang , X. C. , Asa-Awuku , A. and Jung , H. S. 2010 . Characterization of a Method for Aerosol Generation from Heavy Fuel Oil (Hfo) as an Alternative to Emissions from Ship Diesel Engines . J. Aerosol Sci., , 41 : 1143 – 1151 .