Abstract
Experimental and simulation results of sulfuric acid aerosol formation during absorption of sulfuric trioxide in a wet flue gas scrubber (quench cooler) are presented. The complete characterization of the volatile aerosol with respect to number concentration and droplet size distribution is only possible by combining experimental and theoretical methods. Both experiments performed in a pilot plant and simulation results, reveal high number concentrations. A simulation tool predicts the formation of very small droplets that remain even submicron after coagulation in typical residence times of scrubbing systems and thus are difficult to precipitate.
Copyright 2013 American Association for Aerosol Research
INTRODUCTION AND STATE OF THE ART
The formation of aerosols during condensation in the presence of inert gases or absorption of gases with strong acid components leads to operational problems in industrial gas cleaning processes as it has been reported, for example, by Schaber (Citation1995) and Gretscher and Schaber (Citation1999). Huge efforts have to be made to precipitate the small mist droplets. The installed precipitation devices, such as baghouse filters or electrostatic precipitators (ESP), increase the pressure drop and the energy consumption.
During condensation or absorption processes, the gas phase may become supersaturated due to simultaneous heat and mass transfer, and subsequently nucleation and aerosol formation occur. For most strong acids, heterogeneous nucleation on pre-existing foreign nuclei is the dominating mechanism of nucleation in flue gas absorption processes. If sulfuric acid is present in the flue gas, homogeneous nucleation only of sulfuric acid and water itself—without the need of foreign nuclei—is the dominating mechanism as predicted theoretically (Wix et al. Citation2010). The reason for this is the phase behavior of the azeotropic sulfuric acid–water system whose dew point lines (isotherms) exhibit extremely low minimum pressures. These steep declining dew point lines at already very low sulfuric acid concentrations can easily be crossed by the absorption path describing the change of state of the gas phase. This leads to higher degrees of supersaturation and consequently homogeneous nucleation resulting in considerably higher number concentrations and very small (submicron) aerosol droplet diameters. This makes them even more complicated to precipitate.
Sulfuric acid aerosol formation in wet flue gas desulfurization (WFGD) may lead to operational problems such as corrosion but also to a lower absorption rate in the scrubber and possible appearance of a distinct plume outside the stack. This may violate the regulation of opacity, which is valid in the United States. In Europe, the only limiting value so far is the sum of SO2 and SO3 emissions (SOX). Anyway, the formation of aerosols can lead to further problems as other harmful component may absorb on those aerosol droplets and get carried out together with the sulfuric acid aerosol.
During the last years, the problem of sulfuric acid aerosol formation during WFGD for the removal of SO2 from flue gases has been addressed by several authors. Jones and Ellison (Citation1998) reported on the formation of submicron mist of sulfuric acid in the quench section of coal-fired units. Brown and Hohne (Citation2001) described the sulfuric acid aerosol formation in wet caustic scrubbers of a coke calciner. They specified the submicron droplets to be difficult to precipitate and to pass spray towers, packed bed scrubbers and venturi scrubbers. In order to avoid a visible plume, the installation of a wet electrostatic precipitator (WESP) was conducted. Buckley and Altshuler (Citation2002) also reported on sulfuric acid aerosol formation in commercial FGD technology and appraised that 30–60% of the aerosol may exit the stack. Srivastava et al. (Citation2004) gave an overview on SO3 emissions from coal-fired plants and referred the WFGD systems to be inefficient in removing SO3/H2SO4 (∼50%) due to the formation of small droplets. Moretti et al. (Citation2006) specified the removal efficiency to increase when the aerosol particle size increases and stated that the capture of H2SO4 on WFGD systems ranges from 30 to 70%. Cao et al. (Citation2010) performed lab scale tests of coal-fired utility boilers and concluded that the SO3 removal efficiency of the WFGD is generally ∼35% or less.
The problem evolves in particular in the presence of DeNOX-systems with selective catalytic reduction (SCR). This phenomenon has been known since a distinctive “blue plume” has emerged shortly after the new SCR system has been installed at a coal-fired power plant in Ohio (Adams and Constance 2006; EPA Citation2006). The operation of the catalyst leads to higher conversion rates of SO2 to SO3, in addition to the primary conversion of 0.1–1.5% in the furnace (Srivastava et al. Citation2004; Cao et al. Citation2010). Values reported for the conversion rate in the SCR unit range from 0.25 to 1.25% (Srivastava et al. Citation2004).
In the Emergency Planning and Community Right-to-Know Act, the Environmental Protection Agency of the United States (EPA) requires certain facilities to report their environmental releases. Since 1995 the listing includes “sulfuric acid (acid aerosols including mists, vapors, gas, fog, and other airborne forms of any particle size” [EPA Citation1998]). In the EPA report of 2006 (EPA Citation2006), the authors estimate that more than half of the reviewed coal-fired units may require mitigation of SO3 emissions in order to meet the US legislation. They also provided a first evaluation of number concentration and droplet size evolution in a wet scrubber and the following stack. The electric power research institute (EPRI) is aware of this problem and has published three reports since 1994 describing the problem and reporting about different procedural approaches to solve it (EPRI Citation1994, Citation2004, Citation2006).
It is of importance to be able to estimate the number concentration, sizes, and composition of the forming aerosol droplets depending on the operating conditions in order to better understand the characteristics of the aerosol. Within the frame of a collaboration between the Institute for Technical Thermodynamics and Refrigeration (ITTK) and the Konrad-Zuse-Zentrum für Informationstechnik, Berlin, a simulation tool has been developed in order to predict aerosol formation via heterogeneous and homogeneous nucleation (Ehrig et al. Citation2002; Wix et al. Citation2007, Citation2010).
First experimental investigations with sulfuric acid on a pilot plant have been performed by Sinanis et al. (Citation2008). The aerosol droplets had to be enlarged in a second column in order to be measured with the in-situ Three-Wavelength-Extinction-Method (3WEM) (Schaber et al. Citation1994). It revealed that this gives too small number concentrations, as not all droplets can be enlarged in the second column and thus cannot be measured. First experiments with a condensation particle counter (CPC) and a dilution cascade of factor 1000 showed already higher concentrations than with the 3WEM, but the number concentration was higher than the measuring range, so only a lower threshold value could be given.
In this article, simulation results for the conditions of the pilot plant are given and compared with measurements at this plant with a CPC and a dilution factor of 10,000. Additionally, a subsequent polydisperse coagulation calculation is given in order to compare the simulation results with the experiments from the pilot plant. Finally, calculations with the simulation tool for typical WFGD conditions are given and the influence of different conditions is highlighted.
SIMULATION TOOL
The simulation tool AerCoDe (Aerosol Formation in Contact Devices) is based on a spatial one-dimensional modeling that implies the rigorous description of the mass and energy transfer between the gas phase, the liquid phase, and the aerosol droplets in a gas-liquid contact device. In the model, the heat and mass transfer between gas and liquid phase takes place in a separate control volume—the phase interface. Additionally, a cooling phase is provided if heat losses should be included in the calculation. Nucleation and growth are taken into account with the help of source and sink terms. The material and energy balances as well as the transport equations are solved simultaneously. The system of partial differential and algebraic equations is solved with the help of the linearly implicit Euler discretization. Thus, the saturation of the gas phase and the growth of the droplets can be calculated along the interfacial area of a contact device. The equations and the numerical scheme have been described in detail in Ehrig et al. (Citation2002) and are not given here in detail.
For the axis of the interfacial area the number of transfer units (NTU) of the acid is chosen. This can be expressed as the following and corresponds to a dimensionless phase interface assuming a mean mole flow ![]() and a mean mass transfer coefficient βOG
acid that are constant along the interfacial area A:
and a mean mass transfer coefficient βOG
acid that are constant along the interfacial area A:
Most of all scrubbing processes (especially in FGD-processes) are chemisorption processes where the equilibrium concentration of the gas-liquid interface can be assumed to be zero. Then the number of transfer units can be correlated easily to the inlet and outlet concentrations of the acid substance.
This expression is important for the absorption of harmful gases, as it provides a rate of the absorbed amount of the contaminant. Applying this for the spatial axis, any gas-liquid contact apparatus can be modeled with the simulation tool. In order to correlate this with the actual length scale, models for the interface of the particular gas-liquid contact device have to be applied. At present, this is possible for packed columns with AerCoDe.
For heterogeneous nucleation, the simulation tool has been validated already by comparison with experimental data (Ehrig et al. Citation2002; Sinanis et al. Citation2013).
The modeling of homogeneous nucleation is based on the use of different discrete droplet classes that are generated as soon as the critical supersaturation is reached (Wix et al. Citation2010). The droplet classes originate at a certain position in the column with the size of the critical nucleus and the composition based on the nucleation theory used. For the critical degree of supersaturation, which is defined as the supersaturation at which the aerosol formation becomes observable, the nucleation rate has to be larger than 104 l/(cm3s) (Schaber Citation1995). For the calculation of the nucleation rate either the calculation based on the classical nucleation theory like described in Ehrler and Schaber (Citation2013) and Wix et al. (Citation2010) or the use of the parameterization of Vehkamäki et al. (Citation2003) is possible in AerCoDe. The latter is as well based on the classical nucleation theory but considers the lower nucleation rates due to the formation of hydrates in the vapor phase that stabilize the vapor as they reduce the free acid molecules. The parameterization gives nucleation rates as a function of temperature, relative humidity, and the number concentration of sulfuric acid molecules in the gas phase. In the simulation tool the supersaturation along the process path in the column is depleted by the growth of the existing droplet classes, by condensation as well as by the nucleation of more droplet classes.
POLYDISPERSE COAGULATION
Because of the high number concentrations, which are predicted from simulation (see simulation results), coagulation must be considered in order to be able to compare with experimental results. With AerCoDe, no inclusion of coagulation is possible so far for the simulation of homogeneous nucleation. This is owing to the modeling with discrete droplet classes—droplets from different classes coagulate and add up to a new droplet that does not necessarily match an existing class. Thus, multiple new classes would originate at every node of the calculation. At every node 10+5*n equations have to be solved with n being the number of droplet classes. It is obvious that the number of equations to be solved would increase rapidly to an unfeasible number. However, the nucleation is taking place in a small nucleation window in the column and covers only 4–17 cm and 19–75 ms. This is a rather small period of time compared to the entire time in the column (1.6–5%). Thus, coagulation calculations are performed as post-processing using the droplet classes from the previous stand-alone AerCoDe simulations as input data. The conditions change from the end of nucleation to the measurement positions. For the coagulation calculation the data of the end of the column for temperature, density, viscosity, and mean free path are taken as these are the conditions prevailing during most of the coagulation time. A sensitivity analysis using the data from the beginning of the coagulation process shows that this influence is minor and can, therefore, be neglected.
Due to coagulation, the particle size distribution of the aerosol changes according to the following continuous integrodifferential equation (Jacobson Citation1999; Seinfeld and Pandis Citation2006):
Here, n(v,t) is the density function of the number concentration of particles with volume v at time t. The first term describes the formation of particles with volume v through coagulation of two smaller particles; the second term describes the loss of particles with volume v by collision and coagulation with particles of other sizes.
The coagulation kernel Ki,j describes the collision frequency of particles i and j. Different physical mechanisms contribute to this collision frequency, but for this work only Brownian motion has been taken into account. The coagulation kernel in this case is the following:
Di is the Stokes Einstein diffusion coefficient including the Cunningham slip correction CC.
The correction factor β i,j accounts for the transition and free-molecular regime, where the mean free path λ of the gas molecules is in the same size range or even larger than the radius r of the particles, as is the case for the present task. Several formulations are proposed in literature (Dahneke Citation1983; Davies Citation1979) but the most common is still the formulation of Fuchs (Citation1964) which is used as well in this work.
The algorithm of the polydisperse coagulation calculation used for this article is based on the publications of Fernández-Díaz et al. (Citation2000) and Kochenburger (Citation2011) and is described here briefly. The density function q(v) for the volume concentration of particles with volume v at time t is derived from EquationEquation (3) by multiplication by the particle volume. Rearranging the equation, followed by the two steps of size discretization and time integration, leads to a discrete semi-implicit coagulation EquationEquation (7):
Here, the continuous particle size distribution is discretized into a finite number N of particle size classes. EquationEquation (7) calculates the change of the total volume concentration Qk
of the particles in each class k from time t to time t + Δt. Like in EquationEquation (3), the first term inside the parenthesis describes the production and the second term the loss of particles by coagulation. ![]() denotes the mean volume of the particles in the class. γ
i,j,k
is called coagulation coefficient; it contains the coagulation kernel and ensures that only the collisions of the particles from classes i and j are taken into account which form particles that are placed in class k. For the computation of coagulation coefficients and mean volumes, the volume distribution density function is assumed to be a linear function of the particle radius with a different slope in each class, which is recalculated in each time step.
denotes the mean volume of the particles in the class. γ
i,j,k
is called coagulation coefficient; it contains the coagulation kernel and ensures that only the collisions of the particles from classes i and j are taken into account which form particles that are placed in class k. For the computation of coagulation coefficients and mean volumes, the volume distribution density function is assumed to be a linear function of the particle radius with a different slope in each class, which is recalculated in each time step.
Different approaches are possible for time integration. In this work, a semi-implicit method has been used as described in Fernández-Díaz et al. (Citation2000). It is a combination of the explicit method where the change of the density function is calculated with the help of the derivative at the present time t and the implicit method during which the derivative from the next step in the future is taken for the calculation. The first method is fast, but may lead to problems when the time steps are chosen too big. The latter is much more exact but requires time-consuming iteration in order to solve the equation. EquationEquation (7) combines both advantages as it can be resolved into an explicit expression for Q t+Δt k that depends on information about the present (superscript t) and classes with smaller particles (Q t+Δt i with i < k). Hence, by calculating the classes with the smaller particles first, the equation can be solved without any iteration in each time step.
The classes used during the coagulation simulation are the AerCoDe droplet classes with additional classes for bigger particles. At the beginning, the volume concentration in each class is calculated from the number concentrations of the AerCoDe results. Then, the change of the particle size distribution is calculated through repeated application of EquationEquation (7) for time steps of Δt = 1 ms.
EXPERIMENTAL APPARATUS AND METHODS
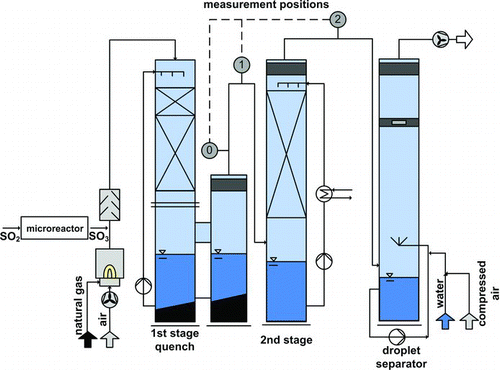
At the Institute for Technical Thermodynamics and Refrigeration, a pilot plant for flue gas cleaning () has been built and enhanced since 1995 in order to investigate the aerosol formation in gas-liquid contact devices (Gretscher and Schaber Citation1999; Wende Citation2003; Sinanis et al. Citation2013). Natural gas is burned in order to provide a flue gas of ∼175 m3(STP)/h. The foreign nucleus concentration due to incomplete burning can be varied between 104 and 106 l/cm3 by earlier/later addition of the air. SO2 is converted in a separate step into SO3. This is realized through a catalytic reaction in a microstructured reactor (Pfeifer et al. Citation2011). Synthetic air and SO2 from gas bottles are mixed with a ratio of 12.5 (O2/SO2 ratio of 2.5). The SO2 flow is adjusted with a mass flow controller, the air with rotameters. The heating cartridges are set that way that the temperature in the entrance is 500°C and descends to 460°C at the outlet. For the applied conditions, a conversion factor of 85% has been estimated with an infrared gas analyzer (Brachert et al. Citation2011). This value has been taken to calculate the sulfuric acid raw gas concentration from the set SO2 flow. This value could not be determined exactly as on the one hand the SO2 concentration in the flue gas is very low (several ppm only) and on the other hand the huge amount of water in the system causes absorption of SO2 which distorts the measurement. However, parameter studies have shown that the variation of this presumed conversion factor does not affect the characteristics of the results essentially which will be addressed later. The SO3 is discharged into the flue gas where it is converted completely into H2SO4 vapor by combining with water vapor from the combustion (Perry Citation1997). The generated flue gas enters a cocurrent (pipe) quench of 300-mm diameter and a height of 1.5 m with 200°C and is rapidly cooled down to the adiabatic saturation temperature (∼46°C). The column is filled with Hiflow-rings (types 20-4 and 25-7). This type of quench has been chosen as the phase interface for packed columns can be described precisely for the simulation tool. The liquid load of the column is 42 m3/(m2h). The column has ∼8 number of transfer units of the acid (NTUacid) and theoretically 99.96% of the acid should be absorbed. In reality, the formation of a sulfuric acid aerosol in the column reduces the absorption rate considerably.
In the second purification stage, the gas enters a countercurrent packed column of 300-mm diameter and 3 m of packing material (Hiflow type 25-7). The liquid load here is 37 m3/(m2h). The circulating liquid can be cooled in an external heat exchanger in order to achieve an additional supersaturation. This mode of operation has not been applied for the experiments of this publication, but will be subject of future investigations. Downstream the second column, an aerosol separator with 300-mm diameter and 6-m height precipitates the formed aerosols in order to clean the gas before exiting the stack. This is realized with a two-component jet by atomizing water with compressed air. In the following growth section, the aerosol droplets attach to the larger water droplets and can be precipitated in the following Multiwir-droplet separator.
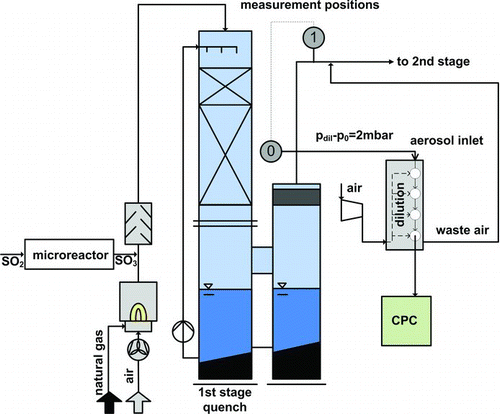
The pilot plant features different measurement access points. Compared to previous experimental investigations, a new measurement position has been used and is labeled with 0. This turned out to be necessary as the expected number concentrations are so high that coagulation reduces rapidly the number concentration. Therefore, it was important to measure the number concentration as close as possible to the outlet of the quench.
A dilution is necessary in order to be able to measure the high number concentration. Here, a dilution cascade from PALAS (DC10000) has been used for the dilution with factor 10,000. The dilution in this ejector system with clean compressed air is only determined by the geometrical dimensions of the ejector jet (Koch et al. Citation1988). In order to get the correct dilution factor, the same pressure at the outlet of the cascade and the mixing zone (= plant pressure at position 0) has to be provided. Because of the pressure drop in the plant, the low pressure to ambient at position 0 is ∼14 mbar. In order to provide a similar pressure level at the outlet of the dilution cascade it is connected to the plant via an access point, a short way downstream the measurement access (). The remaining pressure difference of 2 mbar in the measuring system is tolerable.
Partial evaporation of water and shrinking will occur to the volatile droplets during the dilution. However, due to the low vapor pressure of sulfuric acid, a complete evaporation is obviated. Nevertheless, the detection of the small droplets by the CPC gets even more difficult. This will be addressed later.
The number concentration of the diluted aerosol is measured with a condensation particle counter (CPC) with butanol as working fluid (PALAS UFCPC with sensor 200). For this device, a counting efficiency of 81% for particles with a mobility diameter of 5 nm and 12.2% for 3 nm particles is declared for a saturation temperature of 44°C (METAS Citation2010).
EXPERIMENTAL RESULTS
The range of conditions, at which the experiments have been conducted, can be seen in . Experiments have been performed for sulfuric acid concentrations in the flue gas from 2.6 to 88.2 mg/m3(STP).
TABLE 1 Range of conditions for experiments
FIG. 3 Aerosol number concentration at different measurement positions at pilot plant with low and high foreign nuclei (FN) number concentration. (Color figure available online.)
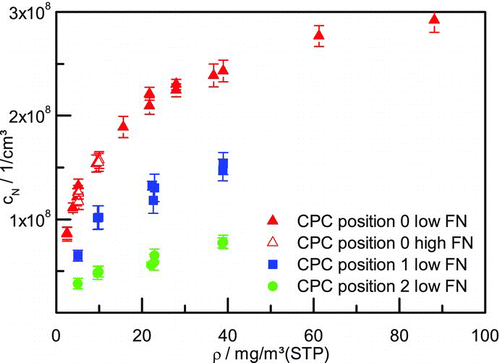
For each measurement point the mean of 5 min has been taken. The standard deviation is indicated in the figures. The resulting number concentrations vary from 8.6 × 107 to 2.9 × 108 l/cm3 at measurement position 0, which is the closest possible to the quench outlet (). Between the quench and this first measurement position the gas flow passes 1-m piping which corresponds to a residence time of 0.9 s. Besides deposition effects, the number concentration of the aerosol reduces relatively fast due to coagulation at this high concentrations. This is further addressed in the section Simulation Results. Position 1 is 6.5 m and 2 s behind position 0 during which only piping is passed. In the meanwhile, the number concentration is reduced further due to deposition and coagulation to values from 6.5 × 107 to 1.5 × 108 l/cm3 at the different sulfuric acid concentrations varying from 5.1 to 38.9 mg/m3(STP). Measurement position 2 is sited after the second packed column. Residence time in the column is 1.3 s. Additionally, 12-m piping is traversed in 3.8 s. Here the measured number concentrations lay between 3.8 × 107 and 7.8 × 107 l/cm3 for the same concentration range as at position 1. All these experiments are performed at low foreign nuclei concentrations of ∼4 × 104 l/cm3. Thereby, the occurrence of homogeneous nucleation due to the extreme phase equilibrium is revealed by the experiments as in the whole range of sulfuric acid concentrations in the flue gas the measured number concentrations exceed the foreign nuclei concentrations by several orders of magnitude. In order to investigate the influence of a higher foreign nuclei level, two experiments at low sulfuric acid concentrations (5.2 and 10 mg/m3(STP)) have been performed at a foreign nuclei level of ∼5 × 106 l/cm3. As it can be seen in , no influence is visible at these conditions. The measured aerosol number concentration by homogeneous nucleation is the same as before, the nucleation is not superimposed observably by heterogeneous nucleation.
SIMULATION RESULTS AND COMPARISON WITH EXPERIMENTAL DATA
Simulations have been performed with the conditions in for six different sulfuric acid concentrations from 5 to 100 mg/m3(STP).
TABLE 2 Conditions for simulations pilot plant
In the results can be seen for the calculation including the parameterization of Vehkamäki et al. (Citation2003) for the nucleation rate. The highest number concentrations of 2.5 × 109 l/cm3 are received for 5 mg/m3(STP). The higher the sulfuric acid concentration, the smaller is the number concentration. However, for 100 mg/m3(STP), still a number concentration of 3.7 × 108 l/cm3 is maintained. This trend can be explained by the two competing mechanisms for the depletion of supersaturation that are nucleation and growth by condensation. The reached supersaturation is extremely high in the whole range of concentrations due to the extreme phase equilibrium of sulfuric acid and water exhibiting a minimum azeotrope with respect to the pressure at isothermal conditions. This leads to high nucleation rates in the whole concentration range. The higher the sulfuric acid concentration, the higher is as well the gradient of partial pressure leading to growth by condensation of sulfuric acid resulting in a faster depletion of supersaturation and less nucleation. Moreover, at 5 mg/m3(STP) the acid concentration in the new-formed droplets is lower than, for example, it is at 100 mg/m3(STP) (68 wt% compared to 77 wt%). This results in a minor lowering of the vapor pressure and, therefore, leads to a reduced growth at lower acid concentrations in the flue gas. Using the classical nucleation theory without considering hydrate formation for the calculation of the nucleation rate, the simulation reveals higher number concentrations changing from 1.6 × 1010 to 109 l/cm3, respectively (Wix et al. Citation2010) but shows the same trend as with the parameterization for hydrate formation.
FIG. 4 Aerosol number concentrations from measurement, simulations from AerCoDe and including coagulation calculations. (Color figure available online.)
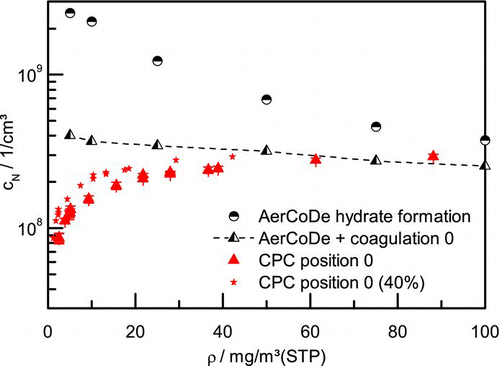
If additional polydisperse coagulation calculations are performed with the droplet classes resulting from the AerCoDe simulations further reduction of the number concentration is predicted before reaching the measurement position 0–2. These predictions can be seen in as half-filled triangles connected with dashed lines. At lower sulfuric acid concentrations, both the higher number concentrations and the smaller droplet sizes lead to faster coagulation. Hereby, the earlier described trend is damped and an almost constant line of number concentrations throughout the whole concentration range is resulting. For example, at position 0 number concentrations from 2.5 × 108 to 4.0 × 108 l/cm3 are predicted. Comparing these values with the actual measured values at position 0, one can see that the calculated values match fine at higher sulfuric acid concentrations. At lower sulfuric acid concentrations, remains still a difference between measurement and simulation even though very high concentrations of 8.6 × 107 l/cm3 are measured even at the lowest sulfuric acid concentrations of 2.6 mg/m3(STP). As reported before, the conversion factor of the microreactor and therewith the actual sulfuric acid concentrations during the experiments could not be determined precisely. In general, a conversion factor of 85% is assumed. In one additional graph with the assumption of a much lower conversion factor (40%) is presented. It can be seen that the general conclusion does not change when comparing with simulation results.
FIG. 5 Cumulative size distributions from simulation and coagulation calculation for 5, 10, and 25 mg/m3(STP). (Color figure available online.)
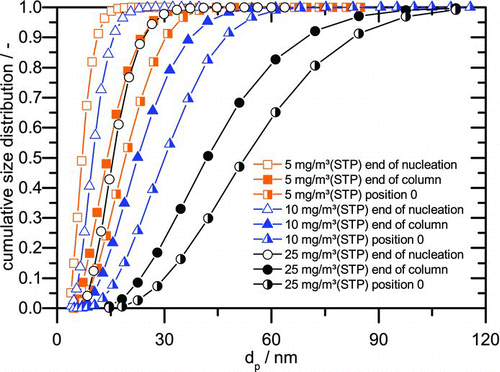
For 5 mg/m3(STP), the AerCoDe simulation predicts an arithmetic mean diameter of 10 nm (when the droplet classes are fit to a lognormal distribution). In the dilution cascade, these droplets shrink further due to the evaporation of water and it may become hard to grow all of them in the condensational particle counter in order to measure them. This becomes obvious when comparing the number concentrations, which are measured when the aerosol is diluted by factor 103 with the measurements with dilution 104. The measurement range of the CPC is reported to be up to 106 l/cm3 so that in principle a dilution of factor 1000 should be sufficient, but in fact more droplets are measured with dilution 10,000. At a lower dilution, more aerosol droplets have to be grown in the condensation part of the CPC and, therefore, the supersaturation in the measurement device is depleted faster. This may cause that the smallest aerosol droplets do not grow enough to be detected by the following optical particle counter. This effect is more pronounced at lower sulfuric acid concentrations where smaller droplets are predicted from the simulation. At 62.7 mg/m3(STP) 11.3% more droplets are counted when measuring with dilution 10,000 whereas at 9.4 mg/m3(STP) even 26% more droplets are detected. This is remarkable as the absolute number concentration at 62.7 mg/m3(STP) is higher. The observed difference in counting efficiency, therefore, can only be explained by the smaller droplets at lower sulfuric acid concentrations. Additionally, no estimation for the depletion by deposition has been done so far which would reduce the expected number concentration from calculations further. As a consequence, the remaining deviation between simulation and experiment at lower sulfuric acid concentrations can be explained by a combination of deposition effects in the pilot plant and by the difficulties of measuring small droplet diameters below 10 nm with a CPC.
The evolution of the droplet classes by means of AerCoDe simulations is shown in and for different concentrations of sulfuric acid in the flue gas. The droplet classes are plotted after the completion of nucleation (nucleation rate drops below 104 l/[cm3s]), at the end of the quench column and at position 0. As an example, the aerosol droplets for 5 mg/m3(STP) with an arithmetic mean diameter of 7.6 nm after nucleation grow to a mean diameter of 15.6 nm at the end of the column by condensation and coagulation. The droplet collective changes further through coagulation up to a diameter of 21 nm at measurement position 0.
FIG. 6 Cumulative size distributions from simulation and coagulation calculation for 5, 50, and 100 mg/m3(STP). (Color figure available online.)
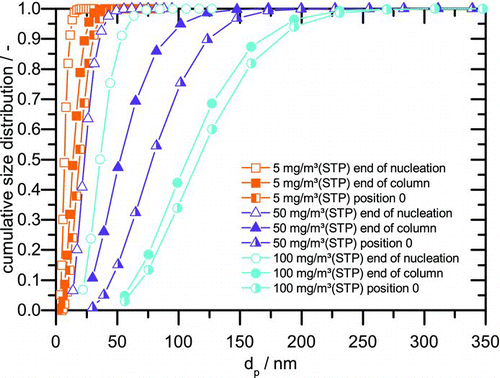
TABLE 3 Conditions for simulations WFGD
TABLE 4 Simulation results for WFGD conditions and after 10 s of coagulation
APPLICATION IN WET FLUE GAS DESULFURIZATION (WFGD) PROCESSES
The problem of aerosol formation evolves frequently during WFGD. In these kinds of applications, the gas inlet temperature is usually lower than that at the pilot plant. Therefore, calculations with gas inlet temperatures of 120°C and 160°C have been performed with AerCoDe. A water content of 0.03 kg/kg inert gas has been chosen as it is prevalent in coal combustion. The used parameters and the resulting number concentrations are summarized in and . The resulting droplet classes without any coagulation calculation have been fit to a lognormal distribution in order to get the arithmetic mean and the standard deviation.
Also for these conditions, high number concentrations are predicted (2.1–3.8 × 109 l/cm3) and the change of the parameters inlet gas temperature and sulfuric acid concentration shows that for different conditions these high number concentrations are preserved. The resulting arithmetic mean diameters are 20.4 and 18.0 nm for 10 mg/m3(STP) at the two different inlet temperatures. At lower sulfuric acid concentrations (3 mg/m3[STP]), the resulting droplet sizes are even smaller (8.6 and 8.2 nm). The aerosol formation results in potential H2SO4 emissions between 0.6 and 3.6 mg/m3(STP) for the different simulations which correspond to separation efficiencies of 64–80%. Due to the small droplet sizes, it may be difficult to precipitate them with common methods as they remain even submicron after coagulation in usual time scales of technical devices (several seconds).
CONCLUSION
The sulfuric acid aerosol formation initiated by homogeneous nucleation during quenching of a humid flue gas has been investigated in experiments and by simulation. Both reveal high number concentrations of >108 l/cm3 over a large range of inlet temperatures and sulfuric acid concentrations in the flue gas. At higher sulfuric acid concentrations, the simulations match very well the experimental results. At lower sulfuric acid concentrations, the remaining gap can be explained by the lower detection efficiency of the CPC at smaller diameters and by deposition. The simulations predict very small droplets diameters. Even after coagulation the resulting droplet collective remains submicron that may result in difficulties to precipitate them. For the present examples for WFGD applications, the droplets sizes grow up to 30–75 nm due to coagulation during typical residence times in scrubbing systems of ∼10 s.
NOMENCLATURE
| A | = |
interface (cm2) |
| CC | = |
Cunningham slip correction |
| cG | = |
molar concentration (mol/cm3) |
| cN | = |
particle number concentration (1/cm3) |
| D | = |
Stokes Einstein diffusion coefficient (cm2/s) |
| Kij | = |
coagulation kernel (cm3/s) |
| kB | = |
Boltzmann constant (cm2kg/(s2K)) |
|
| = |
mass flow (kg/s) |
|
| = |
mole flow (mol/s) |
| n(v) | = |
density function of number concentration of particles with volume v per reference volume (1/(μm3cm3)) |
| NTUOG acid | = |
number of transfer units |
| p | = |
pressure (mbar) |
| Qk | = |
total volume of particles in class k per reference volume (μm/cm3) |
| q(v) | = |
density function of volume concentration of particles (1/cm3) |
| r | = |
radius (μm) |
| T | = |
temperature (K) |
| t | = |
time (s) |
| u | = |
mean velocity of a particle (cm/s) |
| v | = |
particle volume (μm3) |
|
| = |
mean volume of particles (μm3) |
| xi | = |
liquid mole fraction of component i |
| x50 | = |
mean diameter in lognormal distribution (nm) |
| Yi | = |
mass of component i per mass inert gas (kg component i/kg inert gas) |
| yi | = |
gas mole fraction of component i |
Greek Letters
| βOG acid | = |
mass transfer coefficient (cm/s) |
| β i,j | = |
correction factor for transition and free-molecular regime |
| γ | = |
coagulation coefficient (cm3/s) |
| δ | = |
Fuchs correction for absorbing sphere (μm) |
| λ | = |
mean free path (μm) |
| μ | = |
dynamic viscosity of the gas (g/cm*s) |
| ρ | = |
mass concentration (mg/m3) |
| σ | = |
standard deviation in lognormal distribution |
| ξ | = |
mass fraction |
Acknowledgments
The support of the Deutsche Forschungsgemeinschaft (DFG) for this work under Grant SCHA 524/19-2 is gratefully acknowledged.
REFERENCES
- Adams , B. and Constance , Sr. 2006 . Curbing the Blue plume: SO3 Formation and Mitigation . Power , May 15
- Brachert , L. , Riede , R. , Pfeifer , P. and Schaber , K. 2011 . Proportioning of Sulfur Trioxide and Generation of a Test Aerosol with a Microstructured Reactor . Chem. Ing. Tech. , 84 : 524 – 529 .
- Brown , C. A. and Hohne , P. A. 2001 . Eliminating a Sulfuric Acid Mist Plume from a Wet Caustic Scrubber on a Petroleum Coke Calciner . Environ. Prog. , 20 : 182 – 186 .
- Buckley , W. P. and Altshuler , B. 2002 . Acid Mist Causes Problems for FGD Systems . Power Eng. , 11 : 132 – 136 .
- Cao , Y. , Zhou , H. , Jiang , W. , Chen , C. and Pan , W. 2010 . Studies of the Fate of Sulfur Trioxide in Coal-Fired Utility Boilers Based on Modified Selected Condensation Methods . Environ. Sci. Technol. , 44 : 3429 – 3434 .
- Dahneke , B. 1983 . “ Simple Kinetic Theory of Brownian Diffusion in Vapors and Aerosols ” . In Theory of Dispersed Multiphase Flow , Edited by: Meyer , R. E. 97 – 133 . New York : Academic Press .
- Davies , C. N. 1979 . Coagulation of Aerosols by Brownian Motion . J. Aerosol Sci. , 10 : 151 – 161 .
- Ehrig , R. , Ofenloch , O. , Schaber , K. and Deuflhard , P. 2002 . Modelling and Simulation of Aerosol Formation by Heterogeneous Nucleation in Gas–Liquid Contact Devices . Chem. Eng. Sci. , 57 : 1151 – 1163 .
- Ehrler , F. and Schaber , K. 2013 . “ Spontane Kondensation und Aerosolbildung, [Spontaneous condensation and aerosol formation. In German.] ” . In VDI-Waermeatlas , J5 Berlin-Heidelberg : Springer-Verlag .
- EPA . 1998 . EPA - 745 - R - 97 - 007: Emergency Planning and Community Right-To-Know Act – Section 313: Guidance for Reporting Sulfuric Acid , Washington , DC : U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics .
- EPA . 2006 . EPA - 600 - R - 06 - 156: Evaluation and Mitigation of Visible Acidic Aerosol Plumes from Coal Fired Power Boilers , U.S. Environmental Protection Agency, Office of Research and Development .
- Electric Power Research Institute (EPRI) . 1994 . SO3 Mitigation Guide . Technical Report TR-104424s
- EPRI . 2004 . SO3 Mitigation Guide Update. Report No. 1004168
- EPRI . 2006 . SO3 Mitigation: Current Utility Operating Experience . Report No. 1010754
- Fernández-Díaz , J. M. , González-Pola Muñiz , C. , Rodríguez Braña , M. A. , Arganza García , B. and García Nieto , P. J. 2000 . A modified Semi-Implicit Method to Obtain the Evolution of an Aerosol by Coagulation . Atmos. Environ. , 34 : 4301 – 4314 .
- Fuchs , N. A. 1964 . The Mechanics of Aerosols , Oxford : Pergamon Press .
- Gretscher , H. and Schaber , K. 1999 . Aerosol Formation by Heterogeneous Nucleation in Wet Scrubbing Processes . Chem. Eng. Process , 38 : 541 – 548 .
- Jacobson , M. Z. 1999 . Fundamentals of Atmospheric Modeling , Cambridge : Cambridge University Press .
- Jones , C. and Ellison , W. 1998 . SO3 Tinges Stack Gas from Scrubbed Coal-Fired Units . Power , 142 : 73 – 75 .
- Kochenburger , T. 2011 . Study, Modification, and Evaluation of a Semi-Implicit Method for the Simulation of Aerosol Coagulation, Studienarbeit , Institut für Technische Thermodynamik und Kältetechnik, Karlsruhe Institute of Technology and Universidad de Oviedo, Oviedo, Spain .
- Koch , W. , Lödding , H. , Mölter , W. and Munzinger , F. 1988 . Verdünnungssysteme für Die Messung Hochkonzentrierter Aerosole mit Optischen Partikelzählern [Dilution systems for the measurement of high concentrated aerosols with optical particle counters. In German.] . Staub Reinhaltung der Luft. , 48 : 341 – 344 .
- METAS Messbericht (Measurement Report) No. 235-10285 . 2010 . “ METAS Bundesamt für Meteorologie (Federal Institute of Metrology) ” .
- Moretti , A. L. , Triscori , R. J. and Ritzenthaler , D. P. A System Approach to SO3 Mitigation . Presented at the “EPRI-DOE-EPA-AWMA Combined Power Plant Air Pollutant Control Mega Symposium,” August 28–31 .
- Perry , R. H. 1997 . Perry's Chemical Engineers’ Handbook , New York : McGraw-Hill .
- Pfeifer , P. , Zscherpe , T. , Haas-Santo , K. and Dittmeyer , R. 2011 . Investigations on a Pt/TiO2 Catalyst Coating for Oxidation of SO2 in a Microstructured Reactor for Operation with Forced Decreasing Temperature Profile . Appl. Catal. A. , 391 : 289 – 296 .
- Schaber , K. 1995 . Aerosol Formation in Absorption Processes . Chem. Eng. Sci. , 50 : 1347 – 1360 .
- Schaber , K. , Schenkel , A. and Zahoransky , R. A. 1994 . Drei-Wellenlängen-Extinktionsverfahren zur Charakterisierung von Aerosolen unter Industriellen Bedingungen [Three-wavelength-extinction method for the characterization of aerosols in industrial conditions. In German.] . Technisches Messen. , 61 : 295 – 300 .
- Seinfeld , J. H. and Pandis , S. N. 2006 . Atmospheric Chemistry and Physics, , 2nd ed , New Jersey : John Wiley & Sons, Inc. .
- Sinanis , S. , Wix , A. , Ana , L. and Schaber , K. 2008 . Characterization of Sulphuric Acid and Ammonium Sulphate Aerosols in Wet Flue Gas Cleaning Processes . Chem. Eng. Process. , 47 : 22 – 30 .
- Sinanis , S. , Wix , A. and Schaber , K. 2013 . Aerosol Formation During Absorption of HBr and HCl in Wet Flue Gas Cleaning Processes . Chem. Eng. Commun. , 200 : 748 – 763 .
- Srivastava , R. K. , Miller , C. A. , Erickson , C. and Jambhekar , R. 2004 . Emissions of Sulfur Trioxide from Coal-Fired Power Plants . J. Air Waste Manage. Assoc. , 54 : 750 – 762 .
- Vehkamäki , H. , Kulmala , K. and Lehtinen , K. E. J. 2003 . Modelling Binary Homogeneous Nucleation of Water-Sulfuric Acid Vapours: Parameterisation for High Temperature Emissions . Environ. Sci. Technol. , 37 : 3392 – 3398 .
- Wende , B. 2003 . Bildung und Verhalten von Ammoniumchloridaerosolen in Gaswaschanlagen [Formation and Behavior of Ammonium Chloride Aerosols in Gas Cleaning Devices. In German.] Fortschritt-Berichte VDI Reihe 3, Nr 773 , Düsseldorf : VDI Verlag GmbH .
- Wix , A. , Brachert , L. , Sinanis , S. and Schaber , K. 2010 . A Simulation Tool for Aerosol Formation During Sulphuric Acid Absorption in a Gas Cleaning Process . J. Aerosol Sci. , 41 : 1066 – 1079 .
- Wix , A. , Schaber , K. , Ofenloch , O. , Ehrig , R. and Deufelhard , P. 2007 . Simulation of Aerosol Formation in Gas-Liquid Contact Devices . Chem. Eng. Commun. , 194 : 565 – 577 .