Abstract
Copyright 2013 American Association for Aerosol Research
1. INTRODUCTION
The Aerosol Particle Mass Analyzer (APM; Ehara et al. Citation1996) and Centrifugal Particle Mass Analyzer (CPMA; Olfert and Collings Citation2005) are instruments that use opposing electric and centrifugal fields to classify aerosol particles according to their mass-to-charge ratio. Unlike their analog for electrical mobility, the Differential Mobility Analyzer (DMA), the peak mass-to-charge depends on only the elementary charge constant and four well defined properties of the classifier: the rotational speed, potential difference, and the inner and outer electrode radii. There is no dependence upon gas properties such as temperature, pressure, and viscosity, and no semi-empirical corrections are required (such as the slip correction factor). As such, it can be argued that aside from the potentially necessary charge correction, they are an appealing fundamental standard for measuring particle mass and have found a role as calibration standards for other instrumentation (Cross et al. Citation2010). It is known that gravimetric filter measurements are susceptible to sampling artifacts including the adsorption of vapor onto the filter. Even if a suitable nonvolatile aerosol is used, collecting sample on a filter paper can take a long time. This letter considers a method using a CPMA with an aerosol electrometer to provide a stream of particles of known mass concentration for real-time instrument calibration using most aerosol materials (regardless of volatility, or morphology), which does not require correction for multiple charges per se—indeed their presence enhances the technique's sensitivity. Whereas from here on, we refer to the instrument type as a CPMA, this method should also apply to the APM.
CPMAs have been used with a DMA and a Condensation Particle Counter (CPC) to measure effective density and mass-mobility exponent (Park et al. Citation2003, Olfert et al. Citation2007). In this situation, consideration has to be made of any multiply charged particles exiting the DMA. We examine here instead a charger directly connected to the inlet of the CPMA and an aerosol electrometer downstream of a CPMA and consider the effect of the multiply charged states from the CPMA on that electrometer measurement. We allow the stream of charged, mass:charge selected particles to be split before the electrometer, and passed into a challenge instrument, which measures total mass concentration ().
Assuming the particle concentration entering the CPMA is uniformly distributed with respect to particle mass,Footnote 1 the total mass concentration present post-CPMA (m total) is given by:
FIG. 1 Proposed scheme—aerosol is charged, classified by mass:charge ratio by the CPMA, producing a stream of particles of total mass concentration m total, which is split and sampled by both an aerosol electrometer and a challenge instrument. To better match instrument flow rates, particle-free make-up air could be added before the splitter.
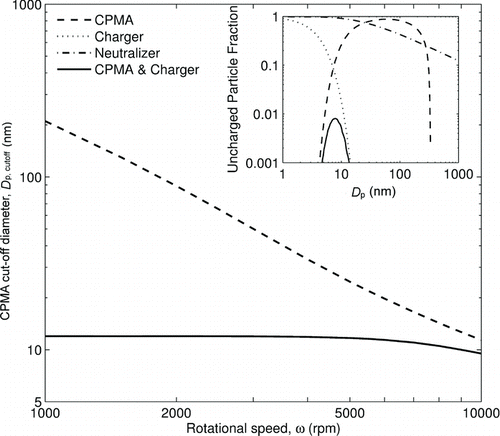
The cutoff diameter of unit density uncharged particles through a CPMA with radii of 60 mm and 61 mm, length of 200 mm, speed ratio of 0.97, and flow rate of 1.5 lpm, as a function of rotational speed, both with and without the unipolar charger described in the text upstream of the CPMA. The inset shows the uncharged particle fraction as a function of particle diameter for a CPMA rotating at 1000 rpm, a unipolar charger, a radioactive neutralizer, and downstream of a unipolar charger and CPMA in series.
2 UNCHARGED PARTICLES
The dependence of Equation (Equation3) upon m 0 requires that this quantity is either accounted for, or minimized. It is possible for small uncharged particles to pass through the CPMA at sufficiently slow speeds. (inset, dashed line) shows the theoretical penetration of uncharged, spherical particles of unit density for a typical CPMA operating at 1000 rpm—the upper cutoff here is due to the centrifugal impaction of uncharged particles. The model accounts for diffusion and is described by Olfert and Collings (Citation2005). The dotted line shows the uncharged particle fraction from a typical unipolar charger. The solid line shows the combined effect of the unipolar charger and CPMA in series: most particles below 10 nm are lost due to diffusion, while the upper cutoff is mostly due to the performance of the charger.
While it is relatively simple to quantify the number concentration of uncharged particles (n 0) by the use of an electrostatic precipitator and CPC, determining the mass concentration of uncharged particles is less straightforward. An upper limit of the uncharged particle mass concentration (m 0,max) can be determined from
It should be borne in mind that these uncharged particles are necessarily small (less than 12 nm in the example), so their mass contribution is small. Ideally, conditions would be chosen to minimize m 0 so that it is insignificant. Some suggestions for this are provided below.
2.1 Use a Unipolar Charger
By using a unipolar charger (i.e., placing a net positive charge on particles, e.g., by a corona-based diffusion charger) it is possible to ensure the population of uncharged particles is small. This has an additional advantage in that the high charge state will increase the signal-to-noise level of the electrometer measurement. We consider here the example of a charger similar to that described by Biskos et al. (Citation2005). The Fuchs (Citation1963) charging model at an ion concentration–time product (n i t) of 5.4 × 1013 ions m−3 s yields a plot of the proportion of uncharged particles exiting this charger versus particle size (dotted line on inset of ). For Dp >20 nm, the population of uncharged particles is negligible. The inset also shows the fraction of uncharged particles in a bipolar equilibrium charge distribution (Wiedensohler Citation1988), as obtained from a radioactive neutralizer. The uncharged fraction of large particles is relatively high. Furthermore, the current measured by the electrometer would be substantially less than when a unipolar charger is used, making a neutralizer poorly suited for this method.
2.2 Operate the CPMA at High Speed
As previously described, small uncharged particles may pass through the CPMA at sufficiently slow speeds. Therefore, running the CPMA at higher speeds will reduce the post-CPMA concentration of uncharged particles. A disadvantage of this is that as the CPMA's resolution depends on the magnitude of the drift force imparted on the particles, increasing the speed leads to higher resolution, and hence a smaller post-CPMA concentration of particles, possibly leading to sensitivity problems with the challenge instrument.
2.3 Choose the Aerosol Source Mass Distribution and Center Mass Carefully
When using a DMA with an electrometer to calibrate a CPC (Liu and Pui Citation1974), one selects aerosol from the side of the underlying distribution larger than the peak, to minimize the population of large particles, which may become multiply charged. Here, where we wish to minimize the number of uncharged particles, we suggest the use of the opposite strategy, that is to select aerosol with the CPMA from the side of the distribution smaller than the peak, to minimize the possible population of small uncharged particles.
3 UNCERTAINTY ANALYSIS
For the CPMA, the mass-to-charge ratio (M/N q) is given by
for rotational speedFootnote
2
ω, voltage V, outer cylinder radius ro
, inner cylinder radius ri
, and elementary charge e. For the commercial CPMA (Cambustion), the radii of the cylinders are 60 mm and 61 mm; these dimensions could be gauged to within ![]() m—therefore, the uncertainties on the absolute radii are insignificant. However, the uncertainty on the gap is significant; given the gap between the cylinders is small compared with their radii, the field between cylinders approximates as the field strength between parallel plates, linear in the gap size. The law of propagation of uncertainties was used for M
+1 as a function of V, ω, ro
, and ri
, with the logarithmic term approximated as (ro
−ri
). The probability distribution of the cylinder radii was assumed uniform; therefore, the standard uncertainty in these was estimated as
m—therefore, the uncertainties on the absolute radii are insignificant. However, the uncertainty on the gap is significant; given the gap between the cylinders is small compared with their radii, the field between cylinders approximates as the field strength between parallel plates, linear in the gap size. The law of propagation of uncertainties was used for M
+1 as a function of V, ω, ro
, and ri
, with the logarithmic term approximated as (ro
−ri
). The probability distribution of the cylinder radii was assumed uniform; therefore, the standard uncertainty in these was estimated as ![]() m (Taylor and Kuyatt Citation1994). Using these assumptions, the combined standard uncertainty in M
+1 is
m (Taylor and Kuyatt Citation1994). Using these assumptions, the combined standard uncertainty in M
+1 is
We now consider the electrometer, where the charge concentration is given by ![]() . The standard uncertainty in I and Q is each assumed to be
. The standard uncertainty in I and Q is each assumed to be ![]() . Again, by using the law of propagation of uncertainties, we calculate the combined standard uncertainty in the aerosol electrometer to be approximately 1.5%. Further propagating this with
. Again, by using the law of propagation of uncertainties, we calculate the combined standard uncertainty in the aerosol electrometer to be approximately 1.5%. Further propagating this with ![]() gives a combined standard uncertainty for the system of ≈2%.
gives a combined standard uncertainty for the system of ≈2%.
The effect of any uncharged particles is dependent upon application, but for particles larger than 20 nm, it should be possible using the means outlined in the previous section to ensure that their population is small, and hence make their overall mass contribution negligible. For example, we charged mini-CAST soot particles using the unipolar charger described above, and used a CPMA to select 0.52 fg particles (100 nm at unit density) at a resolution parameter ![]() . For these conditions, and a sample flow of 4 lpm, ω=7225 rpm, and D
p,cutoff is calculated to be 26.0 nm. Using an electrostatic precipitator and a CPC, the number concentration of uncharged particles downstream of the CPMA was measured to be 5.14 cm−3. Application of Equation (Equation4) gives a maximum uncharged mass concentration of 0.047 fg cm−3. Comparing this to
. For these conditions, and a sample flow of 4 lpm, ω=7225 rpm, and D
p,cutoff is calculated to be 26.0 nm. Using an electrostatic precipitator and a CPC, the number concentration of uncharged particles downstream of the CPMA was measured to be 5.14 cm−3. Application of Equation (Equation4) gives a maximum uncharged mass concentration of 0.047 fg cm−3. Comparing this to ![]() , which was 7.6×104 fg cm−3, yields a maximum uncertainty due to uncharged particles of 0.62 ppm.
, which was 7.6×104 fg cm−3, yields a maximum uncertainty due to uncharged particles of 0.62 ppm.
The CPMA transfer function has a finite width and the accuracy of this technique depends upon it being symmetric in mass space, such that equal populations of masses above and below the peak are present. Part S1 (see online supplementary materials) discusses this effect and shows for the conditions given in the experiments below that this effect creates a systematic error of approximately 0.8–1.5%. Part S2 (see online supplementary materials) also discusses the reproducibility of the commercial CPMA.
4 EXPERIMENTAL VALIDATION
The challenge instrument was a TX40 filter paper with flow controlled to 2 lpm. A 10 mCi Kr-85 neutralizer was placed upstream to minimize the possibility of artifacts caused by the charging of the filter paper by the highly charged aerosol. The challenge aerosol was silicone diffusion pump oil (Kurt J. Lesker 704), chosen for its very low vapor pressure (1.1×10−5 Pa at 25°C) to eliminate gravimetric artifacts caused by volatiles. The oil was nebulized with filtered, dried compressed air, and passed through a cyclone, with d
50
![]() m (removing the largest particles, which would otherwise increase contamination of the CPMA), producing the aerosol spectrum shown in the inset of . The charger used was a prototype Unipolar Diffusion Aerosol Charger (UDAC; Cambustion, of the same type described by Biskos et al. Citation2005), at n
i
t=4.5×1013 ions m−3 s. The CPMA flow was 4 lpm, and R=5. The aerosol electrometer was a HEPA filter within a Faraday cage connected to a Keithley 6514 electrometer, with flow controlled to 2 lpm. Differing mass concentrations were achieved by varying M
+1. An electrostatic precipitator and CPC were used to verify that the proportion of uncharged particles exiting the CPMA was ≪1%, and with the electrometer, that the mean number of charges per particle was around 8–10. Filters were weighed before and after loading, using a Mettler–Toledo UMX2 balance, and the mean mass concentration calculated from the difference in mass, the filter flow and the loading time. A CPC was used to verify that the concentration downstream of the filter paper was ≪1% of the upstream concentration. The results are shown in .
m (removing the largest particles, which would otherwise increase contamination of the CPMA), producing the aerosol spectrum shown in the inset of . The charger used was a prototype Unipolar Diffusion Aerosol Charger (UDAC; Cambustion, of the same type described by Biskos et al. Citation2005), at n
i
t=4.5×1013 ions m−3 s. The CPMA flow was 4 lpm, and R=5. The aerosol electrometer was a HEPA filter within a Faraday cage connected to a Keithley 6514 electrometer, with flow controlled to 2 lpm. Differing mass concentrations were achieved by varying M
+1. An electrostatic precipitator and CPC were used to verify that the proportion of uncharged particles exiting the CPMA was ≪1%, and with the electrometer, that the mean number of charges per particle was around 8–10. Filters were weighed before and after loading, using a Mettler–Toledo UMX2 balance, and the mean mass concentration calculated from the difference in mass, the filter flow and the loading time. A CPC was used to verify that the concentration downstream of the filter paper was ≪1% of the upstream concentration. The results are shown in .
FIG. 3 Mass concentration of silicone oil measured by the CPMA-electrometer method compared with gravimetric measurements. Error bars for the CPMA method are as taken from the analysis in the text, those for the filter paper assume 1% uncertainty for the flow and for each weighing—a coverage factor of 2 is assumed. The inset shows the spectral form of the oil aerosol.
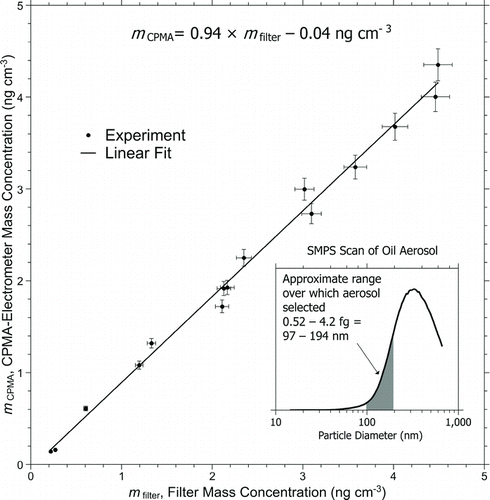
The linear fit to the data shows a systematic 6% disagreement with the gravimetric method—more than the uncertainty analysis above would suggest—and further investigation is needed to find the source. This is partially accounted for by the small systematic error caused by the nonsymmetric output of the CPMA (0.8–1.5% as shown in the online supplementary materials). However, even this level of uncertainty would make this method a very useful tool for aerosol metrology and demonstrates the basic principle.
5 CONCLUSIONS
In the proposed scheme, the total mass concentration sampled by the challenge instrument is equal to the center of the CPMA transfer function for a single charge (M +1) multiplied by the charge concentration measured by the electrometer (I/Qe), provided steps are taken to minimize the number of uncharged particles post-CPMA. All of these parameters can be measured with traceably calibrated instruments. Therefore, the technique is suitable as a suspended mass concentration standard for absolute instrument calibration. Furthermore, electrometer measurements are real-time, which allows rapid calibration.
Part 3 of the supplemental materials discusses the practical throughput of the system and minimum mass concentration for an example aerosol. This modeling work shows that the mass spectrum from the charger-CPMA system consists of a series of narrow spikes, quantized to the individual charge states from the corona charger, rather than a smooth distribution. The overall envelope of this distribution is pseudo-lognormal; however, the applicability of this technique to instruments that assume a lognormal distribution remains to be investigated. We see the main application as a calibration source of particles for instruments such as aerosol mass spectrometers and those that use Laser Induced Incandescence (LII) or photoacoustic techniques.
Supplemental Information.zip
Download Zip (110.7 KB)Acknowledgments
[Supplementary Materials are available for this article. Go to the publisher’s online edition of Aerosol Science and Technology to view the free supplementary files.]
Notes
Section 3 and the online Supplementary Materials (Part S1) discuss the error when this assumption does not hold.
For the APM, ω is the speed of the electrodes and in the CPMA ω is the speed of the gas at the mid-point between the electrodes.
REFERENCES
- Biskos , G. , Reavell , K. and Collings , N. 2005 . Unipolar Diffusion Charging of Aerosol Particles in the Transition Regime . J. Aerosol Sci. , 36 : 247 – 265 .
- Cross , E. S. , Onasch , T. B. , Ahern , A. , Wrobel , W. , Slowik , J. G. Olfert , J. 2010 . Soot Particle Studies—Instrument Inter-Comparison—Project Overview . Aerosol Sci. Technol. , 44 : 592 – 611 .
- Ehara , K. , Hagwood , C. and Coakley , K. J. 1996 . Novel Method to Classify Aerosol Particles According to Their Mass-to-Charge Ratio—Aerosol Particle Mass Analyser . J. Aerosol Sci. , 27 : 217 – 234 .
- Fuchs , N. A. 1963 . On the Stationary Charge Distribution on Aerosol Particles in a Bipolar Ionic Atmosphere . Pure Appl Geophys. , 56 : 185 – 193 .
- Liu , B. Y. H. and Pui , D. Y. H. 1974 . A Submicron Aerosol Standard and the Primary, Absolute Calibration of the Condensation Nuclei Counter . J. Colloid Inter. Sci. , 47 : 155 – 171 .
- Olfert , J. S. and Collings , N. 2005 . New Method for Particle Mass Classification—The Couette Centrifugal Particle Mass Analyzer . J. Aerosol Sci. , 36 : 1338 – 1352 .
- Olfert , J. S. , Symonds , J. P. R. and Collings , N. 2007 . The Effective Density and Fractal Dimension of Particles Emitted from a Light-Duty Diesel Vehicle with a Diesel Oxidation Catalyst . J. Aerosol Sci. , 38 : 69 – 82 .
- Park , K. , Cao , F. , Kittelson , D. B. and McMurry , P. H. 2003 . Relationship Between Particle Mass and Mobility for Diesel Exhaust Particles . Environ. Sci. Technol. , 37 : 577 – 583 .
- Taylor , B. N. and Kuyatt , C. E. 1994 . Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results . Tech. Rep. 1297, NIST, Gaithersburg, MD
- Wiedensohler , A. 1988 . An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range . J. Aerosol Sci. , 19 ( 3 ) : 387 – 389 .