Abstract
The knowledge of yields and properties of soot from combustion of hydrocarbon fuels is crucial for accurate evaluation of the impacts of primary aerosols on air quality and climate. This study presents measurements of soot generated from combustion of propane in a shock tube, using independently adjustable fuel equivalence ratio (φ), temperature, and pressure. The characterization of soot yields inside the shock tube by in situ laser extinction is complemented with a set of comprehensive measurements of soot transferred into a fluoropolymer chamber, including particle size distributions, elemental carbon (EC) mass fraction, effective density, mass fractal dimension (Dfm), dynamic shape factor (χ), and optical properties. The properties of soot particles and the soot yield are sensitive to combustion conditions and the duration of the combustion experiment. High-temperature combustion with φ = 2.5 produces small fractal (Dfm = 2) soot particles composed mainly of EC (up to 90%), at a low mass yield. Particles from lower temperature combustion contain a significant fraction of organic material (∼50%). Using rich fuel mixtures (φ = 4.0 and 8.0) significantly increases particle size and soot mass yield. At lower temperatures, compact (Dfm = 3) and nearly spherical (χ = 1.1) aggregates with high organic content are formed, whereas at higher temperatures, the particles are fractal and closely resemble those obtained using φ = 2.5. Single scattering albedo (SSA) varies from 0.15 for fractal particles to 0.75 for compact particles. For soot generated at high equivalence ratios, SSA can be used as a proxy for particle morphology and EC content.
Copyright 2012 American Association for Aerosol Research
1. INTRODUCTION
Soot particles produced from incomplete combustion of hydrocarbon fuels by transportation represent a significant fraction of primary atmospheric aerosols (Kittelson Citation1998; Bond et al. Citation2004). A major constituent of freshly emitted soot is elemental carbon (EC), which is often associated with variable amounts of organic carbon (OC) and inorganic sulfate, depending on the source and combustion regime (Adachi and Buseck Citation2008). Atmospheric transformations of primary soot aerosols considerably modify their chemical compositions and lead to formation of secondary aerosols (Shiraiwa et al. Citation2007). The transformation pathways of soot particles include oxidation by the hydroxyl radical (OH) or ozone, condensation or heterogeneous reactions with organic and inorganic species, or coagulation with other aerosols (Zuberi et al. Citation2005; Zhang et al. Citation2008; Riemer et al. Citation2009). In particular, the physical and chemical properties of soot particles can be largely altered because of coating of inorganic and organic species. For example, internal mixing of soot may change particle morphology (Pagels et al. Citation2009; Xue et al. Citation2009a) and chemical reactivity (Khalizov et al. Citation2010). Furthermore, primary soot particles are mainly hydrophobic, but coating of inorganic salts and water-soluble organics enhances their hygroscopicity (Khalizov et al. Citation2009b).
Because of the high-absorption cross-section of EC over a broad range of the solar spectrum, soot contributes significantly to climate change by direct radiative forcing (Forster et al. Citation2007) and is the second most important climate warming agent after carbon dioxide (Jacobson Citation2001). Furthermore, model calculations show that, when associated with other nonabsorbing aerosol constituents (i.e., internally mixed), soot may absorb solar light more efficiently and exert a higher positive direct radiative forcing (Jacobson Citation2001). Internal mixing also significantly increases light scattering and this may offset the positive forcing of the light-absorbing EC (Zhang et al. Citation2008; Xue et al. Citation2009b; Ramana et al. Citation2010). In addition, internally mixed soot particles can impact cloud formation (Zhang et al. Citation2007; Fan et al. Citation2008) and atmospheric photochemistry (Tie et al. Citation2003; Li et al. Citation2005; Tie et al. Citation2005). Currently, the direct and indirect forcing due to soot-containing particles represents a large uncertainty in climate predictions (Forster et al. Citation2007).
Both real and surrogate hydrocarbon combustion sources have been widely used to quantify soot emissions and measure the properties of soot particles. The real sources include aircraft (Petzold et al. Citation2011), diesel (Burtscher Citation2005; Kittelson et al. Citation2006a), and spark ignition engines (Kittelson et al. Citation2006b). Direct sampling from aircraft engines is conducted almost exclusively on a test-rig (Petzold et al. Citation2003) or from a grounded airplane (Onasch et al. Citation2009). Soot emissions by internal combustion engines have been investigated both on road (Schneider et al. Citation2005) and in laboratory settings (Weingartner et al. Citation1997; Park et al. Citation2003b; Maricq and Ning Citation2004). Since EC in an internal combustion engine is formed and exhausted along with vapors of partially burned fuel and lubricating oil, the properties of soot aerosol may change significantly on the way through the exhaust system because of vapor condensation on existing EC particles to form organic coatings, vapor nucleation to form new organic particles, and particle coagulation. For this reason, the use of a sophisticated high-volume dilution system is often required for tailpipe soot sampling to reproduce on-road conditions and its design may largely determine measured particle properties, i.e., the size, morphology, and composition. Surrogate combustion soot sources include diffusion flame burners (Stipe et al. Citation2005; Zhang and Zhang Citation2005; Bento et al. Citation2006; Pagels et al. Citation2009), premixed flame burners (Maricq and Ning Citation2004; Slowik et al. Citation2004; Slowik et al. Citation2007), and the spark discharge generator (Horvath and Gangl Citation2003). The benefit of these sources is that they are relatively inexpensive, show little hourly fluctuations and day-to-day variations in performance, and provide precise control of the combustion conditions.
The use of a shock-tube technique (Petersen et al. Citation2005; Eremin Citation2012) to study the formation of soot combines the advantages of the engine and flame approaches. On one hand, the high temperature and pressure behind the shock wave can be adjusted to closely mimic conditions inside an internal combustion engine. On the other hand, combustion experiments can be carried out for a very broad range of fuels and fuel-to-oxygen ratios, at precisely controlled and reproducible temperature and pressure. Also, unlike in flame soot sources, where combustion temperature depends on the fuel equivalence ratio, in the shock tube temperature and the equivalence ratio can be varied independently (the fuel equivalence ratio is the actual fuel–oxygen ratio divided by stoichiometric fuel–oxygen ratio).
Application of time-resolved fluorescence and laser extinction measurements in the shock tube allows one to obtain details of the fuel combustion and soot formation processes on a submillisecond timescale (Petersen et al. Citation2005). Because of these advantages, shock tubes have been extensively used to study the reaction chemistry preceding the inception of soot (Kern and Xie Citation1991), the formation of soot upon combustion of saturated, unsaturated, and aromatic hydrocarbons (Frenklach et al. Citation1983; Kellerer et al. Citation1996; Agafonov et al. Citation2011), and also the effects of various additives, such as oxygenated organics or ceria oxide nanoparticles on the soot yield (Alexiou and Williams Citation1996; Hong et al. Citation2009; Rotavera et al. Citation2009). Although the laser light extinction and scattering diagnostics can be used to quantify the minute and time-dependent details of the soot generation process, such as the growth of primary soot spherules (Kellerer et al. Citation1996), it is often not possible to observe late processes, such as coagulation and condensation, which may significantly change the chemical composition, mixing state, and morphology of particles when soot in the test section of the shock tube is cooled by the driver gas. Also, absolute soot concentrations derived from optical measurements are subject to significant uncertainties because of poorly constrained complex refractive index and material density of soot (Eremin Citation2012). Finally, in situ measurements may fail when soot is formed in insufficient concentrations.
In this study, we have integrated shock-tube combustion experiments with postshock measurements by several complementary aerosol techniques. Following the approach introduced by Glick et al. (Citation1957) for analysis of gaseous combustion products, after the shock-wave experiment is over, soot aerosol is transferred from the shock tube into a fluoropolymer chamber and characterized to obtain soot concentration, mixing state, morphology, effective density, optical properties, and the total yield. A major focus of our study is to derive properties of airborne soot particles on the basis of aerosol number concentration and mass-mobility measurements, which are direct and involve no assumptions regarding poorly constrained parameters, such as the complex refractive index of soot.
2. EXPERIMENTAL SETUP AND PROCEDURES
2.1. Shock-Tube Combustion Experiments
The high temperature and pressure for the combustion of test mixtures were created near the end-wall of the shock tube behind the reflected shock wave (Figure S1). The shock wave was initiated by rupturing a lexan diaphragm which separated the high-pressure driver section from the lower pressure driven section. Helium was used as the driver gas in most of the runs; in some experiments, the driver gas was “tailored” by mixing nitrogen and helium in specific ratios. Experimental conditions were selected by adjusting the initial pressure of the test mixture and/or by changing the thickness of the diaphragm. During a typical experiment, a shock wave is produced, the mixture combusts, generating soot, and the soot formation process is monitored from laser extinction, which is used to calculate soot concentration in the shock tube, C soot, as described in the supplementary materials. The soot yield is calculated according to Equation (1)
2.2. MEASUREMENTS OF COMBUSTION SOOT PROPERTIES
After the driver gas had filled the test section and the postshock gases equilibrated, a known fraction of the gas mixture containing soot aerosol was transferred from the shock tube into a ∼1 m3 fluoropolymer chamber (Figure S1). From the chamber, aerosol was sampled and analyzed using a suite of instruments, including a Sizing Mobility Particle Scanner (SMPS), a Tandem Differential Mobility Analyzer (TDMA), an Aerosol Particle Mass analyzer (APM), a Thermal Denuder, a Cavity Ring-Down Spectrometer (CRDS), and a Nephelometer to measure particle number size distributions, mass, light extinction, and scattering. In selected experiments, soot particles from the chamber were collected on copper grids (200 mesh with amorphous formvar/carbon films, Ted Pella, Inc.) using a cascade impactor (PIXE International Corp.) and their morphology was examined using a JEOL 2010 transmission electron microscope (TEM) at magnifications ranging from 5000 to 100,000.
2.2.1. Particle Size and Mass
An integrated system consisting of two DMAs, an APM, a CPC, and a Thermal Denuder was used to measure the mobility size and mass of soot particles, and to derive information about their morphology and mixing state (McMurry et al. Citation2002; Khalizov et al. Citation2009b; Pagels et al. Citation2009). To measure the diametric growth factor, Gfd, monodisperse aerosol produced by the first DMA was processed in the thermal denuder maintained at 300°C, and the change in the particle size was measured by the second DMA. The diametric growth factor corresponds to the maximum count in the TDMA distribution, where Dp is the processed and Do is the fresh particle diameter
To determine particle mass, size-classified nascent, mo , or heated, mp , aerosol was measured by the APM. The mass fraction of EC
To increase accuracy, effective density (ρ eff) was measured relative to the Polystyrene latex (PSL) aerosol according to
The mass concentrations of soot (C soot) and EC (C EC) were calculated by integrating the particle number size distribution, n(D), taking into account the size dependence of the effective density and, in the case of C EC, also the size dependence of the EC mass fraction
2.2.2. Optical Properties of Soot Aerosol
The optical properties of soot aerosol in the chamber were investigated from measurements of light scattering and extinction, as described previously (Khalizov et al. Citation2009a). The scattering coefficient at 532 nm, b sca, was derived from measurements by a commercial integrating nephelometer (TSI 3563). Aerosol extinction coefficient at 532 nm, b ext, was measured by the CRDS (Khalizov et al. Citation2009a), as described in the Supplementary material. The absorption coefficient, b abs, was calculated from the difference between extinction and scattering coefficients. Single scattering albedo (SSA) was calculated from the ratio of scattering and extinction coefficients.
The mass concentration of EC was estimated from absorption measurements, assuming a uniform value of the mass absorption cross-section (MAC) α = 7 m2 g−1 (Bond and Bergstrom Citation2006)
3. RESULTS
3.1. Combustion Experiments
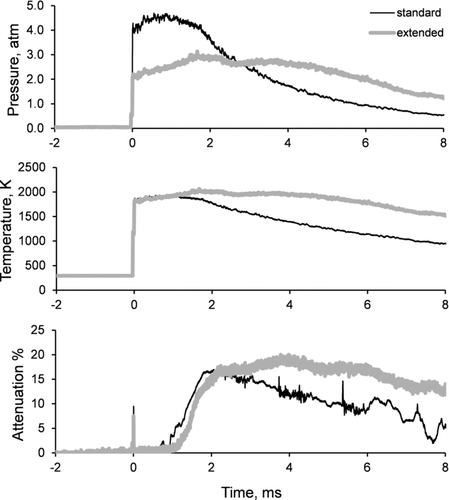
A summary of combustion experiments, including test conditions in the shock tube and major results, is provided in . Combustion was studied for propane/oxygen mixtures with equivalence ratios of 2.5, 4.0, and 8.0, highly diluted in argon (96.5% argon by volume), and in the temperature range of ∼1400 to ∼2200 K. While most of the experiments were conducted using a standard test time of about 2–3 ms, some additional runs with φ = 2.5 and 8.0 were performed using tailored driver gas, resulting in an extended, 4–5 ms, test time. Also, for experiments with φ = 2.5, the effect of pressure behind the reflected shockwave on the yield and properties of soot was explored.
TABLE 1 Summary of experimental conditions and major results obtained in shock-tube experiments; Ar dilution: 96.5%
shows time profiles of pressure and temperature behind the reflected shock wave and also a corresponding increase in the laser signal attenuation caused by the formation of light-absorbing soot. Pressure and laser extinction were measured directly, while the temperature time history was inferred from the measured pressure history assuming an isentropic relationship between the pressure and temperature behind the reflected shock wave. Initiation of soot formation was observed about 0.75 ms after the passing of the reflected shock. The short test time in standard (untailored) experiments is caused by the arrival of the expansion gas that results in a rapid temperature and pressure decrease, quenching soot formation. The short test time combined with the slow initiation of soot formation from propane, as compared to longer chain hydrocarbons, prevents complete transformation of fuel to soot, resulting in a relatively low maximum soot yield, i.e., 0.034 at φ = 8.0 and T = 1876 K (), as calculated from the laser signal attenuation. The arrival of the expansion fan can be delayed by tailoring the driver gas to extend the test conditions behind the reflected shock wave. Use of longer test times increases the amount of soot, with a maximum yield reaching 0.073 (φ = 8.0, T = 1861 K). For mixtures of lower equivalence ratios, i.e., φ = 2.5–4.0, the amount of soot produced by combustion was too low to be detected from in situ attenuation of the laser signal, even in tailored experiments.
3.2. Soot Aerosol Properties
3.2.1. Size Distributions and Number Concentrations
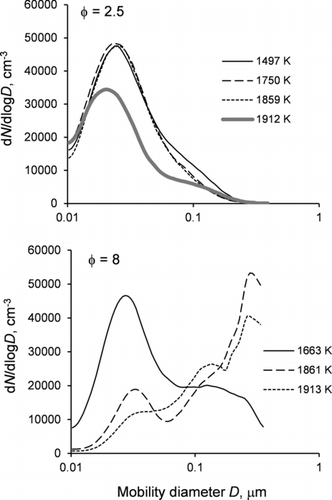
shows that size distributions of soot transferred into the chamber from the shock tube can be represented by two major modes. The first mode is at 15–30 nm and its position varies only moderately with pressure, temperature, and the fuel equivalence ratio. Under leaner combustion conditions (φ = 2.5), the amplitude of this mode is strongly dependent on the pressure behind the reflected shock wave, being very weak at 1.5 atm and becoming progressively stronger as the pressure increases to 6.5 atm (Figure S2). Also, the intensity of the first mode is practically independent of temperature at φ = 2.5 and 4.0, but increases with decreasing combustion temperature at φ = 8.0. Position of the second mode is sensitive to the fuel equivalence ratio and, in high equivalence ratio experiments, to combustion temperature. For lean fuel mixtures (φ = 2.5), the second mode appears in the 50–100 nm range. For rich fuel mixtures (φ = 4.0 and 8.0), it is in the 150–700 nm range, with larger sizes typically corresponding to higher temperatures (Figure S3). The amplitude of the second mode typically increases with increasing temperature. In some of the experiments with the richer fuel mixtures, size distributions extend beyond 800 nm, which is the cutoff size of our aerosol instruments. The total number concentration of soot in the chamber varies between 2×103 and 4×104particle cm−3, corresponding to 1 × 105–3 × 106 particle cm−3 of undiluted soot in the shock tube.
3.2.2. EC Mass Fraction
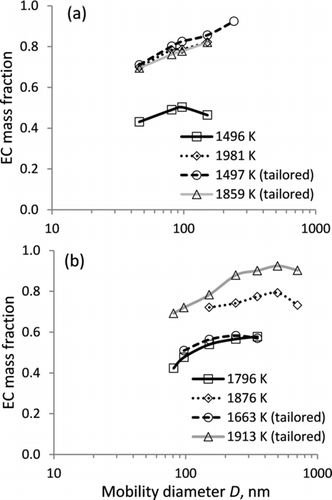
A series of DMA-APM particle mass measurements were conducted on size-classified soot using a thermal denuder to remove organic particle constituents from inorganic carbon cores and thus to quantify the EC mass fraction (f EC), according to Equations (3) and (4). It was assumed that all refractory particle mass after heating to 300°C corresponds to EC. shows that in most experiments f EC increases with increasing particle size and reaches a plateau or drops slightly for the largest soot particles, being in the ranges of 0.4–0.6 and 0.7–0.9 at lower and higher combustion temperatures, respectively. Tailoring leads to a significant increase in f EC for both leaner and richer combustion experiments, and at φ = 2.5 the values of f EC from high-temperature standard experiments and all tailored experiments are practically indistinguishable (). For each individual combustion experiment, the dependence of thermally denuded particle mass versus fresh soot particle mass is linear (Figure S4), with a correlation coefficient r 2 > 0.99. The slope of this dependence, summarized in for different combustion conditions, can be used to represent a size-averaged EC mass fraction of soot, as proposed by Cross et al. (Citation2010). The average f EC shows a trend similar to that of f EC for size-selected particles.
3.2.3. Effective Particle Density, Fractal Dimension, and Dynamic Shape Factor
By combining the measurements of the particle mass and mobility diameter, the effective density, fractal dimension, and dynamic shape factor were determined for fresh and thermally processed soot. Examples of effective densities obtained from lean and rich propane/oxygen mixtures at different combustion temperatures are illustrated in . For soot produced using the leanest fuel mixture (φ = 2.5), the effective density decreases from 1.08 to 0.27 g cm−3 as the mobility size increases from 46 to 240 nm, and there is little difference among the data obtained at different temperatures (). Fitting these density data according to Equation (6) produces mass fractal dimensions in the range of 1.9–2.5 with an average Dfm = 2.08 ± 0.14 (). Although heating reduces the particle mobility diameter and mass, the net change in the effective density is small, especially for larger particles. The average mass fractal dimension of heated soot, Dfm = 2.09 ± 0.11, is very close to that of nascent soot. In experiments with rich fuel mixtures (φ = 8.0), the effective density and mass fractal dimension of soot are sensitive to combustion temperature (). For soot produced at the lowest temperature (T = 1796 K), the effective density is the highest (ρ eff = 1.24 g cm−3) and constant for all particle sizes, resulting in Dfm = 3.0. With the increase in combustion temperature, the effective density of soot is reduced and its dependence on the particle diameter becomes steeper. For instance, at 1904 K, the effective density decreases from 0.40 to 0.11 g cm−3 as the mobility size increases from 240 to 700 nm, resulting in a mass fractal dimension, Dfm = 1.77.
FIG. 4 Size dependence of the effective density of soot generated from combustion of C3H8/O2/Ar mixtures with fuel equivalence ratios of (a) 2.5 and (b) 8.0 at different temperatures (nontailored experiments). Solid and open symbols correspond to measurements of nascent (fresh) and thermally denuded (heated to 300°C) soot aerosol. Solid and gray lines correspond to power fits to the data for fresh and heated soot, respectively. Fractal dimensions derived from the fits are provided in the form (fresh/heated).
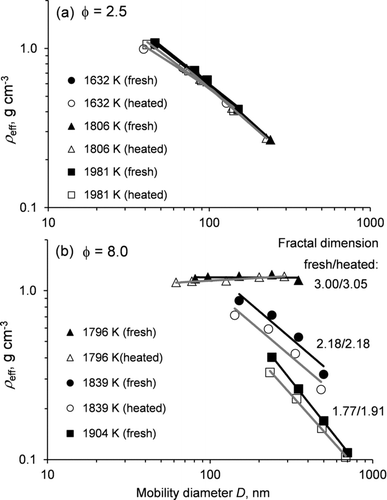
FIG. 5 Size dependence of the dynamic shape factor for soot generated from combustion of C3H8/O2/Ar mixtures with fuel equivalence ratios of (a) 2.5 and (b) 8.0 at different temperatures (nontailored experiments). Solid and open symbols correspond to measurements of nascent (fresh) and thermally denuded (heated to 300°C) soot aerosol.
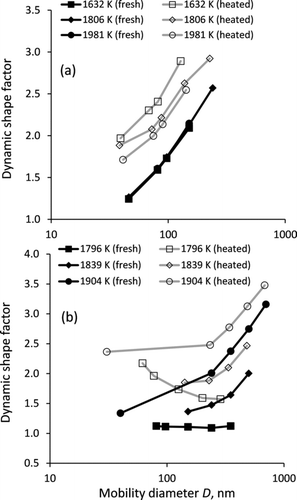
shows the dependence of the dynamic shape factor χ on the particle mobility diameter, which agrees with previous measurements for nascent propane soot under similar conditions (Slowik et al. Citation2004). The dynamic shape factor reflects the increased drag on the particle due to its nonspherical shape, and measured values of χ > 1 indicate that the particles are irregular. At φ = 2.5, the dynamic shape factor increases from 1.2 to 2.6 as the mobility size increases from 46 to 240 nm (). Hence, larger particles become progressively less spherical. The values of χ obtained at different temperatures practically overlap, which is very similar to the trend observed for Dfm. Thermal denuding of soot aggregates removes the organic coating from the EC backbone, resulting in an increased χ. This effect is more pronounced for soot that was produced at lower combustion temperatures and initially contained higher nonrefractory (organic) fraction. At φ = 8.0, for soot produced at T ≤ 1796 K, the dynamic shape factor is constant at 1.10 ± 0.01 (). Such a low value of χ corresponds to compact, nearly-spherical particles. On the contrary, particles produced at higher combustion temperatures are irregular, as follows from higher values of χ, which increase with increasing particle mobility diameter. Thermal denuding of soot results in higher χ, which increases with the particle mobility diameter for soot produced at T ≥ 1939 K, but decreases for soot produced at T = 1796 K. A similar decreasing trend is also observed in low-temperature combustion experiments with φ = 4 and φ = 8.0 (tailored). Such a trend may indicate that EC cores of larger particles are more spherical than EC cores of smaller particles, for instance, due to more extensive restructuring. This trend could also be an artifact, caused by incomplete removal of organics from larger particles upon thermal denuding.
FIG. 6 TEM images of soot particles generated from combustion of C3H8/O2/Ar mixtures, φ = 8.0, tailored experiment: (a, b) 1631 K, 2.9 atm, 57% EC; (c) 1883 K, 2.4 atm, 91% EC.
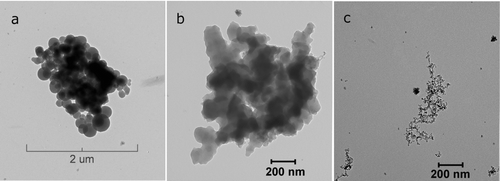
TEM images of soot particles confirm findings of mass-mobility measurements. For instance, soot produced from the most fuel-rich mixtures (φ = 8.0) at low combustion temperature (T = 1631 K) consists of large, nonuniform (d = 70–300 nm), closely packed primary spherules coated with organic material ( and b). On the contrary, for soot produced at high combustion temperatures (T = 1883 K), the primary spherules are small, more uniform (d = 10–15 nm), and arranged in loosely connected aggregates (). Small 30–50 nm compact aggregates clearly visible in all TEM images likely correspond to the first mode in the size distributions shown in .
3.2.4. Light Extinction and Single Scattering Albedo
summarizes some of the results of optical measurements, including extinction coefficients and single scattering albedos for soot aerosol in the chamber. The magnitude of light extinction depends strongly on the number concentration, size, and composition of particles, which vary broadly with combustion conditions. For experiments with φ = 2.5, where smaller particles are produced, the extinction is low, being in the ranges of <1–9 Mm−1 and 8–37 Mm−1 in standard and tailored experiments, respectively. For experiments with φ = 8.0, extinction is in the range of 630–1500 Mm−1, and tailoring shows no discernible effect on the extinction magnitude. SSA, being an intensive property, reflects the relationship of the scattering/extinction ratio on the particle size and composition, but does not depend on the aerosol number concentration. Combustion of the leaner propane/oxygen mixtures produces soot with SSA in the range of 0.4–0.5 in standard and 0.3–0.4 in tailored experiments, without notable temperature dependence. On the contrary, SSA of soot produced using the more fuel-rich mixtures shows a strong dependence on the combustion temperature. In standard experiments, SSA decreases from 0.75 to 0.37 when temperature increases from 1796 to 1876 K. In tailored experiments, SSA is 0.73 at 1663 K and becomes as low as 0.15 at T ≥ 1861 K. There is a linear relationship between SSA and f EC for soot from the richest mixtures (r 2 = 0.99), but not from the leaner mixtures (Figure S5).
3.2.5. Soot Yield
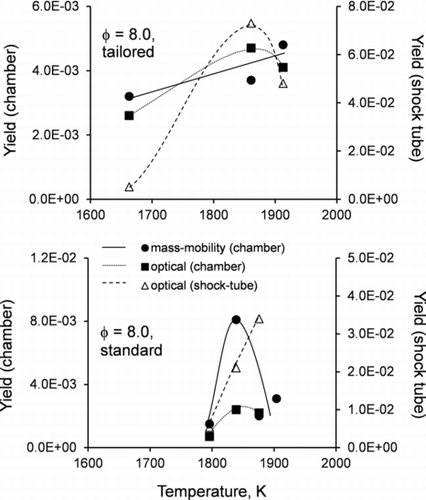
Mass-mobility and optical measurements of aerosol in the chamber were used to estimate the yields of soot (EC) according to Equation (1) in combination with Equations (9) and (11), respectively. These yields along with the yield from direct laser extinction measurements in the shock tube are summarized in . The yields derived from optical and mass-mobility measurements in the chamber are linearly related (r2 = 0.94), with a slope of 0.96 (Figure S6a). Some variance between individual data points can be caused by several factors, including the complex dependence of MAC on the size of primary spherules in soot aggregates (Bond et al. Citation2006), the enhancement of MAC by transparent coatings (Bond and Bergstrom Citation2006), the underestimation of the soot mass concentration caused by the instrument size cutoff in some experiments, and the overestimation of the particle EC mass fraction caused by the assumption that all refractory particle mass at 300°C corresponds to EC.
The slope of 0.059 for the linear dependence between the soot yields measured in the chamber and in the shock tube deviates significantly from unity (Figure S6b). Such large scaling factor is likely caused by an assumption that soot is produced uniformly throughout the shock tube. Actually, the highest concentration of soot is located in the small volume near the end-wall, where the high temperature and pressure are maintained for the longest time during the combustion experiment behind the reflected shock wave. The assumption of a uniform axial soot concentration, when accounting for the dilution of soot upon transfer from the shock tube to the chamber, causes a significant underestimation of the soot yield. Another factor leading to lower concentrations of soot in the chamber and hence lower light extinctions could be due to precipitation losses of supermicrometer soot particles.
Temperature dependences of the soot yields obtained by three independent methods have characteristic nearly Gaussian shapes (a “bell shape”). Detectable soot formation is initiated at ∼1650 K in tailored experiments () and ∼1800 K in standard experiments (). With increasing temperature, the soot production peaks at around 1850–1900 K and then decreases at higher temperatures. Extending the combustion time by tailoring the driver gas significantly broadens the range of temperatures corresponding to detectable formation of soot.
5. DISCUSSION
The mechanism of soot formation is highly complex and involves a large number of chemical and physical processes (Glassman Citation1989; Kern and Xie Citation1991; Mansurov Citation2005). In a shock tube, the high temperature behind the reflected shock wave initiates the pyrolysis of propane, producing small radicals which react to form acetylene, benzene, naphthalene, and increasingly large PAH. Once sufficiently large PAH molecules are formed, dimerization may result in inception of small soot nuclei, which continue to grow by acetylene addition. During the growth process, the high temperature causes dehydrogenation of PAH in the growing nuclei, forming primary spherules consisting mostly of graphitic elemental carbon. The rates of these processes and the properties of the generated soot are highly sensitive to combustion temperature, the fuel equivalence ratio, and also the duration of the combustion experiment. As shown in , the yield of soot decreases when combustion temperature is too low or too high. This occurs because at lower temperatures the growth and dehydrogenation reactions are slow, whereas at higher temperatures, the reverse reactions become dominant. Also, at high temperatures, soot precursors (e.g., acetylene and PAH) and primary spherules can be oxidized in reactions with O2, O, and OH. The oxidation becomes complete at equivalence ratios below ∼1.8 when soot particles are no longer produced in detectable quantities (Slowik et al. Citation2004).
When sufficiently high concentration of primary spherules is reached in the combustion zone, they coagulate through random collisions, forming larger aggregated particles. The fractal dimension of such aggregates is expected to be around 1.80–1.95 (Meakin Citation1986; Slowik et al. Citation2004), which is in agreement with the values obtained in our experiments using φ = 2.5 and also φ = 4.0 and 8.0 at higher temperatures (). The measured low effective densities confirm the aggregated, fractal structure of these particles. It should be noted that the slopes between the effective density and the mobility diameter become less negative for the smallest sizes, reflecting a higher Dfm for the smallest particles of both fresh and heated soot (). A similar behavior has been observed previously for the propane soot generated in premixed (Slowik et al. Citation2004) and diffusion flames (Pagels et al. Citation2009). As soot particles are not true fractals in the mathematical sense, their self-similarity breaks down in the limit of small number of primary spherules Npp < 60 (or Dp < 170 nm) (DeCarlo et al. Citation2004; Slowik et al. Citation2004). This phenomenon leads to a lower apparent fractal dimension for smaller aggregates, which contain fewer primary spherules.
At relatively low combustion temperatures, some of the PAH and aliphatic hydrocarbons from pyrolysis of propane can survive the combustion process and remain in the vapor phase. When the driver gas arrives at the test section and cools the combustion mixture, these organic vapors can condense on soot aggregates and individual primary spherules, contributing up to half of the particle mass (). Organic vapors, when sufficiently supersaturated, may also nucleate small organic particles, which subsequently grow by condensation and coagulate with soot aggregates. This process may explain the presence of primary spherules of different sizes, ranging from 70 to 300 nm, within a single aggregate (). Whereas soot aggregates produced with φ = 2.5 remain fractal despite the high organic content, soot aggregates obtained with φ = 4.0 and 8.0 are compact (Dfm ∼ 3) and nearly spherical (χ = 1.1).
Even the highest effective density measured in this study is significantly lower than the inherent material density of EC, ρ EC = 1.77 g cm−3 (Park et al. Citation2004). The reduced ρ eff is likely caused by interstitial space between primary spherules. Also, in some cases, nonuniform sizes of primary spherules () may prevent the closest packing, producing voids. The densities of heated particles converge to ρ eff ∼ 1.1 g cm−3 at the smallest sizes. This value is very close to 1.13 g cm−3, the maximum effective density of compact aggregates that would be formed as a result of complete restructuring of fractal aggregates (Khalizov et al. Citation2009a), and confirms that even the most compact particles have an aggregated structure. The maximum effective density is estimated as a product of the inherent material soot density (1.77 g cm−3) and a random close packing factor of 0.637 (Feder Citation1988).
A transition from compact to fractal aggregates occurs within a very narrow range of combustion temperatures (<50 K). A similar sharp transition between two types of soot has also been observed in previous studies (Slowik et al. Citation2004; Khalizov et al. Citation2009b), where the fuel equivalence ratio was varied rather than the combustion temperature. As hypothesized by Slowik et al. (Citation2004), condensing PAH vapors initially fill the voids and irregularities within the fractal aggregate before forming a layer on its outer surface. As the PAH vapor concentration increases with decreasing combustion temperature, a point is reached when the interior of the porous soot is filled completely, individual primary spherules lose their identity, and the aggregate becomes a nearly smooth sphere. In our experiments, the mass fractal dimension of such an aggregate remains practically unchanged when the organic coating is removed by heating, indicating a very close arrangement of primary spherules. Such an arrangement is unlikely to be formed from random collisions of primary spherules, but could be produced by restructuring of a more fractal aggregate, as shown previously in experiments with different coating materials (Slowik et al. Citation2007; Zhang et al. Citation2008; Pagels et al. Citation2009; Xue et al. Citation2009a). Whereas coatings composed of solid PAH are expected to cement the spherules more firmly in place, preventing rearrangement, the presence of aliphatic hydrocarbons, which have a structure very similar to that of oleic acid that is known to cause restructuring, may allow the spherules to become less attached to one another and lead to a more compact rearrangement (Slowik et al. Citation2007).
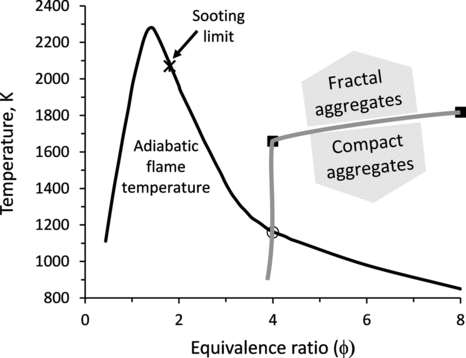
Our results on size distributions and properties of soot particles agree with previous measurements of soot from flame sources when obtained under similar conditions (Slowik et al. Citation2004, Citation2007; Pagels et al. Citation2009). Also, soot particles produced in the shock tube using φ = 2.5 are representative of soot from diesel engine exhaust, and show similar decreasing trends in the mass fractal dimension and effective density with increasing combustion temperature, which corresponds to higher engine loads (Park et al. Citation2003a). Additionally, the shock tube has allowed us to explore the combustion conditions corresponding to high equivalence ratios and high temperatures occurring simultaneously. Such conditions are unattainable in flame sources, where temperature cannot be precisely controlled and decreases with increasing equivalence ratio (Law Citation2006), as illustrated in . We find that a sharp boundary between fractal and compact soot, occurring at an equivalence ratio of about 4.0 in premixed flames (Slowik et al. Citation2004), can be shifted toward higher equivalence ratios in shock-tube experiments by increasing the combustion temperature. For instance, fractal aggregates can be produced at T > 1700 K and T > 1839 K, when using φ = 4.0 and 8.0, respectively. The range of temperatures and equivalence ratios that produce either fractal or compact soot aggregates upon combustion of propane/oxygen mixtures is schematically illustrated in . In experiments with φ > 4, very high concentration of PAH is produced, and if the combustion temperature is sufficiently low PAH remain in the gas phase and condense on aggregates upon cooling, leading to compact particles. At φ < 4, significantly less PAH is formed, and even at lower combustion temperatures soot aggregates remain fractal.
FIG. 8 A phase space diagram illustrating the range of temperature and equivalence ratio conditions that produce either fractal or compact soot aggregates upon combustion of propane/oxygen mixtures. Solid squares and an open circle mark the transition from fractal to compact soot aggregates measured in shock tube (this study) and premixed flame (Slowik et al. Citation2004) experiments, respectively. An asterisk marks the flame sooting limit φ ∼ 1.8, when soot particles are no longer produced in detectable quantities in premixed flames. Adiabatic flame temperature is adapted from Law (Citation2006) and corresponds to real fuel–air mixtures.
The shock tube allows one to control the length of time the soot is maintained at a known combustion temperature, by essentially changing the arrival time of the main expansion front through gas-dynamic tailoring. We find that the degree of PAH graphitization and oxidation depends not only on temperature and the equivalence ratio, but also on duration of the combustion experiment. This effect can be most clearly seen in the experiments conducted with the lower equivalence ratio, where doubling the combustion time allows graphitization and oxidation of the PAH to occur almost completely, resulting in fractal particles with high mass content of EC.
6. CONCLUSIONS
The integration of shock-tube combustion experiments with postshock aerosol mass-mobility measurements allowed us to characterize the properties of soot produced under a broad range of conditions, including high equivalence ratio–high temperature combinations, which cannot be achieved using flame soot sources. A notable advantage of the shock-tube technique is that the temperature, pressure, fuel equivalence ratio, and duration of the combustion experiment can be controlled and varied independently of each other. On the other hand, postshock aerosol measurements provide a wealth of information on soot morphology and mixing state that cannot be obtained from typically used in situ shock-tube diagnostics.
The properties of soot particles and the total mass yield of soot are very sensitive to combustion conditions, and can be additionally varied by tailoring the shock experiment, i.e., by changing the time delay between the passage of the shock wave and the arrival of the expanding gas or expansion wave front from the driver section. Soot particles are composed of elemental carbon and organics, which are present at a ratio that depends strongly on combustion temperature. Soot particles formed above the peak soot yield temperature, which occurs at around 1850–1900 K, contain a higher fraction of elemental carbon when compared to soot formed below the peak soot yield temperature; typically, the higher the temperature, the higher the elemental carbon fraction.
The mass yield of soot is low and particles are small (<100 nm) in experiments with lean propane/oxygen mixtures (φ = 2.5). High-temperature combustion produces highly aggregated soot particles (Dfm = 2.1, χ = 1.2–2.6) that are composed mainly of EC (up to 90%). At lower temperatures, particles contain a significant fraction of organic material (∼50%). Using richer fuel mixtures (φ = 4.0 and 8.0) significantly increases the particle size and the total mass yield of soot. At lower temperatures, compact (Dfm = 3.0) and nearly spherical (χ = 1.1) particles are formed, whereas at higher temperatures, the particles are fractal and their properties closely resemble those obtained using φ = 2.5. Single scattering albedo of soot aerosol varies from 0.15 for aggregated particles composed mostly of EC to 0.75 for compact particles with high organic fraction. For soot generated using rich fuel/oxygen mixtures, SSA can be used as a proxy for particle morphology and composition.
The integrated soot generation and characterization approach developed in this study can be extended for investigation of soot formation from long-chain hydrocarbon fuels, such as toluene, diesel, and diesel surrogates, both in vapor and aerosol forms. The knowledge of yields and properties of soot from combustion of hydrocarbon fuels is crucial for accurate evaluation of soot emissions from various combustion sources. Furthermore, generation of soot particles under controlled conditions allows for investigation of their atmospheric transformation pathways. For example, atmospheric measurements reveal ambient aerosols often contain a soot core (Hasegawa and Ohta Citation2002), which may be formed from condensation or heterogeneous reactions of inorganic and organic species (Zhao et al. Citation2005, Citation2009; Levitt et al. Citation2006; Zhao et al. Citation2006; Levitt et al. Citation2007; Zhang et al. Citation2008). In particular, an enormous amount of organic compounds, produced from oxidation of biogenic and anthropogenic volatile organic compounds (Suh et al. Citation2002; Zhang et al. Citation2002; Fan and Zhang Citation2004), can contribute to aging and transformation of soot particles and modification of their optical and cloud-forming properties. Both the production of soot particles under different combustion conditions and their atmospheric transformation need to be quantified for accurate evaluation of the impacts of soot on air quality and climate.
uast_a_683839_sup_25510570.zip
Download Zip (319.1 KB)Acknowledgments
The authors thank Ann Ellis of the Microscopy and Imaging Center at Texas A&M University for help with acquiring TEM images. The authors acknowledge Sarah Brooks for the use of a cascade impactor. This work was supported by the National Science Foundation (CBET-0932705) and the Robert A. Welch Foundation (A-1417).
[Supplemental materials are available for this article. Go to the publisher's online edition of Aerosol Science and Technology to view the free supplementary files.]
REFERENCES
- Adachi , K. and Buseck , P. R. 2008 . Internally Mixed Soot, Sulfates, and Organic Matter in Aerosol Particles from Mexico City . Atmos. Chem. Phys. , 8 : 6469 – 6481 .
- Agafonov , G. L. , Smirnov , V. N. and Vlasov , P. A. 2011 . Shock Tube and Modeling Study of Soot Formation During the Pyrolysis and Oxidation of a Number of Aliphatic and Aromatic Hydrocarbons . Proc. Combust. Inst. , 33 : 625 – 632 .
- Alexiou , A. and Williams , A. 1996 . Soot Formation in Shock-Tube Pyrolysis of Toluene, Toluene–Methanol, Toluene–Ethanol, and Toluene–Oxygen Mixtures . Combust. Flame , 104 : 51 – 65 .
- Bento , D. S. , Thomson , K. A. and Gulder , O. L. 2006 . Soot Formation and Temperature Field Structure in Laminar Propane–Air Diffusion Flames at Elevated Pressures . Combust. Flame , 145 : 765 – 778 .
- Bond , T. C. and Bergstrom , R. W. 2006 . Light Absorption by Carbonaceous Particles: An Investigative Review . Aerosol Sci. Technol. , 40 : 27 – 67 .
- Bond , T. C. , Habib , G. and Bergstrom , R. W. 2006 . Limitations in the Enhancement of Visible Light Absorption Due to Mixing State . J. Geophys. Res. , 111 : D20211
- Bond , T. C. , Streets , D. G. , Yarber , K. F. , Nelson , S. M. , Woo , J. H. and Klimont , Z. 2004 . A Technology-Based Global Inventory of Black and Organic Carbon Emissions from Combustion . J. Geophys. Res. , 109 : D14203
- Burtscher , H. 2005 . Physical Characterization of Particulate Emissions from Diesel Engines: A Review . J. Aerosol Sci , 36 : 896 – 932 .
- Cross , E. S. , Onasch , T. B. , Ahern , A. , Wrobel , W. , Slowik , J. G. Olfert , J. 2010 . Soot Particle Studies Instrument Inter-Comparison Project Overview . Aerosol Sci. Technol. , 44 : 592 – 611 .
- Davidson , D. F. , Bates , R. , Petersen , E. L. and Hanson , R. K. 1998 . Shock Tube Measurements of the Equation of State of Argon . Int. J. Thermophys. , 19 : 1585 – 1594 .
- DeCarlo , P. F. , Slowik , J. G. , Worsnop , D. R. , Davidovits , P. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory . Aerosol Sci. Technol. , 38 : 1185 – 1205 .
- Eremin , A. V. 2012 . Formation of Carbon Nanoparticles from the Gas Phase in Shock Wave Pyrolysis Processes . Prog. Energy Combust. Sci. , 38 : 1 – 40 .
- Fan , J. and Zhang , R. 2004 . Atmospheric Oxidation Mechanism of Isoprene . Environ. Chem. , 1 : 140 – 149 .
- Fan , J. W. , Zhang , R. Y. , Tao , W. K. and Mohr , K. I. 2008 . Effects of Aerosol Optical Properties on Deep Convective Clouds and Radiative Forcing . J. Geophys. Res. , 113 : D08209 doi: 08210.01029/02007JD009257
- Feder , J. 1988 . Fractals , New York : Plenum Press .
- Forster , P. , Ramaswamy , V. , Artaxo , P. , Berntsen , T. , Betts , R. Fahey , D. W. 2007 . “ Changes in Atmospheric Constituents and in Radiative Forcing ” . In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K. B. , Tignor , M and Miller , H. L. Cambridge, UK and New York , NY : Cambridge University Press .
- Frenklach , M. , Taki , S. , Durgaprasad , M. B. and Matula , R. A. 1983 . Soot Formation in Shock-Tube Pyrolysis of Acetylene, Allene, and 1,3-Butadiene . Combust. Flame , 54 : 81 – 101 .
- Glassman , I. 1989 . Soot Formation in Combustion Processes . Symp. (Int.) Combust. , 22 : 295 – 311 .
- Glick , H. S. , Klein , J. J. and Squire , W. 1957 . Single-Pulse Shock Tube Studies of the Kinetics of the Reaction N2+O2 Reversible 2NO between 2000–3000-degrees-K . J. Chem. Phys. , 27 : 850 – 857 .
- Hasegawa , S. and Ohta , S. 2002 . Some Measurements of the Mixing State of Soot-Containing Particles at Urban and Non-Urban Sites . Atmos. Environ. , 36 : 3899 – 3908 .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : Wiley .
- Hong , Z. , Davidson , D. F. , Vasu , S. S. and Hanson , R. K. 2009 . The Effect of Oxygenates on Soot Formation in Rich Heptane Mixtures: A Shock Tube Study . Fuel , 88 : 1901 – 1906 .
- Horvath , H. and Gangl , M. 2003 . A Low-Voltage Spark Generator for Production of Carbon Particles . J. Aerosol Sci. , 34 : 1581 – 1588 .
- Jacobson , M. Z. 2001 . Strong Radiative Heating Due to the Mixing State of Black Carbon in Atmospheric Aerosols . Nature , 409 : 695 – 697 .
- Kellerer , H. , Muller , A. , Bauer , H. J. and Wittig , S. 1996 . Soot Formation in a Shock Tube under Elevated Pressure Conditions . Combust. Sci. Technol. , 113 : 67 – 80 .
- Kern , R. D. and Xie , K. 1991 . Shock-Tube Studies of Gas-Phase Reactions Preceding the Soot Formation Process . Prog. Energy Combust. Sci. , 17 : 191 – 210 .
- Khalizov , A. F. , Cruz-Quinones , M. and Zhang , R. 2010 . Heterogeneous Reaction of NO2 on Fresh and Coated Soot Surfaces . J. Phys. Chem. A , 114 : 7516 – 7524 .
- Khalizov , A. F. , Xue , H. , Wang , L. , Zheng , J. and Zhang , R. 2009a . Enhanced Light Absorption and Scattering by Carbon Soot Aerosol Internally Mixed with Sulfuric Acid . J. Phys. Chem. A , 113 : 1066 – 1074 .
- Khalizov , A. F. , Zhang , R. , Zhang , D. , Xue , H. , Pagels , J. and McMurry , P. H. 2009b . Formation of Highly Hygroscopic Soot Aerosols upon Internal Mixing with Sulfuric Acid Vapor . J. Geophys. Res. , 114 : D05208
- Kittelson , D. B. 1998 . Engines and Nanoparticles: A Review . J. Aerosol Sci. , 29 : 575 – 588 .
- Kittelson , D. B. , Watts , W. F. and Johnson , J. P. 2006a . On-Road and Laboratory Evaluation of Combustion Aerosols—Part 1: Summary of Diesel Engine Results . J. Aerosol Sci. , 37 : 913 – 930 .
- Kittelson , D. B. , Watts , W. F. , Johnson , J. P. , Schauer , J. J. and Lawson , D. R. 2006b . On-Road and Laboratory Evaluation of Combustion Aerosols—Part 2: Summary of Spark Ignition Engine Results . J. Aerosol Sci. , 37 : 931 – 949 .
- Law , C. K. 2006 . Combustion Physics , New York : Cambridge University Press .
- Levitt , N. P. , Zhang , R. Y. , Xue , H. X. and Chen , J. M. 2007 . Heterogeneous Chemistry of Organic Acids on Soot Surfaces . J. Phys. Chem. A , 111 : 4804 – 4814 .
- Levitt , N. P. , Zhao , J. and Zhang , R. Y. 2006 . Heterogeneous Chemistry of Butanol and Decanol with Sulfuric Acid: Implications for Secondary Organic Aerosol Formation . J. Phys. Chem. A , 110 : 13215 – 13220 .
- Li , G. H. , Zhang , R. Y. , Fan , J. W. and Tie , X. X. 2005 . Impacts of Black Carbon Aerosol on Photolysis and Ozone . J. Geophys. Res. , 110 : D23206
- Mansurov , Z. 2005 . Soot Formation in Combustion Processes (Review) . Combust., Explo., Shock Waves , 41 : 727 – 744 .
- Maricq , M. M. and Ning , X. 2004 . The Effective Density and Fractal Dimension of Soot Particles from Premixed Flames and Motor Vehicle Exhaust . J. Aerosol Sci. , 35 : 1251 – 1274 .
- McMurry , P. H. , Wang , X. , Park , K. and Ehara , K. 2002 . The Relationship between Mass and Mobility for Atmospheric Particles: A New Technique for Measuring Particle Density . Aerosol Sci. Technol. , 36 : 227 – 238 .
- Meakin , P. 1986 . “ Computer Simulation of Growth and Aggregation Processes ” . In On Growth and Form: Fractal and Non-Fractal Patterns in Physics. NATO ASI Series. Series E, Applied Scienes , Edited by: Stanley , H. E. and Ostrowsky , N. 308 Dordrecht, The Netherlands : M. Nijhoff .
- Onasch , T. B. , Jayne , J. T. , Herndon , S. , Worsnop , D. R. , Miake-Lye , R. C. Mortimer , I. P. 2009 . Chemical Properties of Aircraft Engine Particulate Exhaust Emissions . J. Propul. Power , 25 : 1121 – 1137 .
- Pagels , J. , Khalizov , A. F. , McMurry , P. H. and Zhang , R. Y. 2009 . Processing of Soot by Controlled Sulphuric Acid and Water Condensation–Mass and Mobility Relationship . Aerosol Sci. Technol. , 43 : 629 – 640 .
- Park , K. , Cao , F. , Kittelson , D. B. and McMurry , P. H. 2003a . Relationship between Particle Mass and Mobility for Diesel Exhaust Particles . Environ. Sci. Technol. , 37 : 577 – 583 .
- Park , K. , Kittelson , D. B. and McMurry , P. H. 2003b . A Closure Study of Aerosol Mass Concentration Measurements: Comparison of Values Obtained with Filters and by Direct Measurements of Mass Distributions . Atmos. Environ. , 37 : 1223 – 1230 .
- Park , K. , Kittelson , D. B. , Zachariah , M. R. and McMurry , P. H. 2004 . Measurement of Inherent Material Density of Nanoparticle Agglomerates . J. Nanopart. Res. , 6 : 267 – 272 .
- Petersen , E. L. , Rickard , M. J. A. , Crofton , M. W. , Abbey , E. D. , Traum , M. J. and Kalitan , D. M. 2005 . A Facility for Gas—and Condensed-Phase Measurements Behind Shock Waves . Meas. Sci. Technol. , 16 : 1716 – 1729 .
- Petzold , A. , Marsh , R. , Johnson , M. , Miller , M. , Sevcenco , Y. Delhaye , D. 2011 . Evaluation of Methods for Measuring Particulate Matter Emissions from Gas Turbines . Environ. Sci. Technol. , 45 : 3562 – 3568 .
- Petzold , A. , Stein , C. , Nyeki , S. , Gysel , M. , Weingartner , E. Baltensperger , U. 2003 . Properties of Jet Engine Combustion Particles During the PartEmis Experiment: Microphysics and Chemistry . Geophys. Res. Lett. , 30 : 1719 doi: 1710.1029/2003gl017283
- Ramana , M. V. , Ramanathan , V. , Feng , Y. , Yoon , S. C. , Kim , S. W. Carmichael , G. R. 2010 . Warming Influenced by the Ratio of Black Carbon to Sulphate and the Black-Carbon Source . Nat. Geosci. , 3 : 542 – 545 .
- Riemer , N. , West , M. , Zaveri , R. A. and Easter , R. C. 2009 . Simulating the Evolution of Soot Mixing State with a Particle-Resolved Aerosol Model . J. Geophys. Res. , 114 10.1029/2008jd011073
- Rotavera , B. , Kumar , A. , Seal , S. and Petersen , E. L. 2009 . Effect of Ceria Nanoparticles on Soot Inception and Growth in Toluene–Oxygen–Argon Mixtures . Proc. Combust. Inst. , 32 : 811 – 819 .
- Schneider , J. , Hock , N. , Weimer , S. and Borrmann , S. 2005 . Nucleation Particles in Diesel Exhaust: Composition Inferred from In Situ Mass Spectrometric Analysis . Environ. Sci. Technol. , 39 : 6153 – 6161 .
- Shiraiwa , M. , Kondo , Y. , Moteki , N. , Takegawa , N. , Miyazaki , Y. and Blake , D. R. 2007 . Evolution of Mixing State of Black Carbon in Polluted Air from Tokyo . Geophys. Res. Lett. , 34 : L16803
- Slowik , J. G. , Cross , E. S. , Han , J. H. , Kolucki , J. , Davidovits , P. Williams , L. R. 2007 . Measurements of Morphology Changes of Fractal Soot Particles Using Coating and Denuding Experiments: Implications for Optical Absorption and Atmospheric Lifetime . Aerosol Sci. Technol. , 41 : 734 – 750 .
- Slowik , J. G. , Stainken , K. , Davidovits , P. , Williams , L. R. , Jayne , J. T. Kolb , C. E. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 2: Application to Combustion-Generated Soot Aerosols as a Function of Fuel Equivalence Ratio . Aerosol Sci. Technol. , 38 : 1206 – 1222 .
- Stipe , C. B. , Higgins , B. S. , Lucas , D. , Koshland , C. P. and Sawyer , R. F. 2005 . Inverted Co-Flow Diffusion Flame for Producing Soot . Rev. Sci. Instrum. , 76 : 023908 doi: 023910.021063/023901.1851492
- Suh , I. , Zhang , D. , Zhang , R. Y. , Molina , L. T. and Molina , M. J. 2002 . Theoretical Study of OH Addition Reaction to Toluene . Chem. Phys. Lett. , 364 : 454 – 462 .
- Tie , X. X. , Madronich , S. , Walters , S. , Edwards , D. P. , Ginoux , P. Mahowald , N. 2005 . Assessment of the Global Impact of Aerosols on Tropospheric Oxidants . J. Geophys. Res. , 110 : D03204
- Tie , X. X. , Madronich , S. , Walters , S. , Zhang , R. Y. , Rasch , P. and Collins , W. 2003 . Effect of Clouds on Photolysis and Oxidants in the Troposphere . J. Geophys. Res. , 108 : 4642 doi: 4610.1029/2003jd003659
- Weingartner , E. , Burtscher , H. and Baltensperger , U. 1997 . Hygroscopic Properties of Carbon and Diesel Soot Particles . Atmos. Environ. , 31 : 2311 – 2327 .
- Xue , H. , Khalizov , A. F. , Wang , L. , Zheng , J. and Zhang , R. 2009a . Effects of Coating of Dicarboxylic Acids on the Mass–Mobility Relationship of Soot Particles . Environ. Sci. Technol. , 43 : 2787 – 2792 .
- Xue , H. X. , Khalizov , A. F. , Wang , L. , Zheng , J. and Zhang , R. Y. 2009b . Effects of Dicarboxylic Acid Coating on the Optical Properties of Soot . Phys. Chem. Chem. Phys. , 11 : 7869 – 7875 .
- Zhang , D. , Lei , W. F. and Zhang , R. Y. 2002 . Mechanism of OH Formation from Ozonolysis of Isoprene: Kinetics and Product Yields . Chem. Phys. Lett. , 358 : 171 – 179 .
- Zhang , D. and Zhang , R. 2005 . Laboratory Investigation of Heterogeneous Interaction of Sulfuric Acid with Soot . Environ. Sci. Technol. , 39 : 5722 – 5728 .
- Zhang , R. , Khalizov , A. F. , Pagels , J. , Zhang , D. , Xue , H. and McMurry , P. H. 2008 . Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols During Atmospheric Processing . Proc. Natl. Acad. Sci. USA , 105 : 10291 – 10296 .
- Zhang , R. Y. , Li , G. H. , Fan , J. W. , Wu , D. L. and Molina , M. J. 2007 . Intensification of Pacific Storm Track Linked to Asian Pollution . Proc. Natl. Acad. Sci. USA , 104 : 5295 – 5299 .
- Zhao , J. , Levitt , N. P. and Zhang , R. Y. 2005 . Heterogeneous Chemistry of Octanal and 2,4-Hexadienal with Sulfuric Acid . Geophys. Res. Lett. , 32 : L09802
- Zhao , J. , Levitt , N. P. , Zhang , R. Y. and Chen , J. M. 2006 . Heterogeneous Reactions of Methylglyoxal in Acidic Media: Implications for Secondary Organic Aerosol Formation . Environ. Sci. Technol. , 40 : 7682 – 7687 .
- Zhao , J. , Khalizov , A. F. , Zhang , R. and McGraw , R. 2009 . Hydrogen Bonding Interaction of Molecular Complexes and Clusters of Aerosol Nucleation Precursors . J. Phys. Chem. , 113 : 680 – 689 .
- Zuberi , B. , Johnson , K. S. , Aleks , G. K. , Molina , L. T. and Laskin , A. 2005 . Hydrophilic Properties of Aged Soot . Geophys. Res. Lett. , 32 : L01807