Abstract
Generation of monomobile molecular standards by electrospray (ES) followed by classification in a differential mobility analyzer (DMA) fails at diameters above ∼2 nm because many clusters in different charge states z crowd in a narrow mobility range. Use of a second DMA (DMA2) in series (tandem) with DMA1 is very helpful because, unexpectedly, many multiply charged ions selected in DMA1 undergo spontaneous transitions, appearing as pure species at different mobilities in DMA2. Remarkably, for salt clusters of composition (CA) n (C+ ) z carrying z elementary charges and n neutral ion pairs, (i) ion evaporation (CA) n (C+ ) z →(CA) n –1(C+ ) z– 1+(CA)C+ and (ii) neutral evaporation transitions (CA) n (C+ ) z →(CA) n –1(C+ ) z+CA affect a substantial fraction of the clusters. Neutral evaporation (fueled by the Kelvin effect) is effective in isolating singly charged clusters, yielding mobility standards easily exceeding 2 nm. Ion evaporation (fueled by large electric fields) produces even larger well-resolved standards. Singly charged clusters of up to 2.5 nm rising in isolation result from metastable doubly charged parent ions (z = 2→1 transition). Isolated doubly charged ions of up to 3.5 nm arise from the z = 3→2 transition, but are harder to resolve from the products of higher initial charge states. We report tandem DMA measurements for electrosprayed nanodrops of two ionic liquids: EMI-Im and EMI-Methide, both based on the small cation EMI+ (1-Ethyl-3-methylimidazolium+) and two relatively large anions: Im− = (CF3SO2)2N−; Methide− = (CF3SO2)3C−. Some exploration on the effect of actively reducing the charge on the clusters as they pass between both analyzers is also included.
Copyright 2013 American Association for Aerosol Research
1. INTRODUCTION
Particle standards play an important role in many aspects of aerosol science, from instrument calibration or evaluation, to testing measurement techniques, to basic research. Paradoxically, nanoaerosol standards have been much easier to produce at ∼1 nm sizes than at 10 or 100 nm, thanks to the existence of pure molecular standards in the 1 nm size range. The development of electrospray (ES) as a means to produce gas phase ions of relatively large and involatile dissolved molecules (Fenn et al. Citation1989), has provided for almost 30 years a source of such ∼1 nm standards. They have been used in a diversity of tasks, including the evaluation of differential mobility analyzers (DMAs) (i.e., references in Fernandez de la Mora Citation2011b) and condensation particle counters (Vanhanen et al. Citation2011); basic nanoparticle research such as the relation between mobility and size, (Larriba et al. Citation2011), nanodrop evaporation (or the Kelvin effect at nanometer dimensions) (Hogan and Fernández de la Mora 2010), nucleation studies (Seto et al. Citation1997; Gamero-Castaño and Fernández de la Mora Citation2002, 2000a), filtration (Heim et al. Citation2010), etc. A striking application was recently given by Winkler et al. (Citation2012) using the nucleation theorem to determine the size of the critical nucleus for heterogeneous nucleation on seed molecular ions. This task requires measuring the very sharp dependence of the nucleation rate on supersaturation, which was precluded prior to the availability of molecular standards by broadening resulting from the polydispersity (in size, shape and composition) of most nanoparticles generated by conventional methods (Fernandez de la Mora Citation2011a,Citationb; Winkler et al. Citation2012). The present work is therefore concerned with methods to generate particles of exactly fixed composition, shape, and size, at diameters above 2 nm.
2. LIMITATIONS OF CURRENT ES METHODS OF GENERATING SIZE STANDARDS
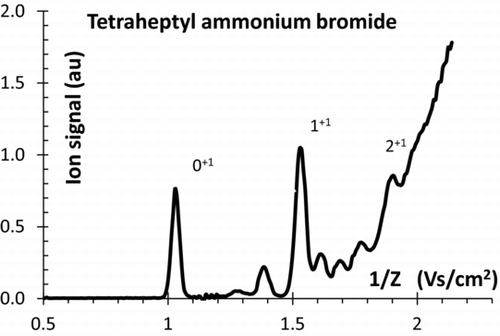
shows a typical mobility spectrum of an electrosprayed salt, in this case tetraheptylammonium bromide [THA+Br−; (C7H15)4N+Br−] dissolved in alcohol. The figure features two dominant peaks labeled 0+1 and 1+1, corresponding respectively to the monomer and the dimer, namely, the bare tetraheptylammonium cation THA+, and that same ion attached to one neutral molecule of tetraheptylammonium bromide, Br−(THA+)2. A third imperfectly resolved peak marked 2+1 is the trimer, attaching two neutral pairs to THA+. The well-defined smaller peaks seen at high mobility Z are associated primarily with multiply charged salt clusters, and possibly also to salt clusters solvated with water vapor or other volatiles in the gas. The apparently continuous background rising approximately at the position of the trimer is associated with multiply charged ions too numerous to produce distinct mobility peaks. This picture is not peculiar to electrosprayed THA-Br clusters, but applies similarly to most ESs of salts, as well as other substances soluble in polar solvents. Many similar spectra can be seen in prior publications (i.e., Gamero-Castaño and Fernández de la Mora Citation2000b; of Ude and Fernández de la Mora 2005; of Ku and Fernández de la Mora Citation2005; Figures 2–7 of Ku et al. 2004). Consequently, ESs are excellent sources of small molecular ions, but do not ordinarily produce isolated ion standards at inverse mobilities (in ambient air) beyond 2 Vs/cm2. Perfect isolation may not seem necessary in many applications. However, in nucleation studies, multiply charged clusters tend to activate nucleation at supersaturations considerably smaller than singly charged ions. Therefore, even a slight level of contamination greatly distorts the picture.
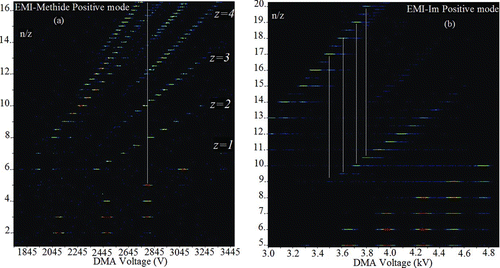
provides a more thorough picture of the complex ion composition of ESs through a two dimensional mobility-mass spectrum of the salts 1-ethyl-3-methylimidazolium+ tris (trifluoromethylsulfonyl)methide (EMI-Methide; left) and EMI+–(CF3SO2)2N− (right). Here, ion abundance is given in a logarithmic color scale, the horizontal and vertical axes representing respectively the DMA voltage, and the mass/charge ratio obtained in a mass spectrometer. For easier interpretation, mass/charge is expressed in terms of n/z, where n is the number of neutral ion pairs in the cluster and z is the net number of elementary charges. All the ions represented by bright regions in the spectrum have the chemical composition (AC) n (C+ ) z , to be referred as n+z , where in this case C + = EMI+ and A − = (CF3SO2)3C− (Methide−) or (CF3SO2)2N− (Im−). The details on how these data are obtained have been described before (Rus et al. Citation2010). For present purposes, is simply a superposition of mass spectra, each corresponding to the ions transmitted into the mass spectrometer at a selected DMA voltage. For instance, the mass spectrum obtained at a DMA voltage of 2785 V is contained in the vertical white line included in . Near its bottom, at n/z = 5, one sees a short horizontal line corresponding to the singly charged ion cluster 5+1. We know its charge state z = 1 through the unit mass interval between isotopic peaks, and also because the neighboring peaks are all separated by a unit vertical n/z shift. Moving upward along that vertical white line one goes slightly to the left of another peak at n/z = 8, associated with the doubly charged ion 16+2. We know similarly that z = 2 because all the members of the inclined band to which it belongs are spaced by ½ n/z units. The third and fourth bands of peaks encountered as one ascends vertically go between the triply charged ions 30+3 and 31+3, and the quadruply charged ions 48+4 and 49+4. Many higher bands not shown in are associated with increasingly large charge states z. The message is therefore clear that, at a particular mobility (the vertical line), there are many more ions in many different charge states than a DMA alone can distinguish. If one repeats this vertical trip along another vertical line displaced more to the left, one sees that the 3+1 ion has far fewer ions on top of it. Hence, while not completely isolated, it rises above a background lower than its own height, and can therefore still be distinguished (as with the trimer of ). For EMI-Methide, the only peaks to be found in almost perfect isolation correspond (very much as for THABr) to the smallest singly charged clusters, typically more mobile than any of the other multiply charged clusters. A similar situation is seen in for EMI-Im.
The picture just drawn serves to illustrate why it is so relatively straightforward to generate ion standards with inverse mobilities between 1 and 1.5 Vs/cm2, and why the perspectives of moving substantially above 1.5 Vs/cm2 by conventional electrospraying seem so grim.
2.1. Charge Reduction
The principal method used in the past to overcome the complexity associated with multiple charging in ESs has relied on partial neutralization, ideally to achieve predominantly singly charged particles. The approach was pioneered by Kaufman et al. (Citation1996), initially with proteins, but including also other materials (Kaufman Citation1998), as well as cluster ions (Kaufman et al. Citation2008). They relied on a specially designed powerful charge-reducing radioactive source (Chen et al. Citation1995), apparently able to bring to unity the charge of the initial ES drops so fast that they had no chance to evaporate enough to undergo Coulomb fissions. Proteins have essentially fixed mass, often adopting a highly reproducible native configuration in neutral aqueous solutions. Many are commercially available in purified form, covering a vast range of sizes. All of this has suggested the use of charge-reduced electrosprayed proteins as ideal nanometer size standards. However, for a variety of reasons, proteins have proven less ideal aerosol standards than expected. First, their mobility peaks are never as sharp as allowed by the resolving power of the instrument, not even for MS selected protein ions, implying that many different conformations coexist. The gas-phase conformation of proteins is also different from the crystal structure (Hogan and Fernández de la Mora Citation2011), and highly variable depending on electrospraying circumstances, (even when electrospraying from neutral aqueous solutions where the proteins are in their native configuration). These problems are compounded by the fact that many solution impurities end up adducted to the dry protein, substantially modifying their theoretical mass. This adduction difficulty is evidently highly dependent on the purity of the solvent and the type of volatile salts used for electrospraying it. It also depends on the details of the charge reduction process, which is rarely a clean step. In particular, the widely used and very efficient neutralizer of Chen et al. (Citation1995) (commercialized by TSI) removes drop charge so effectively that, by design, it suppresses secondary drop atomization. This has some analytical advantages, but it also leads to much larger drops than the usual series of secondary Coulombic explosions, resulting in considerably more impurity adduction than otherwise.
One way of alleviating the impurity problem is to greatly increase the solute concentration. This however leads to clustering (aggregation of several sample molecules), not just in the case of the salt molecules discussed earlier, but also in the case of proteins. That this approach to minimize adduction is very effective is evident in , where the vast majority of the peaks seen correspond exactly to clusters of the salt introduced in solution (at tens of mM concentrations versus the 1 μM concentrations typical of protein work). For this reason, charge reduction of electrosprayed salt clusters is one possible approach to produce nanoparticle standards. This method was exploited by Gamero-Castaño and Fernández de la Mora (2000, 2002) in their studies of heterogeneous nucleation on size-resolved molecular ions from ESs of THA-Br. Their work shows partially resolved peaks of singly charged clusters with 1/Z larger than 6 Vs/cm2 (∼3.4 nm mobility diameter). However, even at substantially smaller 1/Z their peaks exhibited low mobility tails indicative of some impurity attachment (perhaps from the radioactive neutralizer), as well as from multiply charged ions surviving through the charge reduction process. Subsequent comparison of the nucleation data of Gamero-Castaño and Fernández de la Mora (Citation2000a) with theoretical predictions has confirmed the presence of a contaminating multiply charged component in their size selected clusters (Fernandez de la Mora Citation2011a). We shall reencounter this problem in the present study, as we have in a related recent article (Attoui et al. Citation2013). It results from the poor transmission of such small ions through the lines connecting the ES source to the neutralizer, the DMA and the detector. To compensate for these large losses one typically needs to use relatively high sample flow rates, which decreases the efficiency of the neutralizer. In conclusion, a number of difficulties have so far thwarted a variety of efforts aimed at increasing the size range of purified molecular standards available for nanoparticle studies.
2.2. Tandem DMA Approach
In the mean time, we have used the tandem DMA method (TDMA; Rader and McMurry Citation1986, equivalently referred here as DMA2) to study various kinetic processes arising in nanodrops of ionic liquids. Analyzing in a second DMA the same clusters selected by a first DMA helps only slightly in purifying them further. However, the TDMA method provides a dramatic simplification of the mobility spectrum when some clusters undergo a change as they proceed from the exit slit of the first DMA into the entry slit of the second. Here, we report our findings when these changes: (i) proceed spontaneously; or (ii) are externally forced by reducing the charge state of the clusters with a radioactive source.
3. EXPERIMENTAL
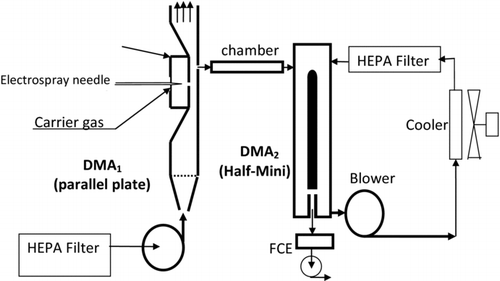
A schematic of the experimental apparatus is given in . The setup is closely related to that of Attoui et al. (Citation2013), except for several key improvements in ion transmission and acquisition speed. The frequency of data acquisition is vital in a system where scanning in two DMA voltages requires typically a 100 × 100 grid (2.77 h at 1 Hz). The acquisition frequency was increased by a factor of the order of 10 by decreasing the value of the resistor used in the electrometer detector. This improvement came at the expense of signal decrease (∼1/10 less amplification), even though the TDMA performance of Attoui et al. (Citation2013) was already limited by insufficient signal. In order to increase drastically the transmission, instead of their cylindrical DMA, we have used a parallel plate DMA as the first analyzer immediately following the ES source (DMA1). Our parallel plate DMA was a loan from the company Sciex, and is based on a SEADM design (Rus et al. Citation2010). The distance between plates is 1 cm, and the streamwise distance between slits is 2 cm. The instrument withstands a maximum voltage difference of 10 kV. The ES chamber is similar to that of Rus et al. (Citation2010), and is such that the electrospraying needle can be brought arbitrarily close to the inlet slit of the DMA. This simple feature permits signal increases of several orders of magnitude, and is the major improvement factor of our TDMA system with respect to that of Attoui et al. (Citation2013). The original outlet slit of the parallel plate DMA was designed to be coupled to the vacuum system of a mass spectrometer, and could pass a maximum flow rate of 3.6 L/min when operated critically (by applying a vacuum downstream from the outlet slit). Under these conditions the resolving power with 1 nm clusters was in the range of 50. Operating critically in a tandem DMA system is not practical, so the sample flow rate in DMA1 was limited to 2 L/min. In order to increase ion transmission through the tandem system, the original outlet slit was made substantially longer (7.6 mm) in the direction orthogonal to the flow, while preserving its original streamwise width. This increase in slit cross section permitted sample flow rates well above 10 L/min with acceptable pressure drops. In order to avoid resolution loss, it is important that the slit extends only through the central region of the channel (excluding lateral boundary layers), such that the gas velocity at the various lateral positions of the slit is highly uniform. For this reason, our lengthened slit occupied less than 50% of the channel width. In spite of this precaution, this change resulted in a substantial decrease of the resolving power of DMA1. The second DMA (DMA2) is cylindrical, of the Half-Mini type (Fernandez de la Mora Citation2011b, Fernandez de la Mora & Kozlowski Citation2013). Its analyzing region is bound externally by a cylinder of radius R 2 = 6 mm. The internal electrode is slightly conical (half angle of 1.5o), ending at the outlet slit with a radius R 1 = 4 mm. The axial distance between slits is 20 mm, suitable for analysis of charge-reduced particles having relatively small electrical mobilities. In spite of the small 2 mm gap between electrodes, DMA2 withstands safely a maximum voltage difference of 5 kV. The closed circuit for DMA2 includes a HEPA filter and two air-cooled refrigeration circuits that bring the air following the pump within the range 23–26°C.
Charge reduction was achieved in some experiments by inserting a Ni tube internally coated with Ni63 (10 mCi; Eckert & Ziegler) in the conduit going from the sample outlet line of DMA1 to the sample inlet line of DMA2. The bipolar ionic atmosphere created by the beta radiation from the Ni63 then reduces the charge state of the particles passing through it. In this arrangement, DMA1 analyzes the particles in their natural charge state, as electrosprayed, and DMA2 analyzes them again after charge reduction. The source was cleaned with solvents to minimize the presence of spurious vapors (Steiner and Reischl Citation2012), which could produce reactive products and condensates when irradiated with the β particles. Baking the whole system would have been more effective, but was precluded by the incompatibility of the DMA and the pumps with baking temperatures. In the experiments relying on natural fragmentation reactions of the clusters, two important characteristics are the residence time tr between the two DMAs and the corresponding gas temperature Tr . These are controlled by inserting a cylindrical chamber (21 cm long, 3 cm ID, tr ∼ 1.16 s at 4 L/min air flow) between the DMAs (the holding chamber). The chamber is heated electrically to a selected temperature with the assistance of a temperature control. In the absence of this chamber, the volume of the lines between both DMAs is minimal (estimated at some 2 cm3). This volume increases only slightly (<1 cm3) on inserting the radioactive element, but substantially (∼150 cm3) with one of the cylindrical (holding or reaction) chambers. Typical sample flow rates were 7 L/min into DMA1 and 10 L/min into and out of DMA2. The 7/10 flow asymmetry in DMA1 has negligible influence on its resolution thanks to the high sheath gas flow rates used.
Salt solutions at typically ∼10 mM concentrations in methanol, ethanol, or acetonitrile were electrosprayed from a sharpened tip of either 20 or 40 μm ID silica capillaries at a liquid flow rate close to the minimal value at which a steady Taylor cone-jet could be stabilized. The high voltage required for electrospraying was applied to an electrode immersed in the solution reservoir.
Two salts were used, both based on the small cation EMI+ (1-ethyl-3-methylimidazolium+). One included the large anion (CF3SO2)3C− (methide), the other the also relatively bulky anion (CF3SO2)2N− (Im). Both were purchased from Covalent Associates. The tetraheptylammonium bromide sample used for mobility calibration was from Sigma Aldrich.
The computer control program used was developed at SEADM by J. Cuevas and G. Vidal. It is a variant of another program they have previously used to control a pair of unique instruments achieving steady mobility separation by means of time dependent electric fields (Vidal-de-Miguel Citation2010). The software scans the voltages V 1, V 2 applied to the two DMAs over a 2-dimensional (2D) rectangular grid, and measures the corresponding nanoparticle current I exiting the second DMA. The Faraday cage electrometer used is commercialized by SEADM. It converts current into voltage with two amplifiers, the first with a trans-impedance estimated at 1010 Ohm. The second magnifies the voltage by one hundredfold. Its overall response time is of the order of 0.1 s. The resulting 3D data set (V 1, V 2, I) is stored as a single file and can be represented via 2D or 3D graphics. We used the computer programs Matlab and Mathematica for preliminary and final data representation.
Stability in the parameters of the DMA is important for our relatively long experiments. The pump driving the sheath gas flow of DMA2 in closed loop was controlled by an active stabilization circuit based on keeping the rotation speed close to an externally set value. This circuit is identical to the one used in our DMA-MS facility (http://www.eng.yale.edu/DMAMSfacility/), and was kindly provided by its commercial manufacturer SEADM. This control system allowed a wide range of flow rates in DMA2. Having only one such variable speed controller available, the pump driving DMA1 in open circuit was based on a universal motor driven by the fixed DC current output of a stabilized power supply. In order to provide a limited range of speeds in DMA1 we used two such power supplies, one providing a stabilized voltage in the range 36 ± 2 V, the other in the range 48 ± 3 V.
4. STANDARDS BASED ON SPONTANEOUS ION EVAPORATION FROM EMI-METHIDE CLUSTERS
shows two representations of a two-dimensional mobility spectrum for EMI-Methide clusters. Many ions fall on a straight line V 2 = kV 1, which we shall for brevity denote the k line, or the main line. This is to be expected for ions whose mobilities in the first and the second DMA are identical. The figure shows also several groups of peaks ordered along other lines different from the main line, and therefore associated with clusters having different mobilities on both DMAs. These peaks are evidently due to unstable particles, having undergone some transformation (decomposition) on their way from the outlet slit of DMA1 to the inlet slit of DMA2. It is important to note that these decomposition or fragmentation reactions do not take place in the analyzing sections of the DMAs, but in the transit between both analyzing sections. This is clear from the fact that the peaks observed are well defined in both dimensions. Yet, if one cluster fragmented into a product within one DMA, the mobility measured would be intermediate between those of the parent and the product. And because the fragmentation may take place at any point within the analyzer, a continuous signal would be seen covering the region between the peaks associated with the parent and the product clusters. No such features are seen in our spectra. The reason why fragmentation takes place primarily between the DMAs rather than within them is that the residence times are much larger in the transit period than in the analyzing period. Both DMAs have an axial length of analysis of 2 cm and operate at sheath gas speeds in the range of 100 m/s. The analysis time is therefore of the order of 0.2 ms. The smallest line volume between both DMAs (when no holding chamber is introduced) can be estimated as greater than 2 cm3, with a residence time of at least 12 ms at our typical sample flow rate of 10 L/min.
FIG. 4 Two representations of the DMA2 spectrum of an electrospray of EMI-Methide. Lines labeled αk have slopes αk. The k line corresponds to untransformed clusters having the same mobility on both DMAs. Peaks marked n+z are from clusters composition (CA) n (C+ ) z containing n ion pairs and z unpaired cations. (Color figure available online.)
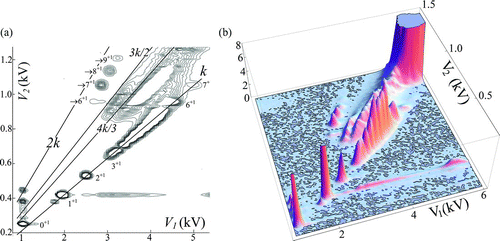
One of the groups of products from unstable clusters lies over a horizontal line, such that the cluster mobility on DMA2 is the same for all the members of the group, and coincides with that of the cluster labeled 1+1. We shall refer to this cluster as the dimer, as it has the second highest mobility among the high abundance clusters within the main line. The reason why many parent ions selected in DMA1 appear at DMA2 at the dimer mobility is that they undergo reactions, one of whose products is this dimer.
Another dominant group of products from unstable clusters falls above the main line, not far below a line going through the origin of coordinates with slope 2k. These product ions are the result of transitions dividing the original mobility approximately by two, which must correspond to the conversion of a cluster from doubly into singly charged. The two product series just discussed appear hence to be both part of the following decomposition process:
includes other groups of ions lying slightly below lines going through the origin of coordinates with slopes kz/(z–1). They would be associated with clusters whose initial charge state shifts from z to z–1, corresponding approximately to ions whose mobility on DMA1 is reduced on DMA2 by a factor z/(z–1). The fact that several groups of product ions group slightly below these lines confirms that they are the products of ion evaporation. The majority of the distinguishable product clusters lying above the main line in can in fact be interpreted as the result of ion evaporation. We shall refer to these various ion evaporation transitions by the shorthand 2→1, 3→2, etc. Each well-defined peak in the main line of represents just one ion, which we will denote n+z when it is formed by n neutral salt molecules (ion pairs) and z EMI+ cations. Well-defined peaks not lying on the main line in are associated with two (parent and product) rather than just one cluster, and could generally be denoted symbolically as n+z →n′ +z′ . The most common peaks created by reaction (2) have n′ = n–1, z′ = z–1 and will more economically be referred to just through the product ion as →n+z .
Notice the peculiarity of the 0+1 ion appearing at several well-defined vertically stacked peaks. Because the parent ion is singly charged, the corresponding increase in size in DMA2 cannot be due to ion evaporation. It is rather due to condensation over the ion of some vapor impurities present in the gas. This we will refer to as solvation. Each of the peaks appearing above a certain bare ion must represent a different state of solvation. The small EMI+ ion is evidently prone to such associations with impurity vapors, so its shifting mobility cannot be used as a reliable mobility standard. This problem is not evident in pure mobility measurements. Nor in DMA-MS measurements, since the relatively energetic process of entry into the vacuum system of contemporary atmospheric pressure ionization mass spectrometers (API-MS) tends to remove the weakly bound solvating neutrals. The solvation process is however quite clear in DMA2 spectra because both analyzers operate under ambient pressures and temperatures. The fact that many well-defined solvation states are apparent in DMA2 shows that their lifetime is longer than their residence time in the DMA. The TDMA approach therefore offers a useful new tool for detailed studies of heterogeneous nucleation. None of the larger clusters of give any indication of a tendency to solvate, so that all but the monomer present themselves as excellent candidates for use as standards.
4.1. Ion Assignment
The identification of ions included in was performed with the help of the DMA-MS data of , which provide unambiguous peak voltages, and n and z values for all peaks present. The voltages V are converted into inverse mobilities 1/Z through a single proportionality constant ξ, appropriate for the operating conditions of the DMA in that experiment:
TABLE 1 Approximate mobilities of singly positively charged EMI-Methide cluster ions determined via DMA-MS
Returning to our peak assignment process, collects the mobilities of all members of the singly charged series identified as such. In addition to the main bands z = 1, 2, 3, …, marked in the DMA-MS spectrum of , one sees other anomalous bands resulting from ion evaporation events. Their origin is readily recognized because the most mobile doubly charged ions seen in are partly transformed by ion evaporation and have above them another singly charged ion in one of these spurious bands (similar transitions are marked in by vertical lines). n and n′ differ in and exactly by unity, corresponding to transition (1) with the loss of a dimer ion. The members of this series of product ions extend in down to n = 7 (n′ = 6), considerably smaller than the smallest surviving member of the legitimate z = 2 series (n = 12), evidently because these smaller z = 2 ions are completely converted. Accordingly, although not included in , the mobilities of the completely converted doubly charged parent ions have been inferred from their products. Unfortunately, the singly charged products of the 2→1 transition interfere with the doubly charged products of the 3→2 transitions, precluding a reliable DMA-MS inference of the mobilities of clusters 10+2 and 11+2. Notice that, due to substantial ion heating in the entry section of the mass spectrometer, the transitions observed in DMA-MS experiments do not correspond necessarily to those taking place at atmospheric pressure between the two DMAs.
We now proceed to analyze the DMA2 spectrum of by compiling in the pairs of voltages in the main line and in the 2→1 transition. No mobility calibration standard was used for these data, but they are calibrated by comparison of the V 1 values for the clusters 0+1, 1+1, and 2+1, with those previously obtained via DMA-MS (). The intermediate clusters from 3+1 to 5+1 are resolved in DMA-MS spectra, but they are mixed with other doubly and triply charged ions in the DMA2 spectrum of . Their positions are hence not indicated in . They coincide with resolved ions observed in along the main line. Larger clusters from 6+1 to 8+1fall beyond the region of multiply charged clusters and are directly recognizable in , though the assignment of their corresponding n is achieved by comparison with the DMA-MS data.
TABLE 2 Approximate mobilities of positively charged EMI-Methide cluster ions determined via DMA2, including originally doubly charged ions
We now proceed to the identification of the clusters in the 2→1 transition. One can see in that the lower members of the series are aligned horizontally with the largest originally singly charged clusters 6+1 and 7+1 in the main line (also 8+1, though its intensity is too low to appreciate in this figure). This provides a first means to assign the 2→1 ions, as labeled in . Although the 2→1 series visible in goes only up to the transition 10+2→9+1, one can unambiguously recognize low intensity transitions up to 12+2→11+1. The horizontal coordinate of this series of metastable ions yields the mobilities of the precursor doubly charged ions. These are known also from the DMA-MS data, directly down to 12+2, and indirectly (via their ion evaporation products) from 9+2 to 7+2. The DMA2 measurement provides also the mobility of the clusters 10+2 and 11+2, unavailable from the DMA-MS spectrum.
Interestingly, the parent ion 7+2 in this first transition is preserved at a small but measurable signal in the DMA2 spectrum. In contrast, as already noted, the smallest doubly charged parent ion visible in the DMA-MS spectrum is 12+2. For completeness, includes the properties n, z, n′, z′ of the parent and the product ions.
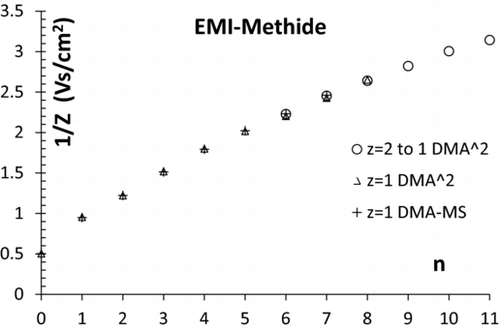
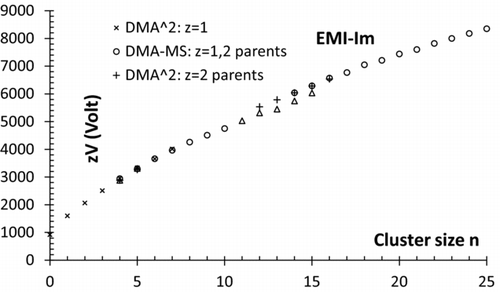
The various mobilities collected in and are represented in versus the number n of neutral pairs in the cluster. The fair agreement seen between the three types of measurements made provides excellent confidence on the correctness of the assignment.
Irrespective of the assignment made, it is noteworthy that the daughters of the z = 2→1 series of metastable ions yield unusually large and exceptionally well resolved clusters of various sizes, which may be used as convenient standards for a variety of applications. The largest member of that series, 11+1 has a mobility diameter in excess of 2.5 nm. Its molecular mass of 5862 Da is similar to that of the protein insulin, but its mobility peak is much better defined than that of any electrosprayed protein ion.
5. STANDARDS BASED ON SPONTANEOUS ION EVAPORATION FROM EMI-IM CLUSTERS
We start by assigning voltages to the various ions n+z from the DMA-MS spectrum previously reported by Hogan and Fernández de la Mora (2009), shown in , where ion evaporation transitions for doubly charged ions are marked by vertical white lines. The smallest initially doubly charged ion that keeps both charges through the MS is 18+2, which transforms almost completely into 17+1. In spite of the limited mobility and mass range covered, shows also the product ions down to 12+1, whose parent is 13+2. The mean peak voltage associated with these ions is shown in (left) as a function of the n value for the parent ion, and is included also in .
TABLE 3 Peak voltages (V) for various parent ions in DMA/MS and DMA2 measurements (multiplied by a rescaling constant) for EMI-Im
shows several DMA2 spectra for ESs of EMI-Im, starting in panel (a) with a global view of limited resolution. Panels (b) and (c) give two different views of the same data, showing a main line exceptionally dominated by singly charged ions, with a slight contamination from multiply charged ions. The products of the 3→2 transition are not well-resolved in most of our data with either of the two salts studied. However, as shown in , these peaks can be isolated when using optimal sweeping and DMA flow parameters. (right) and include several DMA voltages derived from these spectra, after determining the proper calibration ratio of DMA-MS versus DMA2 data.
FIG. 7 DMA2 spectra for EMI-Im: (a) global low-resolution view. (b, c) Two detailed views of the main line, dominated by well-resolved singly charged ions, and also displaying neutral evaporation products. (d) Exceptional conditions where isolated peaks are resolved for the 3→2 transitions, also showing lines with slopes k, 4k/3, 3k/2, and 2k. (Color figure available online.)
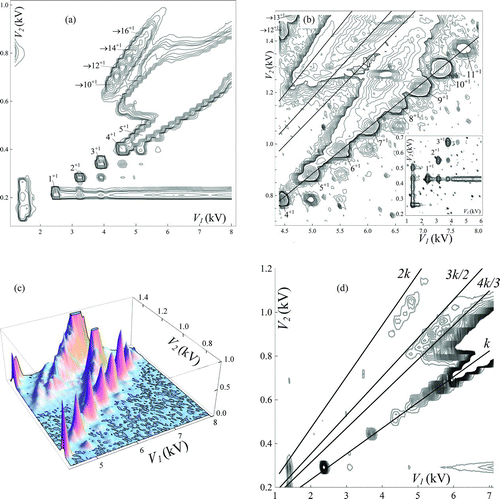
No mobilities are reported for EMI-Im as no direct calibration was performed. An approximate indirect calibration based on the (not truly reliable) EMI+ peak (1/Z = 0.503 Vs/cm2; ), appearing at 922 V, indicates that the largest well-defined product ion (16+1) of 2→1 transition has 1/Z ∼ 3.45 Vs/cm2 (mobility diameter of ∼2.65 nm). The largest resolvable ion in the 3→2 transition has z/Z ∼ 6.17 Vs/cm2, with a mobility diameter of ∼3.53 nm.
6. STANDARDS BASED ON SPONTANEOUS NEUTRAL EVAPORATION FROM EMI-IM CLUSTERS
So far we have obtained isolated clusters pushed above the main line by ion evaporation. – show a lower abundance series of clusters appearing below the main line, just underneath each of the singly charged clusters. This alternative means of isolating clusters away from the main line results from the neutral evaporation reaction
FIG. 8 ES-DMA2 spectra of EMI-Methide with neutralization between DMA1 and DMA2. (a) Low resolution, including continuous lines with slope k (=0.1388); 4k/3; 3k/2; 2k; 5k/2; 3k. (b) Increased vertical resolution, including continuous lines with slope k (=0.168) and 2k. (Color figure available online.)
7. EXPERIMENTS WITH A RADIOACTIVE CHARGE-REDUCING SOURCE
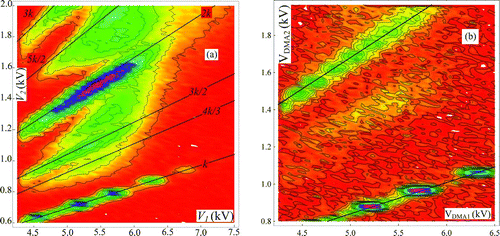
Because only a few exploratory experiments of this type were performed, their inclusion here is intended simply to provide a qualitative indication of the merits of this approach versus that based on spontaneous cluster decomposition. includes two spectra from a spray of EMI-Methide. The contour lines shown on the left figure correspond to amplifier voltage increments of 75 mV. The lowest continuous line shown (the k line) corresponds to particles having the same mobility (same charge state) on both DMAs. Its slope k = 0.1388 is chosen such that it passes through the least mobile peak, which assures that it also goes through all the peaks in the lowest series. The major ions in the k line are all singly charged. They can be identified based on our prior discussion, but they may remain anonymous for present purposes. It is clear that there are more than just singly charged peaks in this series, indicating that the neutralization process is not complete. An even more intense series of ions can be seen slightly below the line of slope 2k (the 2k line), corresponding to ions that had originally two charges, and have reached the second DMA with only one. Unlike the k line, which included remnants of multiply charged species, the 2k line includes only singly charged particles (other than perhaps the tails of other ion classes represented in the figure by peaks centered away from the 2k line). This approximate charge-purity feature is of interest, particularly for nucleation studies. The advantage, however, is balanced by a potential composition impurity disadvantage. Notice that these artificially discharged ions are closer to the 2k line than was the case with naturally discharged ions. The reason is that now they are discharged by attachment of a relatively small gas phase anion G − (perhaps O2 −), according to the reaction
The upper region of includes a short line segment with slope 3k. The series of ions slightly below it is evidently associated with clusters going through DMA1 with three charges and reaching DMA2 with only one. The discussion just included on the ions below the line of slope 2k applies similarly to those below the 3k line, except that the discharged product may now incorporate two discharging anions instead of one. Besides the clusters discussed, falling near the k, 2k, and 3k lines, shows several other groups of ions, also ordered approximately near straight lines going through the origin with slopes ik/j, with i/j = 3/2, 4/3, 5/2, etc. This proximity indicates that these groups of clusters correspond to insufficiently discharged particles having originally i elementary charges, which are reduced to j charges in the neutralizer. This complexity (as well as the presence of multiply charged ions on the k line) would be avoided with a better neutralization process, calling for a smaller sample flow rate. This solution, however, makes the signal harder to detect. was taken for the same salt but within a narrower range of mobilities and a higher vertical mobility resolution. One sees similar features as in , though now ions in the 2k line are well resolved. The limited horizontal resolution resulting from the excessively long slit in the parallel plate DMA is manifest.
8. DISCUSSION
In view of the large fraction of naturally charged ions spontaneously undergoing ion evaporation, even at room temperature, and even in sub-millisecond times, this phenomenon offers the most promising among the various approaches explored to achieve unusually large isolated standard clusters. A good part of the advantage of spontaneous charge reduction over its radioactive source analog is the possibility to use relatively large sample flow rates, leading to relatively large currents of purified clusters. Based on the 2→1 transition, the method readily produces singly charged isolated clusters larger than 2.5 nm. With some effort, even the products from the 3→2 transition may be resolved, yielding pure doubly charged particles as large as 3.5 nm. While not impossible, proceeding to even larger sizes and higher charge states will certainly be limited by resolution. Also, the charge state cannot be widely or easily controlled independently of the size. These limitations, however, must be strongly dependent on the nature of the salt (as hinted by the diversity of behaviors found in the single DMA work of Ude and Fernández de la Mora 2005) and the temperature upstream and downstream from the first DMA.
The method based on artificial charge reduction followed by a single DMA has been used before. In this approach, it is possible to avoid survival of multiply charged particles by operating at moderate flow rates, although this limits greatly the transmission. Artificial charge reduction also provides little flexibility to select charge states beyond z = 1. The method nonetheless offers much wider size flexibility than spontaneous charge reduction. The DMA-neutralizer-DMA combination offers some interesting new possibilities, even though the purity of the resulting standards is not guaranteed. When using relatively large sample flow rates (as in ), even though many charge states may survive in the main line, singly charged particles will be found primarily in the 2k, 3k, … lines. There is nonetheless the potential of interferences from the z different possible final charges originating from a given initial charge z. Accordingly, it seems to us that the only sure way to obtain reliable standards with artificial charge reduction is to achieve complete removal of charge states beyond z = 1. Whenever this desirable condition is achievable one might more conveniently use a single DMA rather than a TDMA.
9. CONCLUSIONS
| 1. | A continuous TDMA system based on a first parallel plate DMA has greatly alleviated the transmission problems previously reported by Attoui et al. (Citation2013), enabling the use of a faster yet less sensitive detector, and improving considerably the resolution. | ||||
| 2. | The investigation of multiply charged clusters electrosprayed from solutions of ionic liquids shows three rich mechanisms leading to spontaneous mobility shifts on going from the first to the second DMA: (i) ion solvation by vapors, (ii) neutral evaporation, and (iii) ion evaporation. These shifts are investigated here as potential sources of pure molecular standards. | ||||
| 3. | Ion solvation affects primarily subnanometer ions and is of limited use for our application. It is however clear from our data that the TDMA method offers a novel tool for ion induced solvation and nucleation studies. | ||||
| 4. | Neutral evaporation helps isolating singly from multiply charged clusters, and increases only moderately the size of accessible molecular standards with respect to those available from single DMAs. | ||||
| 5. | Ion evaporation from initially doubly charged clusters offers with relative ease pure size standards with mobility diameters up to 2.5 nm. Doubly charged ions decaying from triply charged species can be isolated up to mobility diameters of 3.5 nm, though with greater difficulty. | ||||
| 6. | A limited exploration with a radioactive neutralizer interposed between both DMAs provides singly charged clusters over a very wide size range. This positive result is not new. However, the TDMA method shows that many of these particles are contaminated by multiply charged ions, except under conditions of moderate sample flow, when a single DMA would be comparably effective. Even under such low flow rates, the charge exchange reaction with uncontrollable atmospheric ions may introduce unknowns unfit of a true standard. | ||||
| 7. | For these reasons, and due to its compatibility with relatively high sample flow rates and high concentrations of purified ions, the most effective source of molecular standards identified is ion evaporation. | ||||
Acknowledgments
Statement on conflict of interest: Following Yale rules, J. Fernandez de la Mora declares that he has a personal interest in the company SEADM manufacturing the mobility analyzer coupled to a mass spectrometer used to generate .
This work was partly supported by the US AFOSR contract FA9550–09-C-0178 to Alameda Applied Sciences, through a subcontract to Yale as part of a Phase II STTR program. We thank Applied Biosystems and SEADM for their loan of our IMS-MS facility, (http://www.eng.yale.edu/DMAMSfacility/), Yale's W. M. Keck Center for hosting it, and Bruce Thomson (Sciex) for his guidance on mass spectrometry and for the loan of the parallel plate DMA used in our DMA2 facility. We are grateful to Chris Hogan, Juan Rus, and Alejandro Casado of SEADM for their contributions to the early study on EMI-Im used here for ion assignment. We finally thank Mr. Juan Fernandez-Garcia for his invaluable help in manipulating two-dimensional files and Mr. Ernesto Criado-Hidalgo for sharing his improved mobility calibration of the EMI-Methide data.
REFERENCES
- Attoui , M. , Fernandez-García , J. , Cuevas , J. , Vidal , G. and Fernandez de la Mora , J. 2013 . Charge Evaporation from Nanometer Polystyrene Aerosols . J. Aerosol Sci. , 55 : 149 – 156 .
- Chen , D. R. , Pui , D. Y. H. and Kaufman , S. L. 1995 . Electrospraying of Conducting Liquids for Monodisperse Aerosol Generation in the 4 nm to 1.8 mm Diameter Range . J. Aerosol Sci. , 26 ( 6 ) : 963 – 977 .
- Earle , M. J. , Esperanca , J. M. S. S. , Gilea , M. A. , Lopes , J. N. C. , Rebelo , L. P. N. Magee , J. W. 2006 . The Distillation and Volatility of Ionic Liquids . Nature , 439 ( 7078 ) : 831 – 834 .
- Fenn , J. B. , Mann , M. , Meng , C. K. , Wong , S. F. and Whitehouse , C. M. 1989 . Electrospray Ionization for Mass Spectrometry of Large Biomolecules . Science , 246 ( 4926 ) : 64 – 71 .
- Fernandez de la Mora , J. 2011a . Heterogeneous Nucleation with Finite Activation Energy and Perfect Wetting: Capillary Theory Versus Experiments with Nanometer Particles, and Extrapolations on the Smallest Detectable Nucleus . Aerosol Sci. Technol. , 45 ( 4 ) : 543 – 554 .
- Fernandez de la Mora , J. 2011b . “ Sub-3 nm Aerosol Measurement with DMAs and CNCs, Chapter 32 ” . In Aerosol Measurement: Principles, Techniques, and Applications, Third Edition , Edited by: Kulkarni , P. , Baron , P. A. and Willeke , K. 697 – 721 . New York : John Wiley & Sons, Inc .
- Fernandez de la Mora , J. and Kozlowski , J. 2013 . Hand-held Differential Mobility Analyzers of High Resolution for 1–30 nm Particles: Design and Fabrication Considerations . J. Aerosol Sci. , 57 : 45 – 53 .
- Gamero-Castaño , M. and Fernandez de la Mora , J. 2000a . A Condensation Nucleus Counter (CNC) Sensitive to Singly Charged Subnanometer Particles . J. Aerosol Sci. , 31 : 757 – 772 .
- Gamero-Castaño , M. and Fernández de la Mora , J. 2000b . Mechanisms of Electrospray Ionization of Singly and Multiply Charged Salt Clusters . Analytica Chimica Acta , 406 : 67 – 91 .
- Gamero-Castaño , M. and Fernandez de la Mora , J. 2002 . Ion-Induced Nucleation: Measurement of the Effect of Embryo's Size and Charge State on the Critical Supersaturation . J. Chem. Phys. , 117 ( 7 ) : 3345 – 3353 .
- Heim , M. , Attoui , M. and Kasper , G. 2010 . The Efficiency of Diffusional Particle Collection Onto Wire Grids in the Mobility Equivalent Size Range of 1.2 to 8 nm . J. Aerosols Sci. , 41 : 207 – 222 .
- Hogan , C. J. Jr. and Fernández de la Mora , J. 2009 . Tandem Ion Mobility-Mass Spectrometry (IMS-MS) Study of Ion Evaporation from Ionic Liquid-Acetonitrile Nanodrops . Phys. Chem. Chem. Phys. , 11 : 8079 – 8090 .
- Hogan , C. J. Jr. and Fernández de la Mora , J. 2010 . Ion-Pair Evaporation from Ionic Liquid Clusters . J. Am. Soc. Mass Spectrom. , 21 : 1382 – 1386 .
- Hogan , C. , Ruotolo , B. , Robinson , C. and Fernandez de la Mora , J. 2011 . Tandem Differential Mobility Analysis-Mass Spectrometry Reveals Partial Gas-Phase Collapse of the GroEL Complex . J. Phys. Chem. B , 115 ( 13 ) : 3614 – 3621 .
- Iribarne , J. V. and Thomson , B. A. 1976 . Evaporation of Small Ions from Charged Droplets . J. Chem. Phys. , 64 ( 6 ) : 2287 – 2294 .
- Kaufman , S. L. 1998 . Analysis of Biomolecules using Electrospray and Nanoparticle Methods: The Gas-Phase Electrophoretic Mobility Molecular Analyzer (GEMMA) . J. Aerosol Sci. , 29 ( 5–6 ) : 537 – 552 .
- Kaufman , S. L. and Dorman , F. D. 2008 . Sucrose Clusters Exhibiting a Magic Number in Dilute Aqueous Solutions . Langmuir , 24 ( 18 ) : 9979 – 9982 .
- Kaufman , S. L. , Skogen , J. W. , Dorman , F. D. , Zarrin , F. and Lewis , L. C. 1996 . Macromolecule Analysis Based on Electrophoretic Mobility in Air: Globular Proteins . Anal. Chem. , 68 : 3703 – 3703 . 1895–1904; corrections in Anal. Chem. 68
- Ku , B. K. and Fernandez de la Mora , J. 2004 . Cluster Ion Formation in Electrosprays of Acetonitrile Seeded with Ionic Liquids . J. Phys. Chem. B , 108 : 14915 – 14923 .
- Ku , B. K. and Fernandez de la Mora , J. 2005 . Evaporation Kinetics of Tetra-Alkylammonium Ions from Charged Formamide Drops . J. Phys. Chem. , 109 ( 22 ) : 11173 – 11179 .
- Larriba , C. , Hogan , C. J. Jr. , Attoui , M. , Borrajo , R. , Fernandez Garcia , J. and Fernandez de la Mora , J. 2011 . The Mobility-Volume Relationship Below 3.0 nm Examined by Tandem Mobility-Mass Measurement . Aerosol Sci. Tech. , 45 ( 4 ) : 453 – 467 .
- Loscertales , I. G. and Fernández de la Mora , J. 1995 . Experiments on the Kinetics of Field-Evaporation of Small Ions from Droplets . J. Chem. Phys. , 103 : 5041 – 5060 .
- McLuckey , S. A. and Stephenson , J. L. 1998 . Ion Ion Chemistry of High-Mass Multiply Charged Ions . Mass Spectrom. Rev. , 17 : 369 – 407 .
- Rader , D. J. and McMurry , P. H. 1986 . Application of the Tandem Differential Mobility Analyzer to Studies of Droplet Growth or Evaporation . J. Aerosol Sci. , 17 ( 5 ) : 771 – 787 .
- Rus , J. , Moro , D. , Sillero , J. A. , Royuela , J. , Casado , A. and Fernández de la Mora , J. 2010 . IMS-MS Studies Based on Coupling a Differential Mobility Analyzer (DMA) to Commercial API-MS Systems . Int. J. Mass Spectrom. , 298 : 30 – 40 .
- Seto , T. , Okuyama , K. and Fernández de la Mora , J. 1997 . Condensation of Supersaturated Vapors on Monovalent and Divalent Ions of Varying Size . J. Chem. Phys. , 107 : 1576 – 1585 .
- Steiner , G. and Reischl , G. P. 2012 . The Effect of Carrier Gas Contaminants on the Charging Probability of Aerosols Under Bipolar Charging Conditions . J.Aerosol Sci. , 54 : 21 – 31 .
- Tammet , H. 1995 . Size and Mobility of Nanometer Particles, Clusters, and Ions . J. Aerosol Sci. , 26 ( 3 ) : 459 – 475 .
- Ude , S. and Fernández de la Mora , J. 2005 . Molecular Monodisperse Mobility and Mass Standards from Electrosprays of Tetra-Alkyl Ammonium Halides . J. Aerosol Sci. , 36 ( 10 ) : 1224 – 1237 .
- Vanhanen , J. , Mikkila , J. , Sipila , M. , Manninen , H. E. , Lehtipalo , K. Siivola , E. 2011 . Particle Size Magnifier for Nano–CN Detection . Aerosol Sci. Technol. , 45 : 533 – 542 .
- Vidal-de-Miguel , G. 2010 . Method and Apparatus to Produce Steady Beams of Mobility Selected Ions via Time-Dependent Electric Fields . US patent application publication 20100243883
- Winkler , P. M. , Vrtala , A. , Steiner , G. , Wimmer , D. , Vehkamaki , H. Lehtinen , K. E. J. 2012 . Quantitative Characterization of Critical Nanoclusters Nucleated on Large Single Molecules . Phys. Rev. Lett. , 108 ( 8 ) : 108.085701