Abstract
In this study, particles generated from a direct-injection (DI) diesel engine fueled with biodiesel, ultra-low-sulfur diesel (ULSD, <10 ppm-wt), and low-sulfur diesel (LSD, <500 ppm-wt) were investigated experimentally for their oxidation properties, using the thermogravimetric analysis (TGA), at five engine loads. Kinetic analysis of particulate oxidation was conducted based on the mass loss curves obtained from the TGA. The activation energy was found to be in the range of 142–175, 76–127, and 133–162 kJ/mol for the particulate samples for ULSD, biodiesel, and LSD, respectively. The particulate oxidation rate decreases with the increase of engine load for each fuel, and at each engine load, the oxidation rate decreases in the order of biodiesel, LSD, and ULSD. The primary particle size, nanostructure, and volatile fraction were also investigated for different particulate samples. The results indicate that the higher oxidation rate of biodiesel particles could be related to the smaller primary particle size, the more disordered nanostructure, and the larger volatile fraction, compared with the ULSD and LSD particles. The increase of sulfur content in a diesel fuel has a limited influence on primary particle size and nanostructure, while inducing a larger volatile fraction, which might be one of the reasons for the stronger oxidative reactivity of the LSD particles, compared with the ULSD particles.
Copyright 2012 American Association for Aerosol Research
1. INTRODUCTION
The oxidative reactivity of diesel particulate is an important factor affecting diesel particulate filter (DPF) regeneration behavior. The relationship between particulate properties and reactivity has been the subject of several previous studies. Müller et al. (Citation2005, Citation2006) investigated the oxidative reactivity of particulate matter from different sources and found that the internal nanostructure controlled the particle oxidative reactivity. Vander Wal and Tomasek (Citation2003) found that the oxidation rate could differ by more than 400% depending on the initial particle nanostructure. Engine-operating conditions are an important factor affecting the nanostructure of the particles and hence the particle oxidative reactivity. Lee et al. (Citation2001) and Zhu et al. (Citation2005) investigated the nanostructure of diesel particulate and found that the particulate appeared to have amorphous structure at low engine loads and graphitic structures at high engine loads. Particulate composition is another important factor affecting the particle oxidative reactivity. Volatile matter such as hydrocarbons that filled the micropores on the particulate is a source of further micropore development and hence creating a variation in particle reactivity (Stanmore et al. Citation2001; Kandas et al. Citation2005).
Fuel properties exhibit a strong influence on particulate emission. It is well known that decreasing the sulfur content in fuels could effectively reduce the number and mass concentration of particulate emission (Ullman et al. Citation1994; Bagley et al. Citation1996; Shi and Harrision Citation1999; Wei et al. Citation2001). Hence, compositional constraints on sulfur have been specified for diesel fuels (Diesel Emission Controls Sulfur Effects [DECSE] Report Citation2001). Oxygenated fuels such as biodiesel also can lead to a reduction in particulate emission. Krahl et al. (Citation1996) reported that using pure biodiesel could reduce diesel particulate emission by up to 50% by mass. Biodiesel can also reduce the demand of fossil fuel and thus reduce the overall life-cycle CO2 emission because it is produced from renewable sources such as vegetable oil, waste cooking oil, and animal fats. Therefore, the use of biodiesel as an alternative to diesel fuel has been well reviewed in the literature (Lapuerta et al. Citation2008; Dwivedi et al. Citation2011).
The above review shows that fuel properties could affect particulate emission and hence might probably affect the oxidation properties of the particulate. A few literatures compared oxidation properties of particulates from biodiesel and diesel fuels (Boehman et al. Citation2005; Jung et al. Citation2006; Song et al. Citation2006; Strzelec et al. Citation2011); however, there is lack of investigation on the effect of fuel sulfur on the oxidation properties of the particulate. In this study, we used a thermal-gravimetric analyzer to investigate the effect of fuel and engine load on the oxidation property of particulate samples collected from a diesel engine. The primary particle size, nanostructure, and volatility of the particulate were also investigated and related to the oxidation property. The fuels investigated include two diesel fuels with different sulfur contents (10-ppm-wt sulfur and 400-ppm-wt sulfur) and a biodiesel fuel.
2. EXPERIMENTAL METHOD
The experimental investigation was performed on a naturally aspirated, water-cooled, four-cylinder direct-injection diesel engine (ISUZU 4HF1). The specifications of the engine are shown in . The engine was connected to an eddy-current dynamometer and a control system was available for adjusting its speed and torque. Three fuels were used in this study, including a diesel fuel with less than 10-ppm-wt sulfur (ultra-low-sulfur diesel [ULSD]), a diesel fuel with 400-ppm-wt sulfur (low-sulfur diesel [LSD]), and a biodiesel fuel produced from waste cooking oil. The major properties of the three fuels are given in . The ULSD is widely used in Hong Kong. Properties of the LSD are similar to the typical diesel fuel available in China. The biodiesel used in this study was produced from waste cooking oil by Dynamic Progress Ltd. and its properties are in compliance with EN14214.
TABLE 1 Specifications of the test engine
TABLE 2 Properties of test fuels
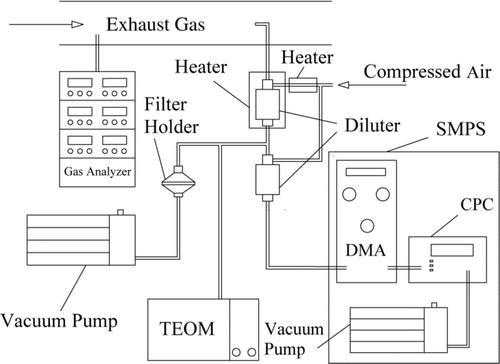
The schematic of the experimental system is shown in . The gaseous species, including hydrocarbon (HC), nitrogen oxides (NO x ), carbon monoxide (CO), and carbon dioxide (CO2), and particulate mass (PM) concentration were measured using online analyzers. HC was measured with a heated flame ionization detector (HFID); NO x was measured with a heated chemiluminescence analyzer (HCLA); while CO and CO2 were measured with nondispersive infrared analyzers (NDIR). Exhaust gas temperature was measured with a K-type thermocouple. All gas analyzers come from California Analytical Instruments, Inc. All gas analyzers were calibrated with standard gases and zero gas before each experiment. Particulate mass concentration was measured with a tapered element oscillating microbalance (TEOM, Series 1105, Rupprecht & Patashnick Co., Inc.). Particle number concentration and size distribution were measured with a scanning mobility particle sizer (SMPS, TSI Model 3934). Before passing through the TEOM and the SMPS, the exhaust gas was diluted with a Dekati mini-diluter. The dilution ratio was determined from the measured CO2 concentrations of background air, undiluted exhaust gas, and diluted exhaust gas. The dilution ratio for the SMPS varied from 67.5 to 89.6, depending on the actual operating conditions. The dilution ratio for the TEOM was one-eighth of that for the SMPS.
Experiments were conducted at a steady engine speed of 1800 rev/min and at five engine loads, corresponding to the brake mean effective pressures (BMEP) of 0.08, 0.2, 0.38, 0.55, and 0.7 MPa. The exhaust gas temperature and the air fuel ratio for each test condition are listed in . To ensure the repeatability and comparability of the measurements, samples were collected after the engine has reached the steady state, as indicated by the cooling water and lubricating oil temperature when they reached steady values. Moreover, upon switching a test fuel, the engine was allowed to operate with the new fuel for 30 min to clean the fuel system.
TABLE 3 Engine operation condition, exhaust gas temperature (exhaust temp.), air fuel ratio (A/F), and gaseous and PM emissions
Particulate samples used for the investigation of particle morphology and oxidation property were collected on 47-mm quartz filter paper (Whatman Corporation) with the same diluted conditions as the TEOM. The transfer line from the exhaust to the dilutor was insulated and heated at 170°C to avoid volatile HC condensation loss. A high-resolution transmission electron microscopy (HRTEM; Tecnai G2 20 S-TWIN Philips) was used to investigate primary particle size and nanostructure. The maximum magnification is up to 910,000× with a resolution of about 0.2 nm. TEM samples were prepared by the extraction method. In this method, a portion of the particulate-loaded filter paper was extracted ultrasonically in ethanol for about 5 min. A droplet of the colloidal solution was placed on the TEM grid and then dried in the atmosphere. Similar method has been used by Vander Wal and Mueller (Citation2006).
A commercial image processing software Image-Pro Plus 6.0 (Media Cybernetics) was used for analyzing the TEM and HRTEM images. From the TEM images, the diameter of a particle with clear boundaries could be measured with the software by putting a circle around it. The nanostructure of primary particles is exhibited in the form of parallel or twisted carbon lamellae. Three parameters, namely fringe length (La ), separation distance (Ds ), and tortuosity (Tf ), of the carbon lamellae can be used to describe the nanostructure of the primary particles (Vander Wal Citation2005). Details on the calculation of the three parameters can be found in Vander Wal (2005) and Shim et al. (Citation2000).
The investigation of particulate composition and oxidation property were conducted through thermogravimetric analysis (TGA) on the same filter samples collected for the TEM measurement. TGA was conducted using the Netzch STA 449 TGA/DSC (thermogravimetric analysis/differential scanning calorimetry) with Al2O3 crucible. The heating program is listed in . When the TG temperature was below 400°C, the particulate samples were heated in argon in order to remove the volatile substance. Then the samples were oxidized in air for the investigation of particle oxidation. In order to keep the TGA results comparable, for each test, the initial mass of particulate samples was maintained at around 2 mg.
TABLE 4 TGA heating program
TABLE 5 Effect of fuel type and engine load on mean size and nanostructure parameters of primary particles
All the gaseous emissions and PM mass concentrations were continuously measured for 5 min and the average results are presented. Each experiment was repeated twice and the results were found to agree with each other within the 95% confidence level. Standard errors in the measurements have been determined based on the method of Kline and McClintock (Citation1953). The standard errors are 7.3%, 2.9%, 4.8%, 1.5%, and 3.3% for HC, NO x , CO, particle mass concentration, and particle number concentration, respectively.
3. RESULTS
3.1. Gaseous and Particulate Emissions
The regulated gaseous emissions (CO, HC, and NO x ) and the PM concentrations are shown in . In general, biodiesel could effectively reduce the emissions of (HC and CO, while induce a slight increase of NO x . On average of five engine loads, PM from the use of biodiesel decreased by 36.0% and 54.9%, while NO x increased by 7.2% and 7.5%, in comparison with ULSD and LSD, respectively. The use of LSD leads to the most abundant PM emission, which might be attributed to the higher sulfur and aromatic content in the LSD.
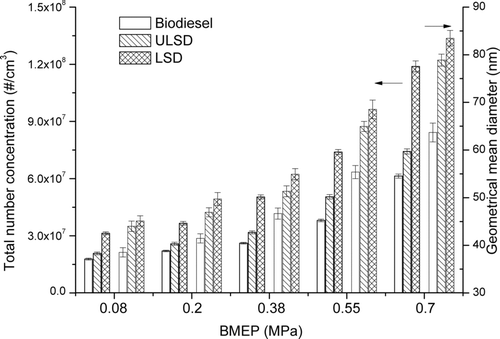
The effects of fuel type and engine load on particle number emission were also investigated in this study. As shown in , the total number concentration (TNC) and the geometric mean diameter (GMD) both increased with the engine load. On the other hand, biodiesel particles exhibit the smallest GMD and TNC, while LSD particles have the highest GMD and TNC. The results indicate that although the usage of biodiesel could induce the decrease of TNC, it also leads to a larger percentage of small size particle aggregates.
3.2. Primary Particle Size and Nanostructure
Primary particle size at high (0.7 MPa) and low (0.08 MPa) engine loads was examined. About 150–250 spherules with clear boundaries were randomly chosen from the TEM images for determining the primary particle diameter. The mean diameters of the primary particles (Dp ) and their standard deviations (SDs) obtained at the two engine loads are listed in . It can be observed that, for each fuel, the primary particle size is larger at the high engine load. At the high engine load, a lower air/fuel ratio promotes particle nucleation and growth, while suppressing particle oxidation, which leads to the formation of more large-sized particles (Neer and Köylü Citation2006). For different fuels, the primary particle diameters are not significantly different between ULSD and LSD, at 95% confidence level, while they are both larger than that from biodiesel with a small but statistically significant difference.
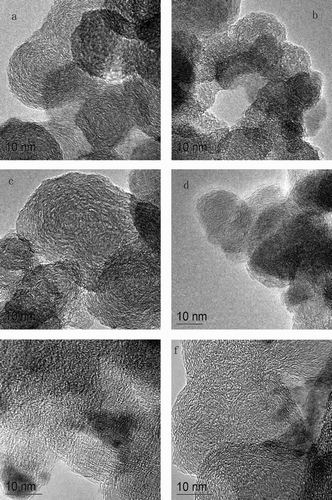
The HRTEM images for the particulate samples collected at 0.7 MPa and 0.08 MPa are shown in . The high engine load particles (left column) exhibit more orderly nanostructure for each fuel, compared with the low engine load particles (right column). Zhu et al. (Citation2005) also examined the particles with Raman spectroscopy and found more ordered graphitic structures with the increase in engine load. For different fuels, at the high engine load, the nanostructure of biodiesel soot shows more disordered graphene segments, while for ULSD () and LSD (), the particles exhibit a typical shell-core structure with one or several nuclei (cores). However, at the low engine load, the particulate samples of the three fuels all exhibit similar amorphous nanostructure ().
For each fuel, about 25–30 HRTEM images of different primary particles were analyzed for their nanostructure parameters. The average values and their SDs are shown in . Biodiesel particles obtained at both the high and the low engine loads all exhibit shorter and more curved graphene layers than the other two fuels, as indicated by the shorter fringe length and higher tortuosity, and the difference is significant at 95% confidence level, while the difference between particles generated from ULSD and LSD is not that significant. The separation distance between two adjacent graphene layers is in the range of 0.36–0.38 nm and the difference is not significant for the three fuels at each engine load. These results indicate that the oxygen content in the fuel could induce a more disordered nanostructure, while the sulfur content in the fuel has a limited effect on particle nanostructure.
3.3. Particle Volatility
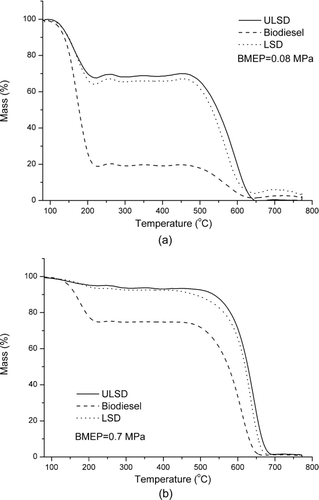
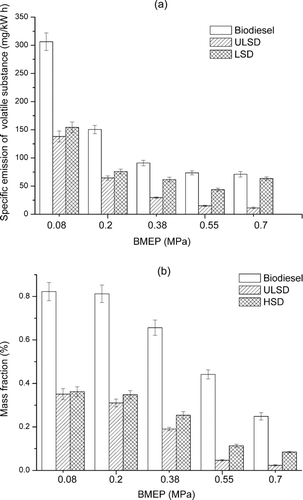
TGA measurements can be used to measure the mass of volatile and nonvolatile substances in the collected particulate samples. Typical curves of PM loss against heating temperature are shown in for samples collected at the engine loads of 0.08 MPa and 0.7 MPa. For each fuel, obvious mass loss occurs at two temperature segments: <300°C and 400°C–800°C, which respectively is mainly caused by the loss of the volatile substances and the oxidation of nonvolatile substances.
shows the effect of fuel type and engine load on the brake-specific emission of volatile substances and the fraction of volatile substances in the particulate matter. In general, with an increase in the engine load, the brake-specific emission of volatile substances and the mass fraction of volatile substances decrease for biodiesel, ULSD, and LSD. However, for LSD, there is a slight increase in the brake-specific emission at 0.7 MPa. For different fuels, both the brake-specific emission and the fraction of volatile substances increase in the order of ULSD, LSD, and biodiesel. The fractions of volatile substances, respectively, are 2.3%–35.1%, 8.4%–36.2%, and 24.9%–82.3% for ULSD, LSD, and biodiesel. Biodiesel particulate contains obviously a larger percentage of volatile substances than those of ULSD and LSD particles.
3.4. Particle Oxidation
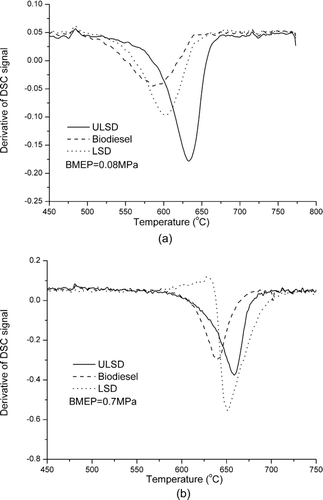
TGA was used to investigate the oxidative reactivity of the particulate samples. As shown in , nonvolatile substances loss their mass sharply with temperature between 500°C and 650°C. When the temperature is high enough, the particulate sample losses almost all the mass and the mass curve becomes level. Based on the TGA results, the analysis of oxidation kinetics for the nonvolatile substances was conducted using a modified form of the Arrhenius expression, as suggested in Stratakis and Stamatelos (Citation2003):
The calculated kinetic parameters are listed in . In this study, the activation energy is in the range of 142–175, 76–127, and 133–162 kJ/mol for the ULSD, biodiesel, and LSD particulate samples, respectively. Biodiesel particles exhibit obviously lower activation energy than those of the two diesel fuels.
TABLE 6 Effect of fuel type and engine load on kinetic parameters of the particulate samples
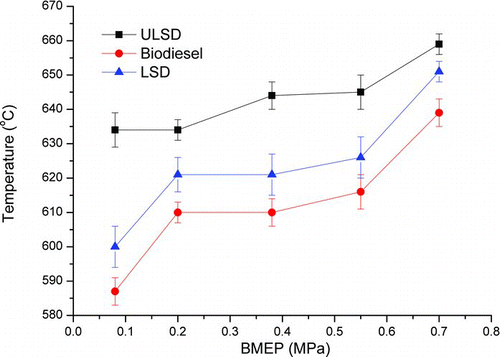
The ignition temperature could be used to quantify the oxidation reactivity of particulate samples (Stratakis and Stamatelos Citation2003). The DSC could provide information about the heat release rate, which could be used as a supplementary approach to determine the ignition temperature (Stratakis and Stamatelos Citation2003) because the rates of both mass loss and heat release reach their maximum at the ignition temperature. shows the derivative of DSC signals when the particulate sample is oxidized in an oxidizing atmosphere. It could be used to countercheck the ignition temperature of the different samples. However, the ignition temperatures thus measured are only indicative and do not correspond to the ignition temperatures of real soot collected on the DPFs. From the figure, the ignition temperatures, respectively, are 639°C, 651°C, and 659°C for the biodiesel, LSD, and ULSD particulate samples at the engine load of 0.7 MPa and 587°C, 600°C, and 634°C at the engine load of 0.08 MPa. The effect of fuel type and engine load on soot ignition temperature is shown in . The ignition temperature increases with engine load for each fuel and at each engine load, it increases in the order of biodiesel, LSD, and ULSD.
4. DISCUSSION
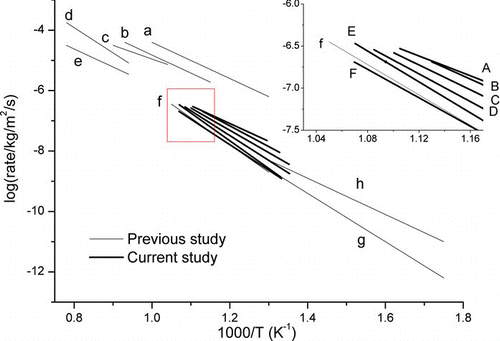
shows an Arrhenius plot of oxidation rates for this study in comparison with results obtained in other studies, which have been shown in Higgins et al. (Citation2002, Citation2003). In order to keep the results comparable, a hypothesis of 120 m2/g surface area of particle samples was used to calculate the surface-specific oxidation from the mass decrease rate, following the same approach in Higgins et al. (Citation2002, Citation2003). For pure biodiesel soot, Jung et al. (Citation2006) reported an activation energy of 89 kJ/mol (line c), which is lower than results obtained in this study. For diesel soot, the following reported lower activation energy than those obtained in this study: Jung et al. (Citation2005) with 25 ppm cerium-dosed case (line a, 107 kJ/mol); Jung et al. (Citation2003) with lube-oil-dosed case (line b, 101 kJ/mol); Higgins et al. (Citation2003) with regular diesel fuel (line e, 108 kJ/mol); Ahlström and Odenbrand (Citation1989) with flow reactor study of diesel particles (line h, 106 kJ/mol). On the other hand, Miyamoto et al. (Citation1988) obtained higher results using TGA for uncatalyzed diesel soot (line f, 191 kJ/mol) and the following two obtained similar results: Higgins et al. (Citation2002) using diffusing flame-generated soot (line d, 164 kJ/mol) and Otto et al. (Citation1980) using TGA for diesel particles (142 kJ/mol). Clearly, the activation energy depends on the type of fuel, the treatment of the fuel or the soot, and the methods of measurement. For example, the higher activation energy obtained in this study in comparison with those reported in Jung et al. (Citation2006), for pure biodiesel, and in Higgins et al. (Citation2003), for diesel soot, might arise from the different measurement methods. In these two studies, suspended particles were oxidized directly using high-temperature oxidation-tandem differential mobility analyzer (HTO-TDMA). The HTO-TDMA has little heat or mass transfer limitation, while in TGA method, there is heat and mass transfer limitation associated with bulk soot samples.
FIG. 8 Kinetic analysis of the mass loss rates. Thick lines, A–F as shown in the inset, show the current work. From top to bottom: (A) biodiesel particle collected at 0.08 MPa; (B) LSD particle collected at 0.08 MPa; (C) ULSD particle collected at 0.08 MPa; (D) biodiesel particle collected at 0.7 MPa; (E) LSD particle collected at 0.7 MPa; (F) ULSD particle collected at 0.7 MPa. Thin lines show previous works: (a) Jung et al. (Citation2005), 25 ppm cerium-dosed case (107 kJ/mol); (b) Jung et al. (Citation2003), lube-oil-dosed case (101 kJ/mol); (c) Jung et al. (Citation2006), pure biodiesel (89 kJ/mol); (d) Higgins et al. (Citation2002) using diffusion flame-generated soot (164 kJ/mol); (e) Higgins et al. (Citation2003), diesel particles with regular diesel fuel (108 kJ/mol); (f) Miyamoto et al. (Citation1988) TGA of uncatalyzed diesel soot (191 kJ/mol); (g) Otto et al. (Citation1980) TGA of diesel particles (142 ± 21 kJ/mol); (h) Ahlström and Odenbrand (Citation1989) flow reactor study of diesel particles (106 ± 7 kJ/mol). (Color figure available online.)
The oxidation rates of biodiesel soot are always higher than those of diesel soot in the present study and in the literature (Jung et al. Citation2006; Song et al. Citation2006; Strzelec et al. Citation2011). For the three fuels, the oxidation rate decreases in the order of biodiesel, LSD, and ULSD, being the highest for biodiesel particulate. The higher reactivity of biodiesel particulate, compared with the LSD and ULSD particulates, is related to the difference in primary particle size, nanostructure, and particle volatility.
The higher viscosity of biodiesel reduces leakages in the pump leading to an increase in the injection pressure (Tsolakis Citation2006), while higher injection pressure can induce an increased proportion of homogeneous combustion and a shorter duration of combustion, and hence the formation of smaller primary particles (Mathis et al. Citation2005). shows that the biodiesel primary particles have a smaller diameter, while shows that biodiesel particle aggregates have a smaller GMD. The smaller size of biodiesel particles induces a larger specific surface area of the primary particles and subsequently the whole particulate agglomerates (Strzelec et al. Citation2011). The larger specific surface area improves the exposure of the particles to oxygen in the oxidation process, leading to the higher oxidative reactivity of biodiesel particles, compared with the ULSD and LSD particles.
The more disordered nanostructure, as indicated by the shorter and more curved graphene layers, is another important characteristic of the biodiesel particles, compared with the ULSD and LSD particles. According to Morjan et al. (Citation2004), the addition of oxygen in fuel during pyrolysis causes a greater presence of curved layers inside the primary particles. The influence of fringe length and tortuosity on particle oxidation reactivity has been explained in Vander Wal and Tomasek (Citation2004). They suggested that both shorter fringe length and higher tortuosity of graphene layers enhance oxidation reactivity.
Moreover, the soot particles generated by biodiesel contain a larger percentage of surface oxygen functional groups, which also leads to more rapid burning of biodiesel soot in TGA (Boehman et al. Citation2005; Song et al. Citation2006) because the surface oxygen functional groups bounded to edge sites can induce more micropore structures during attack by air on the edge sites (Smith and Chughtai Citation1995).
Particle volatility also affects the oxidation process. Volatile substances filled the micropores of particulate aggregates. During the early stage of the TGA heating program, volatile substances are removed, which promotes the development of porosity and provides more internal surface area for subsequent soot oxidation. The correlation between volatile fraction and particulate ignition temperature is shown in . Roughly, particle reactivity increases with the volatile fraction. The different volatile fractions of the three fuels can be used to explain their variation in particle reactivity.
FIG. 9 Correlation between particulate ignition temperature and volatile mass fraction. (Color figure available online.)
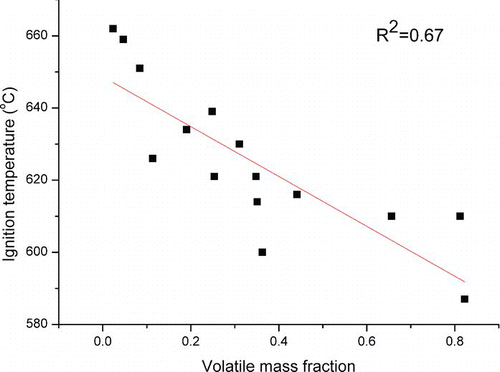
In this study, LSD particles exhibit a higher oxidation rate than ULSD particles. Due to the similar primary particle size and nanostructure between ULSD and LSD particles, the larger percentage of volatile substances is an important reason for the higher reactivity of the LSD particles. The higher sulfur content in LSD can be converted to sulfuric acid or sulfate in the combustion and dilution processes. These compounds can be removed by a thermal denuder and are considered as the volatile substances (Maricq et al. Citation2002; Ristovski et al. Citation2006). Moreover, sulfuric acid can also promote the nucleation of organic compounds, leading to more volatile substances in the particulate samples (Shi and Harrision Citation1999).
Another reason for the higher reactivity of LSD particles is the higher aromatic content in LSD, compared with ULSD. De Soete (Citation1988) found that the oxidation rate of soot from an aromatic source was higher than that from an aliphatic source. Vander Wal and Tomasek (Citation2003) compared the oxidation of combustion-generated soot using benzene and acetylene, and found much stronger reactivity of benzene-derived soot. These results all indicate that the combustion of a fuel with higher aromatic content can generate more reactive soot.
5. CONCLUSIONS
In this study, the effects of fuel type and engine load on physicochemical properties of particulate samples from a DI engine were investigated. Analysis of the TEM images indicates that at low engine load, the primary particles exhibit smaller diameter and more disordered nanostructure, compared with that at high engine load. For different fuels, biodiesel induces smaller primary particle size and the primary particles contain a larger number of short and curved graphene layers than those from the other two fuels, while the difference is not significant between the ULSD and the LSD primary particles.
TGA was used to investigate the volatility and oxidation property of particulate samples. For each fuel, both the volatile fraction and the oxidative reactivity increase with the decrease in engine load. For different fuels, the volatile fraction and the oxidative reactivity decrease in the order of biodiesel, LSD, and ULSD. The smaller primary particle size, more disordered nanostructure, and larger volatile fraction can be used to explain the stronger oxidative reactivity of biodiesel particles, compared with ULSD and LSD particles, while the larger volatile fraction can be the major reason for the higher oxidation rate of LSD particles, compared with the ULSD particles. Thus, it can be concluded that the biodiesel and the sulfur content of a diesel fuel can induce stronger oxidative reactivity, for different reasons.
Acknowledgments
The authors would like to thank the Hong Kong Polytechnic University (GU-790) and the Co-Project of China–U.S. Clean Energy (contract no. 2010DFA72760-201) for financial support.
REFERENCES
- Ahlström , A. F. and Odenbrand , C. U. I. 1989 . Combustion Characteristics of Soot Deposits from Diesel Engines . Carbon , 3 : 475
- Bagley , S. T. , Baumgard , K. J. , Gratz , L. D. , Johnson , J. J. and Leddy , D. G. 1996 . Characterization of Fuel and Aftertreatment Devices on Diesel Emissions , Boston , MA : Health Effects Institute Research Report No. 88. Health Effects Institute .
- Boehman , A. L. , Song , J. and Alam , M. 2005 . Impact of Biodiesel Blending on Diesel Soot and the Regeneration of Particulate Filters . Energy Fuel , 19 : 1857 – 1864 .
- De Soete , G. 1988 . Catalysis of Soot Combustion by Metal Oxides , Salt Lake City , UT : Western States Section Meeting of the Combustion Institute .
- Diesel Emission Controls Sulfur Effects (DECSE) Program . 2001 . Final Report: Diesel Oxidation Catalysts and Lean-NOx Catalysts , Washington , DC : Sponsored by the US Department of Energy .
- Dwivedi , G. , Jain , S. and Sharma , M. P. 2011 . Impact Analysis of Biodiesel on Engine Performance—A Review . Renew. Sustain. Energy Review. , 15 : 4633 – 4641 .
- Higgins , K. J. , Jung , H. , Kittelson , D. B. , Roberts , J. T. and Zachariah , M. R. 2002 . Size-Selected Nanoparticle Chemistry: Kinetics of Soot Oxidation . J. Phys. Chem. A , 106 : 96 – 103 .
- Higgins , K. J. , Jung , H. , Kittelson , D. B. , Roberts , J. T. and Zachariah , M. R. 2003 . Kinetics of Diesel Nanoparticle Oxidation . Environ. Sci. Technol. , 37 : 1949 – 1954 .
- Jung , H. , Kittelson , D. B. and Zachariah , M. R. 2003 . The Influence of Engine Lubricating Oil on Diesel Nanoparticle Emissions and Kinetics of Oxidation , SAE Technical Paper Series No. 2003–2001-3179 Warrendale , PA : SAE International .
- Jung , H. , Kittelson , D. B. and Zachariah , M. R. 2005 . The Influence of a Cerium Additive on Ultrafine Diesel Particle Emissions and Kinetics of Oxidation . Combust. Flame , 142 : 276 – 288 .
- Jung , H. , Kittelson , D. B. and Zachariah , M. R. 2006 . Characteristics of SME Biodiesel-Fueled Diesel Particle Emissions and the Kinetics of Oxidation . Environ. Sci. Technol. , 40 : 4949 – 4955 .
- Kandas , A. W. , Senel , I. G. , Levendis , Y. and Sarofim , A. F. 2005 . Soot Surface Area Evolution During Air Oxidation as Evaluated by Small Angle X-Ray Scattering and CO2 Adsorption . Carbon , 43 : 241 – 251 .
- Kline , S. J. and McClintock , F. A. 1953 . Describing Uncertainties in Single Sample Experiments . Mech. Eng. , 75 : 3 – 8 .
- Krahl , J. , Munack , A. , Bahadir , M. , Schumacher , L. and Elser , N. 1996 . Review: Utilization of Rapeseed Oil, Rapeseed Oil Methyl Ester or Diesel Fuel: Exhaust Gas Emissions and Estimation of Environmental Effects , SAE Technical Paper Series No. 962096 Warrendale , PA : SAE International .
- Lapuerta , M. , Armas , O. and Rodríguez-Fernández , J. 2008 . Effect of Biodiesel Fuels on Diesel Engine Emissions . Prog. Energy Combust. Sci. , 34 : 198 – 223 .
- Lee , K. O. , Cole , R. , Sekar , R. , Choi , M. Y. , Zhu , J. Kang , J. 2001 . Detailed Characterization of Morphology and Dimensions of Diesel Particulates via Thermophoretic Sampling , SAE Technical Paper Series No. 2001–01-3572 Warrendale , PA : SAE International .
- Maricq , M. M. , Chase , R. E. , Xu , N. and Laing , P. M. 2002 . The Effects of the Catalytic Converter and Fuel Sulfur Level on Motor Vehicle Particulate Matter Emissions: Light Duty Diesel Vehicles . Environ. Sci. Technol. , 36 : 283 – 289 .
- Mathis , U. , Mohr , M. , Kaegi , R. , Bertola , A. and Boulouchos , K. 2005 . Influence of Diesel Engine Combustion Parameters on Primary Soot Particle Diameter . Environ. Sci. Technol. , 39 : 1887 – 1892 .
- Miyamoto , N. , Hou , Z. and Ogawa , H. 1988 . Catalytic Effects of Metallic Fuel Additives on Oxidation Characteristics of Trapped Diesel Soot , SAE Technical Paper Series No. 881224 Warrendale , PA : SAE International .
- Morjan , I. , Voicu , I. , Alexandrescu , R. , Pasuk , I. , Sandu , I. Dumitrache , F. 2004 . Gas Composition in Laser Pyrolysis of Hydrocarbon-Based Mixtures: Influence on Soot Morphology . Carbon , 42 : 1269 – 1273 .
- Müller , J. O. , Su , D. S. , Jentoft , R. E. , Krohnert , J. , Jentoft , F. C. and Schlögl , R. 2005 . Morphology-Controlled Reactivity of Carbonaceous Materials Towards Oxidation . Catal. Today , 102 : 259 – 265 .
- Müller , J. O. , Su , D. S. , Jentoft , R. E. , Wild , U. and Schlögl , R. 2006 . Diesel Engine Exhaust Emission: Oxidative Behavior and Microstructure of Black Smoke Soot Particulate . Environ. Sci. Technol. , 40 : 1231 – 1236 .
- Neer , A. and Köylü , U. O. 2006 . Effect of Operating Condition on the Size, Morphology and Concentration of Submicrometer Particulates Emitted from a Diesel Engine . Combust. Flame , 146 : 142 – 154 .
- Neeft , J. P. A. , Nijhuis , T. X. , Makkee , M. and Moulijn , J. A. 1997 . Kinetics of the Oxidation of Diesel Soot . Fuel , 76 : 1129 – 1136 .
- Otto , K. , Sieg , M. H. , Zinbo , M. and Bartosiewicz , L. 1980 . The Oxidation of Soot Deposits from Diesel Engines , SAE Technical Paper Series No. 800336 Warrendale , PA : SAE International .
- Ristovski , Z. D. , Jayaratne , E. R. , Lim , M. , Ayoko , G. A. and Morawska , L. 2006 . Influence of Diesel Fuel Sulfur on Nanoparticle Emissions from City Buses . Environ. Sci. Technol. , 40 : 1314 – 1320 .
- Shi , J. P. and Harrision , R. M. 1999 . Investigation of Ultrafine Particle Formation During Diesel Exhaust Dilution . Environ. Sci. Technol. , 33 : 3730 – 3736 .
- Shim , H. S. , Hurta , R. H. and Yang , N. Y. C. 2000 . A Methodology for Analysis of 002 Lattice Fringe Images and Its Application to Combustion-Derived Carbons . Carbon , 38 : 29 – 45 .
- Smith , D. M. and Chughtai , A. R. 1995 . The Surface Structure and Reactivity of Black Carbon . Colloids Surf. A , 105 : 47 – 77 .
- Song , J. , Alam , M. , Boehman , A. L. and Kim , U. 2006 . Examination of the Oxidation Behavior of Biodiesel Soot . Combust. Flame , 146 : 589 – 604 .
- Stanmore , B. R. , Brilhac , J. F. and Gilot , P. 2001 . The Oxidation of Soot: A Review of Experiments, Mechanisms and Models . Carbon , 39 : 2247 – 2268 .
- Stratakis , G. A. and Stamatelos , A. M. 2003 . Thermogravimetric Analysis of Soot Emitted by a Modern Diesel Engine Run on Catalyst-Doped Fuel . Combust. Flame , 132 : 157 – 169 .
- Strzelec , A. , Toopsa , T. J. and Daw , C. S. Impact of Biodiesel on the Oxidation Kinetics and Morphology of Diesel Particulate . Proceedings of the 7th US National Technical Meeting of the Combustion Institute, Atlanta, GA .
- Tsolakis , A. 2006 . Effects on Particle Size Distribution from the Diesel Engine Operating on RME-Biodiesel with EGR . Energy Fuel , 20 : 1418 – 1424 .
- Ullman , T. L. , Spreen , K. B. and Mason , R. L. 1994 . Effects of Cetane Number, Cetane Improver, Aromatics, and Oxygenates on 1994 Heavy-Duty Diesel Engine Emissions , SAE Technical Paper Series No. 941020 Warrendale , PA : SAE International .
- Vander Wal , R. L. 2005 . Soot Nanostructure: Definition, Quantification and Implications , SAE Technical Paper Series No. 2005–01-0964 Warrendale , PA : SAE International .
- Vander Wal , R. L. and Mueller , C. J. 2006 . Initial Investigation of Effects of Fuel Oxygenation on Nanostructure of Soot from a Direct-Injection Diesel Engine . Energy Fuel , 20 : 2364 – 2369 .
- Vander Wal , R. L. and Tomasek , A. J. 2003 . Soot Oxidation: Dependence upon Initial Nanostructure . Combust. Flame , 134 : 1 – 9 .
- Vander Wal , R. L. and Tomasek , A. J. 2004 . Soot Nanostructure: Dependence upon Synthesis Conditions . Combust. Flame , 136 : 129 – 140 .
- Wei , Q. , Kittelson , D. B. and Watts , W. F. 2001 . Single Stage Dilution Tunnel Performance , SAE Technical Paper Series No. 2001–01-0201 Warrendale , PA : SAE International .
- Zhu , J. , Lee , K. O. , Yozgatligil , A. and Choi , M. Y. 2005 . Effects of Engine Operating Conditions on Morphology, Microstructure, and Fractal Geometry of Light-Duty Diesel Engine Particulates . Proc. Combust. Inst. , 30 : 2781 – 2789 .